The Application of Cinnamon Twig Extract as an Inhibitor of Listeriolysin O against Listeria monocytogenes Infection
Abstract
1. Introduction
2. Results and Discussion
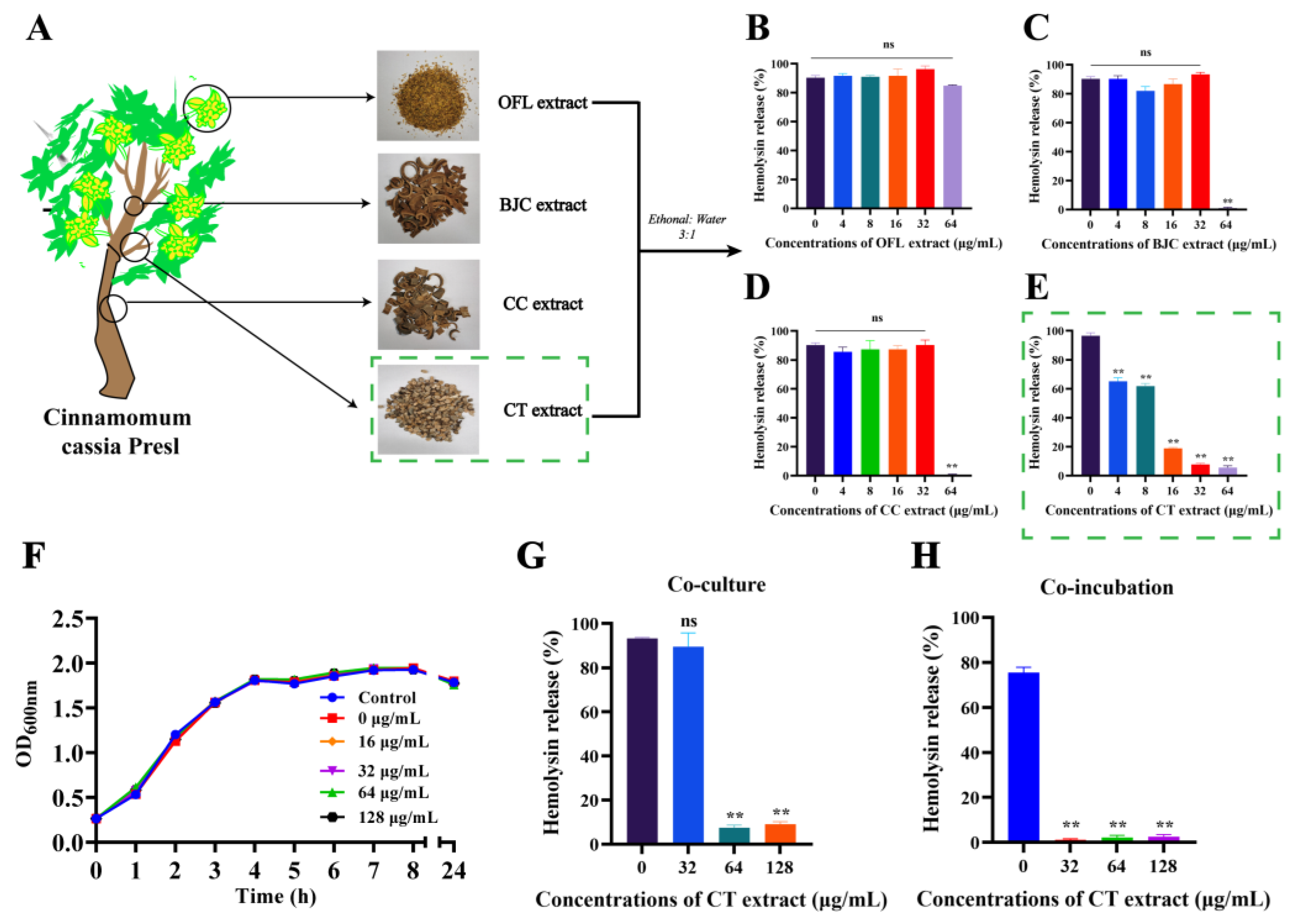
2.1. Hemolysis Inhibition Assay
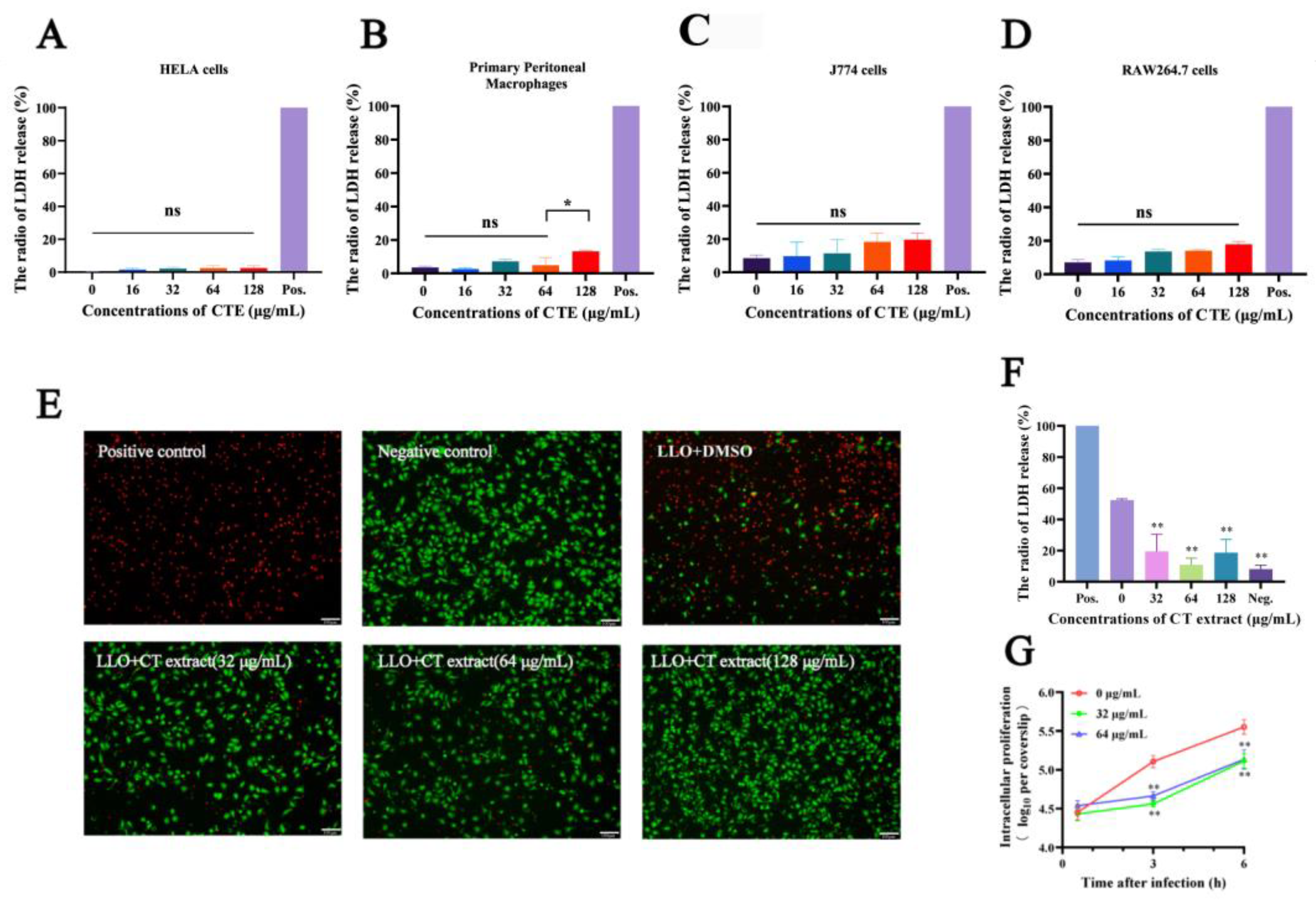
2.2. Cell Protection Experiments
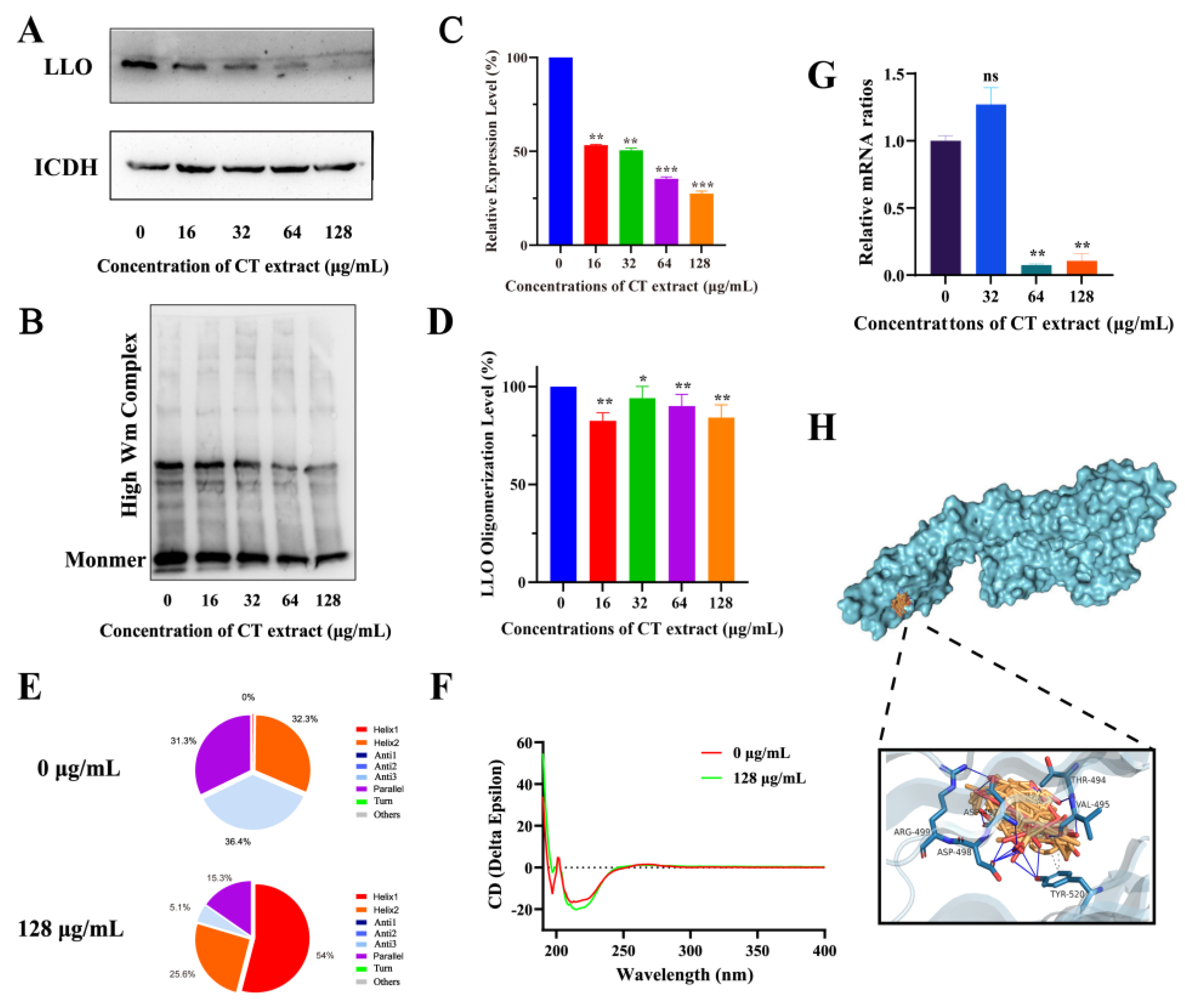
2.3. Action Mechanism Assay
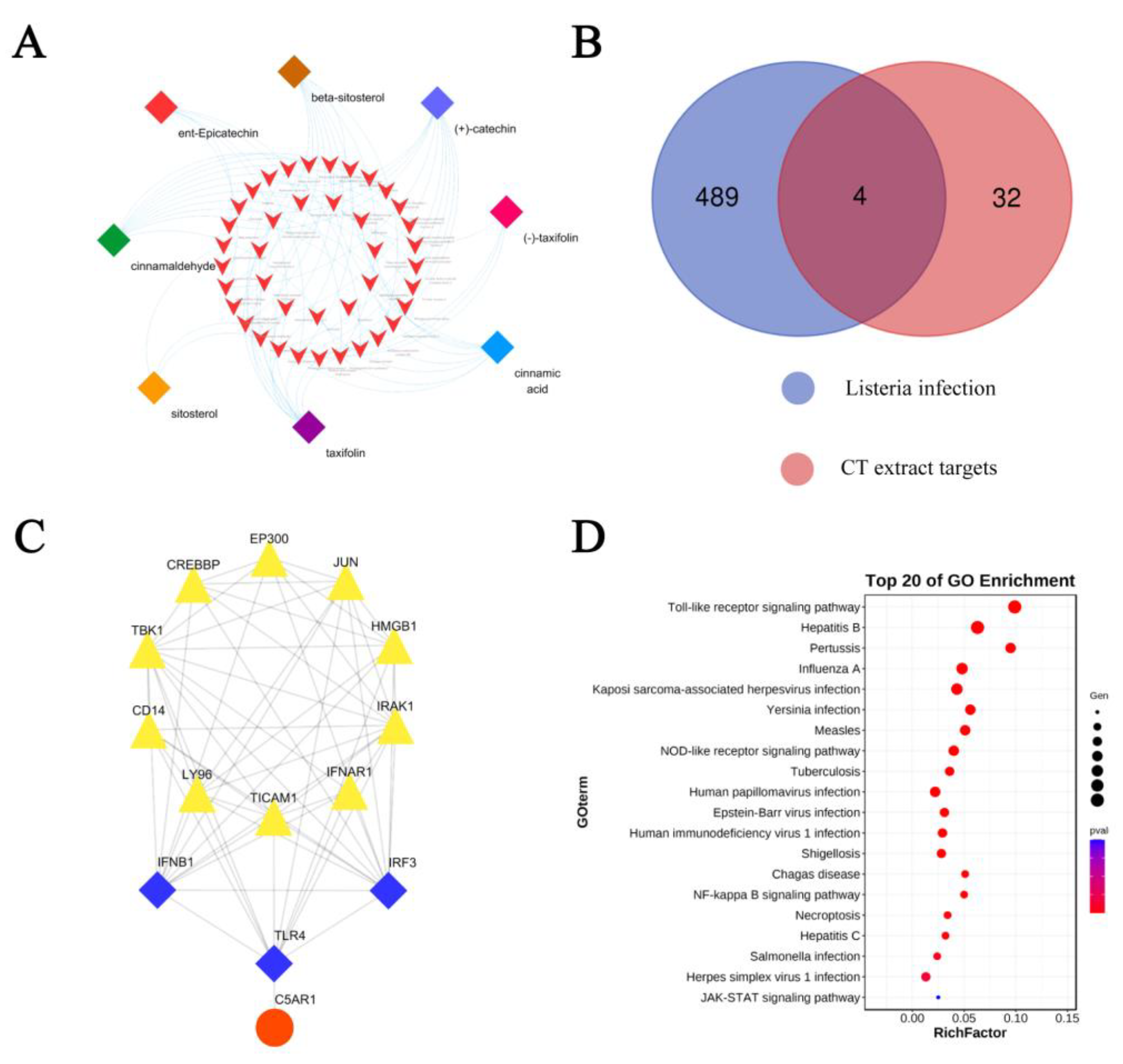
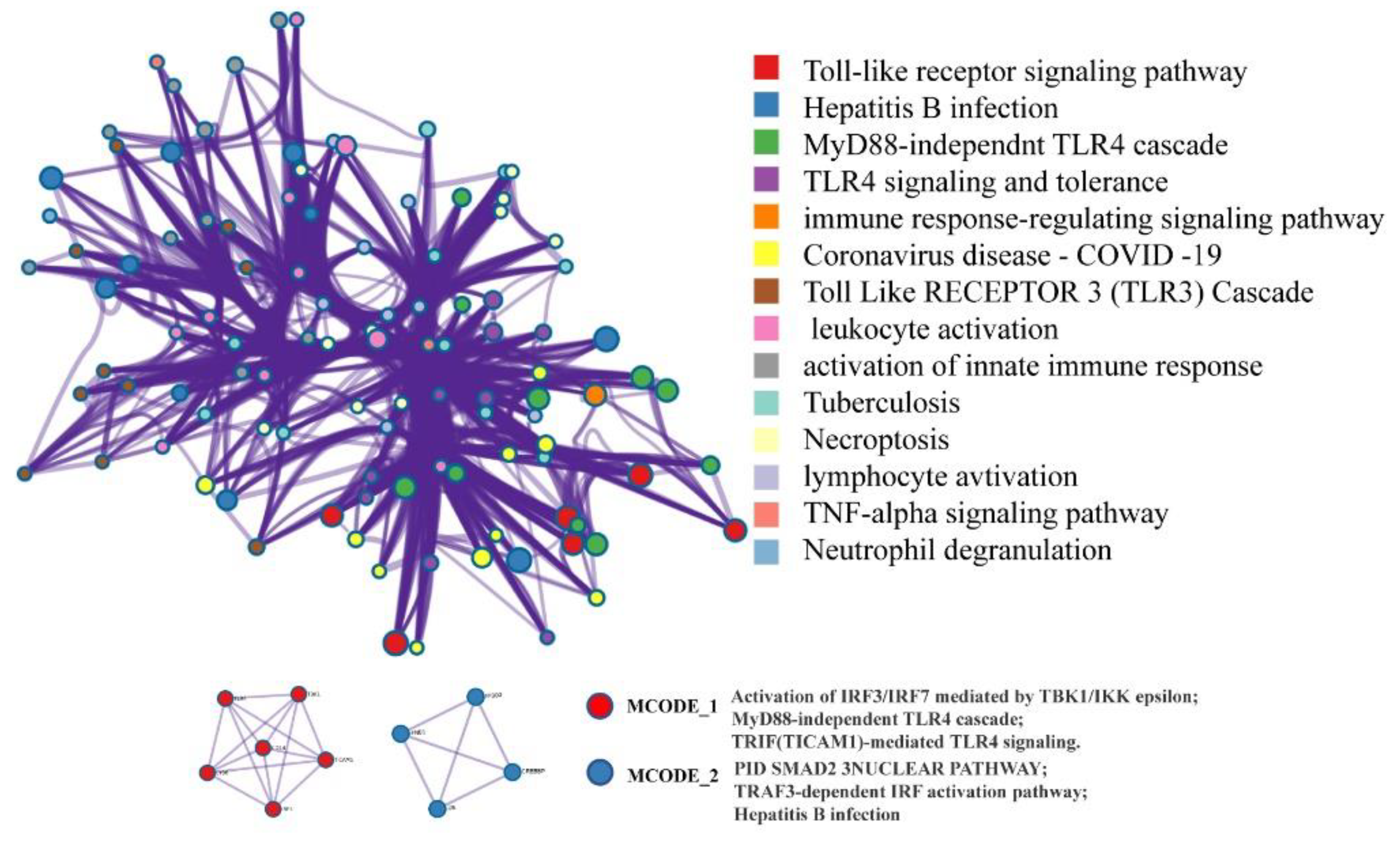
2.4. Network Pharmacology Analysis
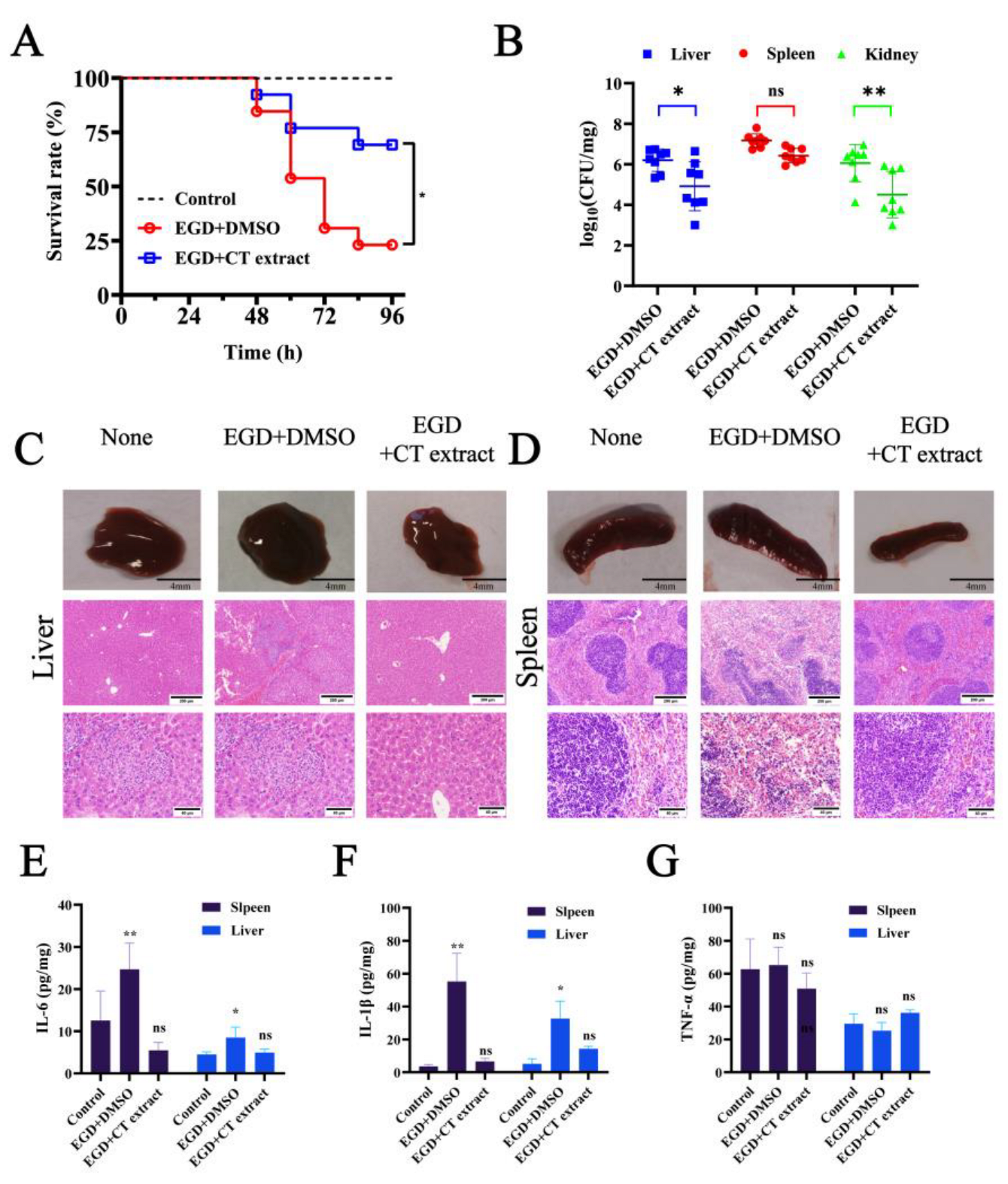
2.5. Animal Experiments
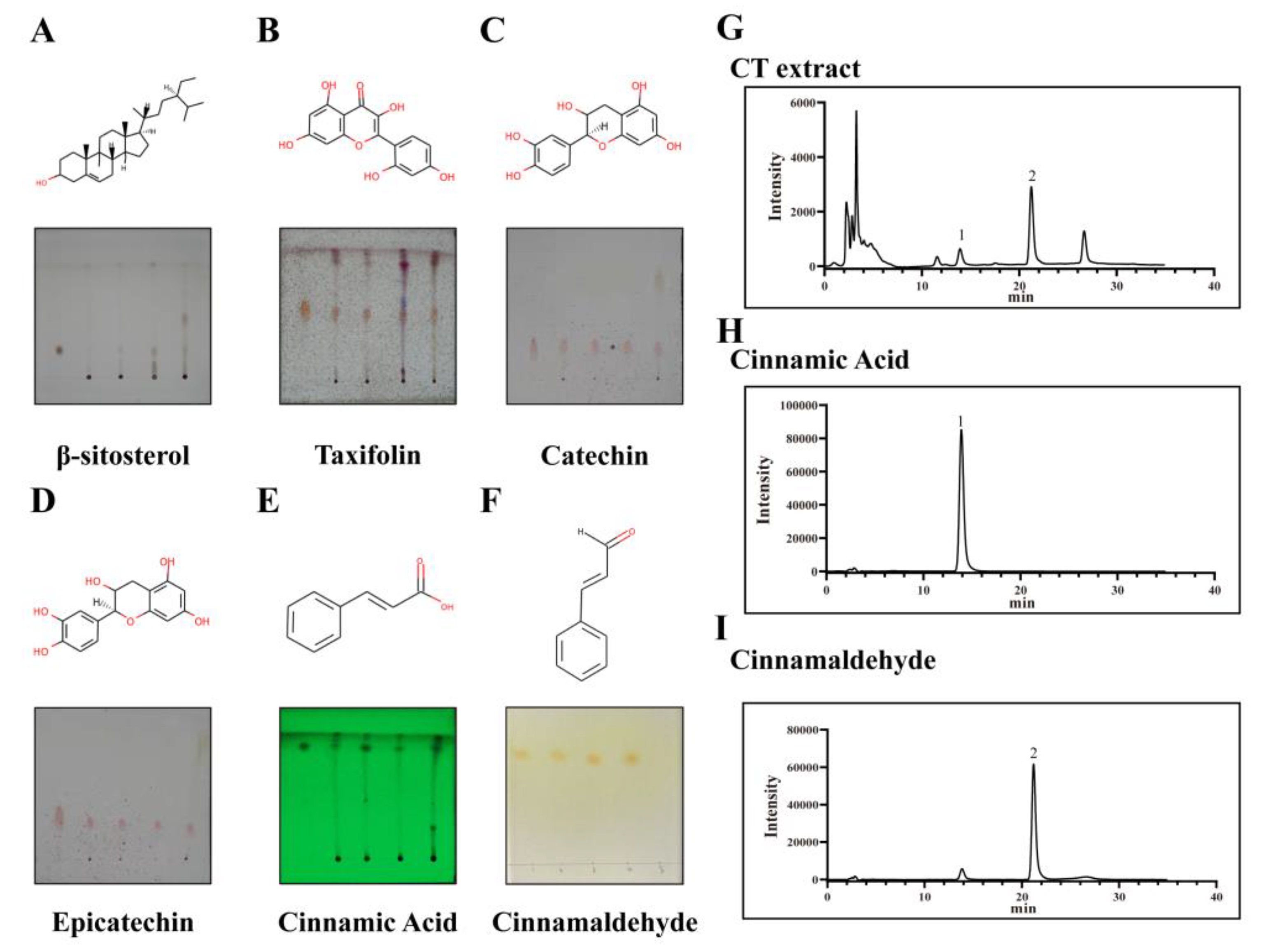
2.6. Component Analysis
3. Materials and Methods
3.1. Materials
3.1.1. Microorganisms
3.1.2. Preparation of Plant Material
3.2. Minimal Inhibitory Concentration (MIC) Assay
3.3. Growth Curve Assay
3.4. Hemolysis Assay
3.5. Cytotoxicity Analysis
3.6. Intracellular Growth Assay
3.7. Cell Viability Determination
3.8. Western Blotting Analysis
3.9. Circular Dichroism (CD) Analysis
3.10. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
3.11. Molecular Docking
3.12. Animal Experiments
3.13. The Detection of the Medicinal Ingredients of Cinnamon Twig and Network Pharma-Cology Analysis
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Charlier, C.; Perrodeau, E.; Leclercq, A. Clinical features and prognostic factors of listeriosis: The MONALISA national prospective cohort study. Lancet Infect. Dis. 2017, 17, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Hearn, J.; Taylor, C.; Wheelhouse, N.; Kaczmarek, M.; Moorhouse, E.; Singleton, I. Listeria monocytogenes isolates from ready to eat plant produce are diverse and have virulence potential. Int. J. Food Microbiol. 2019, 299, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P.; et al. Multistate Outbreak of Listeriosis Associated with Cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Pensinger, D.A.; Aliota, M.T.; Schaenzer, A.J.; Boldon, K.M.; Ansari, I.U.; Vincent, W.J.; Knight, B.; Reniere, M.L.; Striker, R.; Sauer, J.D. Selective pharmacologic inhibition of a PASTA kinase increases Listeria monocytogenes susceptibility to beta-lactam antibiotics. Antimicrob. Agents Chemother. 2014, 58, 4486–4494. [Google Scholar] [CrossRef]
- Magalhaes, R.; Ferreira, V.; Santos, I.; Almeida, G.; Teixeira, P.; Team, R. Genetic and Phenotypic Characterization of Listeria monocytogenes from Human Clinical Cases That Occurred in Portugal Between 2008 and 2012. Foodborne Pathog. Dis. 2014, 11, 907–916. [Google Scholar] [CrossRef]
- Hansen, J.M.; Gernep-Smidt, P.; Bruun, B. Antibiotic susceptibility of Listeria monocytogenes in Denmark 1958–2001. APMIS 2005, 113, 31–36. [Google Scholar] [CrossRef]
- Jamali, H.; Thong, K.L. Genotypic characterization and antimicrobial resistance of Listeria monocytogenes from ready-to-eat foods. Food Control 2014, 44, 1–6. [Google Scholar] [CrossRef]
- de las Heras, A.; Cain, R.J.; Bielecka, M.K.; Vazquez-Boland, J.A. Regulation of Listeria virulence: PrfA master and commander. Curr. Opin. Microbiol. 2011, 14, 118–127. [Google Scholar] [CrossRef]
- Song, L.; Xie, Y.C.; Li, C.; Wang, L.D.; He, C.L.; Zhang, Y.; Yuan, J.Y.; Luo, J.J.; Liu, X.; Xiu, Y.; et al. The Legionella Effector SdjA Is a Bifunctional Enzyme That Distinctly Regulates Phosphoribosyl Ubiquitination. Mbio 2021, 12, e02316-21. [Google Scholar] [CrossRef]
- Alouf, J.E. Pore-forming bacterial protein toxins: An overview. Curr. Top. Microbiol. Immunol. 2001, 257, 1–14. [Google Scholar]
- Nguyen, B.N.; Peterson, B.N.; Portnoy, D.A. Listeriolysin O: A phagosome-specific cytolysin revisited. Cell Microbiol. 2019, 21, e12988. [Google Scholar] [CrossRef]
- Koster, S.; van Pee, K.; Hudel, M.; Leustik, M.; Rhinow, D.; Kuhlbrandt, W.; Chakraborty, T.; Yildiz, O. Crystal structure of listeriolysin O reveals molecular details of oligomerization and pore formation. Nat. Commun. 2014, 5, 3690. [Google Scholar] [CrossRef]
- Schnupf, P.; Portnoy, D.A. Listeriolysin O: A phagosome-specific lysin. Microb. Infect. 2007, 9, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Hudel, M.; Chakraborty, T.; Yildiz, O. Crystallization and X-ray crystallographic analysis of the cholesterol-dependent cytolysin listeriolysin O from Listeria monocytogenes. Acta Crystallogr. Sect. F-Struct. Biol. Commun. 2013, 69, 1212–1215. [Google Scholar] [CrossRef]
- Gekara, N.O.; Westphal, K.; Ma, B.; Rohde, M.; Groebe, L.; Weiss, S. The multiple mechanisms of Ca2+ signalling by listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Cell Microbiol. 2007, 9, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Vadia, S.; Arnett, E.; Haghighat, A.C.; Wilson-Kubalek, E.M.; Tweten, R.K.; Seveau, S. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Path. 2011, 7, e1002356. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, S.J.; Goldfine, H. Mobilization of protein kinase C in macrophages induced by Listeria monocytogenes affects its internalization and escape from the phagosome. Infect. Immun. 2002, 70, 4650–4660. [Google Scholar] [CrossRef]
- Osborne, S.E.; Brumell, J.H. Listeriolysin O: From bazooka to Swiss army knife. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160222. [Google Scholar] [CrossRef]
- Wang, J.F.; Liu, S.; Liu, B.W.; Niu, X.D.; Deng, X.M. Luteolin Inhibits Listeriolysin O Translation by Directly Targeting the Coding Region of the hly mRNA. Front. Microbiol. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Shen, X.; Niu, X.D.; Li, G.; Deng, X.M.; Wang, J.F. Amentoflavone Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Appl. Environ. Microbiol. 2018, 84, e01804-18. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhang, P.; Lv, H.F.; Deng, X.M.; Wang, J.F. A Natural Dietary Flavone Myricetin as an alpha-Hemolysin Inhibitor for Controlling Staphylococcus aureus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef]
- Wang, J.F.; Qiu, J.Z.; Tan, W.; Zhang, Y.; Wang, H.S.; Zhou, X.; Liu, S.; Feng, H.H.; Li, W.H.; Niu, X.D.; et al. Fisetin Inhibits Listeria monocytogenes Virulence by Interfering With the Oligomerization of Listeriolysin O. J. Infect. Dis. 2015, 211, 1376–1387. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Cui, Y.M.; Chen, S.Y.; Teng, Z.H.; Lu, G.J.; Wang, J.F.; Deng, X.M. Curcumin Promotes the Clearance of Listeria monocytogenes both In Vitro and In Vivo by Reducing Listeriolysin O Oligomers. Front. Immunol. 2017, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, B.W.; Teng, Z.H.; Zhou, X.; Wang, X.Y.; Zhang, B.; Lu, G.J.; Niu, X.D.; Yang, Y.J.; Deng, X.M. Phloretin Attenuates Listeria monocytogenes Virulence Both In vitro and In vivo by Simultaneously Targeting Listeriolysin O and Sortase A. Front. Cell. Infect. Microbiol. 2017, 7, 9. [Google Scholar] [CrossRef]
- Lu, G.J.; Xu, L.; Zhang, T.; Deng, X.M.; Wang, J.F. A potential bio-control agent from baical skullcap root against listeriosis via the inhibition of sortase A and listeriolysin O. J. Cell Mol. Med. 2019, 23, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; You, J.R.; Kim, Y.S.; Kim, S.H.; Cho, E.Y.; Yoon, J.H.; Kwon, E.; Jang, J.J.; Park, J.S.; Kim, H.C.; et al. In vitro and in vivo safety studies of cinnamon extract (Cinnamomum cassia) on general and genetic toxicology. Regul. Toxicol. Pharmacol. 2018, 95, 115–123. [Google Scholar] [CrossRef]
- Hwa, J.S.; Jin, Y.C.; Lee, Y.S.; Ko, Y.S.; Kim, Y.M.; Shi, L.Y.; Kim, H.J.; Lee, J.H.; Ngoc, T.M.; Bae, K.H.; et al. 2-Methoxycinnamaldehyde from Cinnamomum cassia reduces rat myocardial ischemia and reperfusion injury in vivo due to HO-1 induction. J. Ethnopharmacol. 2012, 139, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.M.; Chen, Y.H.; Yen, P.L.; Chang, S.T. Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. J. Tradit Complement. Med. 2016, 6, 281–288. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Polansky, M.M.; Anderson, R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000, 48, 849–852. [Google Scholar] [CrossRef]
- Carrero, J.A.; Vivanco-Cid, H.; Unanue, E.R. Granzymes drive a rapid listeriolysin O-induced T cell apoptosis. J. Immunol. 2008, 181, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Terahara, K.; Takahashi, K.G. Mechanisms and immunological roles of apoptosis in molluscs. Curr. Pharm. Des. 2008, 14, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Ribet, D.; Stavru, F.; Cossart, P. Listeriolysin O: The Swiss army knife of Listeria. Trends Microbiol. 2012, 20, 360–368. [Google Scholar] [CrossRef]
- Mulvihill, E.; van Pee, K.; Mari, S.A.; Muller, D.J.; Yildiz, O. Directly Observing the Lipid-Dependent Self-Assembly and Pore-Forming Mechanism of the Cytolytic Toxin Listeriolysin O. Nano Lett. 2015, 15, 6965–6973. [Google Scholar] [CrossRef] [PubMed]
- Ciaston, I.; Dobosz, E.; Potempa, J.; Koziel, J. The subversion of toll-like receptor signaling by bacterial and viral proteases during the development of infectious diseases. Mol. Asp. Med. 2022, 88, 101143. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Wang, T.; Liu, B.; Zhang, C.; Fang, T.; Ding, Y.; Song, G.; Zhang, S.; Shi, L.; Deng, X.; Wang, J. Kaempferol-Driven Inhibition of Listeriolysin O Pore Formation and Inflammation Suppresses Listeria monocytogenes Infection. Microbiol. Spectr. 2022, 10, e0181022. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schioth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B. 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
- Pan, X.; Hou, X.; Zhang, F.; Tang, P.; Wan, W.; Su, Z.; Yang, Y.; Wei, W.; Du, Z.; Deng, J.; et al. Gnetum montanum extract induces apoptosis by inhibiting the activation of AKT in SW480 human colon cancer cells. Pharm. Biol. 2022, 60, 915–930. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Qiu, J.; Li, H.; Meng, H.; Hu, C.; Li, J.; Luo, M.; Dong, J.; Wang, X.; Wang, J.; Deng, Y.; et al. Impact of luteolin on the production of alpha-toxin by Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Wang, C.Y.; Luo, J.J.; Hua, S.C.; Li, D.; Peng, L.P.; Liu, H.M.; Song, L. The protective role of Zingerone in a murine asthma model via activation of the AMPK/Nrf2/HO-1 pathway. Food Funct. 2021, 12, 3120–3131. [Google Scholar] [CrossRef]
- Song, L.; Luo, J.J.; Wang, H.O.; Huang, D.; Tan, Y.H.; Liu, Y.; Wang, Y.W.; Yu, K.W.; Zhang, Y.; Liu, X.Y.; et al. Legionella pneumophila regulates host cell motility by targeting Phldb2 with a 14-3-3 zeta-dependent protease effector. Elife 2022, 11, e73220. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhou, X.; Liu, S.; Li, G.; Zhang, B.; Deng, X.M.; Niu, X.D. Novel inhibitor discovery and the conformational analysis of inhibitors of listeriolysin O via protein-ligand modeling. Sci. Rep. 2015, 5, 8864. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Refregiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Peng, L.P.; Hua, S.C.; Li, X.P.; Ma, L.J.; Jie, J.; Chen, D.; Wang, Y.; Li, D. miR-144-5p Enhances the Radiosensitivity of Non-Small-Cell Lung Cancer Cells via Targeting ATF2. BioMed Res. Int. 2018, 2018, 5109497. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Barthel, M.; Hapfelmeier, S.; Quintanilla-Martinez, L.; Kremer, M.; Rohde, M.; Hogardt, M.; Pfeffer, K.; Russmann, H.; Hardt, W.D. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003, 71, 2839–2858. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Q.; Zhou, M.W.; Gritsenko, M.A.; Nakayasu, E.S.; Song, L.; Luo, Z.Q. Legionella pneumophila modulates host energy metabolism by ADP-ribosylation of ADP/ATP translocases. Elife 2022, 11, e73611. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Sheng, Q.; Zhang, J.; Du, R.; Wang, N.; Zhu, H.; Deng, X.; Wen, Z.; Wang, J.; Zhou, Y.; et al. The Application of Cinnamon Twig Extract as an Inhibitor of Listeriolysin O against Listeria monocytogenes Infection. Molecules 2023, 28, 1625. https://doi.org/10.3390/molecules28041625
Hou X, Sheng Q, Zhang J, Du R, Wang N, Zhu H, Deng X, Wen Z, Wang J, Zhou Y, et al. The Application of Cinnamon Twig Extract as an Inhibitor of Listeriolysin O against Listeria monocytogenes Infection. Molecules. 2023; 28(4):1625. https://doi.org/10.3390/molecules28041625
Chicago/Turabian StyleHou, Xiaoning, Qiushuang Sheng, Jichuan Zhang, Runbao Du, Nan Wang, Haoyu Zhu, Xuming Deng, Zhongmei Wen, Jianfeng Wang, Yonglin Zhou, and et al. 2023. "The Application of Cinnamon Twig Extract as an Inhibitor of Listeriolysin O against Listeria monocytogenes Infection" Molecules 28, no. 4: 1625. https://doi.org/10.3390/molecules28041625
APA StyleHou, X., Sheng, Q., Zhang, J., Du, R., Wang, N., Zhu, H., Deng, X., Wen, Z., Wang, J., Zhou, Y., & Li, D. (2023). The Application of Cinnamon Twig Extract as an Inhibitor of Listeriolysin O against Listeria monocytogenes Infection. Molecules, 28(4), 1625. https://doi.org/10.3390/molecules28041625





