Oxidative Stability of Cottonseed Butter Products under Accelerated Storage Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Color Profile of Cottonseed Butter
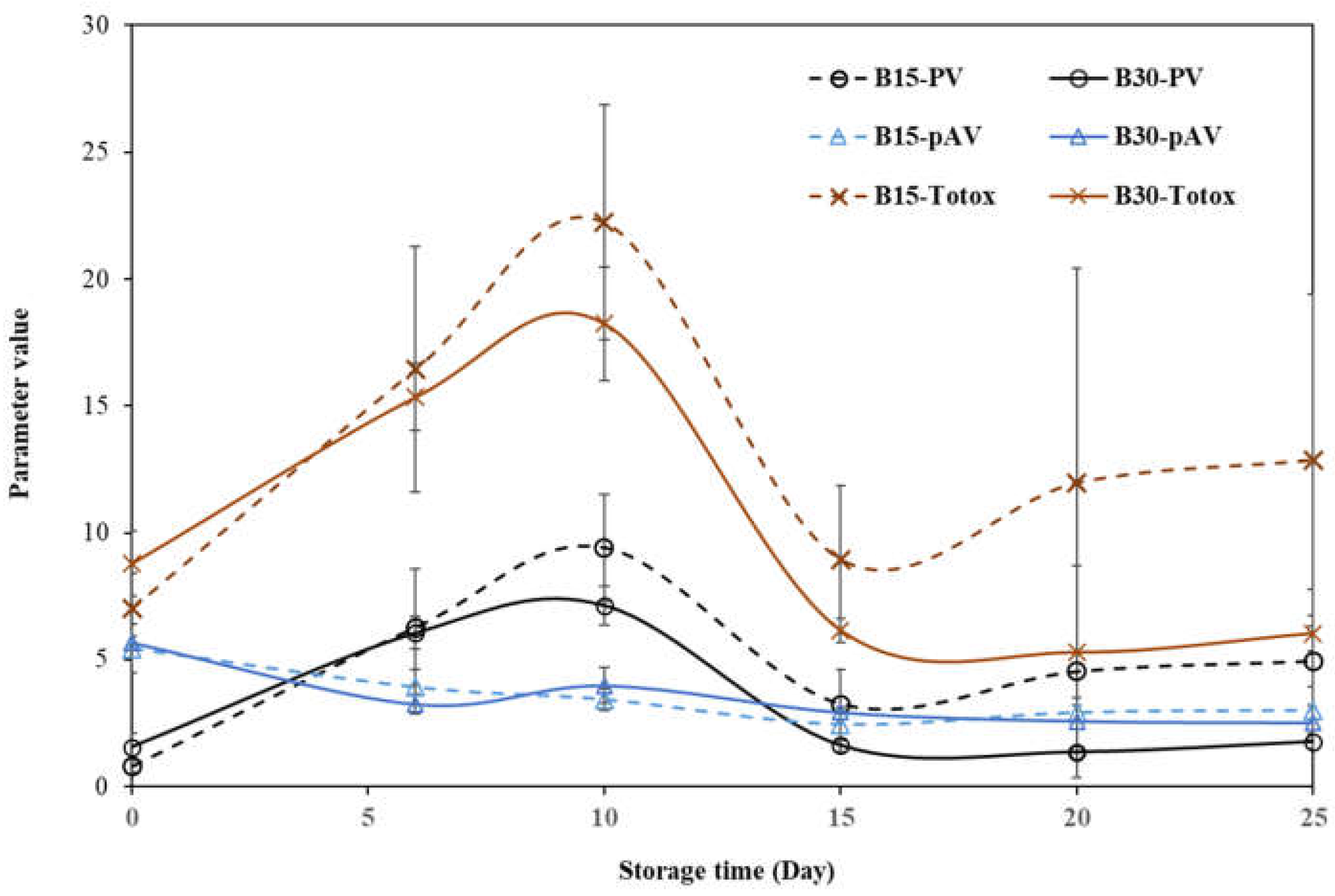
2.2. Impact of Accelerated Storage on the Oxidative Stability Parameters
2.3. Tocopherol Contents in Cottonseed Butter and Impact of Storage Times
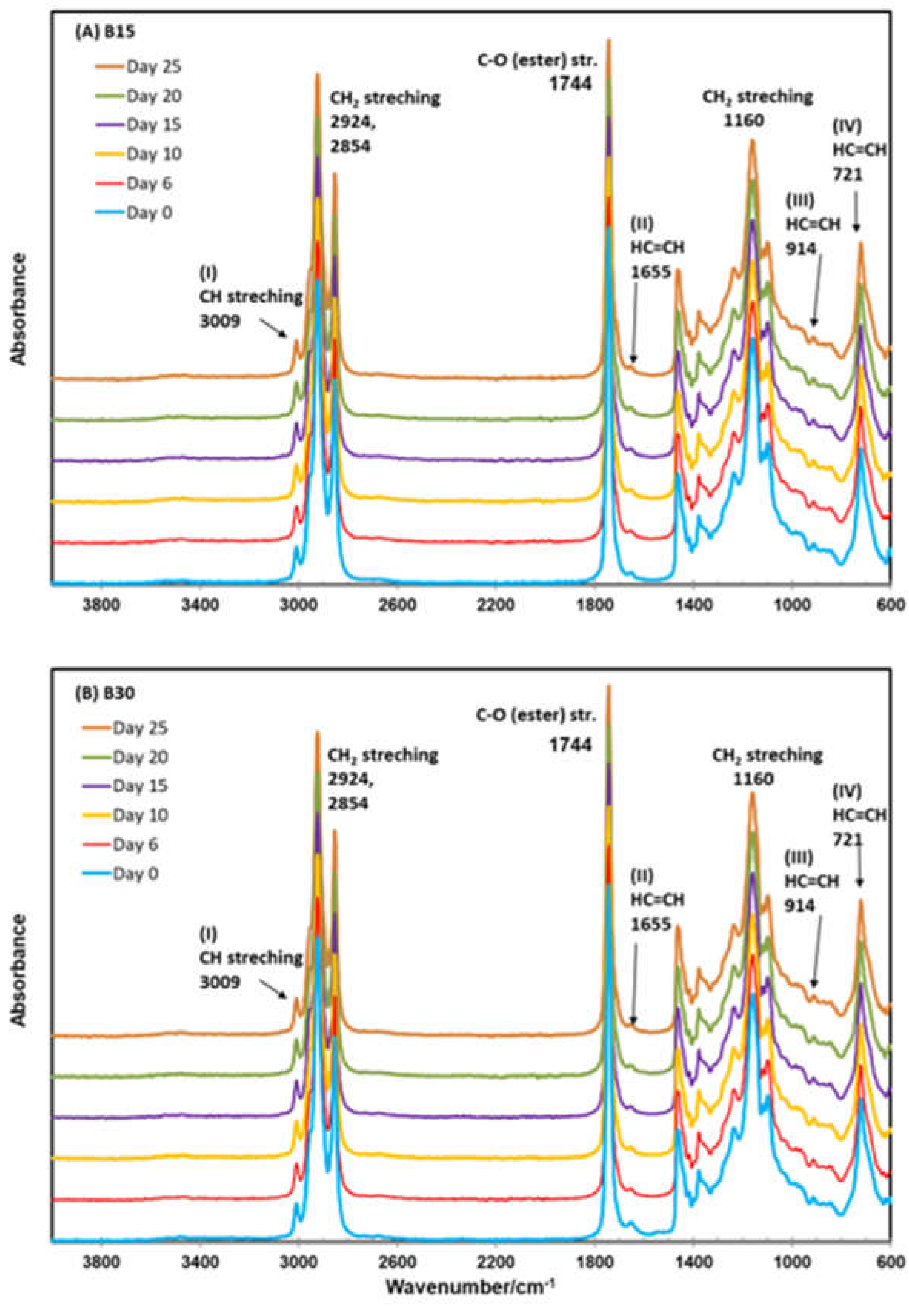
2.4. ATR–FTIR Observations
3. Materials and Methods
3.1. Cottonseed Source and Peanut-Butter-like Product Making
3.2. Accelerated Storage Experiment
3.3. Cottonseed Color Determination
3.4. Extraction of Oil Fractions from Butter Products
3.5. Oxidative Stability Measurement
3.6. HPLC Determination of Tocopherol
3.7. ATR–FTIR Spectroscopy
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Risco, C.; Chase, C. Gossypol. In Handbook of Plant and Fungal Toxicants; D’Mello, J.P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 87–98. [Google Scholar]
- He, Z.; Waldrip, H.M.; Wang, Y. Application of capillary electrophoresis in agricultural and soil chemistry research. In Capillary Electrophoresis: Fundamentals, Techniques and Applications; He, Z., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 131–151. [Google Scholar]
- He, Z.; Zhang, D.; Mattison, C.P. Quantitative comparison of the storage protein distribution in glandless and glanded cottonseeds. Agric. Environ. Lett. 2022, 7, e20076. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, X.; Ding, L.; Xu, B.; Gao, Y.; Cheng, Y.; Dai, F.; Liu, B.; Si, Z.; Fang, L. Development of the engineered “glanded plant and glandless seed” cotton. Food Chem. Mol. Sci. 2022, 5, 100130. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Mattison, C.P.; Zhang, D.; Grimm, C.C. Vicilin and legumin storage proteins are abundant in water and alkali soluble protein fractions of glandless cottonseed. Sci. Rep. 2021, 11, 9209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Hasan, M.; Choyal, P.; Tomar, M.; Gupta, O.P.; Sasi, M.; Changan, S.; Lorenzo, J.M.; Singh, S.; Sampathrajan, V. Cottonseed feedstock as a source of plant-based protein and bioactive peptides: Evidence based on biofunctionalities and industrial applications. Food Hydrocoll. 2022, 131, 107776. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Zhang, H.; Olanya, O.M. Chemical composition and thermogravimetric behaviors of glanded and glandless cottonseed kernels. Molecules 2022, 27, 316. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sethumadhavan, K.; Bland, J.M. Isolation of cottonseed extracts that affect human cancer cell growth. Sci. Rep. 2018, 8, 10458. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K. Identification of bcl2 as a stably expressed qpcr reference gene for human colon cancer cells treated with cottonseed-derived gossypol and bioactive extracts and bacteria-derived lipopolysaccharides. Molecules 2022, 27, 7560. [Google Scholar] [CrossRef]
- He, Z.; Zhang, D.; Olanya, O.M. Antioxidant activities of the water-soluble fractions of glandless and glanded cottonseed protein. Food Chem. 2020, 325, 126907. [Google Scholar] [CrossRef]
- Delgado, E.; Valverde-Quiroz, L.; Lopez, D.; Cooke, P.; Valles-Rosales, D.; Flores, N. Characterization of soluble glandless cottonseed meal proteins based on electrophoresis, functional properties, and microscopic structure. J. Food Sci. 2019, 84, 2820–2830. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S.-k. Preparation and identification of antioxidant peptides from cottonseed proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; He, J. Initial formulation of novel peanut butter-like products from glandless cottonseed. Foods 2023, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Shakerardekani, A.; Karim, R.; Ghazali, H.M.; Chin, N.L. Textural, rheological and sensory properties and oxidative stability of nut spreads—a review. Int. J. Mol. Sci. 2013, 14, 4223–4241. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, V.; Danthine, S.; Bolboacă, S.D.; Racolţa, E.; Muste, S.; Socaciu, C.; Blecker, C. Roasted sunflower kernel paste (tahini) stability: Storage conditions and particle size influence. J. Am. Oil Chem. Soc. 2015, 92, 669–683. [Google Scholar] [CrossRef]
- Shakerardekani, A.; Karim, R.; Ghazali, H.M.; Chin, N.L. Oxidative stability of pistachio (Pistacia vera L.) paste and spreads. J. Am. Oil Chem. Soc. 2015, 92, 1015–1021. [Google Scholar] [CrossRef]
- Hou, L.; Li, C.; Wang, X. The colloidal and oxidative stability of the sesame pastes during storage. J. Oleo Sci. 2020, 69, 191–197. [Google Scholar] [CrossRef]
- Valdés García, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the oxidative stability and volatiles in blanched, roasted and fried almonds under normal and accelerated storage conditions by dsc, thermogravimetric analysis and atr-ftir. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213. [Google Scholar] [CrossRef]
- Wall, M.M. Functional lipid characteristics, oxidative stability, and antioxidant activity of macadamia nut (Macadamia integrifolia) cultivars. Food Chem. 2010, 121, 1103–1108. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Choe, E. Temperature dependence of the autoxidation and antioxidants of soybean, sunflower, and olive oil. Eur. Food Res. Technol. 2007, 226, 239–246. [Google Scholar] [CrossRef]
- Valdés García, A.; Beltrán Sanahuja, A.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Effect of frying and roasting processes on the oxidative stability of sunflower seeds (Helianthus annuus) under normal and accelerated storage conditions. Foods 2021, 10, 944. [Google Scholar] [CrossRef]
- Shafiei, G.; Ghorbani, M.; Hosseini, H.; Mahoonak, A.S.; Maghsoudlou, Y.; Jafari, S.M. Estimation of oxidative indices in the raw and roasted hazelnuts by accelerated shelf-life testing. J. Food Sci. Technol. 2020, 57, 2433–2442. [Google Scholar] [CrossRef]
- Vieira, T.M.; Regitano-d’Arce, M.A. Canola oil thermal oxidation during oven test and microwave heating. LWT 2001, 34, 215–221. [Google Scholar] [CrossRef]
- Xie, C.; Ma, Z.F.; Li, F.; Zhang, H.; Kong, L.; Yang, Z.; Xie, W. Storage quality of walnut oil containing lycopene during accelerated oxidation. J. Food Sci. Technol. 2018, 55, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Muttagi, G.C.; Joshi, N.; Shadakshari, Y.G.; Chandru, R. Storage stability of value added products from sunflower kernels. J. Food Sci. Technol. 2014, 51, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.B.C.; Liu, J.L.; Jamilah, B.; Rahman, R.A. Quality changes of refined-bleached-deodorized (rbd) palm olein, soybean oil and their blends during deep-fat frying. J. Food Lipids 1999, 6, 181–193. [Google Scholar] [CrossRef]
- Ye, Y.; Khushvakov, J.; Boboev, A.; Akramova, R.; Yunusov, O.; Dalimova, D.; Turdikulova, S.; Mirzaakhmedov, S.; Engelsen, S.B.; Khakimov, B. Effect of refinement and production technology on the molecular composition of edible cottonseed oils from a large industrial scale production. J. Funct. Foods 2022, 99, 105326. [Google Scholar] [CrossRef]
- Marzocchi, S.; Pasini, F.; Verardo, V.; Ciemniewska-Żytkiewicz, H.; Caboni, M.F.; Romani, S. Effects of different roasting conditions on physical-chemical properties of polish hazelnuts (Corylus avellana L. Var. Kataloński). LWT 2017, 77, 440–448. [Google Scholar] [CrossRef]
- He, Z.; Liu, S.; Nam, S.; Klasson, K.T.; Cheng, H.N. Molecular level characterization of the effect of roasting on the extractable components of glandless cottonseed by fourier transform ion cyclotron resonance mass spectrometry. Food Chem. 2023, 403, 134404. [Google Scholar] [CrossRef] [PubMed]
- Kouser, S.; Mahmood, K.; Anwar, F. Variations in physicochemical attributes of seed oil among different varieties of cotton (Gossypium hirsutum L.). Pak. J. Bot. 2015, 47, 723–729. [Google Scholar]
- Sharif, I.; Farooq, J.; Chohan, S.M.; Saleem, S.; Kainth, R.A.; Mahmood, A.; Sarwar, G. Strategies to enhance cottonseed oil contents and reshape fatty acid profile employing different breeding and genetic engineering approaches. J. Integr. Agric. 2019, 18, 2205–2218. [Google Scholar] [CrossRef]
- Javidipour, I.; Tüfenk, R.; Baştürk, A. Effect of ascorbyl palmitate on oxidative stability of chemically interesterified cottonseed and olive oils. J. Food Sci. Technol. 2015, 52, 876–884. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed oil: A review of extraction techniques, physicochemical, functional, and nutritional properties. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, K.A.; White, B.L.; Dean, L.L.; Sanders, T.H.; Davis, J.P. Compositional and mechanical properties of peanuts roasted to equivalent colors using different time/temperature combinations. J. Food Sci. 2012, 77, C1293–C1299. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Y. Fourier transform infrared spectroscopic analysis in applied cotton fiber and cottonseed research: A review. J. Cotton Sci. 2021, 25, 167–183. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Kim, H.J.; Tewolde, H.; Zhang, H. Fourier transform infrared spectral features of plant biomass components during cotton organ development and their biological implications. J. Cotton Res. 2022, 5, 11. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Shankle, M.; Tewolde, H. Compositional features of cotton plant biomass fractions characterized by attenuated total reflection fourier transform infrared spectroscopy. Ind. Crop. Prod. 2016, 79, 283–286. [Google Scholar] [CrossRef]
- Arslan, F.N.; Akin, G.; Elmas, Ş.N.K.; Yilmaz, I.; Janssen, H.-G.; Kenar, A. Rapid detection of authenticity and adulteration of cold pressed black cumin seed oil: A comparative study of atr–ftir spectroscopy and synchronous fluorescence with multivariate data analysis. Food Control 2019, 98, 323–332. [Google Scholar] [CrossRef]
- Beltrán Sanahuja, A.; Prats Moya, M.; Maestre Pérez, S.; Grané Teruel, N.; Martín Carratalá, M. Classification of four almond cultivars using oil degradation parameters based on ftir and gc data. J. Am. Oil Chem. Soc. 2009, 86, 51–58. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Some of the most significant changes in the fourier transform infrared spectra of edible oils under oxidative conditions. J. Sci. Food Agric. 2000, 80, 2028–2036. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, S.; Wang, L.; Li, Q.; Gao, Y.; Yu, X. New method for the determination of the induction period of walnut oil by fourier transform infrared spectroscopy. Food Anal. Met. 2022, 15, 833–843. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y. Application of ftir spectroscopy for monitoring the stabilities of selected vegetable oils during thermal oxidation. Int. J. Food Prop. 2013, 16, 1594–1603. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Moigradean, D.; Dumbrava, D.-G.; Radulov, I.; Raba, D.N.; Rivis, A. Exploring the potential of grape pomace extract to inhibit thermo-oxidative degradation of sunflower oil: From routine tests to atr-ftir spectroscopy. Foods 2022, 11, 3674. [Google Scholar] [CrossRef]
- Song, W.; Kong, X.; Hua, Y.; Li, X.; Zhang, C.; Chen, Y. Antioxidant and antibacterial activity and in vitro digestion stability of cottonseed protein hydrolysates. LWT 2020, 118, 108724. [Google Scholar] [CrossRef]
- Zhang, J.; Wedegaertner, T.; Idowu, O.J.; Flynn, R.; Hughs, S.E.; Jones, D.C. Registration of ‘numex cot 15 gls’ glandless cotton. J. Plant Regist. 2016, 10, 223–227. [Google Scholar] [CrossRef]
- Nikolić, I.; Dokić, L.; Rakić, D.; Tomović, V.; Maravić, N.; Vidosavljević, S.; Šereš, Z.; Šoronja-Simović, D. The role of two types of continuous phases based on cellulose during textural, color, and sensory characterization of novel food spread with pumpkin seed flour. J. Food Process. Preserv. 2018, 42, e13684. [Google Scholar] [CrossRef]
- Wagener, E.A.; Kerr, W.L. Effects of oil content on the sensory, textural, and physical properties of pecan butter (Carya illinoinensis). J. Texture Stud. 2018, 49, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ben-Youssef, S.; Fakhfakh, J.; Breil, C.; Abert-Vian, M.; Chemat, F.; Allouche, N. Green extraction procedures of lipids from tunisian date palm seeds. Ind. Crop. Prod. 2017, 108, 520–525. [Google Scholar] [CrossRef]
- AOCS. Peroxide value: Acetic acid-isooctane method. In AOCS Official Method Cd 80-90; AOCS: Urbana, IL, USA, 1997. [Google Scholar]
- Rozalli, N.H.M.; Chin, N.L.; Yusof, Y.A.; Mahyudin, N. Quality changes of stabilizer-free natural peanut butter during storage. J. Food Sci. Technol. 2016, 53, 694–702. [Google Scholar] [CrossRef]
- AOCS. P-anisidine value. In AOCS Official Method Cd 18-90; AOCS: Urbana, IL, USA, 1997. [Google Scholar]
- Shahidi, F.; Wanasundara, U.N. Methods for evaluation of the oxidative stability of lipid-containing foods. Food Sci. Technol. Inter. 1996, 2, 73–81. [Google Scholar] [CrossRef]
- AOCS. Determination of tocopherols and tocotrienols in vegetable oils and fats by hplc. In AOCS Offical Method Ce 8-89; AOCS: Urbana, IL, USA, 2013. [Google Scholar]

| (A) B15 | |||||
|---|---|---|---|---|---|
| Day | L* | a* | b* | C* | h |
| 0 | 59.52 ± 0.01 a | 3.19 ± 0.02 f | 25.60 ± 0.04 d | 25.80 ± 0.04 e | 82.89 ± 0.05 a |
| 6 | 55.21 ± 0.02 f | 4.08 ± 0.01 d | 24.68 ± 0.01 f | 25.01 ± 0.01 f | 80.61 ± 0.02 e |
| 10 | 55.30 ± 0.02 e | 4.44 ± 0.01 a | 25.40 ± 0.01 e | 25.87 ± 0.01 d | 80.11 ± 0.01 f |
| 15 | 57.67 ± 0.02 d | 4.21 ± 0.01 b | 26.42 ± 0.01 b | 26.75 ± 0.01 bc | 80.94 ± 0.01 d |
| 20 | 59.01 ± 0.01 b | 3.99 ± 0.01 e | 26.11 ± 0.01 c | 26.41 ± 0.01 c | 81.32 ± 0.01 b |
| 25 | 58.54 ± 0.00 c | 4.18 ± 0.00 c | 26.73 ± 0.00 a | 27.05 ± 0.01 a | 81.12 ± 0.01 c |
| (B) B30 | |||||
| Day | L* | a* | b* | C* | h |
| 0 | 56.58 ± 0.01 b | 3.60 ± 0.01 f | 26.15 ± 0.04 d | 26.41 ± 0.01 d | 82.16 ± 0.00 a |
| 6 | 56.35 ± 0.01 d | 4.30 ± 0.01 d | 26.06 ± 0.00 e | 26.42 ± 0.01 d | 80.62 ± 0.00 e |
| 10 | 55.47 ± 0.05 e | 4.52 ± 0.01 a | 25.68 ± 0.01 f | 26.08 ± 0.00 e | 80.01 ± 0.02 f |
| 15 | 54.78 ± 0.01 f | 4.04 ± 0.01 e | 26.37± 0.01 c | 26.68± 0.01 cc | 81.28 ± 0.00 b |
| 20 | 57.77 ± 0.01 a | 4.46 ± 0.01 b | 27.14 ± 0.01 a | 27.51 ± 0.01 a | 80.66 ± 0.02 d |
| 25 | 56.45 ± 0.01 c | 4.34 ± 0.01 c | 26.78 ± 0.00 b | 27.13 ± 0.01 b | 80.79 ± 0.01 c |
| (A) B15 | |||||
|---|---|---|---|---|---|
| Day | a- | γ- | δ- | b- | Total |
| 0 | 23.30 ± 10.30 ab | 62.28 ± 15.12 a | 0.66 ± 0.05 a | 0.73 ± 0.27 c | 87.18 ± 15.14 a |
| 6 | 14.23 ± 11.16 ab | 43.49 ± 16.81 ab | 0.52 ± 0.11 a | 1.24 ± 0.37 ab | 59.48 ± 28.41 a |
| 10 | 9.68 ± 8.63 b | 34.30 ± 18.15 ab | 0.54 ± 0.06 a | 1.16 ± 0.29 abc | 49.70 ± 31.19 a |
| 15 | 31.01 ± 8.33 a | 61.97 ± 2.82 a | 0.69 ± 0.04 a | 1.10 ± 0.07 bc | 94.78 ± 10.89 a |
| 20 | 24.27 ± 8.84 ab | 52.30 ± 11.81 ab | 0.68 ± 0.13 a | 0.98 ± 0.27 bc | 78.22 ± 20.66 |
| 25 | 15.09 ± 17.66 ab | 28.52 ± 27.32 b | 0.52 ± 0.15 a | 1.58 ± 0.21 a | 45.72 ± 44.87 a |
| (B) B30 | |||||
| Day | a- | γ- | δ- | b- | Total |
| 0 | 30.28 ± 17.24 b | 66.24 ± 1.43 a | 0.74 ± 0.06 b | 0.77 ± 0.07 bc | 98.03 ± 8.71 a |
| 6 | 21.36 ± 3.39 c | 43.27 ± 4.54 b | 0.65 ± 0.09 bc | 0.80 ± 0.09 b | 66.09 ± 7.13 b |
| 10 | 14.06 ± 5.91 c | 29.70 ± 7.79 c | 0.57 ± 0.05 c | 0.68 ± 0.05 c | 45.01 ± 13.55 c |
| 15 | 45.45 ± 0.81 a | 66.85 ± 1.68 a | 0.92 ± 0.03 a | 1.07 ± 0.01 a | 114.29 ± 2.24 a |
| 20 | 45.33 ± 0.66 a | 64.63 ± 0.19 a | 0.97 ± 0.04 a | 1.10 ± 0.02 a | 112.03 ± 0.80 a |
| 25 | 41.25 ± 2.03 ab | 63.35 ± 0.35 a | 0.96 ± 0.04 a | 1.08 ± 0.03 a | 106.64 ± 2.25 a |
| Tocopherol | Oxidative Stability | |||||||
|---|---|---|---|---|---|---|---|---|
| α- | g- | d- | b- | Total | PV | pAV | ||
| Tocopherol | g- | 0.851 *** | ||||||
| d- | 0.974 *** | 0.802 ** | ||||||
| b- | −0.069 | −0.275 | −0.129 | |||||
| Total | 0.957 *** | 0.967 *** | 0.916 *** | −0.179 | ||||
| Oxidative stability | PV | −0.815 *** | −0.873 *** | −0.747 ** | 0.163 | −0.880 *** | ||
| pAV | −0.316 | 0.059 | −0.314 | −0.543 | −0.123 | −0.121 | ||
| Totox | −0.881 *** | −0.866 *** | −0.812 ** | 0.06 | −0.909 *** | 0.981 *** | 0.074 | |
| Major Component (g kg−1) | ||||||
|---|---|---|---|---|---|---|
| Protein | Oil | Sugar | Starch | ADF | ADL | Gossypol |
| 315.8 | 430.5 | 75.0 1 | 12.5 | 81.8 | 50.9 | 0.0045 |
| Macro Element (g kg−1) | ||||||
| P | Ca | K | Mg | Na | S | Cl |
| 8.6 | 1.7 | 9.6 | 4.6 | 3.2 | 3.7 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Nam, S.; Klasson, K.T. Oxidative Stability of Cottonseed Butter Products under Accelerated Storage Conditions. Molecules 2023, 28, 1599. https://doi.org/10.3390/molecules28041599
He Z, Nam S, Klasson KT. Oxidative Stability of Cottonseed Butter Products under Accelerated Storage Conditions. Molecules. 2023; 28(4):1599. https://doi.org/10.3390/molecules28041599
Chicago/Turabian StyleHe, Zhongqi, Sunghyun Nam, and K. Thomas Klasson. 2023. "Oxidative Stability of Cottonseed Butter Products under Accelerated Storage Conditions" Molecules 28, no. 4: 1599. https://doi.org/10.3390/molecules28041599
APA StyleHe, Z., Nam, S., & Klasson, K. T. (2023). Oxidative Stability of Cottonseed Butter Products under Accelerated Storage Conditions. Molecules, 28(4), 1599. https://doi.org/10.3390/molecules28041599







