Abstract
One of the challenges in developing practical CO2 photoconversion catalysts is the design of materials with a low cost, high activity and good stability. In this paper, excellent photocatalysts based on TiO2, WO3, ZnO, Cu2O and CeO2 metal oxide materials, which are cost-effective, long-lasting, and easy to fabricate, are evaluated. The characteristics of the nanohybrid catalysts depend greatly on their architecture and design. Thus, we focus on outstanding materials that offer effective and practical solutions. Strategies to improve CO2 conversion efficiency are summarized, including heterojunction, ion doping, defects, sensitization and morphology control, which can inspire the future improvement in photochemistry. The capacity of CO2 adsorption is also pivotal, which varies with the morphological and electronic structures. Forms of 0D, 1D, 2D and 3DOM (zero/one/two-dimensional- and three-dimensional-ordered macroporous, respectively) are involved. Particularly, the several advantages of the 3DOM material make it an excellent candidate material for CO2 conversion. Hence, we explain its preparation method. Based on the discussion, new insights and prospects for designing high-efficient metallic oxide photocatalysts to reduce CO2 emissions are presented.
1. Introduction
According to the report from Carbon Emission Accounts and Datasets for emerging economies (CEADs), China has emitted more than 10 billion tons of CO2 per year since 2018. It is inextricably linked to people’s desire for a more comfortable and convenient life. Nearly 60% of total emissions emanated from the production of cement, steel and coal-fired power generation processes. As a result of the greenhouse effect caused by ever-growing CO2, the global average temperature is continuously increasing [1] and various types of severe weather will incur more frequently, such as ice melting, land drought, typhoons, hurricanes, earthquakes and tsunamis [2]. It is, therefore, imperative to convert and utilize the CO2 present in the atmosphere.
The transformation of CO2 can be driven by illumination, electricity and heat [3]. Solar energy is a safe, clean, renewable and inexhaustible energy source, so it is ingenious to achieve this conversion with sunlight [4]. Additionally, the reaction conditions in photocatalytic processes are mild [5]; thus, it is easy to conduct experimental tests. A new world opened up since H2 and O2 were obtained after radiating TiO2 with light. Extensive research about photocatalyst were conducted until the year of 2000. Since then, a growing number of materials have been designed and studied to absorb solar energy, including oxide semiconductors, sulfides (ZnS, CdS and MoS2), and polyoxometalates (Bi2WO6, Bi2MoO6 and BiFeO3) [6,7]. Organics, organometallic complexes, covalent organic polymers and noncovalent self-assembled supramolecular organic matter are also involved. Among them, inorganic metal oxide materials are widely studied for establishing efficient artificial photosystems due to their low cost, facile synthesis, stable crystal structures and environmental friendliness. TiO2, Cu2O, ZnO, WO3 and CeO2 show promising research value as the most common materials.
Despite the significant progress that has been achieved, researchers still have difficulties in developing highly active catalysts because of poor light-harvesting capacity, low CO2 adsorption capacity and rapid recombination of charge carriers. To this end, theoretical foundation and strategies to upgrade photocatalysts are described in detail in this article. Firstly, the advantages of exposed facets adjustment, and the morphology of 0D, 1D, 2D and 3DOM materials are summarized. Then, common co-catalyst materials are introduced. Defects, ion doping and sensitization engineering are also discussed. We provide a basic understanding on these approaches to inspire the future improvement in photocatalytic field. Furthermore, as inorganic metal oxide materials are more suitable for large-scale production, this paper can serve as a model for future industrialization.
2. Theoretical Foundation and Strategies of Photocatalytic CO2 Reduction
The photocatalytic process in semiconductors can generally be described as shown in Figure 1. Upon being excited by an incident photon with energy equal to or higher than the bandgap (Eg), charge carriers are generated. Electrons (e−) at the bottom of conduction band (CB) migrate to the surface of the catalyst to initiate reduction reactions with CO2. Holes (h+) at the top of valence band (VB) conduct oxidative reactions.
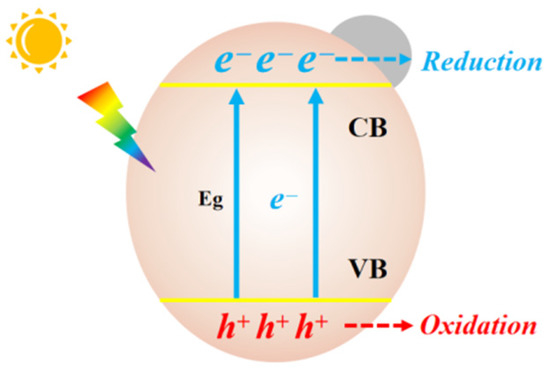
Figure 1.
Fundamentals of photocatalytic reduction on a semiconductor catalyst.
The photocatalytic process of CO2 conversion on semiconductors can be divided into five general steps: (1) Formation of electron–hole pairs under light radiation, (2) Separation and migration of the electrons and positive holes, (3) Adsorption and activation of CO2, (4) Redox reactions that between surface-adsorbed species and electron–hole pairs, and (5) Desorption of the product. The electrochemical reactions with standard oxidation–reduction potentials (at pH 7 vs. NHE) are as follows:
H2O + h+ → OH• + H+
H+ + e− → H•
H• + H• → H2
CO2 + e− → CO2−
CO2 + 2H+ + 2e− → HCOOH, E0 = −0.61 V
CO2 + 2H+ + 2e− → CO + H2O, E0 = −0.53 V
CO2 + 4H+ + 4e− → HCHO + H2O, E0 = −0.48 V
CO2 + 6H+ + 6e− → CH3OH + H2O, E0 = −0.38 V
CO2 + 8H+ + 8e− → CH4 + 2H2O, E0 = −0.24 V
To carry out the conversion, it is necessary to comprehend the structure of CO2. Due to the great symmetry and high bond energy of 750 kJ/mol, it is particularly difficult to break the C=O double bond. Therefore, the transmutation between CO2 and bent radical anion of CO2•− on the surface of the catalyst is widely recognized as the first step to activate CO2 for subsequent reaction. Additionally, photoreactions occur favorably only when the CB position of the catalyst presents a more negative potential than the target reduction and the VB position is more positive than the oxidation reaction. Figure 2A displays the CO2 reduction potentials of some common semiconductors, along with the Eg positions. From a molecular perspective, the adsorbed CO2 is combined with e−, H+ or other intermediates on the surface of the catalyst. On the basis of newly-released reports, the photocatalytic mechanism of CO2 reduction to CH4/CO is graphically explained in Figure 2B [8,9,10].
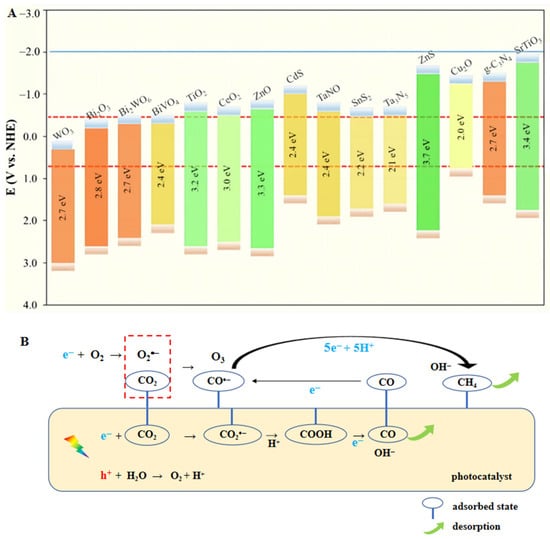
Figure 2.
(A) Band edge positions of various semiconductors in relation to the redox potential of different products at pH = 7 [11]. (B) Schematic illustration of the mechanism for CH4/CO generation.
Therefore, enough e− and H+ are requisites for the entire photoreduction process. A series of fuels can be obtained by means of well-designed catalysts, such as CH4 [12,13], CH3OH [14] and HCOOH [15]. It makes sense to create valuable solar fuel from CO2, which provides a solution to environmental pollution. Products, alcohols, hydrocarbons and even carbon monoxide can be used as feedstock for energy reserves or high-value compounds.
Figure 3 summarizes the current strategies that can boost the photocatalytic CO2 reduction pace, with detailed information being presented sequentially below.
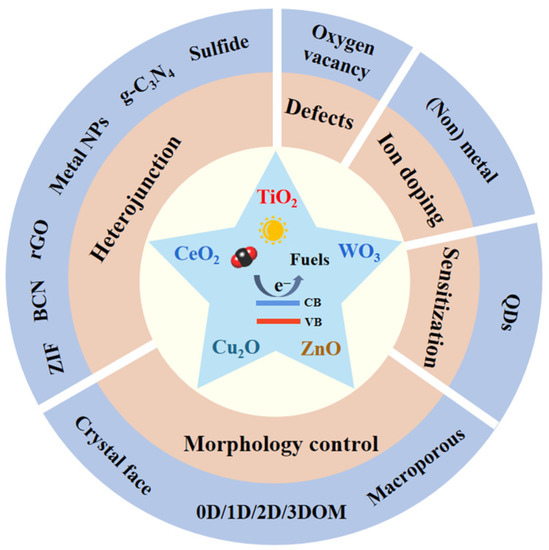
Figure 3.
Strategies to boost the photocatalytic CO2 reduction.
2.1. Morphology Control
The surface topography of nanocrystals could evidently alter the electronic structure, surface energy and chemical properties of catalysts. Therefore, morphology control is one of the most important issues that concerns researchers in nanoscience, chemistry and physics. Open facets and edges determine the shape of nanocrystals. Thus, the preferential adsorption of additives on certain crystal surfaces provides a good opportunity to tune the surface of nanomaterials.
2.1.1. Exposed Facet Adjustment
Exploring and figuring out the variation that is connected with exposed surfaces is crucial to elucidate shape-related chemical and physical properties. In semiconductor crystals, different facets have distinct electronic band structures that influence the transport of photoexcited carrier charges. It is wise to expose active facets to tune the CO2 photoreduction efficiency [16].
2.1.2. Quantum Dots (QDs)
Zero-dimensional (0D) semiconductor quantum dots (QDs) have many unique properties, such as quantum confinement effect, high extinction coefficient and multiple exciton generation [17]. Hence, QDs show much better photoactivity in the visible light region. Unlike bulk materials, surface atoms make up the majority of QD semiconductors. The abundant surface sites enhance the interaction between electron donors and acceptors, thus facilitating the photocatalytic charge transfer rate.
2.1.3. One-Dimensional and Two-Dimensional Structures
One-dimensional nanostructured catalysts have high aspect ratios, such as nanowires, nanorods and nanotubes. The morphological tuning of the material makes a difference to their thermal, optical, electrical and magnetic properties [18]. For instance, the TiO2 nanotube can act as a channel for electron transfer and build up the chemical reactions rate.
Two-dimensional layered materials can protect a tiny particle component from aggregating. The CO2 adsorption capacity on the surface of 2D photocatalysts can be enhanced due to the large specific surface area and bountiful surface defects [19]. Two-dimensional lamellar nanosheets are widely used in photocatalysts, such as g-C3N4, MoS2 and WO3.
2.1.4. Macroporous and Three-Dimensional Ordered Macroporous (3DOM) Structures
Macroporous materials are being widely used in massive photocatalytic materials, owing to their excellent properties [20]. Unlike dispersed particles, sunlight can penetrate the pore wall easily and scatter widely inside the hollow structure, thus increasing the efficiency of illumination. Subsequently, the slender walls of pore reduce the transfer length of photo-generated charge carriers. Electrons (e−) and holes (h+) are separated more efficiently when heterojunctions are loaded on porous materials. The specific surface it provides is so large that more CO2 molecules have a chance to contact the catalyst for reduction reactions.
Growing attention has been paid to hierarchical composite pores, including photonic crystal catalysis and separation of sub-microns. The slow light effect of photons associated with 3DOM materials have been considered to increase solar radiation absorption and enhance photocatalyst performance [21]. 3DOM products with periodic macrostructures [22], known as inverse opal, have been applied in battery materials, sensors, separation engineering and heterogeneous catalysis. Thanks to the periodic dielectric constants, Bragg diffraction permits certain wavelengths of light to radiate, leading to stop-band reflection. The limited photons reflected back will slow down at the edge of the stop bands, from which it received its name of “slow photons” [23,24]. It accelerates the light absorption rate when the photon energy is consistent with the absorption spectrum of the 3DOM materials [25]. In addition, the light scattering properties are optimized due to the relation between the macrospores and pore walls. For example, a 3D macro mesoporous Mo: BiVO4 architecture [26] was designed and fabricated, which had a superior photocurrent density.
It has been demonstrated that gas absorption and separation functions benefit from orderly porous architectures [27]. 3DOM chemicals show superior CO2 absorption/desorption rates than other commercial products, as the special structure can capture CO2 from the ambient air by utilizing humidity variation. It is a great way to use microporous catalysts because it not only increases visible light absorption but also shortens the transport time of CO2. They are available at a low cost through simple and rapid methods.
2.1.5. Preparation of 3DOM Materials
Specifically, 3DOM materials are produced by the colloidal crystal template (CCT) method (Figure 4). A uniform and close-connected organic sphere template can be obtained, firstly, through three key processes. Then, seep metallic salt sol into the void of microspheres. After heat treatment, the organic microsphere template fades away and leaves a metal oxide frame. (i) The polymerizable monomer (methyl methacrylate, styrene) and initiator are mixed and heated under the protection of Ar; (ii) the earlier reaction liquid is filtered with microfiltration membrane; (iii) the microsphere mixture is centrifuged at a high speed for a long time, yielding the polymethylmethacrylate (PMMA) or polystyrene (PS) template; (iv) the template is immersed in the precursor solution and (v) is calcinated.
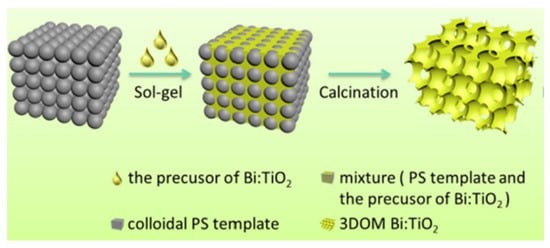
Figure 4.
The fabrication process of the 3DOM Bi-doped TiO2 [26].
In the absence of ultra-high temperature and expensive equipment, a fantastic 3DOM structure was obtained. Various materials of frame can also be synthesized by altering the precursor formula.
2.2. Heterojunction
Photogenerated charge carriers in a single material tend to recombine rapidly due to coulombic force and they can be separated efficiently in the multi-materials that are in close contact [28]. The heterojunction is an interface between various semiconductors with different energy band structures. The heterojunction structure can reduce the probability of charge recombination and is therefore considered an effective method to enhance the photocatalytic activity [29,30].
2.3. Defects
All semiconductors have surface defects, which are rooted in the absence of host atoms. The amount of oxygen vacancies can be altered by ion doping and nanocrystal modifications. Oxygen defects and other defects originating from the generated vacancy [31] can facilitate the separation of h+/e−. Over and above, defects can also activate CO2 molecules, reducing the activation energy of the reaction [32].
2.4. Ion Doping
Elemental doping is a common strategy to modulate the surface electronic structure. Then, the band gap of the semiconductors make a difference, nonmetal doping (such as N, C and O) mainly alters the VB and metal-doping (e.g., Mo, Co and Ni) influences the CB [33,34]. For example, Cu-doped TiO2 absorbs more visible light due to the Cu 3d-Ti 3d optical transition [35].
2.5. Sensitization
Sensitization means coupling the quantum dots, dyes, etc., with semiconductors to increase the photogenerated carriers and promote the absorption of light by taking advantage of their receptivity in UV, visible or infrared light.
In the following, exuberant measures to optimize TiO2-, WO3-, ZnO-, Cu2O- and CeO2-based photocatalysts for CO2 reduction are demonstrated.
3. Photocatalysts with Different Basis Matrices
3.1. TiO2-Based Photocatalysts
Titanium dioxide (TiO2) is notable in photochemistry, with advantages such as non-toxic, cheapness, corrosion resistant, good physical and chemical stability. However, owing to the wide Eg of 3.0–3.2 eV, TiO2 only absorbs energy in the ultraviolet region (3–5% of the solar energy), and photoexcited charge pairs are easy to combine, resulting in quantum inefficiency. Usually, TiO2 is divided into the rutile phase and anatase phase on the basis of atomic arrangement modes. Rutile TiO2 is thermodynamically stable and does not distort or decompose at high temperatures. It has a narrower energy gap (3.0 eV) and a wider spectral response than the anatase phase (3.2 eV). Rutile TiO2 seeds generally grow larger in size and tend to form an agglomerated structure. Smaller anatase TiO2 particles have a wider lattice gap and abundant surface oxygen defects, which make it favorable for ion doping and photoreactions.
3.1.1. Morphology Control
In hollow nanotube-shaped catalysts, the transport speed of CO2 and photoproducts can be facilitated. Ru phase is inclined to form methane in CO2 hydrogenation process [36] and Yang et al. [37] entrapped Ru nanoparticles in TiO2 nanotubes. Restricted Ru nanoparticles were resistant to sintering and leaching in the Ru-in/TNT catalyst channel (Figure 5). Electrons tend to gather in the tubes because of a confinement effect, which leads to an abundant, accessible metallic phase. It is easier for high-priced Ru species to combine with free electrons and then exhibit a superb CH4 and CH3OH yield.
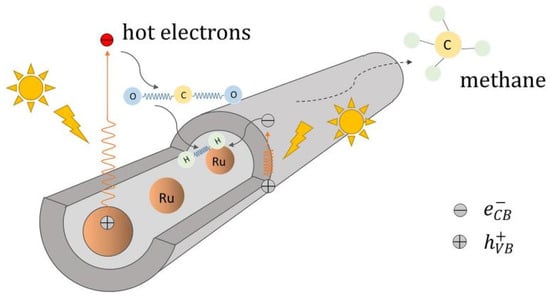
Figure 5.
The reaction pathway for the Ru entrapped in the TiO2 nanotubes catalyzes CO2 methanation [37].
On the contrary, Kar et al. [38] loaded metal nanoparticles onto vertically oriented 1D TiO2 nanotube arrays (TNAs) platforms via the graft method. In the synthesis process, Au, Ru and ZnPd NPs grow anodically on transparent glass substrates. There is no band bending phenomenon in Au NP-grafted TiO2, which can be observed from ultraviolet photoelectron spectroscopy (UPS). TPD experiments proved that all NP-grafted samples absorb more CO2 than TNAs. It is worth noting that, in nanoparticle-grafted TNAs, blue photons close to and below the TiO2 band edge were excited to drive CO2 photoreduction process. Ru-TNAs, ZnPd-TNAs and Au-TNAs had the CH4 formation rates of 26, 27 and 58 µmol·g–1·h–1, respectively. Pt and CoOx growing on the outer and inner layers of a porous TiO2-SiO2 frame, respectively, can act as “warehouses” for e− and h+, and the selectivity of the new system for reducing CO2 to CH4 can reach 94% [39].
Metal–organic frameworks (MOFs), also known as porous coordination polymers, composed of organic linkers and metal nodes (metal ions or clusters), are a kind of porous crystalline inorganic–organic hybrid materials. The unique advantages of MOFs, such as extremely high surface area, uniform adjustable porous structure and high density coordination unsaturated metal sites, have been extensively researched. They have been applied in many fields, such as gas adsorption and separation, sensing, catalysis, capture and conversion of CO2. The high specific surface area and uniform porous structure of MOFs make it possible to incorporate metal nanoparticles (as electron acceptors) into their frameworks, leading to efficient charge separation. In addition, the eminent CO2 adsorption capacity of various MOFs resulted in higher concentration of CO2 in the pores, which helped to accelerate the photoreaction. In [40], a porous material-zirconium-based organic skeleton (UiO-66) was introduced to a TiO2 photocatalyst as an effective CO2 adsorbent. The designed two-step strategy endowed the TiO2/UiO-66 composite with abundant graded pore structure, thus ensuring sufficient catalytic sites and high CO2 adsorption capacity (78.9 cm3 g−1). The ultrafine TiO2 nanoparticles were loosely loaded on the UiO-66 surface rather than tightly packed due to the electrostatic repulsion, thus ensuring the exist of microporous of the MOF. Finally, in the weak gas–solid catalytic system with water as the electron donor, the yield of CH4 was up to 17.9 μmol g−1 h−1, and the selectivity was 90.4%. Additionally, the photocatalytic efficiency was comparable to that of pure CO2 atmosphere even under low CO2 concentration conditions (≤2%).
Exposed Facet Adjustment
A Pt-TiO2 single atomic site catalyst (PtSA/Def-s-TiO2) was prepared [41] by the “thermal solvent-argon treatment and hydrogen reduction” method. In order to construct Ti–Pt–Ti structures, TiO2 nanosheets with oxygen deficient sites were used to anchor monatomic Pt particles, which can retain the stability of isolated single atomic Pt and improve photocatalytic performance. The exposed (101) and (001) crystalline of TiO2 nanosheets (Figure 6A,B) were determined by transmission electron microscopy, and a thickness of 6.9 nm was observed through atomic force microscope. The EPR spectra of the samples confirm that the rich oxygen defect structure can be obtained by heating TiO2 nanosheets in argon atmosphere. They also indicated that single-atom Pt junctions were formed by occupying the oxygen defect sites, which provides a benchmark for the rational design of highly active and stable single-atom catalysts on metal oxide carriers with defect structures. CH4 product can be detected when copper oxide nanoparticles are mixed with mesoporous TiO2 nanorods in close contact [42]. Both the special crystal plane and porous structure contribute to furthering the CH4 yield.
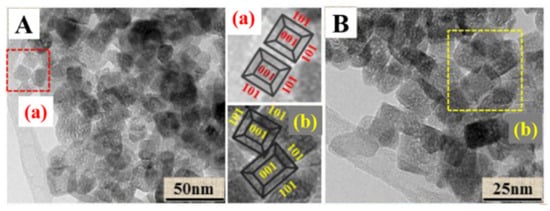
Figure 6.
(A,B) HRTEM images of s-TiO2 (a,b) partial enlarged drawing [41].
3DOM Structure Ti-Based Materials
A ternary 3DOM Bi-doped TiO2 photocatalyst decorated with carbon dots (CDs) was obtained, whose pore engineering of the 3DOM skeleton greatly promoted the response in the whole solar spectrum range [26]. It exhibits enhanced photocatalytic performance because of its excellent exquisite structure and high charge transfer efficiency. Similarly, a BiVO4/3DOM TiO2 nanocomposite [43] was synthesized as a highly efficient photocatalytic catalyst for the degradation of dye pollutants. Further studies on its textural, optical and surface properties revealed that connecting pores not only improve the electron transfer rate between coupled materials, but also provide abundant active sites for reactant molecules.
3DOM TiO2 were prepared [44] by the CCT method (Figure 7A) and CeO2/3DOM TiO2 samples (Figure 7B) were obtained by the original bubble-assisted membrane precipitation method. The introduction of CeO2 nanolayers broadened the photo-absorption range and facilitated the separation of photogenerated electron−hole pairs. The mesoporous structure provided a larger surface area and the catalyst exhibited a higher CO2 reduction activity.
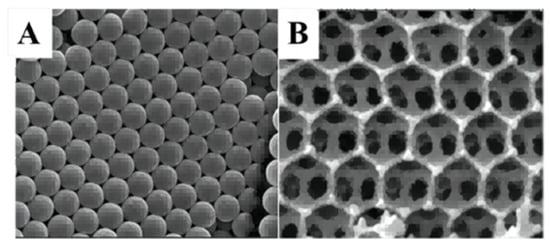
Figure 7.
(A,B) SEM images of colloidal crystal template and CeO2/3DOM TiO2 [44].
Pt-particle-decorated 3DOM carbon-coated TiO2 and g-C3N4 were combined [45] to construct an all-solid-state catalyst for CO2 artificial photoconversion. Slow photon effect and carbon coat optimized the absorption capability of light. The exquisite design drove vectorial electrons from TiO2@C to Pt particles and then fell to g-C3N4, which facilitated the carrier separation. The Z-scheme that consist of two isolated systems has three components and converts CO2 to CH4 with H2O at a yield of 65.6 μmol g−1 h−1. To enhance the surface enrichment of CO2, Wu et al. [46] fabricated 3DOM perovskite-type Ptn/SrTiO3, in which Pt nanoparticles can take in photoelectrons from SrTiO3 and transfer CO2 to CO and CH4 (Figure 8). Pt2/3DOM SrTiO3 exhibited the highest CH4 yield of 26.7 μmol g−1 h−1.
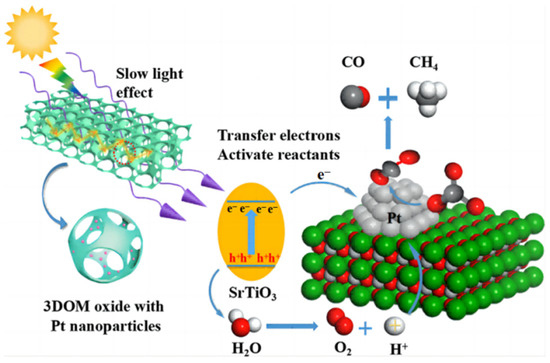
Figure 8.
The possible mechanisms of the photocatalytic CO2 reduction over Pt/3DOM SrTiO3 [46].
A two-dimensional MoS2 layers/3DOM TiO2 photocatalyst was prepared [47] to form heterojunctions, which had a higher performance in the range of 420–900 nm. Cu single atoms were uniformly distributed in 3DOM TiO2 via the in situ method [48], which provides active sites for CO2 photoconversion. The main products tested in the gas–solid two-phase system were CH4 and C2H4 with the corresponding generation rates of 43.15 and 6.99 μmol g−1 h−1. Chen’s work provided a new perspective for improving the catalytic efficiency by regulating reaction conditions. Jiao et al. [49] loaded core-shell structure AuPd NPs in 3DOM TiO2 via one pot of the gas bubbling-assisted membrane reduction method to form a functional photocatalyst. The low Fermi level of AuPd NPs empowered the catalyst system to trap electrons and enhance the separation of charge pairs. Carbon-quantum-dot-decorated 3DOM CaTiO3 photocatalysts were duly obtained [50], exhibiting an apparent quantum efficiency (QAY). The macro–meso–microporosity structure provided improved charge carrier separation and transport, and it was explored in depth through density functional theory calculations (DFT) and finite difference time-domain simulations.
3.1.2. Heterojunction
p–n heterojunction: A p–n heterojunction is formed by combining p-type and n-type semiconductors. Even without light irradiation, electrons can diffuse from an n-type semiconductor to a nearby p-type one, in the case of the combination of two materials. Correspondingly, the holes on the surface of a p-type semiconductor are transferred to the n-type one, which results in an efficient separation of charge carriers. A ZnFe2O4-modified TiO2 was synthesized by the hydrothermal method [51], and the p-n heterojunction system could reduce CO2 to methanol at a yield of 75.34 μmol g−1 h−1.
rGO composite: In recent years, graphene materials have been widely used because of their large specific surface area, unique thermal stability and excellent electrical conductivity. Graphite nanomaterials are visible-light-responsive materials with appropriate band gaps, and the energy levels of CB and VB are in optimal positions relative to ordinary hydrogen electrodes. These unique photocatalytic properties have made them prime candidates for photocatalytic CO2 reduction. Fortunately, tightly contacted ultra-thin graphene layers and TiO2 compounds and can be prepared with some additives [52]. Seeharaj et al. [53] employed high-intensity ultrasonic waves (ultrasonic horn, 20 kHz, 150 W/cm2) to exfoliate the TiO2 surface, which led to a highly specific active area and highly reactive nanosheets. The modification of tiny rGO and CeO2 on the rGO nanosheet surface can improve the CO2 absorptivity and the charge carriers’ migration efficiency of the catalyst (Figure 9). A kind of d–π electron orbital overlap was formed between TiO2′s d orbitals and rGO’s π orbitals, which provides a good environment for activated CO2 and electrons. The complex heterojunction photocatalysts TiO2/rGO/CeO2 exhibited high yields of CH3OH (641 μmol/gcath) and C2H5OH (271 μmol/gcath).
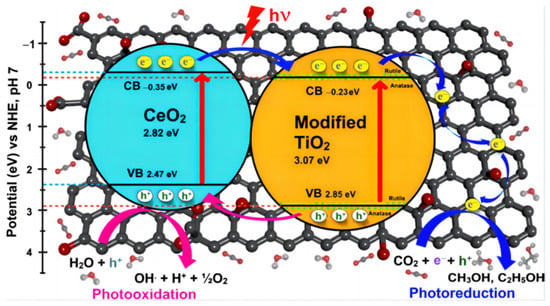
Figure 9.
Mechanism for the photocatalytic conversion of CO2 with H2O to methanol and ethanol over TiO2/rGO/CeO2 photocatalysts [53].
BCN composite: The boron carbon nitride (BCN) composite BCN is an adjustable band gap material that has been applied in CO2 reduction, water splitting and as a detoxification catalyst. Highly negative CB potential of BCN materials makes them suitable for the construction of S-scheme heterojunction, and Kumar et al. [54] designed a novel S-scheme Fe@TiO2/BCN composite. In situ XPS technology, DFT calculations and finite difference time-domain simulations were adopted to verify the S-scheme transfer mechanism. Under visible light irradiation, the intimately contacted heterojunction reduced the probability of charge recombination. The sample exhibited excellent photocatalytic activity; in addition to converting CO2 selectively, it also degraded tetracycline antibiotics.
TMS composite: Some transition metal sulfides (TMSs) exhibit the same properties as Pt or Pd in photocatalyst. They form longer-lived charged carriers within the S3p orbital [55] with a highly negative reduction potential. However, the self-oxidation of metal sulfides confuses researchers and limits their application. It can be alleviated by constructing a Z-scheme, and thus researchers introduced it to Bi-modified TiO2 [56]. Meanwhile, a spatially coupled heterojunction was enhanced by regularly capsulated CuCo2S4 yolk–shell hollow sphere.
3.1.3. Ion Doping
In recent years, elements such as B, N, Co and Bi have been widely applied in TiO2 doping. A carbon-based hybrid nanocomposite reduced graphene oxide (rGO), belonging to the narrow band gap, with oxygen-containing functional groups on the surface that can be enhanced by π interactions [57]. Laminar graphene carriers not only prevent TiO2 repolymerization, but also hybridize the function of the catalytic system. Co-doped TiO2 was loaded on the rGO [58], and the Co peak in EDX spectra and C-O peak in FT-IR spectra confirmed the successful doping and the presence of graphene support, respectively. The size of TiO2 particles decreased from 48–80 nm to 23–28 nm, which is consistent with earlier reports of changes in titanium doping with transition metal ions.
3.1.4. Sensitization
A growing number of semiconductor materials are being used to modify TiO2 dioxide, but randomly mixed catalysts are not stable enough to achieve reproducibility. Therefore, Lee [59] grew well dispersed p-type NiS nanoparticles on the surface of a highly aligned n-type TiO2 film to obtain the NiS-sensitized TiO2 films. The band gaps of two components were estimated by wavelength relation. Some inferences can be drawn when considering the results of both the ultraviolet and visible spectra. It indicates that more electrons are subpoenaed from the short-Eg NiS and transferred to TiO2 conduction band. The spectra results reconfirmed the electron contribution of the NiS and the design of a catalyst that produced 3.77-fold CH4 compared to the TiO2 film.
3.1.5. Summary
Overall, some of the photocatalytic systems that use TiO2 is presented in Table 1.

Table 1.
Summary of the recent results of the photoreduction of CO2 using TiO2-based materials.
3.2. WO3-Based Photocatalysts
Tungsten-based oxides (WO3) have been extensively studied in recent decades and various morphologies have been presented. In the WO3 structure, the crystal in the stoichiometric ratio is connected with a twisted WO6 octahedra to form a perovskite crystal structure. It has monoclinic, orthorhombic and hexagonal crystal forms. At the same time, the oxygen lattice can be lost easily, resulting in oxygen vacancies and unsaturated, coordinated W atoms. Therefore, tungsten oxide has many non-stoichiometric compounds, such as WO2.72, WO2.8, WO2.83 and WO2.9. Of these, WO3 is the most common and has been widely studied as a typical photocatalytic water oxidation semiconductor material. WO3 is a typical narrow-band gap indirect semiconductor with a forbidden band width of 2.6–2.8 eV, which can absorb part of the visible light [64]. In addition, WO3 is a research hotspot in the field of photoelectrochemical water splitting because of its high carrier mobility, stability in acidic electrolytes and resistance to photocorrosion.
3.2.1. Morphology Control
Bi2WO6 is one of the tungsten-based materials that belongs to Aurivillius crystal oxides. Its crystal has an orthorhombic system, and its narrow band gap (2.7–2.9 eV) structure allows it to meet the response absorption of visible light. Moreover, its stable structure and eco-friendly properties have attracted many scientific researchers to study it. Since the valence band of Bi2WO6 is composed of O2p and Bi6p, and the conduction band is composed of W5d-assisted Bi6p orbitals, the VB energy levels can be dispersed broadly. By employing the Kirkendall effect in ion exchange and BiOBr precursor, Huang et al. [65] prepared a bowl-shaped Bi2WO6 HMS material. Based on the large specific surface area of the material, its adsorption capacity for CO2 reaches 12.7 mg g−1 at room temperature and pressure. The material adsorbs a large number of HCO3− and CO32− species on the surface during the reaction, which makes the catalytic reaction easier. The Bi2WO6 HMS thus has a high catalytic activity, and the methanol yield is 25 times higher than that of the Bi2WO6 SSR.
Iron phthalocyanine FePc is neatly assembled on porous WO3 under induction and coupled with surface atoms by H-bonding [66]. The optimized FePc/porous WO3 nanocomposites exhibit enhanced CO2 photoreduction activity, which is attributed to the synergistic effects of a high specific surface area, a better charge separation and proper central metal cation. A series of mesoporous WO3 with interconnected networks were synthesized by the silica KIT-6 hard template method [67], which became oxygen-deficient after hydrogenation treatment. Both the ordered porous structure and oxygen vacancies contributed to the increased yield of CH4 and CH3OH.
WO3 with a hollow nest morphology with hierarchical micro/nanostructures (HNWMs) was synthesized [68] by the one-step hydrothermal method (Figure 10), with a particle diameter of about 2.5 μm. The 2D nanosheets, which have an average thickness of 30–40 nm, were assembled to build a distinctive hollow nest structure with a good stability and reusability under visible light. Hao et al. [69] prepared core–shell heterojunctions of two-dimensional lamellar WO3/CuWO4 by the in situ method. After the modification of amorphous Co-Pi co-catalyst, the photoanode of ternary homogeneous core–shell structure exhibited a high photocurrent of 1.4 mA/cm2 at 1.23 V/RHE, which was 6.67 and 1.75 times higher than that of the pristine WO3 and 2D homogeneous heterojunction. Ren et al. [70] synthesized unique flower-like Bi2WO6/BiOBr catalysts by the simple one-step solvothermal method, and showed that the photocatalytic activity of the composites was significantly enhanced due to the construction of type II heterojunctions. The presence of Br source enhanced the light absorption and improved charge-carrying spatial transfer and separation.
Figure 10.
Schematic diagram of the HNWM formation process [68].
Ti atoms in ultrathin Ti-doped WO3 nanosheets promoted the charge transfer [71], as they accelerate the generation of key intermediates COOH*, which was revealed by in situ characterization. Furthermore, Gibbs free energy calculations were calculated to verify that ion doping can reduce the CO2 activation energy barrier and CH3OH desorption energy barrier by 0.22 eV and 0.42 eV, respectively, thus promoting the formation of CH3OH. The ultrathin Ti-WO3 nanosheets showed an excellent CH3OH yield of 16.8 μmol g −1 h −1. Two-dimensional bilayered WO3@CoWO4 were prepared [72] via a facile interface-induced synthesis method. The optical energy conversion efficiency can be improved by both p–n heterojunctions and interfacial oxygen vacancies. The narrow band gap of the WO3@CoWO4 heterojunction was proved by DFT calculations and some characterizations, which allows a better visible light absorption. A tree-like WO3 film was prepared [73] by the hydrothermal process, which has a large specific surface area. The WO3 product was a unity of hexagonal/monoclinic crystals, which contained W5+ defects and oxygen vacancies. The products were further subjected to a mild reduction solution at the lower temperature of 333 K to introduce more defects. It turns out that the intermediate state induced by defects diminished the band gap. A reasonable amount of defects benefits the photocatalytic activity of WO3, while too many defects impair its catalytic capacity. The performance of the treated WO3 films increased 2.1 times in 48 h compared to that of the annealed WO3 samples.
Preferentially Exposed Facets
According to studies, infrared (IR) light makes up nearly 50% of solar energy, and it is challenging to make use of the majority of the light. Liang et al. [74] fabricated 2D ultrathin WO3 with an intermediate band gap. They achieved the first complete decomposition of CO2 driven by infrared light without the addition of sacrificial agents. Theoretical calculations indicated that the generation of the intermediate energy band resulted from the critical density of the generated oxygen vacancies, which has also been verified by synchrotron valence band spectroscopy, photoluminescence spectroscopy, ultraviolet-visible-near-infrared spectroscopy and synchrotron infrared reflection spectroscopy. The results showed that the WO3 atomic layer containing oxygen vacancies can achieve the complete decomposition of CO2 and generate CO and O2 under infrared light.
Microscopic WO3 nanocrystals were formed by Chen et al. [75] through solid–liquid phase arc discharge in an aqueous solution. Then, they synthesized ultrathin single-crystal WO3 nanosheets via a laterally oriented attachment method. The quantization effect of this nanostructure altered the bandgap width of WO3 nanosheets, enabling the semiconductor to exhibit a high performance at a wide range of nanometer sizes. It is beneficial to control the activity and selectivity of the photoconverted CO2 products. As a consequence, WO3 with a strong visible light response has enormous potential in the field of photocatalytic CO2 reduction.
Bi2WO6 has a positive conduction band potential, which is not enough to excite and reduce CO2 molecules, thus limiting its application in the photocatalytic reduction of CO2. Therefore, researchers have been devoted to modifying the surface of Bi2WO6 to improve its photocatalytic activity in order to obtain a high-efficiency CO2 reduction ability. Zhou et al. [76] successfully prepared Bi2WO6 nanosheets with a monolayered structure by introducing the surfactant CTAB (hexadecyltrimethylammonium bromide) into the precursor solution. A large number of unsaturated Bi atoms were produced, which provided sufficient active sites for photocatalytic reactions. Bi2WO6 is composed of a [BiO]+-[WO4]2−-[BiO]+ sandwiched layer structure. Under irradiation, holes and electrons are generated in different [BiO]+ layers. This structure promotes the spatial separation of photogenerated electron–holes and greatly reduces the carrier recombination rate of the monolayer Bi2WO6 material.
With the assistance of an oil-based primary amine (C18H37N) surfactant, Zhou et al. [77] conducted a hydrothermal reaction at 200 °C for 20 h to prepare an ultra-thin and uniform Bi2WO6 nanosheet. The material has a strong response under visible light, and its forbidden band width was about 2.44 eV through theoretical calculations, with a conduction band potential of −0.31 e V. Then, CO2 could be easily reduced to CH4, and its yield was 20 times higher than that of SSR Bi2WO6. The light-absorbing capacity will decrease if the nanosheets are too thin because of the quantum size effect. Therefore, scholars need to consider roundly when designing fresh material [78].
DOM Structure W-Based Materials
Unexpectedly, it was found that the resistance of 3DOM-WO3(270) and the Ag3PO4 electron absorption band were comparable. By depositing Ag3PO4 nanoparticles in the micropores of 3DOM-WO3, Chang et al. [79] achieved a higher photocatalytic activity and more efficient light harvesting at the wavelengths of 460–550 nm. A Z-scheme g-C3N4/3DOM-WO3 catalyst designed by Tang et al. [80] also has a high CO2 photoreduction activity. The separation way of the photogenerated electron–hole pairs determined its Z structure, and 3DOM framework heightened the light collecting efficiency. Therefore, an excellent photocatalyst exhibited a high CO evolution rate of 48.7 μmol g−1 h −1.
3.2.2. Heterojunction
Quantum dot composite: CuO quantum dots (QDs) were combined with WO3 nanosheets by a self-assembly method and the diameter of 6%CuO QDs/WO3 NSs was mainly located at 1.6 nm [81]. The bandgap energy of CuO/WO3 fell in 2.28 eV and the complex catalyst possessed a lower resistance for charge carrier transfer that showed in UV-vis DRS and EIS analysis. Due to the low CB position, CO cannot be obtained when using pure WO3. However, the photogenerated electrons gathered in the WO3 CB position was able to reach the CuO VB position when the Z-scheme (Figure 11) was formed by intimate heterojunctions. At the same time, the reduction reaction that transformed CO2 into CO occurred at the CuO CB position. The high yield rate of about 1.58 mmol g−1 h−1 also benefited from a longer fluorescence lifetime, and reduced the overlap of electron pore pairs.
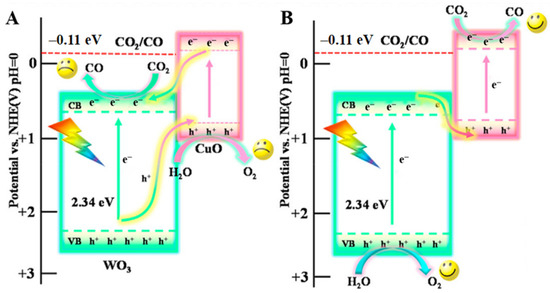
Figure 11.
The proposed charge transfer mechanisms: (A) Ⅱ-scheme and (B) Z-scheme for CuO/WO3 [81].
Perovskite composite: For WO3, the negative potential energy of the sheet shape (−31.5 mV) was lower than that of the rod (−21.0 mV). In addition to its potential advantages, S–WO3 offers a higher specific surface area. Defects occurred because of the exposed interior atoms in nanosheets surface, which promoted the CO2 adsorption. Positively charged perovskite cesium lead tribromide (CsPbBr3, CPB) with a long electrically diffused length was selected to be combined with S–WO3, affording a high field rate of CO and CH4. Zhang and others [82] employed a three-dimensional hydrophobic porous melamine foam to support the mixture, not only to protect the CPB from dissolution but also to reduce toxic Pb2+.
3.2.3. Ion Doping
Molybdenum with a similar ionic size was chosen to dope the WO3 as a low-valence metal species. The W5+/W6+ ratio of the catalyst was increased, making it easier to exchange electrons with reactants. The conductivity of protons was enhanced by the presence of hydrogen bronze, which originated from a chemical reaction between WO3 and Brønsted protons and excess electrons in their lattices. Wang et al. [83] prepared molybdenum-doped WO3·0.33 H2O by the hydrothermal method. The Ecb and Evb energy were both higher at 3%Mo-WO than WO3. After 20 min of the FTIR spectra, rare intermediate CO2− was observed, verifying the activation of CO2, which was more common in low-valent meta species. The content of potassium hydroxide in an aqueous solution obtained by photocatalytic water oxidation was higher and the CH4 yield was 4.2 times higher than WO3.
3.2.4. Summary
To date, Bi2WO6 semiconductor photocatalytic materials have made great progress in the field of environmental management. Some photocatalytic systems using WO3-based materials in CO2 conversion are listed in Table 2.

Table 2.
Summary of the recent results on the photoreduction of CO2 by WO3-based materials.
3.3. ZnO-Based Photocatalysts
ZnO, a common metal oxide, is a n-type semiconductor with an Eg value of 3.37 eV. It is a kind of amphoteric oxide that has the advantages of nontoxic harmlessness, low cost, abundant reserves, convenient preparation, low dielectric constant and low optical coupling rate. ZnO has three main lattice structures: wurtzite structure, zinc-blended structure and tetragonal rock salt structure. The wurtzite structure is considered the most stable and common structure in nature. It is a kind of hexagonal crystal, in which the O and Zn atoms are aligned with the hexagonal density stacking. The photodegradation process of ZnO is similar to TiO2 and it has been widely used in photocatalysts, solar cells and conducting materials.
3.3.1. Morphology Control
The 3nm Pt particles were uniformly dispersed over ZnS@ZnO with a mesoporous heterostructure [90] and more CH3OH was obtained. Reactant charge carriers entered the pore channels of the porous heterozygous layer, thus reducing the likelihood of flow resistance and electron–hole recombination. The S-scheme photocatalyst delivered a high CH3OH formation rate of 81.1 μmol g−1 h−1, which is roughly 40 and 20 times larger than that of bare ZnO (3.72 μmol g−1 h−1) and ZnO–ZnS (4.15 μmol g−1 h−1). On the other hand, a porous ZnO@ZnSe core/shell nanosheet array material (Figure 12A) was prepared in a controlled manner [91]. The final n-type semiconductor composites had a proper negative CB band edge. In comparison to ZnO or ZnSe, more pairs of electron–holes were formed under visible light. Electrons tend to land on ZnO, which is aimed at methanol production. Mei et al. prepared a ZnO microsphere with different numbers of shells [92] and the photoelectric performance of ZnO was optimal when the number of shells reached three.
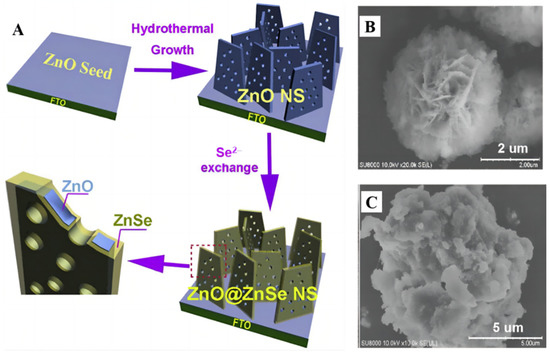
Figure 12.
(A) Schematic illustration of the fabrication process of ZnO@ZnSe photocathode [88]. (B) ZnO/ZnS (C) ZnO/ZnS/g-C3N4 SEM images [91].
It is challenging to coat uniform 2D g-C3N4 nanofilm on the surface of 3D materials because of the difficulty in exfoliation process. Thus, Wang et al. [93] proposed an electrostatic method and incorporated g-C3N4 nanofilm with porous ZnO nanospheres that has a strong interaction. The heterojunction was then anchored on 3D graphene aerogels (GAs). The compound g-C3N4/ZnO/GA has extraordinary stability, maintaining a high CO2 conversion rate, which is 92% of its original activity after 100 h.
ZnO/ZnS nanoflowers (Figure 12B) were combined with g-C3N4 nanosheets (Figure 12C) to construct a double Z-scheme structure [94]. ZnO/ZnS nanoflowers provide a large specific surface area and g-C3N4 helps to absorb more photons under solar light irradiation. Optimized interfacial charge transfer dynamics in ternary heterostructure can be characterized by photocurrent measurements. As a result, the formation rate of H2 product over the novel double Z-scheme mixture increases to 301 μmol g−1 h−1 on water splitting.
Hierarchical CuO/ZnO nanocomposites with p-n heterojunction were prepared [95] by the modified hydrothermal method. The photocatalysts are found to converse CO2 to methanol in aqueous solution containing dimethylformamide (DMF) and triethylamine (TEA) as electron donor under visible light irradiation. ZnO/NiO porous hollow spheres with sheet-like subunits were obtained [96] by calcination of Ni-Zn MOFs. Numerous p-n heterojunctions with n-type ZnO and p-type nickel monoxide were formulated in mixed ZnO/NiO. The porous hollow structure with the large specific surface area can increase the absorption capacity of CO2 and light.
Based on the vapor to solid mechanism, a novel ternary Ag/CeO2/ZnO nanocomposite [97] was synthesized by the facile thermal decomposition method. Oxygen vacancies introduced by line structure contributied to a narrow band gap of 2.66 eV, and this was further confirmed by DRS characterization. After the preparation of the ZnO/TiO2 nano-tree arrays, Ag2S and ZnS were synthesized to modify the nano-tree arrays by cation exchange methods [98]. The core-shell structure of ZnO@ZnS prevented the decomposition of ZnO, and the modification of Ag2S reduced the Eg value of the composite and promoted the red-shifted of light absorption.
3.3.2. 3DOM Structure Zn-Based Materials
Wang et al. [99] published metal-organic-framework-derived 3DOM N-C doped ZnO (Figure 13) for efficient CO2 reduction. The ultra-tiny CoOx clusters were anchored on the surface of catalyst and no Co-Co peak was found in CoOx/N-C-ZnO. The charge transfer rate was jacked up by ion doping and the recombination of electron-hole pairs was tamed because of the CoOx clusters. Furthermore, CoOx on the orderly connected channels can act as an electron trap to capture electrons, which makes a contribution to photoreaction efficiency. The density theory calculations (DFT) was also used to detect the CO2 adsorption ability, and CoOx/N-C-ZnO exhibited the most negative CO2 binding energy due to improved electron structure of adsorption site.
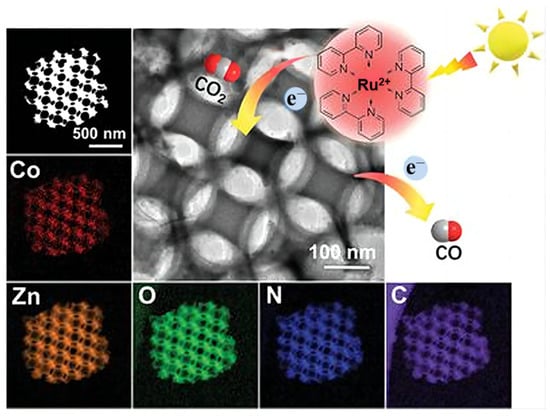
Figure 13.
HAADF-STEM image of CoOx/N-C-ZnO and related elemental mapping images [99].
3.3.3. Heterojunction
Recently, zeolitic framework (ZF) composite fabricated by the microwave-hydrothermal synthesis method (MWH) has attracted attention, which can provide a fast heating-speed and produce morphologically uniform samples. With biodegradable template, the zeolitic framework (ZF) was synthesized via MWH method from volcanic ashes. The NaAlSiO4 (NAS) framework was composed of 50 nm circular channels and has a large surface area. Hip’olito et al. [18] embedded ZnO/CuO hybrid structure in the NAS channels, resulting in a ternary composite. The synergistic effect among ZnO, CuO and ZF support accelerated the photocatalytic process of water splitting and CO2 reduction, which offers higher H2 and HCOOH evolution rate.
The imidazole framework-8 (ZIF-8) molecular as a widely used zeolite (ZIF) composite, has an excellent competence of absorbing CO2, which is apt for CO2 converting. Selective-breathing effect was wielded to boost CO2 conversion efficiency through monolithic NF@ZnO/Au@ZIF-8 (Figure 14A) catalyst [100]. Au particles were loaded on ZnO nanorods that grown on Ni foam (NF), the mixture was immersed in methylimidazole solution (Figure 14B). Based on the results of electrochemical impedance spectroscopy (EIS) Nyquist plots, the alternating magnetic field was introduced to create magnetic heat, which leading to increased carrier density and improved photocatalytic performance. It is found that the selectivity of CH4 cheeringly achieved 89% from 61% under photo-thermal-magnetic coupling effect.
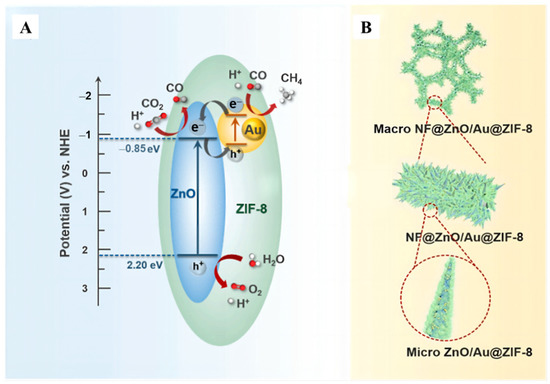
Figure 14.
(A) Illustration of the band structure and charge transfer process of NF@ZnO/Au@ZIF-8 catalyst under UV− vis light. (B) The structural diagram the monolithic NF@ZnO/Au@ZIF-8 photocatalyst [100].
3.3.4. Ion Doping
Many metal elements have been doped in ZnO to minish its band gap, such as Sb, Cu and Mn, among them Co distinguished itself because of the similar ion radius. In addition, the conversion of Co3+ and Co2+ results in oxygen vacancies, which greatly enhances photocatalytic efficiency. Xie et al. [101] introduced Co3+ with different mole ratio to ZnO microspheres precursor (including Zn2+, urea, and PVP), and the introduction of Co3+ did not disrupt lattice structure as seen in the XRD model. It was revealed that the presence of both Co2+ and Co3+ in Co-ZnO from high-resolution spectrum, and Zn2p exhibited higher binding energy due to Zn2+ charge transfer in 7% Co-ZnO. As the ratio of Co/Zn increased, the conversion from Co2+ to Co3+ decreased, and the light response range gradually expanded. The results showed that the electrochemical impedance of 7% Co-ZnO sample was the lowest band gap of 2.56 eV.
3.3.5. Summary
Great efforts have been made to design catalysts for CO2 reduction on ZnO and the relevant research data are summarized in Table 3.

Table 3.
Summary of the recent results on the photoreduction of CO2 by WO3-based materials.
3.4. Cu2O-Based Photocatalysts
Cuprous oxide (Cu2O) is a potential p-type semiconductor with a wide visible-light response range and high photo-electric conversion efficiency (18%) [106], and it displays attractive prospects in solar energy conversion and heterogeneous photocatalysis. Although Cu2O possesses many excellent properties, photocorrosion and the rapid recombination of e−/h+ pairs affect its activity and limit its application. The photocorrosion is believed to occur in two ways: (1) self-reduction caused by generated electrons and (2) self-oxidation caused by the generated holes.
Self-reducing photocorrosion: Cu2O + H2O + 2e− → 2Cu + 2OH−
Self-oxidative photocorrosion: Cu2O + 2OH− + 2h+ → 2CuO + H2O
Therefore, developing Cu-based catalysts with excellent activity, selectivity and stability has become the research hotspot in the area of the photocatalytic reduction of CO2. Many successful attempts have been made to improve the photostability and photocatalytic performance of Cu2O. In general, most studies focus on enhancing the charge transfer from Cu2O to reactants or cocatalysts to prevent charges from accumulating within the particles. A series of methods for improving the performance of Cu2O are discussed in detail in what follows.
3.4.1. Morphology Control
A branch-like CdxZn1-xSe nanostructure was obtained [107] by the cation-exchange method, which was then mixed with Cu2O@Cu to form heterojunctions. Selenium (Se) vacancies were created during the ion exchange process and the crystal growth was limited due to the additive diethylenetriamine (DETA), leading to insufficient coordination of the surface atoms, which then become active adsorption sites. Highly hierarchical branching-like structures assembled by one-dimensional structural materials not only facilitate electron accumulation at their tips but also increase the light-accepting area, and characterization results show that branching structures can effectively absorb visible light. Cd0.7Zn0.3Se/Cu2O@Cu step-scheme heterojunction exhibited a CO release yield of 50.5 μmol g−1 h−1.
Ultrafine cuprous oxide U-Cu2O (<3 nm) was grown on the polymeric carbon nitride (PCN) (Figure 15) by the in situ method [108]. PCN has a narrow band gap of 2.7 eV that can capture visible light. Both ultrafine nanoclusters and Z-scheme heterojunction can protect U-Cu2O from degradation. The photocatalyst U-Cu2O-LTH@PCN has high stability, maintaining more than 95% activity after five cycles of testing, while bare Cu2O grades completely within three cycles. A large number of heterojunctions were formed by U-Cu2O particles and lamellar PCN, expediting the electron transfer efficiency. The product can convert CO2 to methanol with water vapor under light irradiation at the high yield of 73.46 μmol g−1 h−1. Ultrathin Ti3C2MXene with a high fraction of coordinated unsaturated surface sites was fabricated by Zhang et al. [109]. Via the HF etching method, different amounts of Cu2O were combined with Ti3C2 nanosheets under the hydrothermal condition. The unique hexagram morphology of Cu2O, the 2D layer structure and excellent conductivity of Ti3C2Tx nanosheets and the synergistic effect between the two composites promote the improvement of photoactivity. Zhang et al. reported the bifunctional catalyst of Cu2O@Fe2O3. Cu2O nanoparticles coated with an Fe2O3@carbon cloth electrode were used for both overall water splitting and CO2 photoreduction [110].
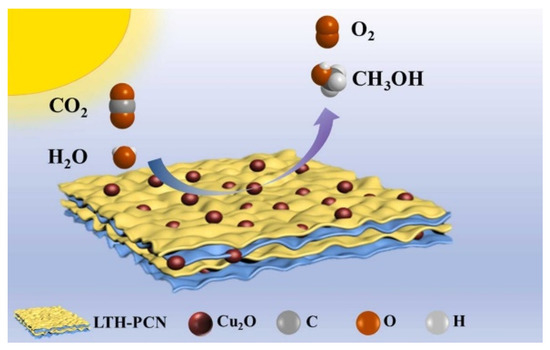
Figure 15.
Ultrafine U-Cu2O nanoclusters anchored on the photosensitizing PCN support [108].
Preferentially Exposed Facets
Cu2O is an ideal compound to study the influence of electron-related effects. The rare occurrence of the O-Cu-O 180o linear coordination of Cu2O makes its (111), (100) and (110) facets chemically active. Zhang et al. [111] successfully achieved the morphology control of Cu2O nanocrystals by utilizing the selective surface stabilization of PVP on the (111) plane of Cu2O. With different amounts of PVP, the surface area ratio of (111) to (100) was subtly tuned, which resulted in the shape evolution of the system and various Cu2O structures (Figure 16). The detailed modification mechanism was elucidated from the structural and kinetic perspectives.
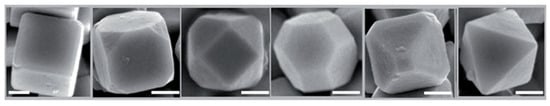
Figure 16.
FESEM images of the Cu2O polyhedrons with different volume ratios of (100) to (111) [111].
Octahedral copper oxide that exposes the (111) crystal faces was decorated with low Fermi energy Ag nanoparticles. After coating with rGO, the ternary heterojunction catalyst [112] exhibits selective photocatalytic superiority towards CH4. The CO* radicals can be characterized via DRIFT spectra and DFT calculations, which is the key intermediate for the conversion of CO2 to CH4. Two types of MoS2 (p-type and n-type) and two shapes of Cu2O (cubic and octahedron) were synthesized and combined with each other [113], and the compositions possessed different electronic and structural properties. The heterostructures formed by the p-type MoS2 with intrinsic conductivity had a higher photocatalytic activity, and the methanol production yield was as high as 76 μmol g−1 h−1. Both Z-type and ii-type charge transfer mechanisms were built using an n-type MoS2 mixture. For Cu2O, the cubes of the exposed (100) crystal plane with a higher binding affinity with MoS2 transferred electrons more efficiently and produced methanol at a higher rate.
A 3D porous Cu was produced by electrodeposition method [114], being transformed into CuO2 after following high-temperature annealing. Three-dimensional Cu2O delivers a 24-fold production of CO compared with the unremarkable and non-porous Cu. Additionally, more CO2 accumulated and took reactions in the hollow space of 3D Cu2O to form C2 products.
3DOM Structure Cu-Based Materials
The 3DOM Cu2O structure was luckily obtained [115] via polystyrene crystal templates. Under the contrived “sunlight” irradiation, incident light was reflected and absorbed around and around again. In the ultra-visible absorption spectra (350 to 800 nm), Cu2O with large orifices absorbs more photons than bulk samples, making it more advisable for solar applications. 3DOM Cu2O was prepared [116] by the electrochemical method to reduce CO2, and its Faraday efficiency was five times higher than that of Cu film. The CO2·− intermediate in 3DOM channels is more stable and leads to the possibility of forming CO and HCOOH products (Figure 17). We look forward to the applications of inverse opal Cu2O in photocatalysis.
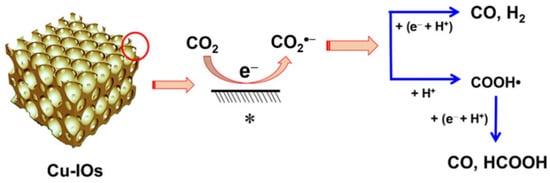
Figure 17.
Proposed mechanism for CO2 reduction to CO and HCOOH on Cu2O-derived Cu-IOs (The symbol “*” represents the surface.) [116].
3.4.2. Heterojunction
Liu et al. [117] reported a facile solution and chemistry route to synthesize rGO-incorporated crystal Cu2O with various facets as visible-light-active photocatalysts for CO2 reduction. The enhanced activity was attributed to the formation of the heterojunction and the existence of rGO as the electron transport mediator. M. Flores et al. [118] adopted the microwave-hydrothermal method to couple the powders of Mg(OH)2, CuO and Cu2O. The synthesis method allowed a sufficient interaction between Mg(OH)2/CuO and Cu2O without inhibiting the gas adsorption capacity of Mg(OH)2. They found that the presence of Cu2O favored the selectivity towards CH3OH production because a higher Cu+ concentration led to better selectivity. Niwesh et al. [119] reported the formation of a p–n heterojunction between Cu2O and the SnS2/SnO2 nanocomposite that offered favorable reductive potentials and high stability, mainly owing to their intimate interfacial contact. In the absence of a sacrificial agent, the generation rate of NH4+ was 66.35 μmol g−1 h−1 for Cu2O/SnS2/SnO2, which is 1.9-fold higher than that of SnS2/SnO2. However, the work of Trang et al. also demonstrated the instability and photo-oxidation of Cu2O heterojunctions. Generally, most p–n heterojunctions are found to reduce the redox capacity of photogenerated charges. This is especially evident for Cu2O p–n type heterojunctions, as CuO is excessively formed on the surface of Cu2O under continued illumination, so there are recombination problems at the heterojunction interface. The construction of Z-scheme heterojunctions overcomes the limitations of p–n heterojunctions, namely the reduction in redox potential and charged carrier recombination at the p–n heterojunction interface. In a Z-type heterojunction, the redox potential can be maintained under the premise of high photo-induced electron transport rate.
Zhang et al. [120] synthesized coal-based CNPs with an sp2 carbon and multilayer graphene lattice structure, and loaded them onto the surface of Cu2O nanoparticles prepared by the in situ reduction of copper chloride. The rapid recombination of electron–hole pairs was suppressed by the introduction of CNPs. The energy gradient formed on the surface of Cu2O/CNPs facilitates the effective separation of electron–hole pairs for CO2 reduction, improving the photocatalytic activity. Atomically dispersed In-Cu bimetallic catalysts were prepared [121] by the in situ pyrolysis method, in which carbon nitride acted as a carrier. The light-harvesting and charge separation efficiency were enhanced by regulating the loading amount of Cu and In, and the supreme generation rate of the photoreduction of CO2 to ethanol reached 28.5 μmol g−1 h−1 with 92% selectivity. The DFT calculations showed that the introduction of an In atom in copper can accelerate electron transfer from carbon nitride to metal, improve the charge separation efficiency and increase the electron density of copper active sites. The presence of In–Cu sites exerted a synergistic effect, which could promote C–C coupling, lower the energy barrier of *COCO generation and increase ethanol yield. Zhao et al. [122] reported the indirect Z-scheme heterojunction of UiO-66-NH2/Cu2O/Cu, which achieved a high CO2 photocatalytic conversion to CO. The SEM results of Cu2O, UiO-66-NH2 and U/C/Cu-0.39 are shown in Figure 18A–C. In this catalytic system, UiO-66-NH2 slowed down the photo-corrosion rate of Cu2O and increased the CO2 adsorption capacity (Figure 18D).
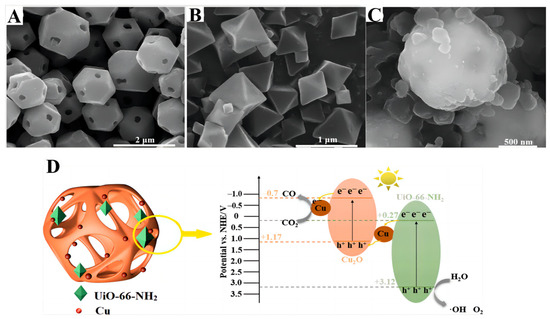
Figure 18.
(A–C) SEM results of Cu2O, UiO-66-NH2 and U/C/Cu-0.39. (D) Illustration of the photocatalytic CO2 reduction in U/C/Cu-0.39 [122].
3.4.3. Summary
In conclusion, photochemical methods offer the opportunity to modulate the persistence and selectivity of Cu-based catalysts photoreduction to value-added compounds. The most recent photocatalytic CO2 reduction outcomes of Cu-based materials are listed in Table 4.

Table 4.
Summary of the recent results on the photoreduction of CO2 by Cu2O-based materials.
3.5. CeO2-Based Photocatalysts
Cerium oxide (CeO2) has an octahedral face-centered cubic fluorite structure, in which the coordination numbers of Ce and O are 8 and 4. When reduced at a high temperature, it can be converted to nonstoichiometric CeO2−x (0 < x < 0.5). Notably, CeO2−x maintains a fluorite crystal structure and forms oxygen vacancies after losing a certain amount of oxygen. CeO2−X materials with different Ce/O ratios were also obtained in different conditions and it could be reconverted to CeO2 again if it returned to an oxidizing environment. Because of the unique electrical structure, cerium oxide (CeO2) is famous for the conversion sates between Ce4+ and Ce3+, which have been studied as oxygen storage catalytic materials and solid oxide full cells by many scholars [124,125]. In summary, CeO2 is a rare-earth metal oxide with a good photochemical stability, low cost and environment friendly characteristics. It is an important n-type semiconductor with a wide bandgap, and credible photocatalysts have been designed to reduce CO2 and degrade pollutants [126].
3.5.1. Morphology Control
Yb-, Er-doped CeO2 hollow nanotubes were synthesized [127] using silver nanowires coated with silica, and the products had a narrower band gap of 2.8 eV. The core–shell structured CeO2 was converted into mesoporous hollow spheres by the Ostwald ripening method in the presence of urea and hydrogen peroxide [128]. CeO2 nanocages can be fabricated by mixing (NH4)2Ce(NO3)2 with templates of Cu2O nanocubes [129], in which Cu2O is finally sacrificed. The photocatalytic results [130] indicated that CeO2 nanocages exhibit higher activity than hollow spheres.
Preferentially Exposed Facets
It was found that molecular CO2 can be distorted and participate in reactions at a low energy on the CeO2 surface [131]. A p-type NiO material was designed to modify the rod-like CeO2 nanostructure [132], allowing electrons and holes to migrate to opposite directions. They then operated the Mott–Schottky test, which showed a typical p–n junction. The presence of hexagon-shaped NiO plates broadened the range of light responses, which can be verified in the UV-Vis absorption spectra. Graphene oxide (rGO) was introduced as a “network” of for photoreduction electron transportation (Figure 19A–C). The impedance can be seen in the EIS Nyquist plot, which shows that the NiO/CeO2/rGO achieved the minimum value. The HCHO production rate of the ultimate catalyst was 421 μmol g−1h−1 with the synergy of several favorable factors. It is worth mentioning that a range of in situ techniques have been used to detect oxygen vacancies, structural changes, free radicals and formate on the surface of CeO2.
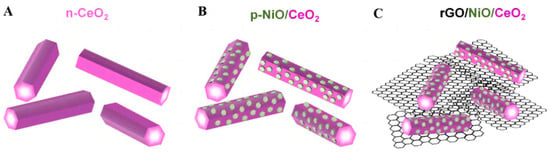
Figure 19.
The structural diagrams of (A) n-CeO2 nanorods, (B) p-NiO/CeO2 composite and (C) NiO/CeO2/rGO hybrid composite [132].
Macroporous
Mesoporous N-doped CeO2(NMCe), a relatively ordered intermediate structure with enhanced CO2-capturing capability, was prepared without any convoluted procedures or expensive equipment [124]. In the Roman spectrum, the bands from 550 to 650 cm−1, which are closely related to oxygen vacancy, were more salient than the MCe band. In addition, N-doped porous CeO2 has a higher CO2 absorption capacity than porous CeO2. All the above results conformed to the photoluminescence spectrum (PL) analysis, and the reduction gross yield of CO and CH4 was 3.5 times higher than that of OMCe.
With the help of a suitable crosslinked and pyrogenic solvent, doped CeO2 was uniformly fixed on transparent polymers by the in situ polymerization pathway [133]. By increasing the Ca/Ce molar ratio (wt.% < 20%), no peaks relating to CaO were observed in the p-XRD of samples and the original diffraction peak intensity became more strident due to the foreign ion’s minuscule radius (Ca2+ 0.1 nm, Ce4+ 0.184 nm). The specific surface areas of three catalysts (CeO2, (20: 80) CaO/CeO2 and CaO/CeO2 NC-dispersed polymers as a whole) were 28.4, 58.3 and 224.7 m2 g−1, respectively, and heterojunction nanocomposites had the highest photocatalytic efficiency.
3DOM Structure Ce-Based Materials
Under the protection of poly alcohol, Zhang et al. [134] synthesized 3DOM CeO2 that was loaded with Au–Pd alloys. 3DOM CeO2 photocatalytic materials are expected to emerge in the field of CO2 emission reduction, which could open up more possibilities for the development of super-catalysts.
3.5.2. Heterojunction
Researchers tried to combine CeO2 with g-C3N4, which is popular for its energy bands and chemical stability. Through hard work, a three-dimensional porous g-C3N4(3DCN) was achieved, with the advantages of multi-channel structure. To accommodate more electrons and heighten the density of photoelectric currents, Zhao et al. [125] loaded Pt nanoparticles (5–6 nm) on CeO2/3DCN using photodeposition techniques that require UV lamp radiation. The photoreduction rate gradually increased as the CeO2 amount rose in the range of 15~45% and the yield rates of 4.69 and 3.03 μmol·h−1·g−1 for CO and CH4 were achieved, respectively, after decorating with Pt crystalline grains.
Under mild reaction conditions, carbon-doped hexagonal boron nitride (h-BN), known as boron carbon nitride (BCN), can reduce more carbon dioxide after dispersing cerium oxides on it. In the BCN/CeO2 heterostructure [135], the N-O-Ce bond was formed by thermal precipitation method. It is of interest that the proportion of Ce3+/(Ce3+ + Ce4+) that influences electron transfer rate fluctuates with CeO2-loading amount, and the yield of CO peaked on 30%CeO2 (selected from 10% to 70%). When exposed to UV light, CeO2 crystals could absorb more photons than visible light, whereas h-BN does not exhibit absorption in the UV range. The establishment of heterojunction expanded the effective wavelengths and improved the absorption capability in ultraviolet and visible light. The •OH species was detected by ESR analysis to identify the type of the heterostructure and the result of no newly generated •OH species was in accord with the II-scheme system.
3.5.3. Summary
As a whole, the wider light harvesting range and longer separation time of charge pairs improved the photoreduction upshot. Overall, recent results on CO2 photoreduction by CeO2-based materials are presented in Table 5.

Table 5.
Summary of the recent results on the photoreduction of CO2 by CeO2-based materials.
4. Other 3DOM Materials
In order to introduce advanced porous structures to slow the self-aggregation of quantum dots, Wang et al. [143] devised 3DOM N-doped carbon (NC) to support CdS and ZnO QDs (Figure 20). They filled the interspace in an ordered PS microsphere template and then employed a pyrolytic treatment and in situ growth methods. Compared to bulky CdS, the 3DOM compounds have a larger cathodic current density and enhanced light harvesting, bearing a satisfactory carbon monoxide yield of 5210 μmol g−1 h−1. A three-dimensional SnO2 inverse opal structure was synthesized as gas sensors, soot oxidation catalyst and photoanode. The 3DOM BiVO4/SnO2 heterostructure was obtained by adding a BiVO4 precursor to fill the space between SnO2 skeleton and periodic PS template. The compatibility of energy states with SnO2 significantly reduced their photoluminescence intensity. Meanwhile, Au nanoparticles enhanced the slow photon effect, which in turn increased the incident light utilization efficiency.
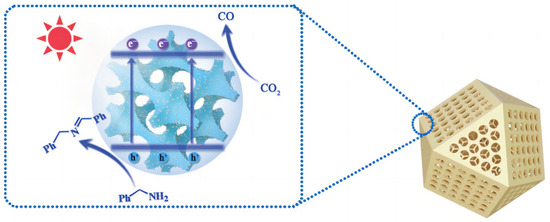
Figure 20.
Proposed mechanism for the photocatalytic CO2 reduction on 3DOM CdS QD/NC. Schematic illustration of the photocatalytic CO2 reduction coupled with selective arylamine oxidation reaction system [143].
Moreover, some photoreduction results on 3DOM materials are listed in Table 6. From the above analysis, we can infer the great potential of macroporous materials for further development in the field of photocatalysis.

Table 6.
Summary of the recent results on the photoreduction of CO2 and H2O by 3DOM materials.
5. Conclusions
The photoconversion of CO2 into solar fuels seems to curb greenhouse effect and resolve the energy crisis. In this review, the major research progresses of different metal oxide materials on solar-light-driven CO2 conversion to fuels were carefully summarized. TiO2, WO3, ZnO, Cu2O and CeO2 are the most common materials for photocatalysts among the numerous semiconductors. Even though considerable progress has been achieved, creating superb catalysts still presents several challenges. Researchers have presented treatment options to boost catalytic activity after apprehending the fundamental principles of objective reactions.
The main formulas for designing photocatalysts are as follows.
- Absorb more photons to produce excitons.
- Improve the migration efficiency of charge carriers.
- Reduce the recombination rate of electron–hole pairs.
- Uplift the absorption capacity of CO2.
In terms of material selection, it is wise to choose metal oxides as one of the active components because of their relatively suitable Eg and CB bands. From the perspective of morphology, compared with the solid and regular morphology, the structure of hollow ordered pores can promote the absorption of reactants and the desorption of products. A larger contact area between the catalyst and reactants and more active sites for the reaction can be provided. From the point of view of light absorption, the CB or VB positions can be adjusted after ion doping, which affects Eg and influences the position of spectral absorption; the slow photon effect in the 3DOM structure can also improve the light utilization. As for charge separation efficiency, it can be promoted at the interface of the heterojunction, and photonic crystals can also improve their separation efficiency by shortening the distance of charge movement to the interface. Numerous attempts have been made in the synthesis of metal oxide photocatalysts, which can yield CO, CH4, HCHO, HCOOH, CH3OH and C2H5OH. However, it remains a great challenge for current photocatalysts to satisfy actual industrial production demands. The efficiency and selectivity for target products cannot meet the requirements for industrial and commercial implementation.
To sum up, the migration–separation efficiency of photoinduced pairs is important to improve the catalytic activity. Scholars should concentrate on the multiple advantages of photonic crystals to design catalysts with better performance based on them. This review provided a wealth of experience and ideas for the exploitation of photocatalyst material selection, morphology control and active site design. There is still a considerable work to conduct in converting CO2 from solar energy to fuel, and we believe more significant breakthroughs can be achieved regarding the efficiency, mechanism and durability of the photocatalyst.
Funding
This research was funded by the National Natural Science Foundation of China (21972166, 22208373), Beijing Natural Science Foundation (2202045), the Technology Development Program of SINOPEC, China (Grant No. 321101) and National Key Research and Development Program of China (2019YFC1907600).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, Y.L.; Wang, Q.W.; Tan, T. Prediction of Carbon Dioxide Emissions in China Using Shallow Learning with Cross Validation. Energies 2022, 15, 8642. [Google Scholar] [CrossRef]
- Xu, X.; Liao, M. Prediction of Carbon Emissions in China’s Power Industry Based on the Mixed-Data Sampling (MIDAS) Regression Model. Atmosphere 2022, 13, 423. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, B.; Hu, H.; Zhang, Y.; Bi, Y. ZnO nanowire arrays decorated with PtO nanowires for efficient solar water splitting. Catal. Sci. Technol. 2018, 8, 2789–2793. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Luo, Y.; Liu, Y.; Ding, J.; Zhou, Z.; Liu, C.; Zou, M.; Lan, J.; Nan, C.-w.; et al. Enhanced CO2 Reduction Performance of BiCuSeO-Based Hybrid Catalysts by Synergetic Photo-Thermoelectric Effect. Adv. Funct. Mater. 2021, 31, 2105001. [Google Scholar] [CrossRef]
- Tang, R.F.; Wang, H.; Dong, X.A.; Zhang, S.H.; Zhang, L.L.; Dong, F. A ball milling method for highly dispersed Ni atoms on g-C3N4 to boost CO2 photoreduction. J. Colloid Interface Sci. 2023, 630, 290–300. [Google Scholar] [CrossRef]
- Shen, H.; Peppel, T.; Stunk, J.; Sun, Z. Photocatalytic Reduction of CO2 by Metal-Free-Based Materials: Recent Advances and Future Perspective. Solar Rrl 2020, 4, 1900546. [Google Scholar] [CrossRef]
- Irfan, S.; Khan, S.B.; Lam, S.S.; Ong, H.C.; Din, M.A.U.; Dong, F.; Chen, D.L. Removal of persistent acetophenone from industrial waste-water via bismuth ferrite nanostructures. Chemosphere 2022, 302, 134750. [Google Scholar] [CrossRef]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Rhimi, B.; Nejad, Z.G.; Karima, S.; Shahsavari, Z.; Wang, C. Multifunctional Ag/AgCl/ZnTiO3 structures as highly efficient photocatalysts for the removal of nitrophenols, CO2 photoreduction, biomedical waste treatment, and bacteria inactivation. Appl. Catal. A 2022, 643, 118794. [Google Scholar] [CrossRef]
- Padervand, M.; Rhimi, B.; Wang, C. One-pot synthesis of novel ternary Fe3N/Fe2O3/C3N4 photocatalyst for efficient removal of rhodamine B and CO2 reduction. J. Alloys Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Wang, C. K4Nb6O17/Fe3N/α-Fe2O3/C3N4 as an enhanced visible light-driven quaternary photocatalyst for acetamiprid photodegradation, CO2 reduction, and cancer cells treatment. Appl. Surf. Sci. 2021, 544, 148939. [Google Scholar] [CrossRef]
- Tran, D.P.H.; Pham, M.T.; Bui, X.T.; Wang, Y.F.; You, S.J. CeO2 as a photocatalytic material for CO2 conversion: A review. Sol. Energy 2022, 240, 443–466. [Google Scholar] [CrossRef]
- Ali, S.; Lee, J.; Kim, H.; Hwang, Y.; Razzaq, A.; Jung, J.-W.; Cho, C.-H.; In, S.-I. Sustained, photocatalytic CO2 reduction to CH4 in a continuous flow reactor by earth-abundant materials: Reduced titania-Cu2O Z-scheme heterostructures. Appl. Catal. B 2020, 279, 119344. [Google Scholar] [CrossRef]
- Li, R.; Luan, Q.; Dong, C.; Dong, W.; Tang, W.; Wang, G.; Lu, Y. Light-facilitated structure reconstruction on self-optimized photocatalyst TiO2@BiOCl for selectively efficient conversion of CO2 to CH4. Appl. Catal. B. 2021, 286, 119832. [Google Scholar] [CrossRef]
- Jiao, W.Y.; Xie, Y.; He, F.; Wang, K.Y.; Ling, Y.; Hu, Y.Y.; Wang, J.L.; Ye, H.; Wu, J.; Hou, Y. A visible light-response flower-like La-doped BiOBr nanosheets with enhanced performance for photoreducing CO2 to CH3OH. Chem. Eng. J. 2021, 418, 129286. [Google Scholar] [CrossRef]
- Luévano, H.E.; Torres, M.L.M.; Fernández, T.A. Ternary ZnO/CuO/Zeolite composite obtained from volcanic ash for photocatalytic CO2 reduction and H2O decomposition. J. Phys. Chem. Solids 2021, 151, 109917. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Huang, H.; Zhang, Y.; Ma, T. Surface sites engineering on semiconductors to boost photocatalytic CO2 reduction. Nano Energy 2020, 75, 126799. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Mao, B.D.; Li, D.; Liu, Y.H.; Li, F.H.; Dong, W.X.; Jiang, T.Y.; Shi, W.D. 0D/2D Z-scheme heterojunctions of Zn-AgIn5S8 QDs/alpha-Fe2O3 nanosheets for efficient visible-light-driven hydrogen production. Chem. Eng. J. 2021, 417, 128275. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.R.; Ayub, I.; Wang, S.L.; Yan, W. In situ decoration of g-C3N4 quantum dots on 1D branched TiO2 loaded with plasmonic Au nanoparticles and improved the photocatalytic hydrogen evolution activity. Appl. Surf. Sci. 2020, 519, 146208. [Google Scholar] [CrossRef]
- Li, M.; Ma, L.N.; Luo, L.; Liu, Y.G.; Xu, M.; Zhou, H.; Wang, Y.; Li, Z.H.; Kong, X.G.; Duan, H.H. Efficient photocatalytic epoxidation of styrene over a quantum-sized SnO(2)on carbon nitride as a heterostructured catalyst. Appl. Catal. B. 2022, 309, 121268. [Google Scholar] [CrossRef]
- Xiong, H.; Dong, Y.; Liu, D.; Long, R.; Kong, T.; Xiong, Y. Recent Advances in Porous Materials for Photocatalytic CO2 Reduction. J. Phys. Chem. Lett. 2022, 13, 1272–1282. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Wu, M.; Van der Schueren, B.; Li, Y.; Deparis, O.; Ye, J.; Ozin, G.A.; Hasan, T.; Su, B.L. Slow Photons for Photocatalysis and Photovoltaics. Adv. Mater. 2017, 29, 1605349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, Z.; Liu, J.; Li, Y.; Wu, M.; Van Tendeloo, G.; Su, B.-L. Blue-edge slow photons promoting visible-light hydrogen production on gradient ternary 3DOM TiO2-Au-CdS photonic crystals. Nano Energy 2018, 47, 266–274. [Google Scholar] [CrossRef]
- Chen, J.I.L.; von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Amplified Photochemistry with Slow Photons. Adv. Mater. 2006, 18, 1915–1919. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Liu, J.; Deng, Z.; Li, Y.; Deparis, O.; Su, B.-L. High photocatalytic activity enhancement of titania inverse opal films by slow photon effect induced strong light absorption. J. Mater. Chem. 2013, 1, 15491–15500. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Deng, Z.; Su, B.L. Three-dimensionally ordered macroporous titania with structural and photonic effects for enhanced photocatalytic efficiency. ChemSusChem 2011, 4, 1481–1488. [Google Scholar] [CrossRef]
- Li, J.F.; Zhong, C.Y.; Huang, J.R.; Chen, Y.; Wang, Z.; Liu, Z.Q. Carbon dots decorated three-dimensionally ordered macroporous bismuth-doped titanium dioxide with efficient charge separation for high performance photocatalysis. J. Colloid Interface Sci. 2019, 553, 758–767. [Google Scholar] [CrossRef] [PubMed]
- He, H.K.; Zhong, M.J.; Konkolewicz, D.; Yacatto, K.; Rappold, T.; Sugar, G.; David, N.E.; Gelb, J.; Kotwal, N.; Merkle, A.; et al. Three-Dimensionally Ordered Macroporous Polymeric Materials by Colloidal Crystal Templating for Reversible CO2 Capture. Adv. Funct. Mater. 2013, 23, 4720–4728. [Google Scholar] [CrossRef]
- Wang, P.F.; Zhan, S.H. Shedding light on the role of interfacial chemical bond in heterojunction photocatalysis. Nano Res. 2022, 15, 10158–10170. [Google Scholar]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Yan, X.W.; Wang, B.; Zhao, J.Z.; Liu, G.P.; Ji, M.X.; Zhang, X.L.; Chu, P.K.; Li, H.M.; Xia, J.X. Hierarchical columnar ZnIn2S4/BiVO4 Z-scheme heterojunctions with carrier highway boost photocatalytic mineralization of antibiotics. Chem. Eng. J. 2023, 452, 139271. [Google Scholar] [CrossRef]
- Ji, M.X.; Feng, J.; Zhao, J.Z.; Zhang, Y.; Wang, B.; Di, J.; Xu, X.Y.; Chen, Z.R.; Xia, J.X.; Li, H.M. Defect-Engineered Bi24O31Cl10 Nanosheets for Photocatalytic CO2 Reduction to CO br. ACS Appl. Nano Mater. 2022, 5, 17226–17233. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y.J. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Shown, I.; Samireddi, S.; Chang, Y.C.; Putikam, R.; Chang, P.H.; Sabbah, A.; Fu, F.Y.; Chen, W.F.; Wu, C.I.; Yu, T.Y.; et al. Carbon-doped SnS2 nanostructure as a high-efficiency solar fuel catalyst under visible light. Nat. Commun. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.P.; Ou, M.; Wang, X.M.; Wang, Y.A.; Zeng, Y.Q.; Ding, J.; Zhang, S.L.; Zhong, Q. Facile fabrication of oxygen and carbon co-doped carbon nitride nanosheets for efficient visible light photocatalytic H2 evolution and CO2 reduction. Dalton Trans. 2019, 48, 12070–12079. [Google Scholar] [CrossRef]
- Zhang, D.P.; Li, Y.X.; Li, Y.; Zhan, S.H. Towards single-atom photocatalysts for future carbon-neutral application. Smartmat 2022, 3, 417–446. [Google Scholar] [CrossRef]
- Kim, A.; Debecker, D.P.; Devred, F.; Dubois, V.; Sanchez, C.; Sassoye, C. CO2 methanation on Ru/TiO2 catalysts: On the effect of mixing anatase and rutile TiO2 supports. Appl. Catal. B. 2018, 220, 615–625. [Google Scholar] [CrossRef]
- Yang, X.; Tan, F.; Wang, D.; Feng, Q.; Qiu, D.; Dang, D.; Wang, X. Entrapping Ru nanoparticles into TiO2 nanotube: Insight into the confinement synergy on boosting pho-thermal CO2 methanation activity. Ceram. Int. 2021, 47, 27316–27323. [Google Scholar] [CrossRef]
- Kar, P.; Farsinezhad, S.; Mahdi, N.; Zhang, Y.; Obuekwe, U.; Sharma, H.; Shen, J.; Semagina, N.; Shankar, K. Enhanced CH4 yield by photocatalytic CO2 reduction using TiO2 nanotube arrays grafted with Au, Ru, and ZnPd nanoparticles. Nano Res. 2016, 9, 3478–3493. [Google Scholar] [CrossRef]
- Dong, C.Y.; Xing, M.Y.; Zhang, J.L. Double-cocatalysts promote charge separation efficiency in CO2 photoreduction: Spatial location matters. Mater. Horiz. 2016, 3, 608–612. [Google Scholar] [CrossRef]
- Ma, Y.J.; Tang, Q.; Sun, W.Y.; Yao, Z.Y.; Zhu, W.H.; Li, T.; Wang, J.Y. Assembling ultrafine TiO2 nanoparticles on UiO-66 octahedrons to promote selective photocatalytic conversion of CO2 to CH4 at a low concentration. Appl. Catal. B. 2020, 270, 118856. [Google Scholar] [CrossRef]
- Hu, X.L.; Song, J.Y.; Luo, J.L.; Zhang, H.; Sun, Z.M.; Li, C.Q.; Zheng, S.L.; Liu, Q.X. Single-atomic Pt sites anchored on defective TiO2 nanosheets as a superior photocatalyst for hydrogen evolution. J. Energy Chem. 2021, 62, 1–10. [Google Scholar] [CrossRef]
- Yang, G.; Qiu, P.; Xiong, J.Y.; Zhu, X.T.; Cheng, G. Facilely anchoring Cu2O nanoparticles on mesoporous TiO2 nanorods for enhanced photocatalytic CO2 reduction through efficient charge transfer. Chin. Chem. Lett. 2022, 33, 3709–3712. [Google Scholar] [CrossRef]
- Zalfani, M.; Hu, Z.Y.; Yu, W.B.; Mahdouani, M.; Bourguiga, R.; Wu, M.; Li, Y.; Van Tendeloo, G.; Djaoued, Y.; Su, B.L. BiVO4/3DOM TiO2 nanocomposites: Effect of BiVO4 as highly efficient visible light sensitizer for highly improved visible light photocatalytic activity in the degradation of dye pollutants. Appl. Catal. B. 2017, 205, 121–132. [Google Scholar] [CrossRef]
- Jiao, J.Q.; Wei, Y.C.; Zhao, Z.; Liu, J.; Li, J.M.; Duan, A.J.; Jiang, G.Y. Photocatalysts of 3D Ordered Macroporous TiO2-Supported CeO2 Nano layers: Design, Preparation, and Their Catalytic Performances for the Reduction of CO2 with H2O under Simulated Solar Irradiation. Ind. Eng. Chem. Res. 2014, 53, 17345–17354. [Google Scholar] [CrossRef]
- Wang, C.J.; Liu, X.; He, W.J.; Zhao, Y.L.; Wei, Y.C.; Xiong, J.; Liu, J.; Li, J.M.; Song, W.Y.; Zhang, X.; et al. All-solid-state Z-scheme photocatalysts of g-C3N4/Pt/macroporous-(TiO2@carbon) for selective boosting visible-light-driven conversion of CO2 to CH4. J. Catal. 2020, 389, 440–449. [Google Scholar] [CrossRef]
- Wu, X.X.; Wang, C.J.; Wei, Y.C.; Xiong, J.; Zhao, Y.L.; Zhao, Z.; Liu, J.; Li, J.M. Multifunctional photocatalysts of Pt-decorated 3DOM perovskite-type SrTiO3 with enhanced CO2 adsorption and photoelectron enrichment for selective CO2 reduction with H2O to CH4. J. Catal. 2019, 377, 309–321. [Google Scholar] [CrossRef]
- Li, Y.F.; Tang, J.J.; Wei, Y.C.; He, W.J.; Tang, Z.L.; Zhang, X.; Xiong, J.; Zhao, Z. The heterojunction between 3D ordered macroporous TiO2 and MoS2 nanosheets for enhancing visible-light driven CO2 reduction. J. CO2 Util. 2021, 51, 101648. [Google Scholar] [CrossRef]
- Chen, C.; Wang, T.; Yan, K.; Liu, S.J.; Zhao, Y.; Li, B.X. Photocatalytic CO2 reduction on Cu single atoms incorporated in ordered macroporous TiO2 toward tunable products. Inorg. Chem. Front. 2022, 9, 4753–4767. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y.; Zhao, Z.; Duan, A.; Liu, J.; Pang, Y.; Li, J.; Jiang, G.; Wang, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B. 2017, 209, 228–239. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, J.; Li, C.F.; Zhang, X.; Li, Y.; Hu, Z.Y.; Li, B.; Chen, Z.X.; Hu, J.G.; Su, B.L. Meso-Microporous Nanosheet-Constructed 3DOM Perovskites for Remarkable Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2022, 32, 2112831. [Google Scholar] [CrossRef]
- Tahir, M.S.; Manzoor, N.; Sagir, M.; Tahir, M.B.; Nawaz, T. Fabrication of ZnFe2O4 modified TiO2 hybrid composites for photocatalytic reduction of CO2 into methanol. Fuel 2021, 285, 119206. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef] [PubMed]
- Seeharaj, P.; Kongmun, P.; Paiplod, P.; Prakobmit, S.; Sriwong, C.; Kim-Lohsoontorn, P.; Vittayakorn, N. Ultrasonically-assisted surface modified TiO2/rGO/CeO2 heterojunction photocatalysts for conversion of CO2 to methanol and ethanol. Ultrason. Sonochem. 2019, 58, 104657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thakur, P.R.; Sharma, G.; Vo, D.N.; Naushad, M.; Tatarchuk, T.; Garcia-Penas, A.; Du, B.; Stadler, F.J. Accelerated charge transfer in well-designed S-scheme Fe@TiO2/Boron carbon nitride heterostructures for high performance tetracycline removal and selective photo-reduction of CO2 greenhouse gas into CH4 fuel. Chemosphere 2022, 287, 132301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Teng, F.; Ye, X.Y.; Zheng, H.J.; Fang, X.S. Photo/Electrochemical Applications of Metal Sulfide/TiO2 Heterostructures. Adv. Energy Mater. 2020, 10, 1902355. [Google Scholar] [CrossRef]
- Hasanvandian, F.; Zehtab Salmasi, M.; Moradi, M.; Farshineh Saei, S.; Kakavandi, B.; Rahman Setayesh, S. Enhanced spatially coupling heterojunction assembled from CuCo2S4 yolk-shell hollow sphere capsulated by Bi-modified TiO2 for highly efficient CO2 photoreduction. Chem. Eng. J. 2022, 444, 136493. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.Y.; Li, N.; Xia, J.H.; Meng, Q.M.; Ding, J.C.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef]
- Mgolombane, M.; Bankole, O.M.; Ferg, E.E.; Ogunlaja, A.S. Construction of Co-doped TiO2/rGO nanocomposites for high-performance photoreduction of CO2 with H2O: Comparison of theoretical binding energies and exploration of surface chemistry. Mater. Chem. Phys. 2021, 268, 124733. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.-I.; Park, S.-M.; Kang, M. A p-n heterojunction NiS-sensitized TiO2 photocatalytic system for efficient photoreduction of carbon dioxide to methane. Ceram. Int. 2017, 43, 1768–1774. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Zakaria, Z.Y. Photo-induced reduction of CO2 to CO with hydrogen over plasmonic Ag-NPs/TiO 2 NWs core/shell hetero-junction under UV and visible light. J. CO2 Util. 2017, 18, 250–260. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Chen, H.; Wang, K.; Wang, X. Photocatalytic CO2 reduction with water vapor to CO and CH4 in a recirculation reactor by Ag-Cu2O/TiO2 Z-scheme heterostructures. J. Alloys Compd. 2022, 896, 163030. [Google Scholar] [CrossRef]
- Wei, Y.C.; Jiao, J.Q.; Zhao, Z.; Zhong, W.J.; Li, J.M.; Liu, J.; Jiang, G.Y.; Duan, A.J. 3D ordered macroporous TiO2-supported Pt@CdS core-shell nanoparticles: Design, synthesis and efficient photocatalytic conversion of CO2 with water to methane. J. Mater. Chem. A 2015, 3, 11074–11085. [Google Scholar] [CrossRef]
- He, W.J.; Wu, X.X.; Li, Y.F.; Xiong, J.; Tang, Z.L.; Wei, Y.C.; Zhao, Z.; Zhang, X.; Liu, J. Z-scheme heterojunction of SnS2-decorated 3DOM-SrTiO3 for selectively photocatalytic CO2 reduction into CH4. Chin. Chem. Lett. 2020, 31, 2774–2778. [Google Scholar] [CrossRef]
- Baserga, A.; Russo, V.; Di Fonzo, F.; Bailini, A.; Cattaneo, D.; Casari, C.S.; Bassi, A.L.; Bottani, C.E. Nanostructured tungsten oxide with controlled properties: Synthesis and Raman characterization. Thin Solid Films 2007, 515, 6465–6469. [Google Scholar] [CrossRef]
- Cheng, H.F.; Huang, B.B.; Liu, Y.Y.; Wang, Z.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. An anion exchange approach to Bi2WO6 hollow microspheres with efficient visible light photocatalytic reduction of CO2 to methanol. Chem. Commun. 2012, 48, 9729–9731. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Bian, J.; Sun, N.; Sun, J.; Chen, L.; Li, Z.; Jing, L. Controlled synthesis of novel Z-scheme iron phthalocyanine/porous WO3 nanocomposites as efficient photocatalysts for CO2 reduction. Appl. Catal. B. 2020, 270, 1188849. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Cheng, Y.; Liu, Z.; Guo, Q.; Minh Ngoc, H.; Zhao, Z. Hydrogen-treated mesoporous WO3 as a reducing agent of CO2 to fuels (CH4 and CH3OH) with enhanced photothermal catalytic performance. J. Mater. Chem. A 2016, 4, 5314–5322. [Google Scholar] [CrossRef]
- Rong, R.C.; Wang, L.M. Synthesis of hierarchical hollow nest-like WO3 micro/nanostructures with enhanced visible light-driven photocatalytic activity. J. Alloys Compd. 2021, 850, 156742. [Google Scholar] [CrossRef]
- Hao, Z.C.; Liu, Z.F.; Li, Y.T.; Ruan, M.N.; Guo, Z.G. Enhanced photoelectrochemical performance of 2D core-shell WO3/CuWO4 uniform heterojunction via in situ synthesis and modification of Co-Pi co-catalyst. Int. J. Hydrog. Energy 2020, 45, 16550–16559. [Google Scholar] [CrossRef]
- Ren, X.Z.; Wu, K.; Qin, Z.G.; Zhao, X.C.; Yang, H. The construction of type II heterojunction of Bi2WO6/BiOBr photocatalyst with improved photocatalytic performance. J. Alloys Compd. 2019, 788, 102–109. [Google Scholar] [CrossRef]
- Ling, P.Q.; Zhu, J.C.; Wang, Z.Q.; Hu, J.; Zhu, J.F.; Yan, W.S.; Sun, Y.F.; Xie, Y. Ultrathin Ti-doped WO3 nanosheets realizing selective photoreduction of CO2 to CH3OH. Nanoscale 2022, 14, 14023–14028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Tian, W.J.; Li, Y.G.; Sun, H.Q.; Tade, M.O.; Wang, S.B. Heterostructured WO3@CoWO4 bilayer nanosheets for enhanced visible-light photo, electro and photoelectro-chemical oxidation of water. J. Mater. Chem. A 2018, 6, 6265–6272. [Google Scholar] [CrossRef]
- Hu, Y.F.; Ping, X.C.; Zhang, Y.; Hao, L.; Liu, T.Y.; Zhao, Q.; Lu, Y.; Liu, J.Z. A nanotree-like WO3 film with adjustable defect concentration and its photocatalytic activity. Mater. Sci. Semicond. Process. 2021, 127, 105737. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.D.; Sun, Y.F.; Tan, Y.L.; Jiao, X.C.; Ju, H.X.; Qi, Z.M.; Zhu, J.F.; Xie, Y. Infrared Light-Driven CO2 Overall Splitting at Room Temperature. Joule 2018, 2, 1004–1016. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhou, Y.; Liu, Q.; Li, Z.D.; Liu, J.G.; Zou, Z.G. Ultrathin, Single-Crystal WO3 Nanosheets by Two-Dimensional Oriented Attachment toward Enhanced Photocatalystic Reduction of CO2 into Hydrocarbon Fuels under Visible Light. ACS Appl. Mater. Interfaces 2012, 4, 3372–3377. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lin, M.; Long, J.; Zhang, Z.; Lin, H.; Wu, J.C.; Wang, X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Z.P.; Zhao, Z.Y.; Liu, Q.; Kou, J.H.; Chen, X.Y.; Gao, J.; Yan, S.C.; Zou, Z.G. High-Yield Synthesis of Ultrathin and Uniform Bi2WO6 Square Nanoplates Benefitting from Photocatalytic Reduction of CO2 into Renewable Hydrocarbon Fuel under Visible Light. ACS Appl. Mater. Interfaces 2011, 3, 3594–3601. [Google Scholar] [CrossRef]
- Zhang, K.F.; Chen, H.X.; Liu, Y.X.; Deng, J.G.; Jing, L.; Rastegarpanah, A.L.; Pei, W.B.; Han, Z.; Dai, H.X. Two-dimensional Bi2WxMo1_xO6 solid solution nanosheets for enhanced photocatalytic toluene oxidation to benzaldehyde. Appl. Catal. B 2022, 315, 121545. [Google Scholar] [CrossRef]
- Chang, Y.; Yu, K.; Zhang, C.; Li, R.; Zhao, P.; Lou, L.-L.; Liu, S. Three-dimensionally ordered macroporous WO3 supported Ag3PO4 with enhanced photocatalytic activity and durability. Appl. Catal. B. 2015, 176–177, 363–373. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, C.; He, W.; Wei, Y.; Zhao, Z.; Liu, J. The Z-scheme g-C3N4/3DOM-WO3 photocatalysts with enhanced activity for CO2 photoreduction into CO. Chin. Chem. Lett. 2022, 33, 939–942. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, Y.; Li, D.; Chen, L.; Meng, S.; Jiang, D.; Chen, M. Construction of CuO quantum Dots/WO3 nanosheets 0D/2D Z-scheme heterojunction with enhanced photocatalytic CO2 reduction activity under visible-light. J. Alloys Compd. 2021, 858, 157668. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Yuan, H.; Sun, X.; Li, X.; Duan, L.; Li, Q.; Huang, Z.; Ban, X.; Zhang, D. Construction of melamine foam–supported WO3/CsPbBr3 S–scheme heterojunction with rich oxygen vacancies for efficient and long–period CO2 photoreduction in liquid–phase H2O environment. Chem. Eng. J. 2022, 430, 132820. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Wang, K.; Sun, X.; Wang, W. Enhanced photocatalytic CO2 reduction to methane over WO3·0.33H2O via Mo doping. Appl. Catal. B. 2019, 243, 771–779. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, W.R.; Chen, C.Y.; Wu, R.J. Preparation of Pd–Au/TiO2–WO3 to enhance photoreduction of CO2 to CH4 and CO. J. CO2 Util. 2018, 28, 247–254. [Google Scholar] [CrossRef]
- Chen, P.; Du, T.; Jia, H.; Zhou, L.; Yue, Q.; Wang, H.; Wang, Y. A novel Bi2WO6/Si heterostructure photocatalyst with Fermi level shift in valence band realizes efficient reduction of CO2 under visible light. Appl. Surf. Sci. 2022, 585, 152665. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Song, X.; Huang, B.; Ma, X.; Xing, R. CdS/WO3 S-scheme heterojunction with improved photocatalytic CO2 reduction activity. J. Photochem. Photobiol. B 2022, 233, 112480. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Sun, L.; Liu, R.; Cui, S. Studies of Z-scheme WO3-TiO2/Cu2ZnSnS4 ternary nanocomposite with enhanced CO2 photoreduction under visible light irradiation. J. CO2 Util. 2020, 37, 260–271. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Nawawi, M.G.M. Highly sTable 3D/2D WO3/g-C3N4 Z-scheme heterojunction for stimulating photocatalytic CO2 reduction by H2O/H2 to CO and CH4 under visible light. J. CO2 Util. 2020, 41, 101270. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, P.; Hu, J.; Pan, J.; Fan, J.; Shao, G. One-dimensional Z-scheme TiO2/WO3/Pt heterostructures for enhanced hydrogen generation. Appl. Surf. Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Alhaddad, M.; Basaleh, A.; Alzahrani, K.A.; Ismail, A.A. Enhanced CO2 photocatalytic conversion into CH3OH over visible-light-driven Pt nanoparticle-decorated mesoporous ZnO–ZnS S-scheme heterostructures. Ceram. Int. 2021, 47, 26779–26788. [Google Scholar] [CrossRef]
- Cai, C.; Xu, Y.F.; Chen, H.Y.; Wang, X.D.; Kuang, D.B. Porous ZnO@ZnSe nanosheet array for photoelectrochemical reduction of CO2. Electrochim. Acta 2018, 274, 298–305. [Google Scholar] [CrossRef]
- Xia, W.W.; Mei, C.; Zeng, X.H.; Chang, S.; Wu, G.Q.; Shen, X.S. Mesoporous multi-shelled ZnO microspheres for the scattering layer of dye sensitized solar cell with a high efficiency. Appl. Phys. Lett. 2016, 108, 113902. [Google Scholar] [CrossRef]
- Wang, P.; Yang, M.; Tang, S.; Li, Y.; Lin, X.; Zhang, H.; Zhu, Z.; Chen, F. Z-scheme heterojunctions composed of 3D graphene aerogel/g-C3N4 nanosheets/porous ZnO nanospheres for the efficient photocatalytic reduction of CO2 with H2O under visible light irradiation. J. Alloys Compd. 2022, 918, 165607. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, Y.; Thirugnanam, N.; Li, G. Double Z-scheme ZnO/ZnS/g-C3N4 ternary structure for efficient photocatalytic H2 production. Appl. Surf. Sci. 2018, 430, 293–300. [Google Scholar] [CrossRef]
- Taraka, T.P.Y.; Gautam, A.; Jain, S.L.; Bojja, S.; Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO p-n heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 2019, 31, 207–214. [Google Scholar] [CrossRef]
- Chen, S.Q.; Yu, J.G.; Zhang, J. Enhanced photocatalytic CO2 reduction activity of MOF-derived ZnO/NiO porous hollow spheres. J. CO2 Util. 2018, 24, 548–554. [Google Scholar] [CrossRef]
- Saravanan, R.; Agarwal, S.; Gupta, V.K.; Khan, M.M.; Gracia, F.; Mosquera, E.; Narayanan, V.; Stephen, A. Line defect Ce3+ induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J. Photochem. Photobiol. A 2018, 353, 499–506. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Z.; Qi, S.H.; Yin, Z.Z.; Deng, S.K.; Zhang, M.; Sun, Z.Q. The quaternary system of Ag2S/ZnS co-modified ZnO/TiO2 nanotree arrays: Excellent photocatalysis and photoelectrochemistry performance. Appl. Surf. Sci. 2021, 538, 148044. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Wang, S.; Li, Y.; Guo, Y.; Luan, D.; Gu, X.; Lou, X.W. Implanting CoOx Clusters on Ordered Macroporous ZnO Nanoreactors for Efficient CO2 Photoreduction. Adv. Mater. 2022, 34, 2204865. [Google Scholar] [CrossRef]
- Tang, Z.; Zhu, F.; Zhou, J.; Chen, W.; Wang, K.; Liu, M.; Wang, N.; Li, N. Monolithic NF@ZnO/Au@ZIF-8 photocatalyst with strong photo-thermal-magnetic coupling and selective-breathing effects for boosted conversion of CO2 to CH4. Appl. Catal. B. 2022, 309, 121267. [Google Scholar] [CrossRef]
- Xie, F.; Guo, J.-F.; Wang, H.-T.; Chang, N. Enhancing visible light photocatalytic activity by transformation of Co3+/Co2+ and formation of oxygen vacancies over rationally Co doped ZnO microspheres. Colloids Surf. A 2022, 636, 128257. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Shi, Z.; Ma, J.; Wang, Z. Cellulose template designed porous ZnO based catalysts with different valence copper for solar photocatalytic CO2 conversion. Ind. Crops Prod. 2022, 186, 115223. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Liu, C.; Hsieh, T.-T. Efficient visible and NIR light-driven photocatalytic CO2 reduction over defect-engineered ZnO/carbon dot hybrid and mechanistic insights. J. Catal. 2020, 391, 298–311. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.-H.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B. 2020, 268, 118380. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Q.; Wang, H.; Liu, Z.; Zhao, Z. Core-shell structured ZnO@Cu-Zn–Al layered double hydroxides with enhanced photocatalytic efficiency for CO2 reduction. Catal. Commun. 2016, 77, 118–122. [Google Scholar] [CrossRef]
- Zhang, P.P.; Zhou, Z.J.; Kou, D.X.; Wu, S.X. Perovskite Thin Film Solar Cells Based on Inorganic Hole Conducting Materials. Int. J. Photoenergy 2017, 2017, 6109092. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Zhang, J.; Dai, K.; Fan, K.; Dawson, G. Branch-like CdxZn1-xSe/Cu2O@Cu step-scheme heterojunction for CO2 photoreduction. Mater. Today Phys. 2022, 26, 100729. [Google Scholar] [CrossRef]
- Yao, S.; Sun, B.Q.; Zhang, P.; Tian, Z.Y.; Yin, H.Q.; Zhang, Z.M. Anchoring ultrafine Cu2O nanocluster on PCN for CO2 photoreduction in water vapor with much improved stability. Appl. Catal. B. 2022, 317, 121702. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Shi, J.J.; Tao, S.; Wu, L.; Lu, J. Cu2O/Ti3C2 MXene heterojunction photocatalysts for improved CO2 photocatalytic reduction performance. Appl. Surf. Sci. 2021, 542, 148685. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.; Xu, C.; Li, Y.; Zhang, J.Y.; Wu, L.; Liu, Y.F.; Fang, Y.Z.; Liu, Z.F. Optional construction of Cu2O@Fe2O3@CC architecture as a robust multifunctional photoelectronic catalyst for overall water splitting and CO2 reduction. Chem. Eng. J. 2021, 426, 131192. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zhang, H.; Guo, L.; Zheng, K.; Han, X.D.; Zhang, Z. Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorption ability. J. Mater. Chem. 2009, 19, 5220–5225. [Google Scholar] [CrossRef]
- Tang, Z.; He, W.; Wang, Y.; Wei, Y.; Yu, X.; Xiong, J.; Wang, X.; Zhang, X.; Zhao, Z.; Liu, J. Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B. 2022, 311, 121371. [Google Scholar] [CrossRef]
- Singh, S.; Punia, R.; Pant, K.K.; Biswas, P. Effect of work-function and morphology of heterostructure components on CO2 reduction photo-catalytic activity of MoS2-Cu2O heterostructure. Chem. Eng. J. 2022, 433, 132709. [Google Scholar] [CrossRef]
- Cui, L.; Hu, L.; Shen, Q.; Liu, X.; Jia, H.; Xue, J. Three-dimensional porous Cu2O with dendrite for efficient photocatalytic reduction of CO2 under visible light. Appl. Surf. Sci. 2022, 581, 152343. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Cai, T.; Kou, L.; Yang, G.; Yan, Z. Preparation and nitrogen-doping of three-dimensionally ordered macroporous TiO2 with enhanced photocatalytic activity. Ceram. Int. 2014, 40, 11213–11219. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Han, J.; Fu, Y.; Deng, Y.; Liu, Y.Y.; Yang, Y.; Wang, T.; Zhang, L.W. Highly efficient CO2 reduction on ordered porous Cu electrode derived from Cu2O inverse opals. Nano Energy 2018, 48, 93–100. [Google Scholar] [CrossRef]
- Liu, S.H.; Lu, J.S.; Pu, Y.C.; Fan, H.C. Enhanced photoreduction of CO2 into methanol by facet-dependent Cu2O/reduce graphene oxide. J. CO2 Util. 2019, 33, 171–178. [Google Scholar] [CrossRef]
- Flores, F.M.; Luevano, H.E.; Torres, M.L.M.; Do, T.O. CO2 adsorption and photocatalytic reduction over Mg(OH)(2)/CuO/Cu2O under UV-Visible light to solar fuels. Mater. Chem. Phys. 2019, 227, 90–97. [Google Scholar] [CrossRef]
- Ojha, N.; Bajpai, A.; Kumar, S. Enriched oxygen vacancies of Cu2O/SnS2/SnO2 heterostructure for enhanced photocatalytic reduction of CO2 by water and nitrogen fixation. J. Colloid Interface Sci. 2021, 585, 764–777. [Google Scholar] [CrossRef]
- Dedong, Z.; Maimaiti, H.; Awati, A.; Yisilamu, G.; Fengchang, S.; Ming, W. Synthesis and photocatalytic CO2 reduction performance of Cu2O/Coal-based carbon nanoparticle composites. Chem. Phys. Lett. 2018, 700, 27–35. [Google Scholar] [CrossRef]
- Shi, H.N.; Wang, H.Z.; Zhou, Y.C.; Li, J.H.; Zhai, P.L.; Li, X.Y.; Gurzadyan, G.G.; Hou, J.G.; Yang, H.; Guo, X.W. Atomically Dispersed Indium-Copper Dual-Metal Active Sites Promoting C-C Coupling for CO2 Photoreduction to Ethanol. Angew. Chem. Int. Ed. 2022, 61, e202208904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, L.; Jin, X.; Xu, M.; Yin, S.; Li, J.; Li, X.; Shen, D.; Yan, Y.; Huo, P. Cu media constructed Z-scheme heterojunction of UiO-66-NH2/Cu2O/Cu for enhanced photocatalytic induction of CO2. Appl. Surf. Sci. 2021, 545, 148967. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, W.; Zhao, L.; Chen, H.; He, X.; Li, W.; Tian, P.; Huang, Z. g-C3N4 foam/Cu2O QDs with excellent CO2 adsorption and synergistic catalytic effect for photocatalytic CO2 reduction. Environ. Int. 2019, 130, 104898. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xia, Q.; Li, Y.; Yin, C.; Ge, Z.; Li, X.; Wang, Y. Adsorption-enhanced nitrogen-doped mesoporous CeO2 as an efficient visible-light-driven catalyst for CO2 photoreduction. J. CO2 Util. 2020, 39, 101176. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.; Li, J.; Li, X.; Wang, H.; Huo, P.; Yan, Y. CeO2/3D g-C3N4 heterojunction deposited with Pt cocatalyst for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 537, 147891. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, P.J.; Wang, Z.W.; Shi, Y.J.; Wang, Z.H.; Peng, Y.; Jing, L.; Liu, Y.X.; Yu, X.H.; Wang, X.; et al. Synergistic Effect of Reactive Oxygen Species in Photothermocatalytic Removal of VOCs from Cooking Oil Fumes over Pt/CeO2/TiO2. Environ. Sci. Technol. 2022, 56, 17341–17351. [Google Scholar] [CrossRef]
- Zhao, R.F.; Huan, L.; Gu, P.; Guo, R.; Chen, M.; Diao, G.W. Yb,Er-doped CeO2 nanotubes as an assistant layer for photoconversion-enhanced dye-sensitized solar cells. J. Power Sources 2016, 331, 527–534. [Google Scholar] [CrossRef]
- Yang, Z.J.; Wei, J.J.; Yang, H.; Liu, L.X.; Liang, H.; Yang, Y.Z. Mesoporous CeO2 Hollow Spheres Prepared by Ostwald Ripening and Their Environmental Applications. Eur. J. Inorg. Chem. 2010, 2010, 3354–3359. [Google Scholar] [CrossRef]
- Ramani, S.; Sarkar, S.; Vemuri, V.; Peter, S.C. Chemically designed CeO2 nanoboxes boost the catalytic activity of Pt nanoparticles toward electro-oxidation of formic acid. J. Mater. Chem. A 2017, 5, 11572–11576. [Google Scholar] [CrossRef]
- Fang, S.; Xin, Y.; Ge, L.; Han, C.; Qiu, P.; Wu, L. Facile synthesis of CeO2 hollow structures with controllable morphology by template-engaged etching of Cu2O and their visible light photocatalytic performance. Appl. Catal. B. 2015, 179, 458–467. [Google Scholar] [CrossRef]
- Kumari, N.; Haider, M.A.; Agarwal, M.; Sinha, N.; Basu, S. Role of Reduced CeO2(110) Surface for CO2 Reduction to CO and Methanol. J. Phys. Chem. C 2016, 120, 16626–16635. [Google Scholar] [CrossRef]
- Park, H.R.; Pawar, A.U.; Pal, U.; Zhang, T.; Kang, Y.S. Enhanced solar photoreduction of CO2 to liquid fuel over rGO grafted NiO-CeO2 heterostructure nanocomposite. Nano Energy 2021, 79, 105483. [Google Scholar] [CrossRef]
- Asu, S.P.; Sompalli, N.K.; Kuppusamy, S.; Mohan, A.M.; Deivasigamani, P. CaO/CeO2 nanocomposite dispersed macro-/meso-porous polymer monoliths as new generation visible light heterogeneous photocatalysts. Mater. Today Sustain. 2022, 19, 2317–2589. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.X.; Deng, J.G.; Yu, X.H.; Han, Z.; Zhang, K.F.; Dai, H.X. Alloying of gold with palladium: An effective strategy to improve catalytic stability and chlorine-tolerance of the 3DOM CeO2 -supported catalysts in trichloroethylene combustion. Appl. Catal. B. 2019, 257, 117879. [Google Scholar] [CrossRef]
- Mei, Y.; Yuan, N.; Liu, Y.; Zhang, X.; Lin, B.; Zhou, Y. Boron Carbon Nitride Semiconductor Modified with CeO2 for Photocatalytic Reduction of CO2 with H2O: Comparative Study. Carbon Capture Sci. Technol. 2021, 1, 100016. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, X.; Li, L.; Du, L.; Tian, G. Sulfur doped In2O3-CeO2 hollow hexagonal prisms with carbon coating for efficient photocatalytic CO2 reduction. Chem. Eng. J. Chem. Eng. J. 2021, 421, 129968. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, Y.; Yan, Y.; Huo, P.; Wang, H. G-C3N4 quantum dots and Au nano particles co-modified CeO2/Fe3O4 micro-flowers photocatalyst for enhanced CO2 photoreduction. Renew. Energy 2021, 179, 756–765. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, M.; Li, Y.; Yang, Y.; Huang, H.; Jiang, Z.; Hu, Q.; Wu, S.; Zhao, X. Novel photoactivation promoted light-driven CO2 reduction by CH4 on Ni/CeO2 nanocomposite with high light-to-fuel efficiency and enhanced stability. Appl. Catal. B. 2018, 239, 555–564. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, W.; Long, J.; Si, Y.; Xu, Y.; Yang, L.; Zou, J.; Dai, W.; Luo, X.; Luo, S. High-throughput lateral and basal interface in CeO2@Ti3C2TX: Reverse and synergistic migration of carrier for enhanced photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 615, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Li, Y.; Wang, Q. Flower-like CoAl layered double hydroxides modified with CeO2 and RGO as efficient photocatalyst towards CO2 reduction. J. Alloys Compd. 2021, 881, 160650. [Google Scholar] [CrossRef]
- Ibarra-Rodriguez, L.I.; Pantoja-Espinoza, J.C.; Luévano-Hipólito, E.; Garay-Rodríguez, L.F.; López-Ortiz, A.; Torres-Martínez, L.M.; Collins-Martínez, V.H. Formic acid and hydrogen generation from the photocatalytic reduction of CO2 on visible light activated N-TiO2/CeO2/CuO composites. J. Photochem. Photobiol. A 2022, 11, 100125. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Malik, A.; Nandal, N.; Pandita, S.; Singh, R.; Bhandari, S.; Saran, S.; Jain, S.L. Morphology controlled Fe and Ni-doped CeO2 nanorods as an excellent heterojunction photocatalyst for CO2 reduction. Appl. Surf. Sci. 2022, 588, 152912. [Google Scholar] [CrossRef]
- Wang, F.; Hou, T.; Zhao, X.; Yao, W.; Fang, R.; Shen, K.; Li, Y. Ordered Macroporous Carbonous Frameworks Implanted with CdS Quantum Dots for Efficient Photocatalytic CO2 Reduction. Adv. Mater. 2021, 33, e2102690. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yu, K.; Zhang, C.; Yang, Z.; Feng, Y.; Hao, H.; Jiang, Y.; Lou, L.-L.; Zhou, W.; Liu, S. Ternary CdS/Au/3DOM-SrTiO3 composites with synergistic enhancement for hydrogen production from visible-light photocatalytic water splitting. Appl. Catal. B. 2017, 215, 74–84. [Google Scholar] [CrossRef]
- Ji, X.; Xu, H.; Liang, S.; Gan, L.; Zhang, R.; Wang, X. 3D ordered macroporous Pt/ZnS@ZnO core-shell heterostructure for highly effective photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2022, 47, 17640–17649. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Liu, J.; Deng, Z.; Li, Y.; Su, B.-L. Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B. 2016, 184, 182–190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).