Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Performance of the cLC-DAD-MS Method
2.2. Chemical Characterization of the Phenolic Extracts from Citrus Peels
2.3. Stability of Polyphenols under Drying Treatments
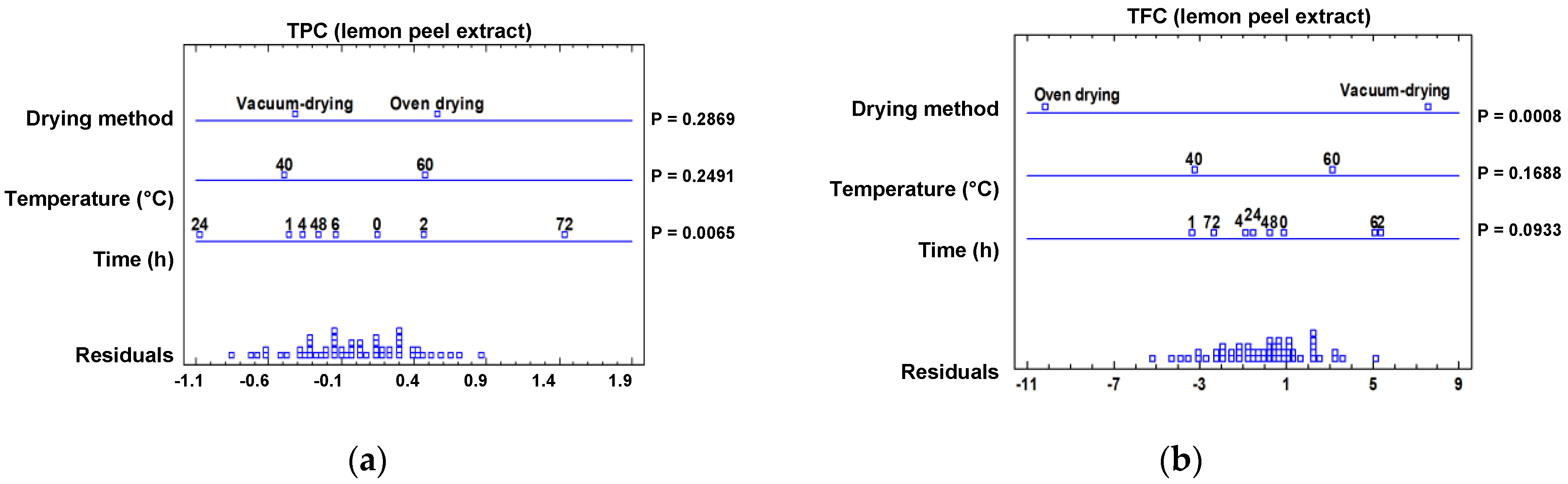
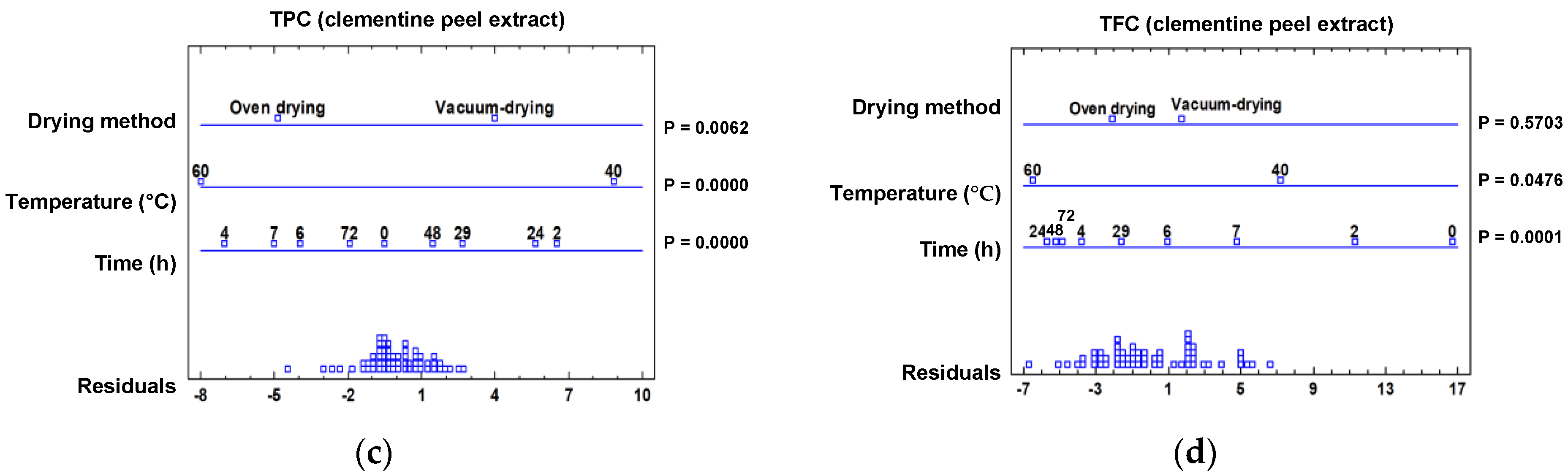
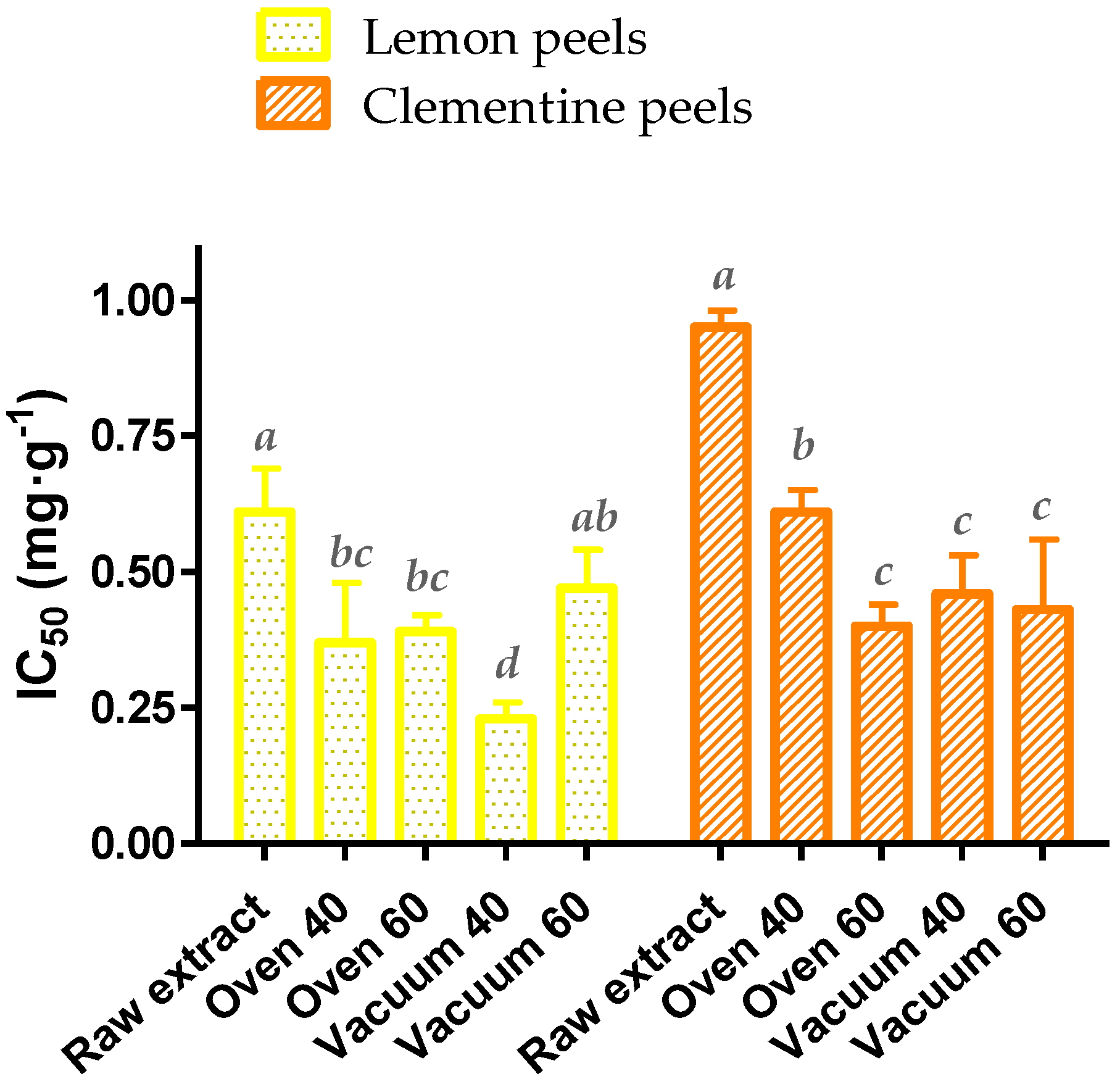
2.3.1. Effect of Drying Treatment on the Spectrophotometric Data
2.3.2. Effect of Drying Treatment on the Individual Phenolic Composition
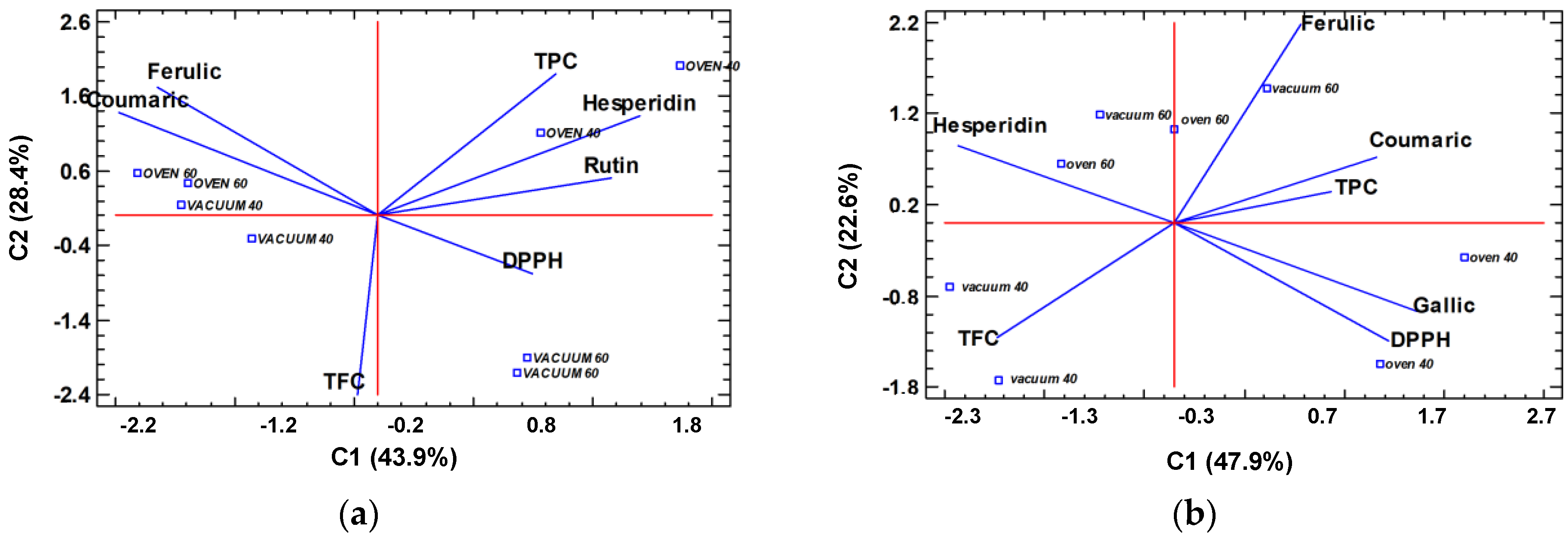
2.3.3. Multifactorial Study of the Drying Treatments by Principal Component Analysis
2.4. Stability of Polyphenols under Storage Conditions
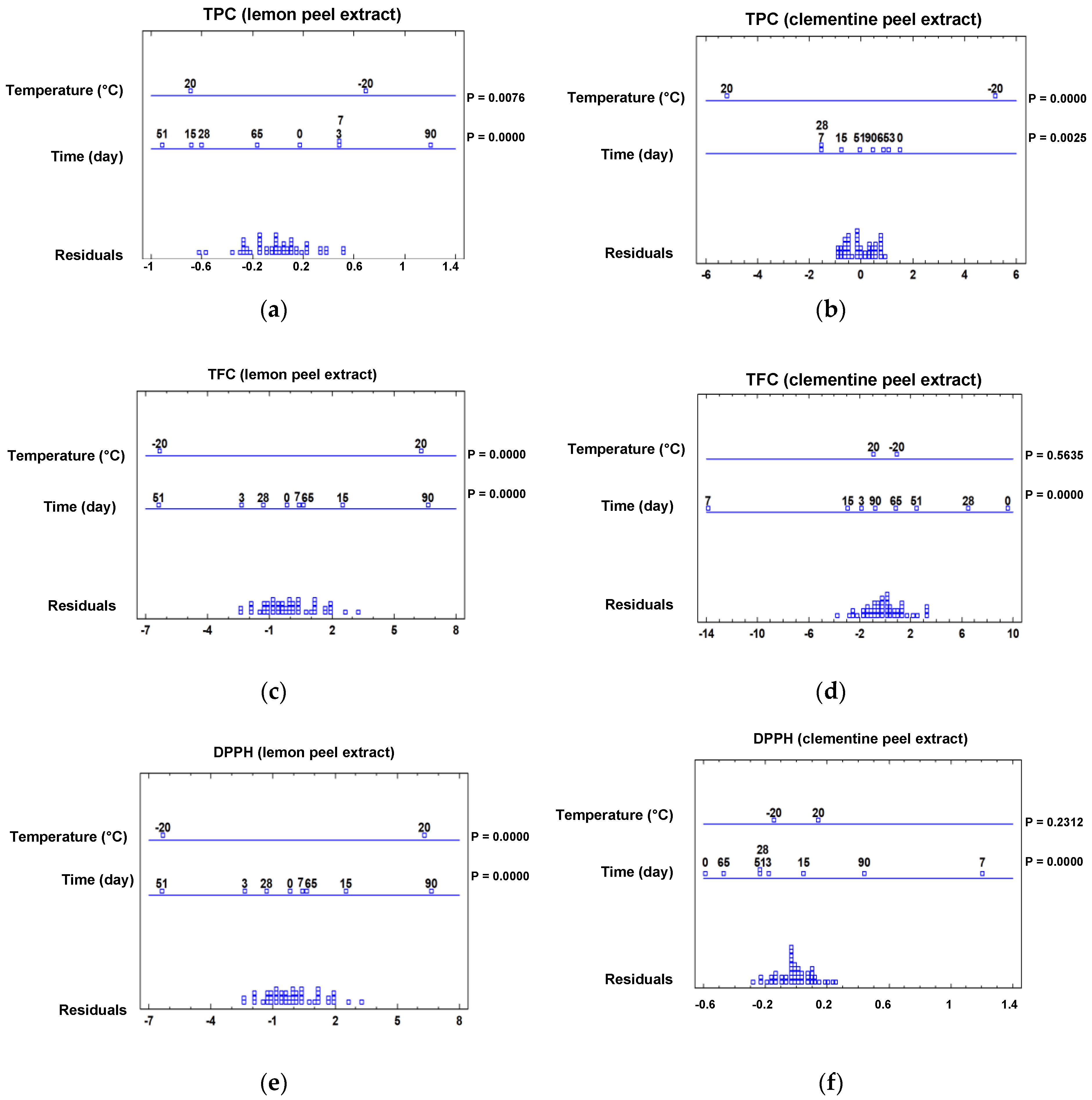
2.4.1. Effect of Storage Temperature on the Spectrophotometric Data
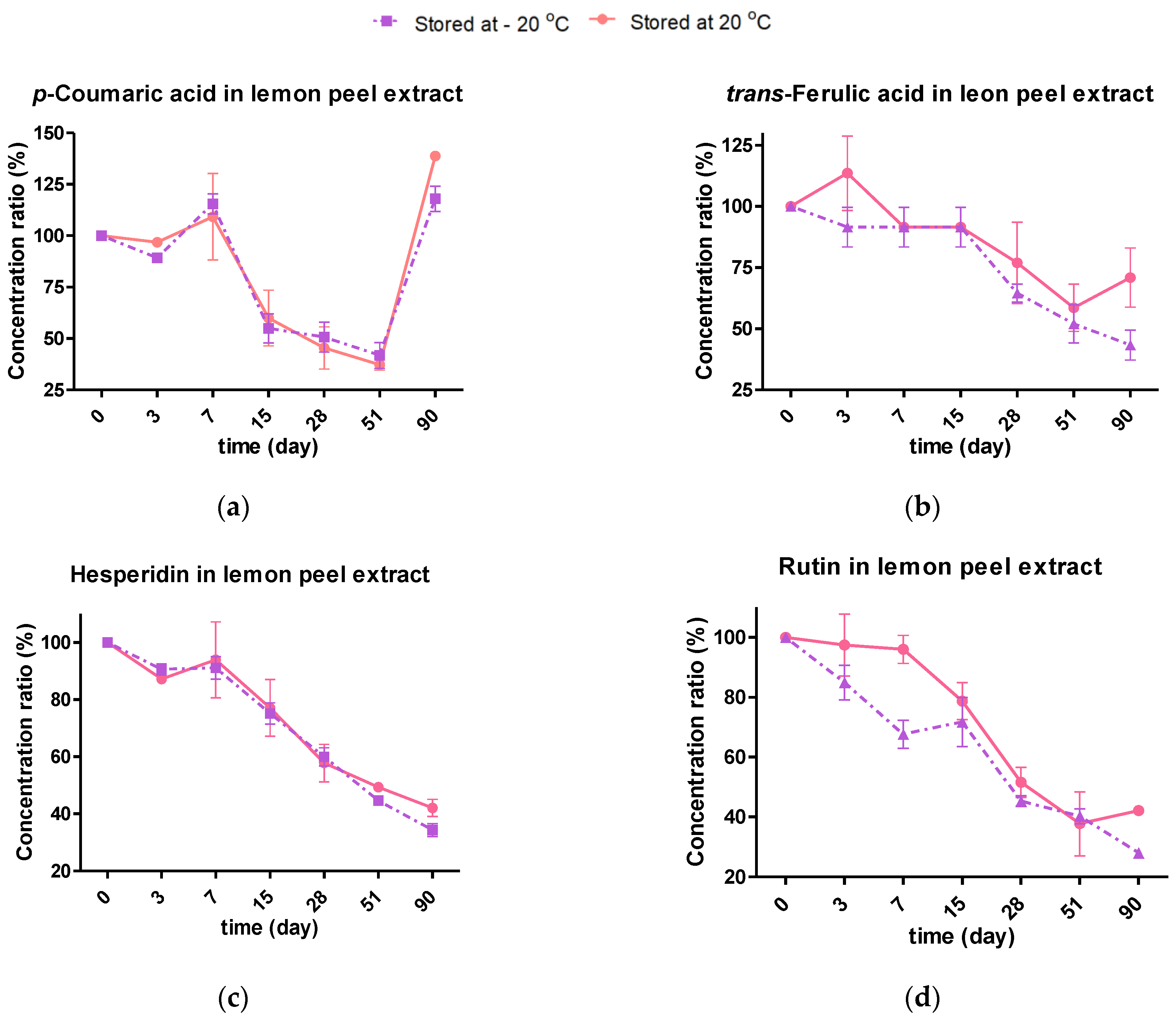
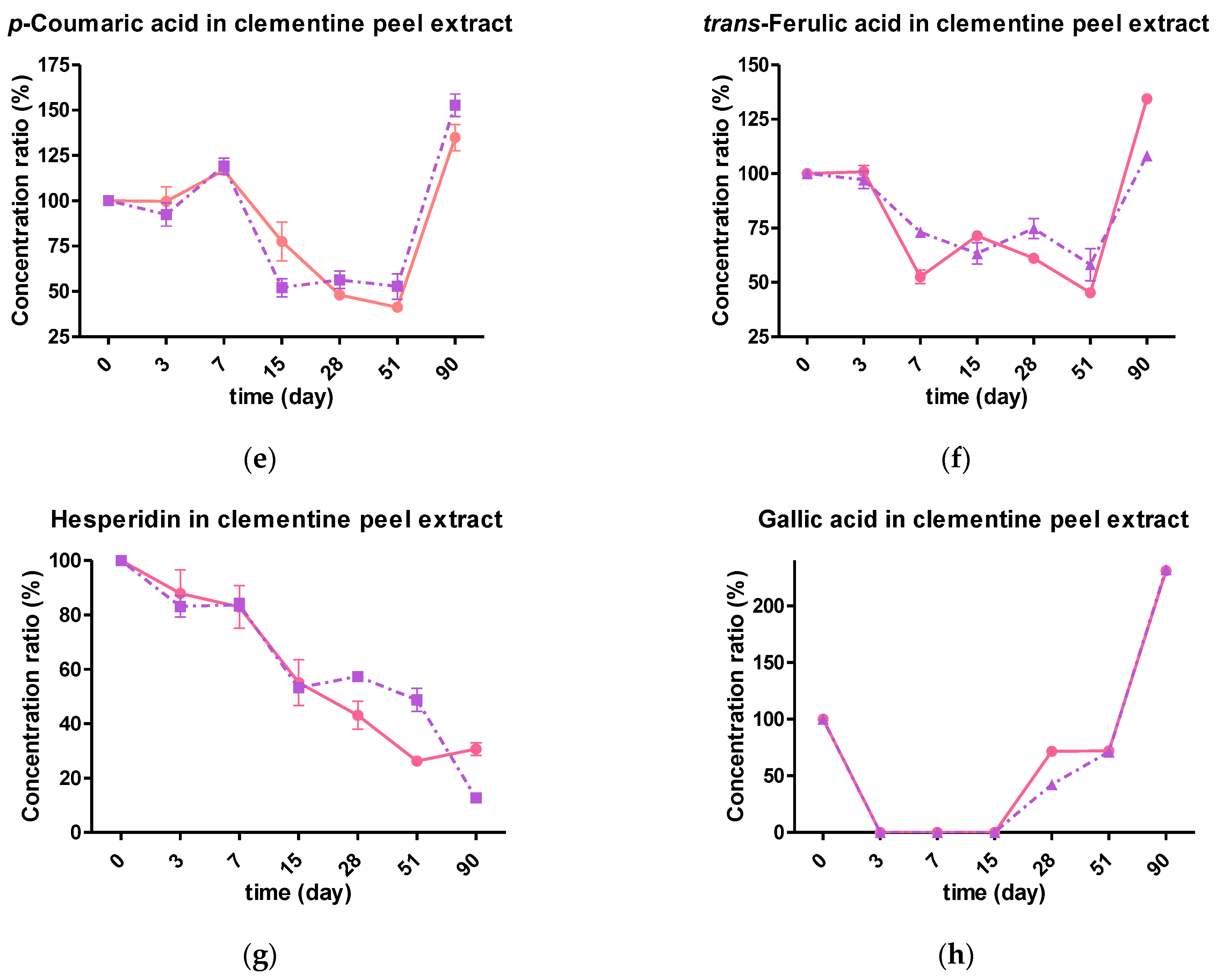
2.4.2. Effect of Storage Temperature on the Individual Phenolic Composition
2.4.3. Kinetic Degradation of Phenolic Extracts during Storage Conditions
3. Material and Methods
3.1. Citrus Samples
3.2. Reagents, Solvents and Polyphenol Standards
3.3. Extraction of Polyphenols from Citrus Peel Residues
3.4. Storage and Drying Treatments
3.5. Determination of Total Polyphenol Content
3.6. Determination of Total Flavonoid Content
3.7. Antioxidant Activity
3.8. Chromatographic Analysis of Polyphenols
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yates, A.A.; Erdman, J.W.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive Nutrients—Time for Tolerable Upper Intake Levels to Address Safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M. Incorporation of Avocado Peel Extract to Reduce Cooking-Induced Hazards in Beef and Soy Burgers: A Clean Label Ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- United Nations (UN). Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://Sustainabledevelopment.Un.Org/Content/Documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.Pdf (accessed on 9 January 2023).
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and Vegetable Waste Management: Conventional and Emerging Approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Citrus: World Markets and Trade; United States Department of Agriculture: Washington, DC, USA, 2022.
- Lee, G.J.; Lee, S.Y.; Kang, N.G.; Jin, M.H. A Multi-Faceted Comparison of Phytochemicals in Seven Citrus Peels and Improvement of Chemical Composition and Antioxidant Activity by Steaming. LWT 2022, 160, 113297. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus sinensis Peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Bi, J.; Ji, Q.; Zhao, X.; Zheng, Q.; Tan, S.; Gao, X. Effect of Hot Air Drying on the Polyphenol Profile of Hongjv (Citrus reticulata Blanco, CV. Hongjv) Peel: A Multivariate Analysis. J. Food Biochem. 2020, 44, e13174. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus Peels Waste as a Source of Value-Added Compounds: Extraction and Quantification of Bioactive Polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.J.; Kim, E.K. Citrus Peel as a Renewable Bioresource: Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Castillo, A.; Celeiro, M.; Rubio, L.; Bañobre, A.; Otero-Otero, M.; Garcia-Jares, C.; Lores, M. Optimization of Bioactives Extraction from Grape Marc via a Medium Scale Ambient Temperature System and Stability Study. Front. Nutr. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological Aspects and Stability of Polyphenols. Polyphen. Prop. Recovery Appl. 2018, 9, 295–323. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus reticulata Blanco Peels as a Source of Antioxidant and Anti-Proliferative Phenolic Compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of Vacuum-Drying, Hot Air-Drying and Freeze-Drying on Polyphenols and Antioxidant Capacity of Lemon (Citrus Limon) Pomace Aqueous Extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- AOAC International AOAC: Official Methods of Analysis. Available online: https://archive.org/details/gov.law.aoac.methods.1980 (accessed on 24 March 2022).
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Screening the Extraction Process of Phenolic Compounds from Pressed Grape Seed Residue: Towards an Integrated and Sustainable Management of Viticultural Waste. LWT 2022, 169, 113988. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A Combined Approach Based on Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles and Liquid Chromatography to Determine Polyphenols from Grape Residues. J. Chromatogr. A 2021, 1644, 462128. [Google Scholar] [CrossRef]
- International Council for Harmonisation. ICH Topic Q2(R2) Validation of Analytical Procedures: Text and Methodology. 1995. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 3 January 2023).
- Rambla-Alegre, M.; Esteve-Romero, J.; Carda-Broch, S. Is It Really Necessary to Validate an Analytical Method or Not? That Is the Question. J. Chromatogr. A 2012, 1232, 101–109. [Google Scholar] [CrossRef]
- Suo, G.; Sun, Z.; Sun, Y.; Xue, W.; Zhou, L.; Zhang, J.; Bao, X.; Zhu, Z.; Zhang, X. Rapid Determination of 30 Bioactive Constituents in XueBiJing Injection Using Ultra High Performance Liquid Chromatography-High Resolution Hybrid Quadrupole-Orbitrap Mass Spectrometry Coupled with Principal Component Analysis. J. Pharm. Biomed. Anal. 2017, 137, 220–228. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; González, M.C. Approach to the Content of Total Extractable Phenolic Compounds from Different Food Samples by Comparison of Chromatographic and Spectrophotometric Methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Bustos, M.C.; Rocha-Parra, D.; Sampedro, I.; de Pascual-Teresa, S.; León, A.E. The Influence of Different Air-Drying Conditions on Bioactive Compounds and Antioxidant Activity of Berries. J. Agric. Food Chem. 2018, 66, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.A.; Mobrami, Z.; Sajedi, R.H. Bioactive Compounds and Antioxidant Capacities in the Flavedo Tissue of Two Citrus Cultivars under Low Temperature. Braz. Soc. Plant Physiol. 2011, 23, 203–208. [Google Scholar] [CrossRef]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Matoba, T. Effects of Thermal Treatment on Radical-Scavenging Activity of Single and Mixed Polyphenolic Compounds. J. Food Sci. 2004, 69, FCT7–FCT10. [Google Scholar] [CrossRef]

| Compound | Retention Time, min | Wavelength (λmax), nm | Molecular Ion [M-H]ˉ (m/z) (Fragmentor, eV) | Calibration Equation, y = a · x + b | |
|---|---|---|---|---|---|
| a, μg·L−1 | b | ||||
| Gallic acid | 3.6 | 260 | 169 (70) | (4.4 ± 0.4) × 102 | (0 ± 8) × 103 |
| DHB 1 | 4.9 | 260 | 153 (70) | (5.4 ± 0.4) × 102 | (0 ± 7) × 103 |
| Caffeic acid | 11.8 | 310 | 179 (150) | (6 ± 1) × 102 | (0 ± 6) × 103 |
| p-Coumaric acid | 14.5 | 310 | 163 (70) | (1.06 ± 0.06) × 103 | (0 ± 3) × 103 |
| trans-Ferulic acid | 15.4 | 310 | 193 (70) | (4.1 ± 0.5) × 102 | (0 ± 2) × 103 |
| Rutin | 15.0 | 260 | - | 0.8 ± 0.1 | (1.8 ± 0.7) × 103 |
| Hesperidin | 16.0 | 292 | - | 0.85 ± 0.03 | (2 ± 2) × 10 |
| Myricetin | 17.7 | 365 | 317 (150) | 0.7 ± 0.1 | (0 ± 1) × 10 |
| Resveratrol | 18.5 | 310 | 227 (150) | 5.0 ± 0.4 | (4 ± 2) × 10 |
| Quercetin | 19.7 | 365 | 301 (150) | (6.2 ± 0.6) × 102 | (0 ± 4) × 103 |
| Kaempferol | 22.7 | 365 | 284 (150) | (1.21 ± 0.07) × 103 | (6 ± 4) × 103 |
| Compound | Linear Range (n), µg∙L−1 | R2 | LOD, µg∙L−1 | LOQ, µg∙L−1 | Intra-Day Repeatability (n = 3) RSD (%) | Inter-Day Repeatability (N = 9) RSD (%) | ||
|---|---|---|---|---|---|---|---|---|
| k | Area | k | Area | |||||
| Gallic acid | 20–450 (8) | 0.9937 | 4 | 13 | 4.8 | 3.5 | 5.1 | 4.5 |
| DHB 1 | 21–450 (9) | 0.9951 | 6 | 20 | 3.8 | 5.3 | 6.1 | 5.8 |
| Caffeic acid | 16–70 (5) | 0.9900 | 4 | 13 | 2.4 | 3.9 | 6.8 | 4.1 |
| p-Coumaric acid | 5–180 (9) | 0.9931 | 0.7 | 2 | 0.77 | 4.0 | 1.0 | 5.9 |
| trans-Ferulic acid | 16–60 (5) | 0.9948 | 4 | 13 | 0.41 | 3.9 | 0.70 | 4.5 |
| Rutin | 70–160 (5) | 0.9986 | 20 | 67 | 0.40 | 3.3 | 1.1 | 3.6 |
| Hesperidin | 50–160 (7) | 0.9992 | 8 | 27 | 0.48 | 1.0 | 1.0 | 1.0 |
| Myricetin | 70–120 (5) | 0.9978 | 20 | 67 | 0.32 | 4.4 | 0.60 | 5.2 |
| Resveratrol | 16–60 (5) | 0.9938 | 4 | 13 | 0.34 | 3.5 | 0.60 | 3.9 |
| Quercetin | 1–150 (8) | 0.9916 | 0.1 | 0.3 | 0.32 | 4.5 | 0.59 | 5.2 |
| Kaempferol | 0.5–150 (9) | 0.9958 | 0.1 | 0.3 | 0.54 | 4.1 | 1.1 | 5.0 |
| Compound (µg·g−1 Sample) | Lemon Peels | Clementine Peels |
|---|---|---|
| Gallic acid | 68 ± 15 | 98 ± 13 ** |
| DHB 1 | n.q. | n.d. |
| Caffeic acid | n.q. | n.d. |
| p-Coumaric acid | 178 ± 23 | 258 ± 90 ** |
| trans-Ferulic acid | 177 ± 15 | 438 ± 125 ** |
| Rutin | (1.33 ± 0.06) × 103 | n.d. |
| Hesperidin | (8.7 ± 0.9) × 103 | (1.5 ± 0.5) × 104 ** |
| Myricetin | n.q. | n.d. |
| Resveratrol | n.q. | n.d. |
| Quercetin | n.q. | n.d. |
| Kaempferol | 5 ± 1 | 2.70 ± 0.02 ** |
| Total Phenolic Acids (mg·g−1 sample) | 0.42 ± 0.01 | 0.6 ± 0.1 ** |
| Total Flavonoids (mg·g−1 sample) | 10.0 ± 0.8 | 15 ± 5 ** |
| Total Polyphenols (mg·g−1 sample) | 10.4 ± 0.8 | 16 ± 5 ** |
| TPC (mg GAE·g−1 sample DW) | 2.1 ± 0.4 | 6.2 ± 0.8 ** |
| TFC (mg QE·g−1 sample DW) | 30 ± 3 | 40 ± 2 ** |
| DPPH (IC50, mg·g−1 sample DW) | 0.61 ± 0.08 | 0.95 ± 0.03 ** |
| Peel Extract | Polyphenol | Temperature, °C | k, Day−1 | t1/2, Day | R2 |
|---|---|---|---|---|---|
| Lemon | Hesperidin | −20 | 0.0122 | 57 | 0.9456 |
| 20 | 0.0098 | 71 | 0.8773 | ||
| Rutin | −20 | 0.0133 | 52 | 0.8963 | |
| 20 | 0.0112 | 62 | 0.7407 | ||
| Clementine | Hesperidin | −20 | 0.0205 | 34 | 0.9216 |
| 20 | 0.0154 | 45 | 0.7301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Mejía, E.; Sacristán, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules 2023, 28, 1624. https://doi.org/10.3390/molecules28041624
Gómez-Mejía E, Sacristán I, Rosales-Conrado N, León-González ME, Madrid Y. Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules. 2023; 28(4):1624. https://doi.org/10.3390/molecules28041624
Chicago/Turabian StyleGómez-Mejía, Esther, Iván Sacristán, Noelia Rosales-Conrado, María Eugenia León-González, and Yolanda Madrid. 2023. "Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts" Molecules 28, no. 4: 1624. https://doi.org/10.3390/molecules28041624
APA StyleGómez-Mejía, E., Sacristán, I., Rosales-Conrado, N., León-González, M. E., & Madrid, Y. (2023). Effect of Storage and Drying Treatments on Antioxidant Activity and Phenolic Composition of Lemon and Clementine Peel Extracts. Molecules, 28(4), 1624. https://doi.org/10.3390/molecules28041624






