Astaxanthin Alleviates Foam Cell Formation and Promotes Cholesterol Efflux in Ox-LDL-Induced RAW264.7 Cells via CircTPP2/miR-3073b-5p/ABCA1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Material
2.2. Cell Cultures
2.3. Oil Red O Staining
2.4. Measurement of Medium Total Cholesterol and Cell Total Cholesterol
2.5. Total RNA Extraction, cDNA Synthesis, and RT-PCR Analysis
2.6. Western Blot Analysis
2.7. RNA Sample Preparation for High-Throughput Sequencing
2.8. Functional Enrichment Analysis
2.9. Differential Expression Analysis of circRNAs
2.10. Target miRNA of circRNAs Prediction
2.11. circTPP2 Knockdown and miR-3073b-5p Mimic/Inhibitor Transfection
2.12. Dual Luciferase Reporter Assay
2.13. Statistical Analysis
3. Results
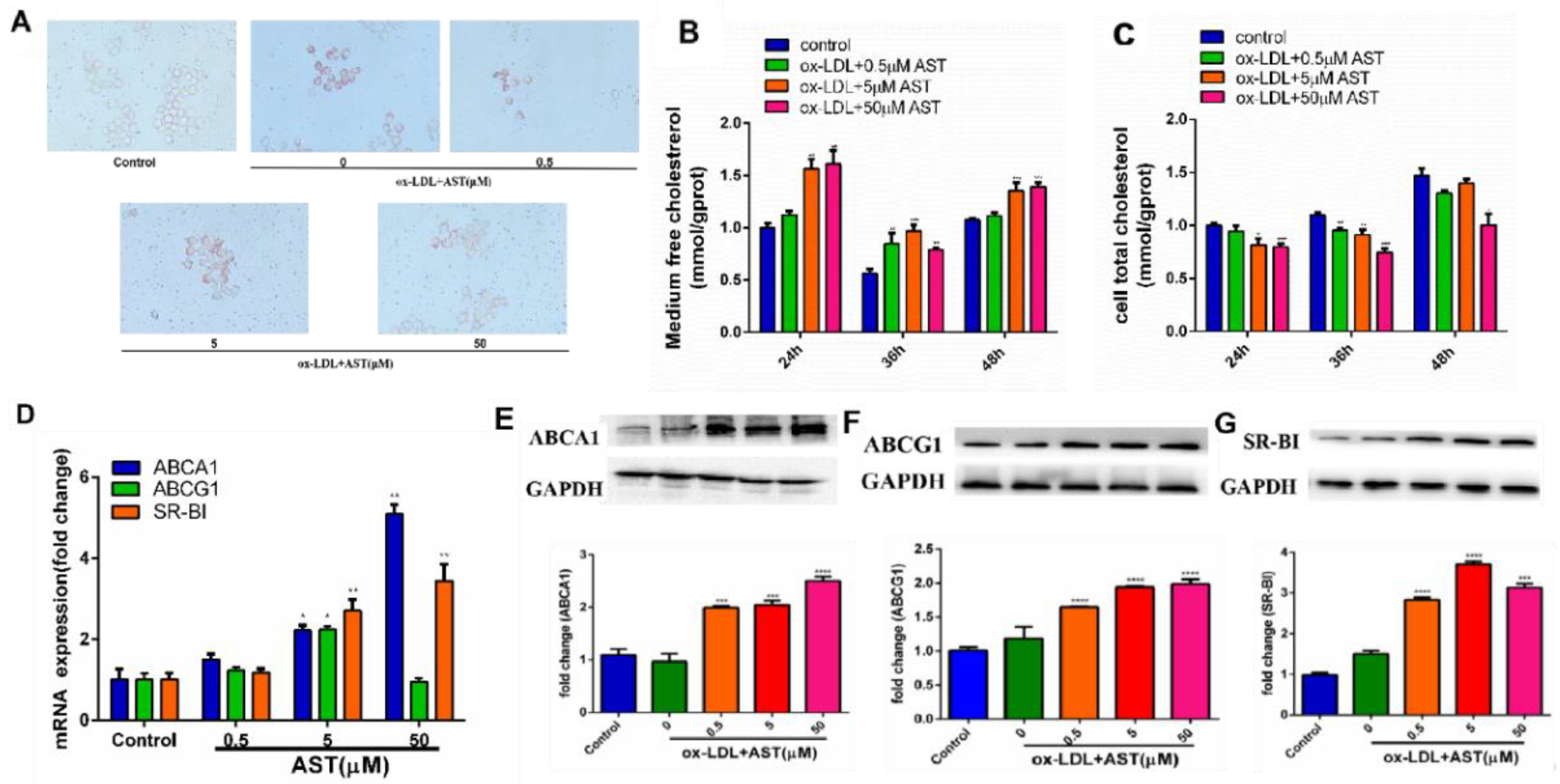
3.1. Effect of AST on ox-LDL-Induced Foam Cell Formation and Promoting Cholesterol Efflux in RAW264.7 Cells
3.2. Differential Expression of circRNAs
3.3. Functional Analysis of Differentially Expressed circRNAs
3.4. Validation of Candidate circRNAs by qRT-PCR
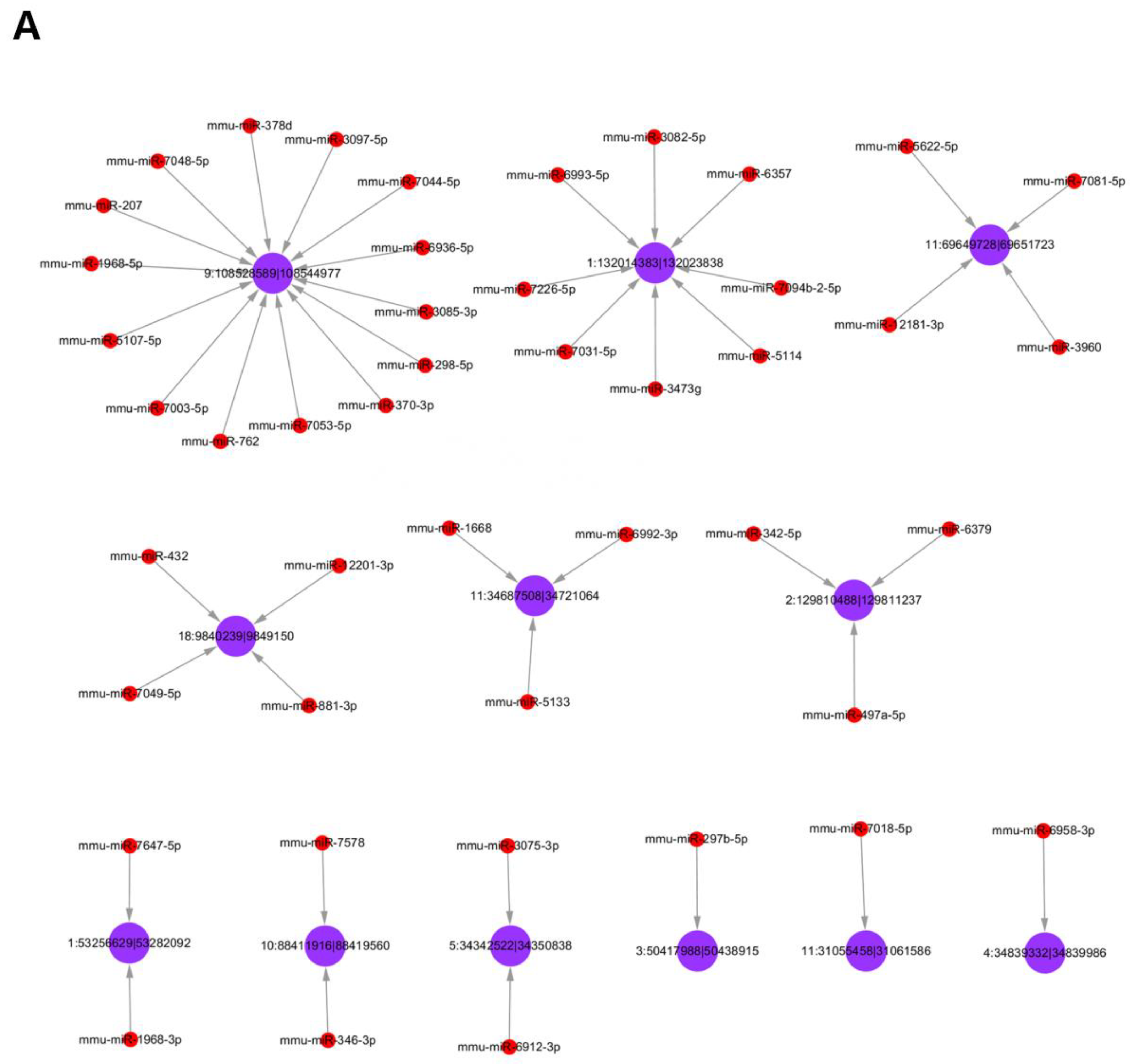
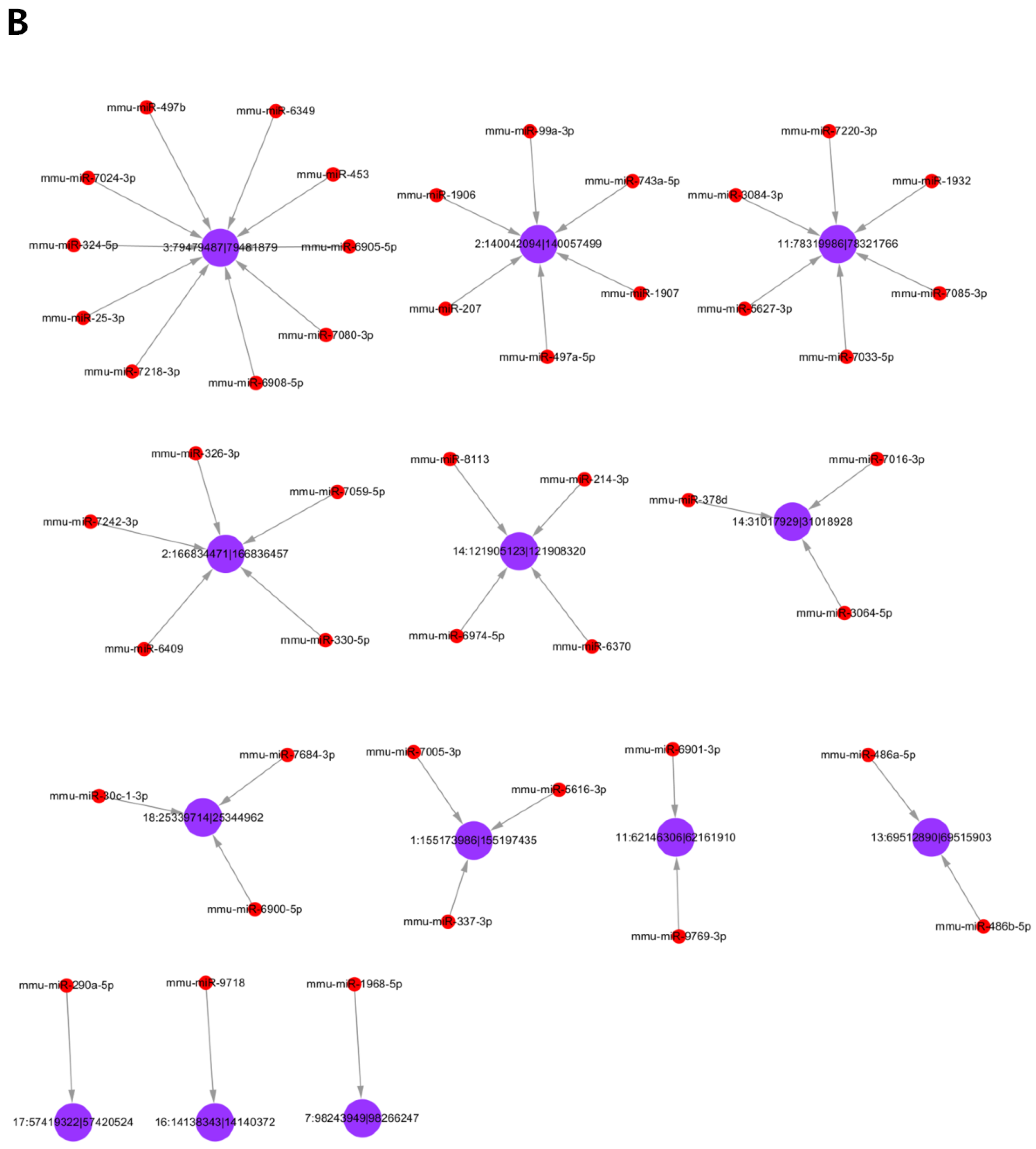
3.5. Analysis of Differentially Expressed circRNAs Targeted miRNAs
3.6. siRNA Interference Screened Out the Most Differentially Expressed circRNAs
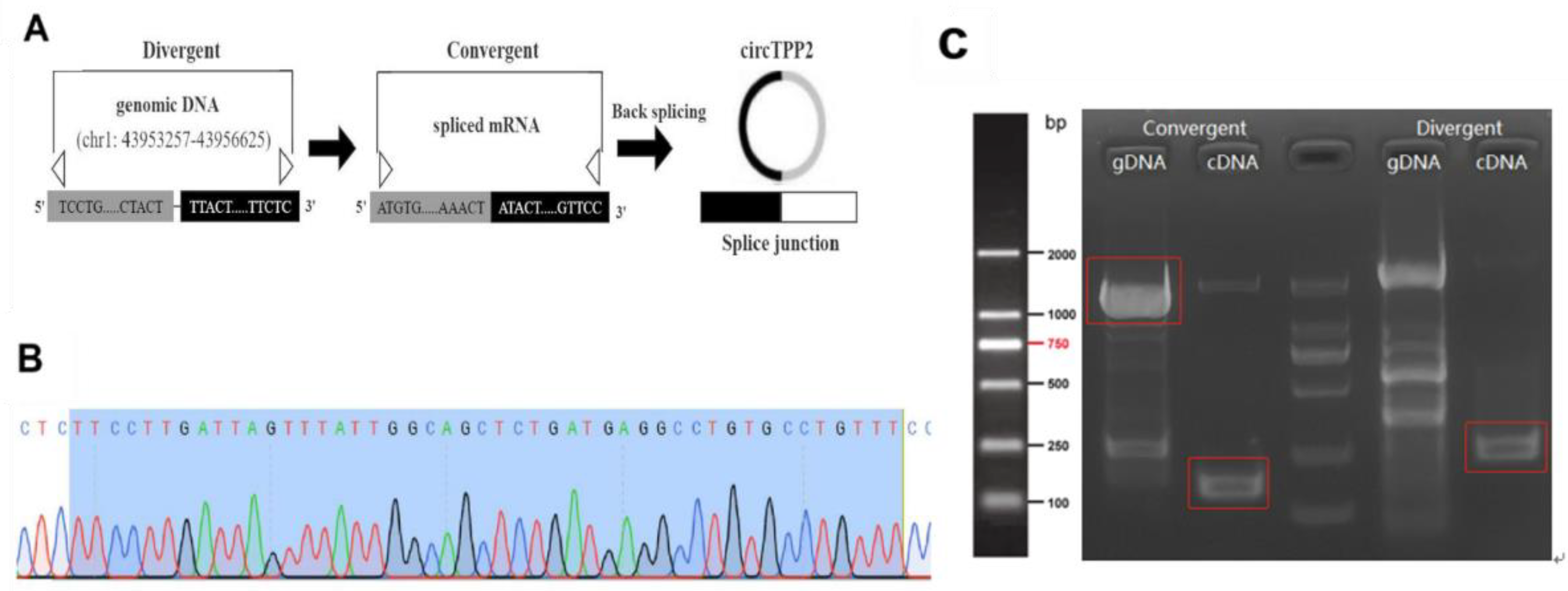
3.7. Identification and Characterization of circTPP2
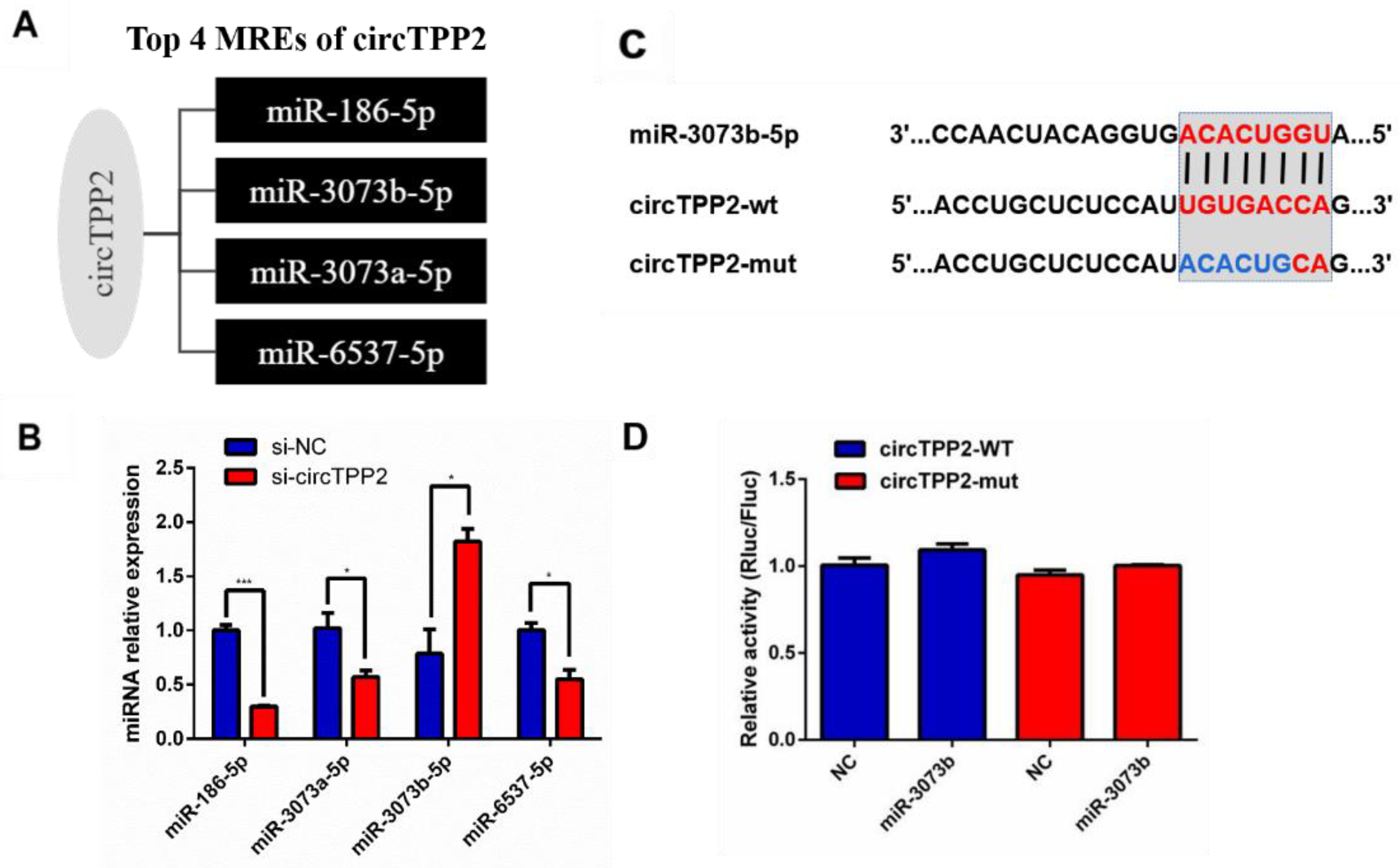
3.8. Validation of Predicted miRNAs Related to circTPP2
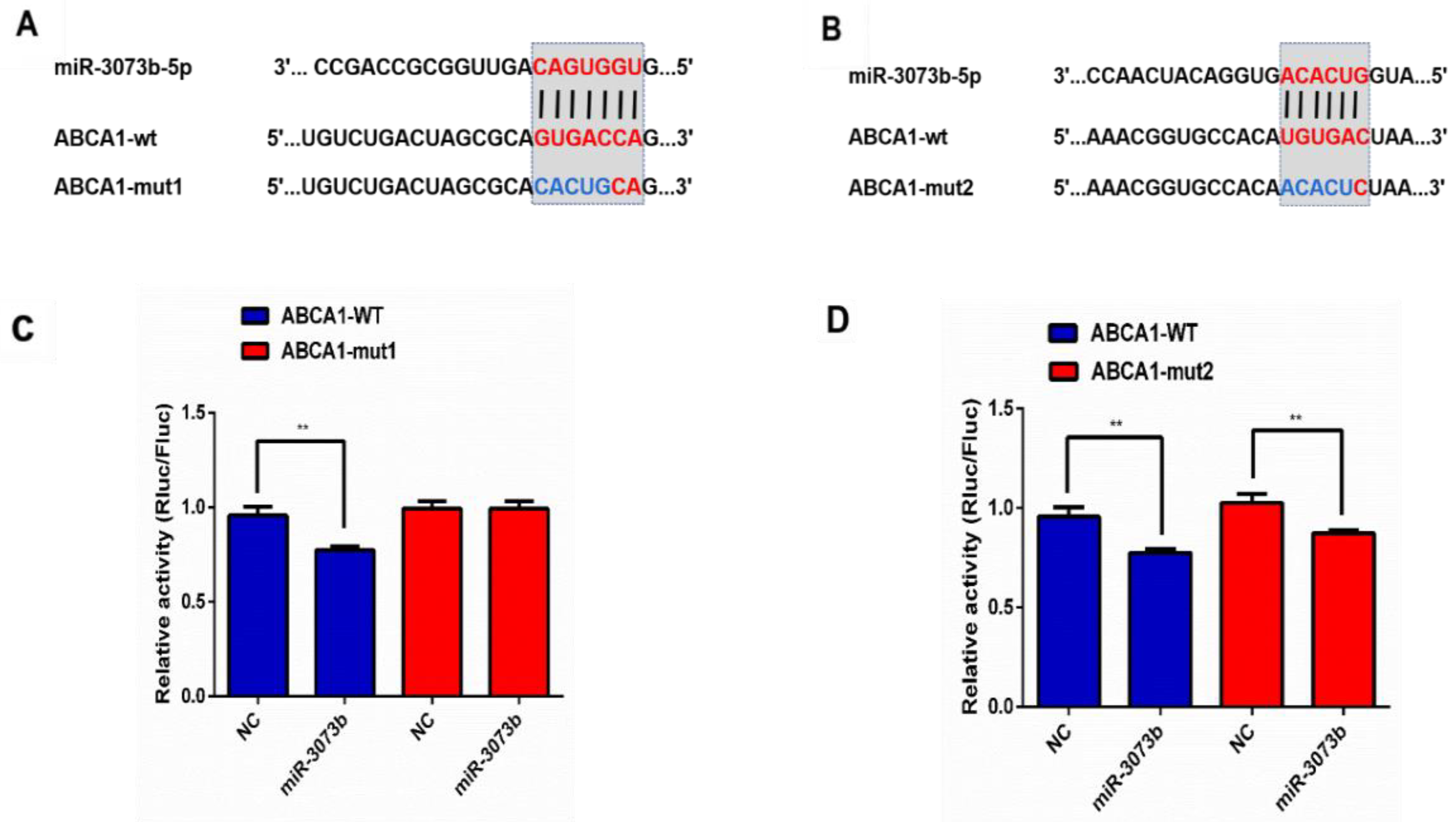
3.9. CircTPP2 Indirectly Bound to miR-3073b-5p to Regulate ABCA1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortesi, P.A.; Fornari, C.; Madotto, F.; Conti, S.; Naghavi, M.; Bikbov, B.; Briant, P.S.; Caso, V.; Crotti, G.; Johnson, C.; et al. Trends in cardiovascular diseases burden and vascular risk factors in Italy: The Global Burden of Disease study 1990–2017. Eur. J. Prev. Cardiol. 2021, 28, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niimi, M.; Watanabe, T.; Wang, Y.; Liang, J.; Fan, J. Treatment of atherosclerosis by traditional Chinese medicine: Questions and quandaries. Atherosclerosis 2018, 277, 136–144. [Google Scholar] [CrossRef]
- Poledne, R.; Lesná, I.K. Inflammation and atherosclerosis. Vnitr. Lek. 2019, 64, 1142–1146. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Meurs, I.; Van Eck, M.; Van Berkel, T.J. High-density lipoprotein: Key molecule in cholesterol efflux and the prevention of atherosclerosis. Curr. Pharm. Des. 2010, 16, 1445–1467. [Google Scholar] [CrossRef]
- Trajkovska, K.T.; Topuzovska, S. High-density lipoprotein metabolism and reverse cholesterol transport: Strategies for raising HDL cholesterol. Anatol. J. Cardiol. 2017, 18, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and reverse cholesterol transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018, 59, 749–763. [Google Scholar] [CrossRef]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A multifunctional receptor in cholesterol homeostasis and atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Agnihotri, N. Role of cholesterol homeostasis and its efflux pathways in cancer progression. J. Steroid Biochem. Mol. Biol. 2019, 191, 105377. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.; Liu, T.; Wang, J.; Dai, W.; Wang, F.; Zheng, Y.; Chen, K.; Li, S.; Abudumijiti, H.; et al. Protective effects of astaxanthin on ConA-induced autoimmune hepatitis by the JNK/p-JNK pathway-mediated inhibition of autophagy and apoptosis. PLoS ONE 2015, 10, e0120440. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364. [Google Scholar] [PubMed]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Karppi, J.; Rissanen, T.H.; Nyyssönen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef]
- Kumar, R.; Salwe, K.J.; Kumarappan, M. Evaluation of antioxidant, hypolipidemic, and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn. Res. 2017, 9, 161–167. [Google Scholar]
- Gasmi, A.; Mujawdiya, P.K.; Shanaida, M.; Ongenae, A.; Lysiuk, R.; Doşa, M.D.; Tsal, O.; Piscopo, S.; Chirumbolo, S.; Bjørklund, G. Calanus oil in the treatment of obesity-related low-grade inflammation, insulin resistance, and atherosclerosis. Appl. Microbiol. Biotechnol. 2020, 104, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M.; et al. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. 2012, 58, 96–104. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, E.; Rojas, A.M.; Andrés-León, E. RNA sequencing and prediction tools for circular RNAs analysis. Adv. Exp. Med. Biol. 2018, 1087, 17–33. [Google Scholar]
- Geng, X.; Jia, Y.; Zhang, Y.; Shi, L.; Li, Q.; Zang, A.; Wang, H. Circular RNA: Biogenesis, degradation, functions and potential roles in mediating resistance to anticarcinogens. Epigenomics 2020, 12, 267–283. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Sun, H.S.; Tsai, S.J. Circular RNA-new member of noncoding RNA with novel functions. Exp. Biol. Med. 2017, 242, 1136–1141. [Google Scholar] [CrossRef]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.; Zhang, J.; Zheng, L. Biogenesis and functions of circular RNAs and their role in diseases of the female reproductive system. Reprod. Biol. Endocrinol. 2020, 18, 104. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Panda, A.C.; Munk, R.; Grammatikakis, I.; Dudekula, D.B.; De, S.; Kim, J.; Noh, J.H.; Kim, K.M.; Martindale, J.L.; et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by circPABPN1. RNA Biol. 2017, 14, 361–369. [Google Scholar] [CrossRef]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Foam cells as therapeutic targets in atherosclerosis with a focus on the regulatory roles of non-coding RNAs. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Lin, Y.; Zhang, P.W.; Zhang, Z.X.; Huang, H.R.; Wu, H.F.; Zou, T.B. Expression of the circular RNAs in astaxanthin promotes cholesterol efflux from THP-1 cells based on RNA-seq. Genes Nutr. 2021, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; Zhu, S.S.; Luo, F.; Li, W.Q.; Sun, X.R.; Wu, H.F. Effects of astaxanthin on reverse cholesterol transport and atherosclerosis in mice. Biomed Res. Int. 2017, 2017, 4625932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, H.; Zhang, Y.; Wu, F.; Mu, Q.; Jiang, M.; Wang, F.; Zhang, W.; Li, L.; Shao, L.; et al. Irisin inhibits atherosclerosis by promoting endothelial proliferation through microRNA126-5p. J. Am. Heart Assoc. 2016, 5, e004031. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, J.; Li, D.; Yu, S.; Ke, J.; Wang, L.; Wang, Y.; Zhang, J.; Huang, L. Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in ox-LDL exposure. Autophagy 2017, 13, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lin, H.Y.; Chan, Y.W.; Li, K.H.; To, O.T.; Yan, B.P.; Liu, T.; Li, G.; Wong, W.T.; Keung, W.; et al. Mouse models of atherosclerosis: A historical perspective and recent advances. Lipids Health Dis. 2017, 16, 12. [Google Scholar] [CrossRef]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q. Cholesterol and lipoprotein metabolism and atherosclerosis: Recent advances in reverse cholesterol transport. Ann. Hepatol. 2017, 16 (Suppl. S1), S27–S42. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Apoprotein E and reverse cholesterol transport. Int. J. Mol. Sci. 2018, 19, 3479. [Google Scholar] [CrossRef]
- Yu, X.H.; Zhang, D.W.; Zheng, X.L.; Tang, C.K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res. 2019, 73, 65–91. [Google Scholar] [CrossRef]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat Biotechnol 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, C.; Braun, S.; Domdey, H. In vitro generation of a circular exon from a linear pre-mRNA transcript. Nucleic Acids Res. 1996, 24, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.; Luo, H.; Zhu, X. Circular RNAs: The star molecules in cancer. Mol. Asp. Med. 2019, 70, 141–152. [Google Scholar] [CrossRef]
- Tomkinson, B. Tripeptidyl-peptidase II: Update on an oldie that still counts. Biochimie 2019, 166, 27–37. [Google Scholar] [CrossRef]
- Tomkinson, B.; Lindås, A.C. Tripeptidyl-peptidase II: A multi-purpose peptidase. Int. J. Biochem. Cell Biol. 2005, 37, 1933–1937. [Google Scholar] [CrossRef]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar] [CrossRef]
- Bortnick, A.E.; Rothblat, G.H.; Stoudt, G.; Hoppe, K.L.; Royer, L.J.; McNeish, J.; Francone, O.L. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J. Biol. Chem. 2000, 275, 28634–28640. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bao, H.; Zhang, S.; Li, R.; Chen, L.; Zhu, Y. miR-186-5p promotes apoptosis by targeting IGF-1 in SH-SY5Y OGD/R model. Int. J. Biol. Sci. 2018, 14, 1791–1799. [Google Scholar] [CrossRef] [PubMed]


| Name | Primer Sequences (5′→3′) |
|---|---|
| GAPDH | F: AAGAAGGTGGTGAAGCAGG |
| R: GAAGGTGGAAGAGTGGGAGT | |
| ABCA1 | F: CCAGAAGGGAGTGTCAGAAAT |
| R: GGGAAACAGCCCAGTCAGTA | |
| ABCG1 | F: GAACTGCCCTACCTACCACAAC |
| R: AAAGAAACGGGTTCACATCG | |
| SR-BI | F: ACCCTAACCCAAAGGAGCAT |
| R: CCACAGCAACGGCAGAACTA | |
| U6 | F: CTCGCTTCGGCAGCACATATACT |
| R: ACGCTTCACGAATTTGCGTGTC | |
| circZfp710 | F: GGACAGCCAAGAAAUGCCCTT |
| R: GGGCAUUUCUUGGCUGUCCTT | |
| circTPP2 | F: CAGAGCUGCCAAUAAACUATT |
| R: UAGUUUAUUGGCAGCUCUGTT | |
| circSclt1 | F: GAAAACAUCUUAGAACAGCTT |
| R: GCUGUUCUAAGAUGUUUUCTT | |
| miR-6537-5p | F: CGCGGTGAGTTTCTCCCACT |
| R: AGTGCAGGGTCCGAGGTATT | |
| 18s RNA | F: GTAACCCGTTGAACCCCATT |
| R: CCATCCAATCGGTAGTAGCG | |
| miR-186-5p | F: CGGGCCAAAGAATTCTCCTT |
| R: CAGCCACAAAAGAGCACAAT | |
| miR-3073a-5p | F: CGGGCGTGGTCACAGTTGGC |
| R: CAGCCACAAAAGAGCACAAT | |
| miR-3073b-5p | F: CGGGCATGGTCACAGTGGAC |
| R: CAGCCACAAAAGAGCACAAT |
| circRNA ID | circBase ID | log2FC | Gene |
|---|---|---|---|
| 7:80081049|80086608 | — | 4.392317423 | Zfp710 |
| 19:16710271|16725693 | — | 4.087462841 | Vps13a |
| 1:43953257|43956625 | — | 3.906890596 | TPP2 |
| 5:100805898|100812202 | mm10_circ_001838 | 3.906890596 | Abraxas1 |
| 17:5886997|5899495 | — | 3.906890596 | Snx9 |
| 1:185266901|185272642 | — | −4.857980995 | Rab3gap2 |
| 2:158035996|158058827 | mm10_circ_016411 | −4.754887502 | Rprd1b |
| 19:37325148|37330612 | — | −4.523561956 | Ide |
| 9:86422841|86448853 | — | −4.392317423 | Ube2cbp |
| 3:41703386|41730919 | — | −4.087462841 | Sclt1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Qiu, Y.; Li, W.; Tang, A.; Huang, H.; Yao, W.; Li, H.; Zou, T. Astaxanthin Alleviates Foam Cell Formation and Promotes Cholesterol Efflux in Ox-LDL-Induced RAW264.7 Cells via CircTPP2/miR-3073b-5p/ABCA1 Pathway. Molecules 2023, 28, 1701. https://doi.org/10.3390/molecules28041701
Zhang Z, Qiu Y, Li W, Tang A, Huang H, Yao W, Li H, Zou T. Astaxanthin Alleviates Foam Cell Formation and Promotes Cholesterol Efflux in Ox-LDL-Induced RAW264.7 Cells via CircTPP2/miR-3073b-5p/ABCA1 Pathway. Molecules. 2023; 28(4):1701. https://doi.org/10.3390/molecules28041701
Chicago/Turabian StyleZhang, Zhexiao, Yunmei Qiu, Wanzhi Li, Anyang Tang, Hang Huang, Wanyi Yao, Huawen Li, and Tangbin Zou. 2023. "Astaxanthin Alleviates Foam Cell Formation and Promotes Cholesterol Efflux in Ox-LDL-Induced RAW264.7 Cells via CircTPP2/miR-3073b-5p/ABCA1 Pathway" Molecules 28, no. 4: 1701. https://doi.org/10.3390/molecules28041701
APA StyleZhang, Z., Qiu, Y., Li, W., Tang, A., Huang, H., Yao, W., Li, H., & Zou, T. (2023). Astaxanthin Alleviates Foam Cell Formation and Promotes Cholesterol Efflux in Ox-LDL-Induced RAW264.7 Cells via CircTPP2/miR-3073b-5p/ABCA1 Pathway. Molecules, 28(4), 1701. https://doi.org/10.3390/molecules28041701




