Graded 2D/3D Perovskite Hetero-Structured Films with Suppressed Interfacial Recombination for Efficient and Stable Solar Cells via DABr Treatment
Abstract
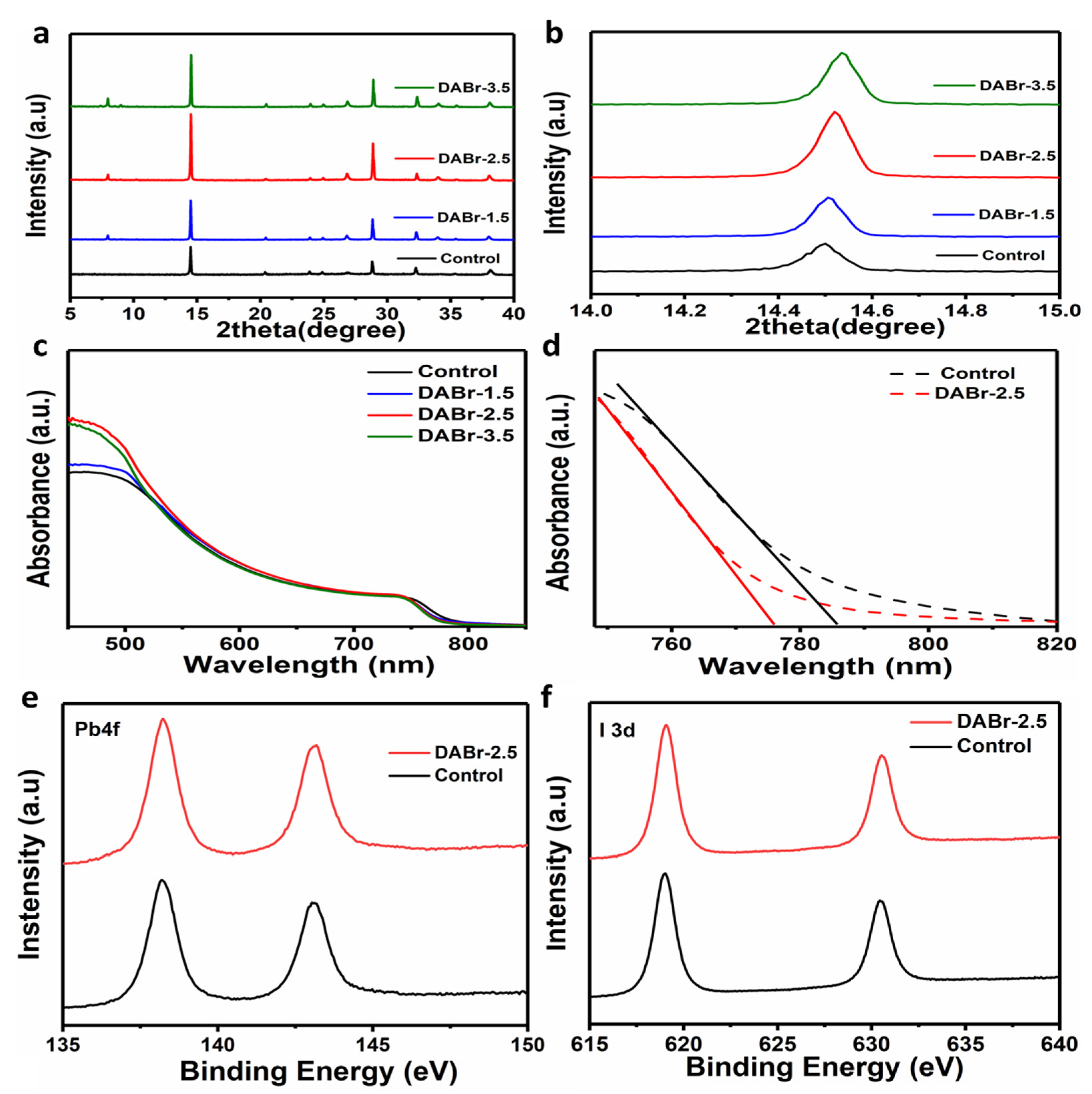
:1. Introduction
2. Results and Discussions
3. Experimental Section
3.1. Materials and Reagents
3.2. Device Fabrication
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy 2016, 1, 16149. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Gandini, M.; Di Stasio, F.; Rastogi, P.; Palazon, F.; Bertoni, G.; Ball, J.M.; Prato, M.; Petrozza, A.; Manna, L. Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nat. Energy 2016, 2, 16194. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Arain, Z.; Liu, C.; Ren, Y.; Yang, Y.; Mateen, M.; Liu, X.; Ding, Y.; Ali, Z.; Liu, X.; Dai, S. Low-temperature annealed perovskite films: A trade-off between fast and retarded crystallization via solvent engineering. ACS Appl. Mater. Interfaces 2019, 11, 16704–16712. [Google Scholar] [CrossRef]
- Bi, E.; Chen, H.; Xie, F.; Wu, Y.; Chen, W.; Su, Y.; Islam, A.; Grätzel, M.; Yang, X.; Han, L. Diffusion engineering of ions and charge carriers for stable efficient perovskite solar cells. Nat. Commun. 2017, 8, 15330. [Google Scholar] [CrossRef]
- Cao, J.; Yan, F. Recent progress in tin-based perovskite solar cells. Energy Environ. Sci. 2021, 14, 1286–1325. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.; Lyu, M.; Yun, J.H.; Wang, L. In situ growth of 2D perovskite capping layer for stable and efficient perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1706923. [Google Scholar] [CrossRef]
- Arain, Z.; Liu, C.; Yang, Y.; Mateen, M.; Ren, Y.; Ding, Y.; Liu, X.; Ali, Z.; Kumar, M.; Dai, S. Elucidating the dynamics of solvent engineering for perovskite solar cells. Sci. China Mater. 2019, 62, 161–172. [Google Scholar] [CrossRef]
- Chi, D.; Huang, S.; Zhang, M.; Mu, S.; Zhao, Y.; Chen, Y.; You, J. Composition and Interface Engineering for Efficient and Thermally Stable Pb–Sn Mixed Low-Bandgap Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1804603. [Google Scholar] [CrossRef]
- Zturk, T.; Akman, E.; Shalan, A.E.; Akin, S. Composition engineering of operationally stable CsPbI2Br perovskite solar cells with a record efficiency over 17%. Nano Energy 2021, 87, 106157. [Google Scholar] [CrossRef]
- Seo, J.Y.; Akin, S.; Zalibera, M.; Preciado, M.A.R.; Kim, H.S.; Zakeeruddin, S.M.; Milić, J.V.; Grätzel, M. Dopant engineering for SPIRO-OMeTAD hole-transporting materials towards efficient perovskite solar cells. Adv. Funct. Mater. 2021, 31, 2102124. [Google Scholar] [CrossRef]
- Fu, L.; Li, H.; Wang, L.; Yin, R.; Li, B.; Yin, L. Defect passivation strategies in perovskites for an enhanced photovoltaic performance. Energy Environ. Sci. 2020, 13, 4017–4056. [Google Scholar] [CrossRef]
- Ma, S.; Cai, M.; Cheng, T.; Ding, X.; Shi, X.; Alsaedi, A.; Hayat, T.; Ding, Y.; Tan, Z.A.; Dai, S. Two-dimensional organic-inorganic hybrid perovskite: From material properties to device applications. Sci. China Mater. 2018, 61, 1257–1277. [Google Scholar] [CrossRef]
- Jin, M.; Li, H.; Lou, Q.; Du, Q.; Huang, Q.; Shen, Z.; Li, F.; Chen, C. Toward high-efficiency sTable 2D/3D perovskite solar cells by incorporating multifunctional CNT: TiO2 additives into 3D perovskite layer. EcoMat 2022, 4, e12166. [Google Scholar] [CrossRef]
- Kore, B.P.; Zhang, W.; Hoogendoorn, B.W.; Safdari, M.; Gardner, J.M. Moisture tolerant solar cells by encapsulating 3D perovskite with long-chain alkylammonium cation-based 2D perovskite. Commun. Mater. 2021, 2, 100. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, W.; Cheng, T.; Zhang, B.; Yao, J.; Alsaedi, A.; Hayat, T.; Ding, Y.; Tan, Z.A.; Dai, S. Optical–electrical–chemical engineering of PEDOT: PSS by incorporation of hydrophobic nafion for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 3902–3911. [Google Scholar] [CrossRef] [PubMed]
- Mateen, M.; Arain, Z.; Liu, X.; Liu, C.; Yang, Y.; Ding, Y.; Ma, S.; Ren, Y.; Wu, Y.; Tao, Y. High-performance mixed-cation mixed-halide perovskite solar cells enabled by a facile intermediate engineering technique. J. Power Sources 2020, 448, 227386. [Google Scholar] [CrossRef]
- Dagar, J.; Fenske, M.; Al-Ashouri, A.; Schultz, C.; Li, B.; Köbler, H.; Munir, R.; Parmasivam, G.; Li, J.; Levine, I. Compositional and interfacial engineering yield high-performance and stable pin perovskite solar cells and mini-modules. ACS Appl. Mater. Interfaces 2021, 13, 13022–13033. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Öz, S.; Jena, A.K. Perovskite solar cells: Can we go organic-free, lead-free, and dopant-free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]
- Mateen, M.; Arain, Z.; Liu, C.; Yang, Y.; Liu, X.; Ding, Y.; Shi, P.; Ren, Y.; Wu, Y.; Dai, S. High-Quality (FA) x (MA) 1–x PbI3 for Efficient Perovskite Solar Cells via a Facile Cation-Intermixing Technique. ACS Sustain. Chem. Eng. 2019, 7, 11760–11768. [Google Scholar] [CrossRef]
- Ren, Y.; Duan, B.; Xu, Y.; Huang, Y.; Li, Z.; Hu, L.; Hayat, T.; Wang, H.; Zhu, J.; Dai, S. New insight into solvent engineering technology from evolution of intermediates via one-step spin-coating approach. Sci. China Mater. 2017, 60, 392–398. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Drop-casting to make efficient perovskite solar cells under high humidity. Angew. Chem. 2021, 133, 11342–11346. [Google Scholar] [CrossRef]
- Ren, Y.; Hao, Y.; Zhang, N.; Arain, Z.; Mateen, M.; Sun, Y.; Shi, P.; Cai, M.; Dai, S. Exploration of polymer-assisted crystallization kinetics in CsPbBr3 all-inorganic solar cell. Chem. Eng. J. 2020, 392, 123805. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Wang, X.; Liu, C.; Chen, S.; Zhang, M.; Li, X.; Yi, W.; Xu, B. Highly efficient and stable GABr-modified ideal-bandgap (1.35 eV) Sn/Pb perovskite solar cells achieve 20.63% efficiency with a record small Voc deficit of 0.33 V. Adv. Mater. 2020, 32, 1908107. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Ding, Y.; Liu, X.-Y.; Ding, X.-H.; Liu, X.-P.; Pan, X.; Dai, S.-Y. Ambient stable FAPbI3-based perovskite solar cells with a 2D-EDAPbI4 thin capping layer. Sci. China Mater. 2020, 63, 47–54. [Google Scholar] [CrossRef]
- Hu, Y.; Schlipf, J.; Wussler, M.; Petrus, M.L.; Jaegermann, W.; Bein, T.; Müller-Buschbaum, P.; Docampo, P. Hybrid perovskite/perovskite heterojunction solar cells. ACS nano 2016, 10, 5999–6007. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, C.; Zhang, S.; Yang, Y.; Du, A.; Waclawik, E.; Yu, X.; Wilson, G.J.; Wang, H. 2D–3D Mixed Organic–Inorganic Perovskite Layers for Solar Cells with Enhanced Efficiency and Stability Induced by n-Propylammonium Iodide Additives. ACS Appl. Mater. Interfaces 2019, 11, 29753–29764. [Google Scholar] [CrossRef]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Jiang, Y.; He, T.; Shi, G.; Fan, Z.; Yuan, M. Two-dimensional perovskite capping layer for stable and efficient tin-lead perovskite solar cells. Sci. China Chem. 2019, 62, 629–636. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, Y.; Tong, L.; Yang, Y.; Wang, X. Ion migration in Br-doped MAPbI 3 and its inhibition mechanisms investigated via quantum dynamics simulations. Phys. Chem. Chem. Phys. 2020, 22, 7778–7786. [Google Scholar] [CrossRef]
- Sirtl, M.T.; Hooijer, R.; Armer, M.; Ebadi, F.G.; Mohammadi, M.; Maheu, C.; Weis, A.; van Gorkom, B.T.; Häringer, S.; Janssen, R.A. 2D/3D Hybrid Cs2AgBiBr6 Double Perovskite Solar Cells: Improved Energy Level Alignment for Higher Contact-Selectivity and Large Open Circuit Voltage. Adv. Energy Mater. 2022, 12, 2103215. [Google Scholar] [CrossRef]
- Sutanto, A.A.; Caprioglio, P.; Drigo, N.; Hofstetter, Y.J.; Garcia-Benito, I.; Queloz, V.I.; Neher, D.; Nazeeruddin, M.K.; Stolterfoht, M.; Vaynzof, Y. 2D/3D perovskite engineering eliminates interfacial recombination losses in hybrid perovskite solar cells. Chem 2021, 7, 1903–1916. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Wu, Y.; Zhang, X.; Zou, J.; Lan, Z.; Sun, W.; Wu, J.; Gao, P. Multifunctional 2D perovskite capping layer using cyclohexylmethylammonium bromide for highly efficient and stable perovskite solar cells. Mater. Today Phys. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Li, B.; Han, X.; Jin, Z.; Wang, Y.; Zhang, Q.; Rui, Y. Interfacial Modification via a 1, 4-Butanediamine-Based 2D Capping Layer for Perovskite Solar Cells with Enhanced Stability and Efficiency. ACS Appl. Mater. Interfaces 2021, 14, 22879–22888. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; He, Y.; Zhang, Y.; Liang, Q.; Liu, F.; Zhou, Z.; Chan, C.C.; Li, G.; Feng, S.-P. Improvement in the Performance of Inverted 3D/2D Perovskite Solar Cells by Ambient Exposure. Solar RRL 2022, 6, 2200224. [Google Scholar] [CrossRef]
- Huang, X.; Bi, W.; Jia, P.; Cui, Q.; Hu, Y.; Lou, Z.; Hou, Y.; Teng, F. Grain Growth of MAPbI3 via Diethylammonium Bromide Induced Grain Mergence. ACS Appl. Mater. Interfaces 2020, 12, 16707–16714. [Google Scholar] [CrossRef]
- Dehghanipour, M.; Behjat, A.; Bioki, H.A. Fabrication of stable and efficient 2D/3D perovskite solar cells through post-treatment with TBABF 4. J. Mater. Chem. C 2021, 9, 957–966. [Google Scholar] [CrossRef]
- Huang, X.; Bi, W.; Jia, P.; Tang, Y.; Lou, Z.; Hu, Y.; Cui, Q.; Hou, Y.; Teng, F. Enhanced efficiency and light stability of planar perovskite solar cells by diethylammonium bromide induced large-grain 2D/3D hybrid film. Org. Electron. 2019, 67, 101–108. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Yuan, Y.; Shao, Y.; Fang, Y.; Wang, Q.; Huang, J. Abnormal crystal growth in CH 3 NH 3 PbI 3− x Cl x using a multi-cycle solution coating process. Energy Environ. Sci. 2015, 8, 2464–2470. [Google Scholar] [CrossRef]
- Xie, F.; Chen, C.-C.; Wu, Y.; Li, X.; Cai, M.; Liu, X.; Yang, X.; Han, L. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- Mateen, M.; Arain, Z.; Yang, Y.; Liu, X.; Ma, S.; Liu, C.; Ding, Y.; Ding, X.; Cai, M.; Dai, S. MACl-induced intermediate engineering for high-performance mixed-cation perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 10535–10543. [Google Scholar] [CrossRef]
- Zhang, W.; Saliba, M.; Moore, D.T.; Pathak, S.K.; Horantner, M.T.; Stergiopoulos, T.; Stranks, S.D.; Eperon, G.E.; Alexander-Webber, J.A.; Abate, A.; et al. Ultrasmooth organic–inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells. Nat. Commun. 2015, 6, 6142. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, F.; Liu, H.; Li, X.; Xiao, Y.; Wang, S. Tuning the crystal growth of perovskite thin-films by adding the 2-pyridylthiourea additive for highly efficient and stable solar cells prepared in ambient air. J. Mater. Chem. A 2017, 5, 13448–13456. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Rakstys, K.; Mahata, A.; Franckevicius, M.; Mosconi, E.; Skackauskaite, R.; Ding, B.; Brooks, K.G.; Usiobo, O.J. Tuning structural isomers of phenylenediammonium to afford efficient and stable perovskite solar cells and modules. Nat. Commun. 2021, 12, 6394. [Google Scholar] [CrossRef]
- Liu, L.; Su, P.; Yao, H.; Wang, J.; Fu, W.; Liu, X.; Yang, H. Optimized interface and recrystallized grains by CsBr treatment for enhanced photovoltaic performance of perovskite solar cells. J. Power Sources 2018, 389, 50–55. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, S.; Zhou, K.; Liu, G.; Zhang, B.; Alsaedi, A.; Hayat, T.; Pan, X. New-type highly sTable 2D/3D perovskite materials: The effect of introducing ammonium cation on performance of perovskite solar cells. Sci. China Mater. 2019, 62, 508–518. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, H.Y.; Qin, Y.; Mu, C.; Li, Q.; Xu, D.; Zhang, J.P. Reduced Defects of MAPbI3 Thin Films Treated by FAI for High-Performance Planar Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1805810. [Google Scholar] [CrossRef]
- Kim, J.; Yun, A.J.; Gil, B.; Lee, Y.; Park, B. Triamine-based aromatic cation as a novel stabilizer for efficient perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1905190. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Vaynzof, Y. The future of perovskite photovoltaics—Thermal evaporation or solution processing? Adv. Energy Mater. 2020, 10, 2003073. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, D.; Liu, S.F. Organic–inorganic hybrid perovskite with controlled dopant modification and application in photovoltaic device. Small 2017, 13, 1604153. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateen, M.; Shi, H.; Huang, H.; Li, Z.; Ahmad, W.; Rafiq, M.; Shah, U.A.; Sajid, S.; Ren, Y.; Park, J.; et al. Graded 2D/3D Perovskite Hetero-Structured Films with Suppressed Interfacial Recombination for Efficient and Stable Solar Cells via DABr Treatment. Molecules 2023, 28, 1592. https://doi.org/10.3390/molecules28041592
Mateen M, Shi H, Huang H, Li Z, Ahmad W, Rafiq M, Shah UA, Sajid S, Ren Y, Park J, et al. Graded 2D/3D Perovskite Hetero-Structured Films with Suppressed Interfacial Recombination for Efficient and Stable Solar Cells via DABr Treatment. Molecules. 2023; 28(4):1592. https://doi.org/10.3390/molecules28041592
Chicago/Turabian StyleMateen, Muhammad, Hongxi Shi, Hao Huang, Ziyu Li, Waseem Ahmad, Muhammad Rafiq, Usman Ali Shah, Sajid Sajid, Yingke Ren, Jongee Park, and et al. 2023. "Graded 2D/3D Perovskite Hetero-Structured Films with Suppressed Interfacial Recombination for Efficient and Stable Solar Cells via DABr Treatment" Molecules 28, no. 4: 1592. https://doi.org/10.3390/molecules28041592
APA StyleMateen, M., Shi, H., Huang, H., Li, Z., Ahmad, W., Rafiq, M., Shah, U. A., Sajid, S., Ren, Y., Park, J., Chi, D., Lu, Z., & Huang, S. (2023). Graded 2D/3D Perovskite Hetero-Structured Films with Suppressed Interfacial Recombination for Efficient and Stable Solar Cells via DABr Treatment. Molecules, 28(4), 1592. https://doi.org/10.3390/molecules28041592








