Implementation of Biopolymeric Nanomaterials to Reduce the Negative Impacts of Salinity on Tomato Quantity and Quality
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biopolymers Characteristic
2.1.1. Surface Analysis and Pore Size Distribution
2.1.2. The FT-IR Spectra
2.2. Incubation Experiment
2.3. Evaluation of the Polymer’s Efficiency in Retaining Sodium Ions (ppm)
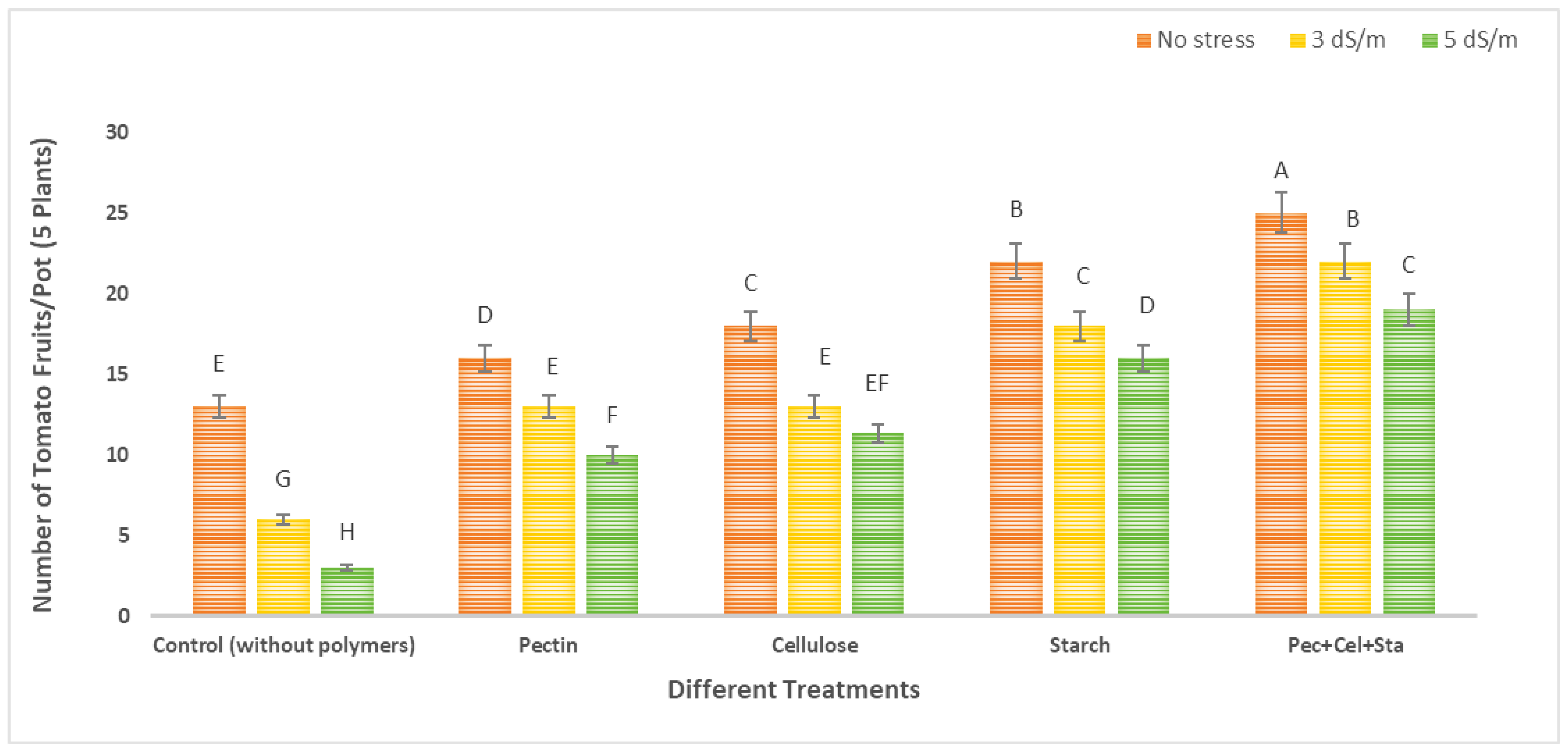
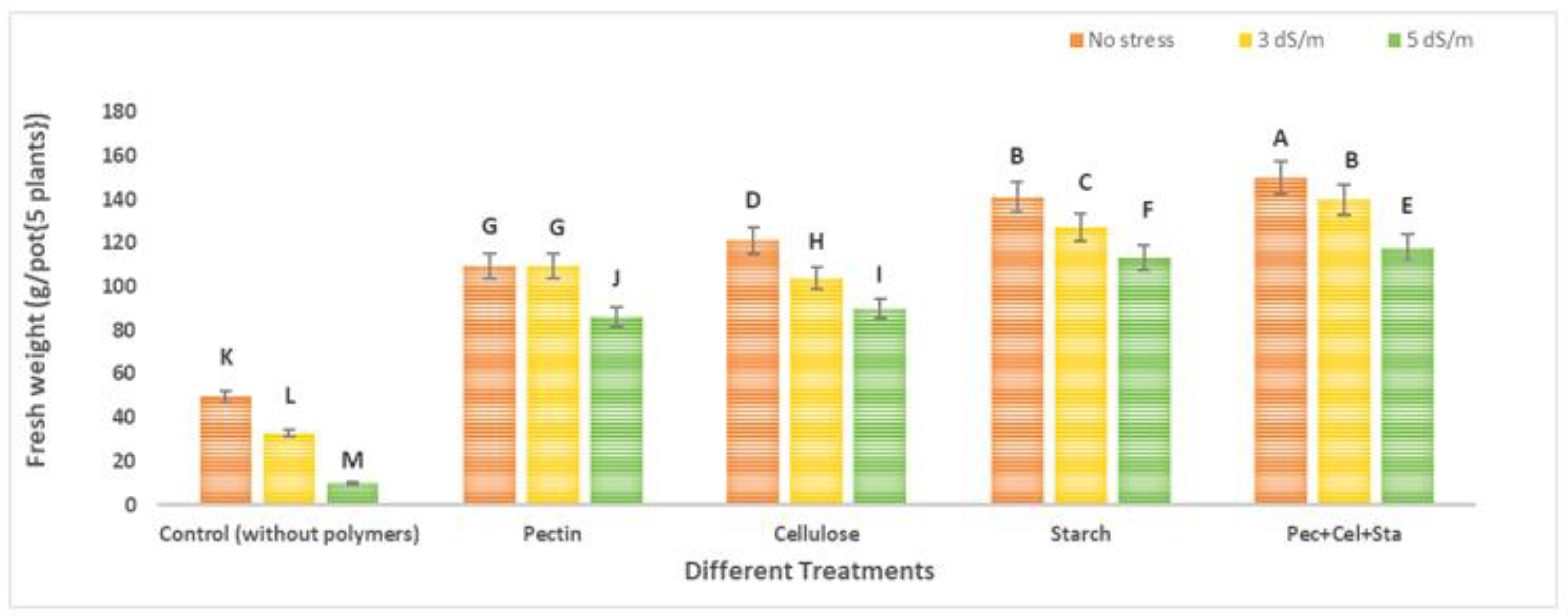
2.4. The Effect of Biopolymers Hydrogel on the Number and Fresh Weight of Fruits
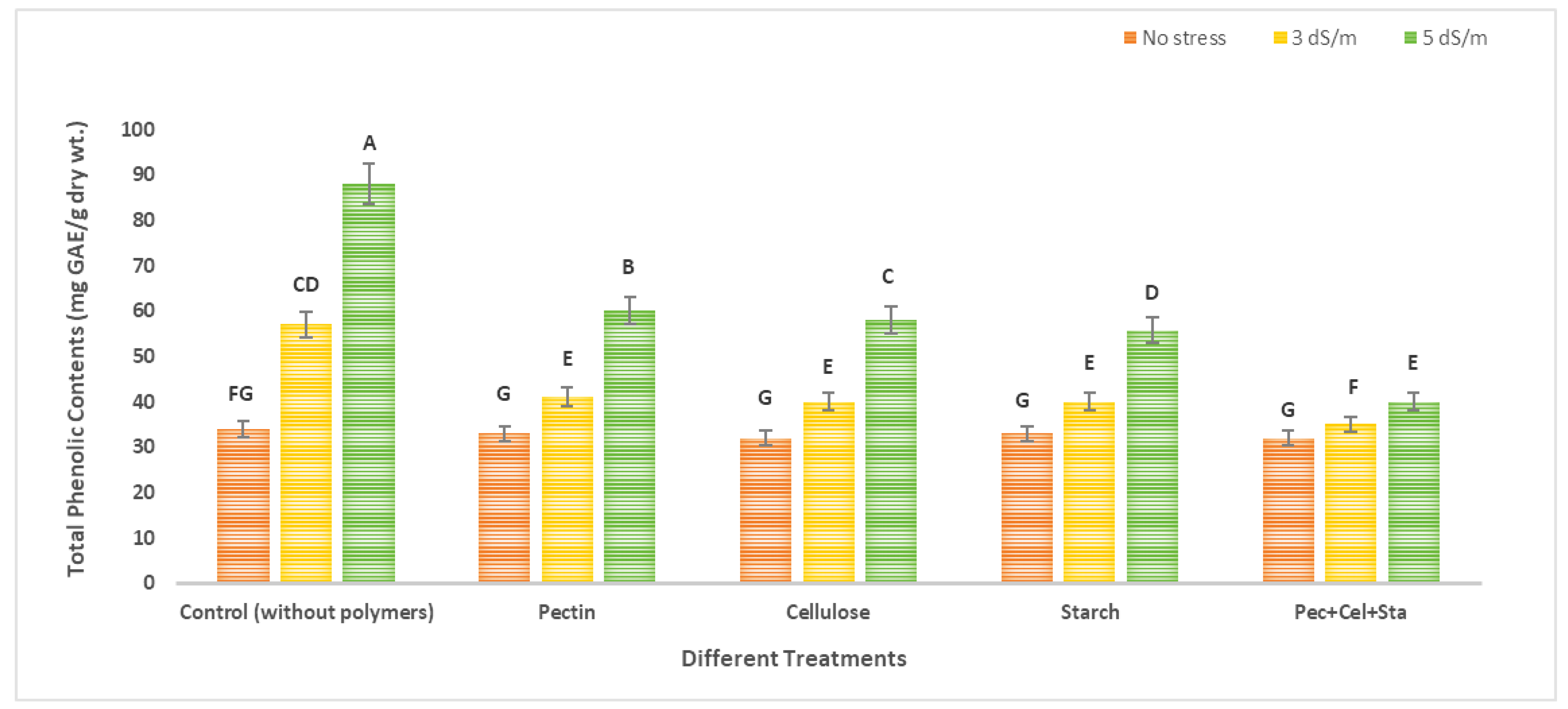
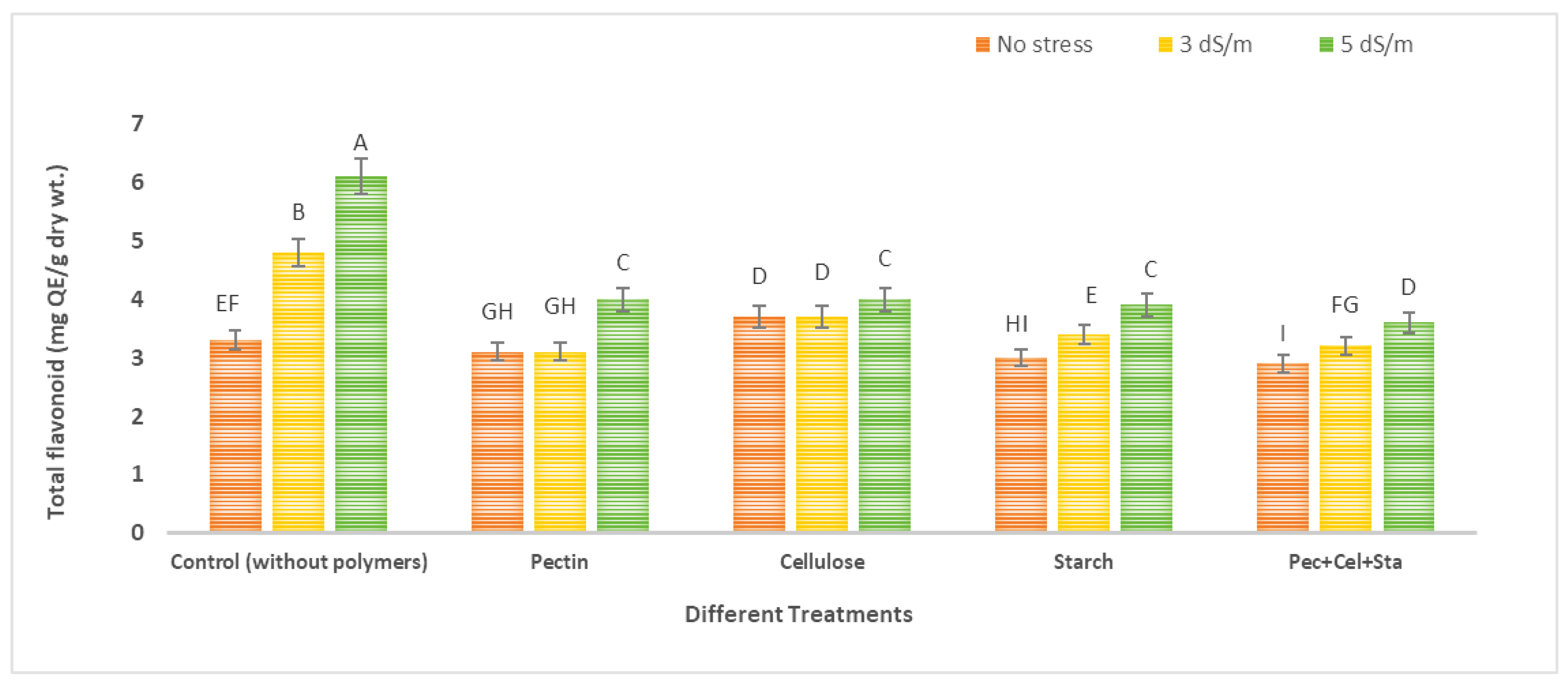
2.5. The Effect of Biopolymers Hydrogel on Phenol and Flavonoid Content of Tomato Fruits under Salinity Stress
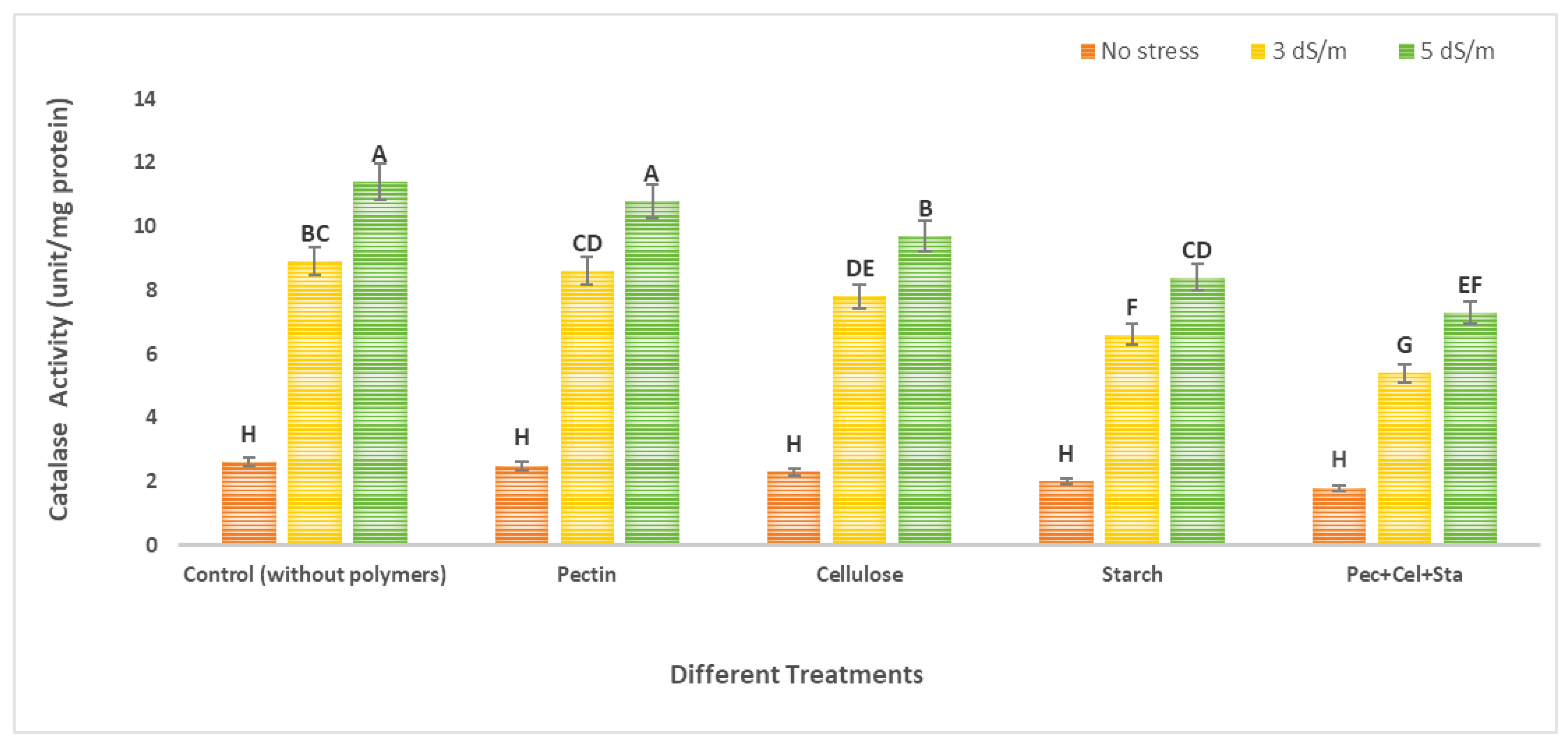
2.6. The Effect of Biopolymers Hydrogel on Tomato Catalase and Peroxidase Activity under Salt Stress
2.7. Impact of Biopolymers Hydrogel on the Quality of Tomato Fruits under Salt Stress
3. Materials and Methods
3.1. Natural Polymer Preparation
3.2. Starch Polymer Preparation
3.3. Preparation of Cellulose Polymer Hydrogel
3.4. Pectin Hydrogel Preparation
3.5. Incubation and Measuring of Soil Electrical Conductivity (EC) Experiment
3.6. Laboratory Experiment to Determine the Polymer’s Ability to Retain Sodium Ions
3.7. Greenhouse Experiment
3.8. Analytical Methods
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. 2021. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 31 March 2021).
- Boari, F.; Cantore, V.; Venere, D.D.; Sergio, L.; Candido, V.; Schiattone, M.I. Pyraclostrobin can mitigate salinity stress in tomato crop. Agric. Water Manag. 2019, 222, 254–264. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2019, 240, 196–204. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Fernandez, M.R. Tomato and salinity. Sci. Hortic. 1999, 78, 83–125. [Google Scholar] [CrossRef]
- Kamrani, M.H.; Khoshvaghti, H.H. Effects of salinity and hydroponic growth media on growth parameters in tomato (Lycopersicon esculentum Mill). Int. J. Agron. Plant Prod. 2013, 4, 2694. [Google Scholar]
- Zhang, P.F.; Senge, M.T.; Yoshiyama, K.H.; Ito, K.G.D. Effects of low salinity stress on growth, yield and water use efficiency of tomato under soilless cultivation. J. Irrig. Drain Rural Eng. 2017, 304, 15–21. [Google Scholar]
- Zhang, P.; Senge, M.; Dai, Y. Effects on salinity stress on growth, yield, fruit quality and water use efficiency of tomato under soilless cultivation. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Feld, M.; Marzocchella, A. Agro-Food Wastes and Innovative Pretreatments to Meet Biofuel Demand in Europe. Chem. Eng. Technol. 2019, 42, 954–961. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture. Food and Agriculture. Moving Forward on Food Loss and Waste Reduction. Rome; FAO: Rome, Italy, 2019; ISBN 978-92-5-131789-1. [Google Scholar]
- Jha, A.; Kumar, A. Biobased Technologies for the Efficient Extraction of Biopolymers from Waste Biomass. Bioprocess Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Testing of Starch-Based Carbohydrate Polymer Coatings for Enhanced Urea Performance. J. Coat. Technol. Res. 2014, 11, 747–756. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J. Chitosan-Starch Beads Prepared by Ionotropic Gelation as Potential Matrices for Controlled Release of Fertilizers. Carbohydr. Polym. 2016, 148, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hong, J.; Liu, Y.; Wang, B.; Hua, Q.; Liu, L.; Ying, D. Urea Controlled-Release Fertilizer Based on Gelatin Microspheres. J. Polym. Environ. 2018, 26, 1930–1939. [Google Scholar] [CrossRef]
- Jiao, G.J.; Xu, Q.; Cao, S.; Peng, P.; She, D. Controlled-Release Fertilizer with Lignin Used to Trap Urea/Hydroxymethylurea/ Urea-Formaldehyde Polymers. BioResources 2018, 13, 1711–1728. [Google Scholar] [CrossRef]
- Akalin, G.O.; Pulat, M. Controlled Release Behavior of Zinc-Loaded Carboxymethyl Cellulose and Carrageenan Hydrogels and Their Effects on Wheatgrass Growth. J. Polym. Res. 2020, 27, 2–11. [Google Scholar] [CrossRef]
- Shreen, S.A.; Fahmy, A.H. Applications of Natural Polysaccharide Polymers to Overcome Water Scarcity on the Yield and Quality of Tomato fruits. J. Soil Sci. Agric. Eng. 2019, 10, 199–208. [Google Scholar]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, Through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 1–26. [Google Scholar] [CrossRef]
- King, T.; Martin, C.; Jeffrey, M.; Gerhard, E.; Dimitrios, Z.; Edward, M.; FoxdJerem, M.; Hille, P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Abobatta, W. Impact of hydrogel polymer in the agricultural sector. Adv. Agric. Environ. Sci. 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Peng, B.; Yao, Z.; Tam, K.C. Sustainable nanomaterials derived from polysaccharides and amphiphilic compounds. Soft Matter 2013, 9, 7905–7918. [Google Scholar] [CrossRef]
- Ramdas, V.M.; Mandree, P.; Mgangira, M.; Mukaratirwa, S.; Lalloo, R.; Ramchuran, S. Review of current and future bio-based stabilisation products (enzymatic and polymeric) for road construction materials. Transp. Geotech. 2021, 27, 7158. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as green binders for soil improvement in geotechnical applications: A review. Geosciences 2021, 11, 1–39. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.M.; Im, J.; Cho, G.C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 1129. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural polysaccharide nanomaterials: An overview of their immunological properties. Int. J. Mol. Sci. 2019, 20, 102. [Google Scholar] [CrossRef]
- Qureshi, M.U.; Chang, I.; Al-Sadarani, K. Strength and durability characteristics of biopolymer-treated desert sand. Geomech. Eng. 2017, 12, 785–801. [Google Scholar] [CrossRef]
- Dorraja, S.; Gdechin, A.; Ahmadi, S. The Effects of Hydrophilic Polymer and Soil Salinity on Corn Growth in Sandy and Loamy Soils. Clean-Soil Air Water 2017, 38, 584–591. [Google Scholar] [CrossRef]
- Dehkordi, G.K.D. Effect of superabsorbent polymer on salt and drought resistance of Eucalyptus. Appl. Ecol. Environ. Res. 2017, 15, 1791–1802. [Google Scholar] [CrossRef]
- Aydin, A.; Canan, K.; Metin, T. Hydrogel substrate alleviates salt stress with increased antioxidant enzyme activity of bean (Phaseolus vulgaris L.) under salinity stress. Afr. J. Agric. Res. 2011, 6, 715–724. [Google Scholar]
- Sánchez, J.; Bryan, B.; Bernabé, L. Biopolymers are applied to remove metal ions through ultrafiltration. J. Chil. Chem. Soc. 2020, 65, 5004–5010. [Google Scholar] [CrossRef]
- Zhu, J.K.; Hasegawa, P.M.; Bressan, R.A. Molecular aspects of osmotic stress. Crit. Rev. Plant Sci. 1997, 16, 253–277. [Google Scholar] [CrossRef]
- Shokuohifar, M.; Nasab, S.B.; Mohammadi, A.S.; Hooshmand, A.R. The effect of salinity of irrigation water and super absorbent polymer on some hydraulic and physical properties of sandy loam soil. Irrig. Sci. Eng. 2016, 39, 101–113. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Handa, A.K. Cellular mechanism of salinity tolerance. Hort Sci. 1986, 21, 1317–1324. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, M.; Lin, C.C.; Kao, C.H. Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci. 2001, 201, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.H.; Hussein, A.H. Effect of polymeric material (Aquastore) on wheat seedlings emergence, soil salinity and available phosphorus in sandy soil. In Proceedings of the Tenth Symposium on the Biological Aspects of Saudi Arabia, Jedda, Saudi Arabia, 1987, Paper presented at the Tenth Symposium on the Biological Aspects of Saudi Arabia, Jedda, Saudi Arabia, 20–24 April 1987. [Google Scholar]
- El Sayed, H.; Kirkwood, R.C. Effect of NaCl salinity and hydrogel polymer treatments on viability, germination and solute contents in maize (Zea mays, L.) Pollen. Phyton J. 1992, 32, 143–157. [Google Scholar]
- Orikiriza, L.J.; Agaba, H.; Tweheyo, M. Amending soils with hydrogels increases the biomass of nine tree species under non-water stress conditions. Clean-Soil Air Water 2009, 37, 615–620. [Google Scholar] [CrossRef]
- Barakat, M.R.; El-Kosary, S.; Borham, T.I. Effect of hydrogel soil addition under different irrigation levels on Grand Nain banana plants. J. Hortic. Sci. Ornam. Plants 2015, 7, 19–28. [Google Scholar]
- Johnson, M.S. Effect of soluble salts on water absorption by gel-forming soil conditioners. J. Sci. Food Agric. 1984, 35, 1063–1066. [Google Scholar] [CrossRef]
- Helalia, A.M.; Letey, J. Effects of different polymer on seedling emergence, aggregate stability and crust hardiness. Soil Sci. 1989, 148, 199–203. [Google Scholar] [CrossRef]
- Yazdani, F.; Allahdadi, I.; Akbari, G.A. Impact of superabsorbent polymer on yield and growth analysis of Soybean (Glycine max L.) under drought stress condition. Pak. J. Biol. Sci. 2007, 10, 4190–4196. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). J. Appl. Phycol. 2015, 27, 1689–1698. [Google Scholar] [CrossRef]
- Bor, M.; Özdemir, F.; Türkan, I. The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci. 2003, 164, 77–84. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Anshuman, S. Harnessing the Potential of Superabsorbent Polymers for Alleviating Drought and Salt Stresses in Fruit Crops. Adv. Agric. Technol. Plant Sci. 2018, 1, 1–10. [Google Scholar]
- Kumaran, S.S. Effect of hydrophilic polymers on yield and quality of tomato. Int. J. Appl. Pure Sci. Agric. (IJAPSA) 2016, 2, 56–60. [Google Scholar]
- Thybo, A.K.; Delenbos, M.E.; Christensen, L.P.; Sørensen, J.N.; Thorup-Kristensen, K. Effect of organic growing systems on sensory quality and chemical composition of tomatoes. LWT-Food Sci. Technol. 2006, 39, 835–843. [Google Scholar] [CrossRef]
- Anthon, G.E.; Lestrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Mahesh, B.; Kathyayani, D.; Nanjundaswamy, G.S.; Channe, G.D.; Sridhar, R. Miscibility studies of plastic-mimetic polypeptide with Hydroxypropylmethylcellulose blends and generation of non-woven fabrics. Carbohydr. Polym. 2019, 212, 129–141. [Google Scholar] [CrossRef]
- Vasanthan, T. Overview of laboratory isolation of starch from plant materials. Food Anal. Chem. 2001, 1, E2.1.1–E2.1.6. [Google Scholar] [CrossRef]
- Pandharipande, S.; Harshal, M. Separation of oil and pectin from orange peel and study of the effect of pH of extracting medium on the yield of pectin. J. Eng. Res. Stud. 2012, 3, 6–9. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Agronomy 9.; American Society of Agronomy: Madison, WI, USA, 1982; p. 1159. [Google Scholar]
- Aloys, H. Effects of Hydrogel Amendment to Different Soils on Plant Available Water and Survival of Trees under Drought Conditions. Clean-Soil Air Water 2010, 38, 328–335. [Google Scholar]
- Wei, L.H.; Intan, S.I. Antioxidant Activity, Total Phenolics and Total Flavonoids of Syzygiumpolyanthum (Wight) Walp Leaves. Int. J. Med. Aromat. Plants 2012, 2, 219–228. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic peroxidases and lignification in needles of Norway Spruce Picea abies L. Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.; Torrie, J.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Duncan, D.B. Multiple ranges and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]


| Polymer | Level of Salinity (dS/m) | Mean of Polymer | ||
|---|---|---|---|---|
| 0 | 3 | 5 | ||
| Soil without polymer | 0.36 ± 0.01 aC | 3.50 ± 0.12 aB | 5.40 ± 0.00 aA | 3.09 ± 0.74 a |
| Soil with cellulose | 0.34 ± 0.01 aC | 3.39 ± 0.01 aB | 5.32 ± 0.68 aA | 3.02 ± 0.67 a |
| Soil with pectin | 0.33 ± 0.01 aC | 3.33 ± 0.01 abB | 5.24 ± 0.01 abA | 2.97 ± 0.82 a |
| Soil with starch | 0.32 ± 0.01 aC | 3.29 ± 0.01 abB | 5.19 ± 0.02 abA | 2.93 ± 0.71 a |
| Soil with mixture polymer | 0.30 ± 0.01 aC | 3.15 ± 0.02 bB | 5.08 ± 0.02 bA | 2.84 ± 0.69 a |
| Mean of level | 0.33 ± 0.01 C | 2.73 ± 0.32 B | 5.11 ± 0.13 A | |
| Correlation | ||||
| Value | ||||
| Corr. | Sig. | |||
| Polymer | −0.025 | 0.872 | ||
| Level | 0.972 *** | 0.000 | ||
| * Salt (3 dS/m) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Polymer | Soluble-Na | Exchange-Na | ||||||
| 0.5 | 1.0 | Mean | 0.5 | 1.0 | Mean | |||
| Cellulose | 26.41 ± 0.24 cB | 35.82 ± 0.26 cA | 31.12 ± 2.12 c | 12.82 ± 0.10 cB | 18.45 ± 0.29 cA | 15.64 ± 1.27 d | ||
| Pectin | 25.14 ± 0.11 dB | 33.91 ± 0.25 dA | 29.53 ± 1.97 d | 11.63 ± 0.06 dB | 16.44 ± 0.13 dA | 14.04 ± 1.08 d | ||
| Starch | 28.36 ± 0.11 bB | 43.20 ± 0.17 bA | 35.78 ± 3.32 b | 14.30 ± 0.10 bB | 20.88 ± 0.11 bA | 17.59 ± 1.47 b | ||
| Mixture | 35.91 ± 0.09 aB | 54.80 ± 0.48 aA | 45.36 ± 4.23 a | 16.62 ± 0.07 aB | 23.21 ± 0.42 aA | 19.92 ± 1.49 a | ||
| Mean | 28.96 ± 0.36 B | 41.93 ± 2.48 A | 13.84 ± 0.16 B | 19.75 ± 0.78 A | ||||
| Correlation | ||||||||
| Soluble-Na | Exchange-Na | |||||||
| Corr. | Sig. | Corr. | Sig. | |||||
| Polymer | 0.634 ** | 0.001 | 0.590 ** | 0.002 | ||||
| Level | 0.705 *** | 0.000 | 0.795 *** | 0.000 | ||||
| Soluble-Na | 0.969 *** | 0.000 | ||||||
| Treatments | Level of salinity | pH | TSS% | Juice% |
|---|---|---|---|---|
| Control (Without polymer) | 0.0 | 4.010 g | 4.200 e | 54.62 d |
| 3 dS/m | 3.640 i | 3.190 k | 50.66 h | |
| 5 dS/m | 3.060 j | 2.950 l | 36.73 j | |
| Pectin | 0.0 | 4.490 c | 4.290 d | 55.44 c |
| 3 dS/m | 4.140 f | 3.640 i | 52.11 f | |
| 5 dS/m | 3.820 h | 3.450 j | 48.93 i | |
| Cellulose | 0.0 | 4.610 b | 4.590 c | 56.74 b |
| 3 dS/m | 4.220 e | 3.953 g | 52.36 f | |
| 5 dS/m | 4.040 g | 3.670 i | 50.19 h | |
| Starch | 0.0 | 4.650 b | 4.830 b | 57.46 a |
| 3 dS/m | 4.290 d | 4.180 e | 53.43 e | |
| 5 dS/m | 4.110 f | 3.900 h | 51.23 g | |
| Mix of all polymers | 0.0 | 4.740 a | 4.940 a | 56.53 b |
| 3 dS/m | 4.300 d | 4.310 d | 53.87 e | |
| 5 dS/m | 4.200 e | 4.110 f | 52.07 f |
| Characteristics | Value |
|---|---|
| Physical properties | |
| Particle size distribution | |
| Sand% | 60 |
| Silt% | 25 |
| Clay% | 15 |
| Texture soil | Sandy loam |
| Chemical properties | |
| Organic matter content% | 1.24 |
| pH (1: 2.5) | 7.80 |
| EC ds/m | 1.61 |
| Cations meq/L | |
| Ca++ | 3.80 |
| Mg++ | 3.31 |
| Na+ | 4.60 |
| K+ | 0.26 |
| Anions meq/L | |
| CO=3 | 0.00 |
| HCO−3 | 1.60 |
| CL | 7.21 |
| SO=4 | 3.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.S.; Khan, T.K.; Abd El-Aziz, G.H.; Shoala, T.; El-Garhy, H.A.S.; Fahmy, A.H. Implementation of Biopolymeric Nanomaterials to Reduce the Negative Impacts of Salinity on Tomato Quantity and Quality. Molecules 2023, 28, 1594. https://doi.org/10.3390/molecules28041594
Ahmed SS, Khan TK, Abd El-Aziz GH, Shoala T, El-Garhy HAS, Fahmy AH. Implementation of Biopolymeric Nanomaterials to Reduce the Negative Impacts of Salinity on Tomato Quantity and Quality. Molecules. 2023; 28(4):1594. https://doi.org/10.3390/molecules28041594
Chicago/Turabian StyleAhmed, Shreen S., Thana K. Khan, Gehan H. Abd El-Aziz, Tahsin Shoala, Hoda A. S. El-Garhy, and Ashraf H. Fahmy. 2023. "Implementation of Biopolymeric Nanomaterials to Reduce the Negative Impacts of Salinity on Tomato Quantity and Quality" Molecules 28, no. 4: 1594. https://doi.org/10.3390/molecules28041594
APA StyleAhmed, S. S., Khan, T. K., Abd El-Aziz, G. H., Shoala, T., El-Garhy, H. A. S., & Fahmy, A. H. (2023). Implementation of Biopolymeric Nanomaterials to Reduce the Negative Impacts of Salinity on Tomato Quantity and Quality. Molecules, 28(4), 1594. https://doi.org/10.3390/molecules28041594









