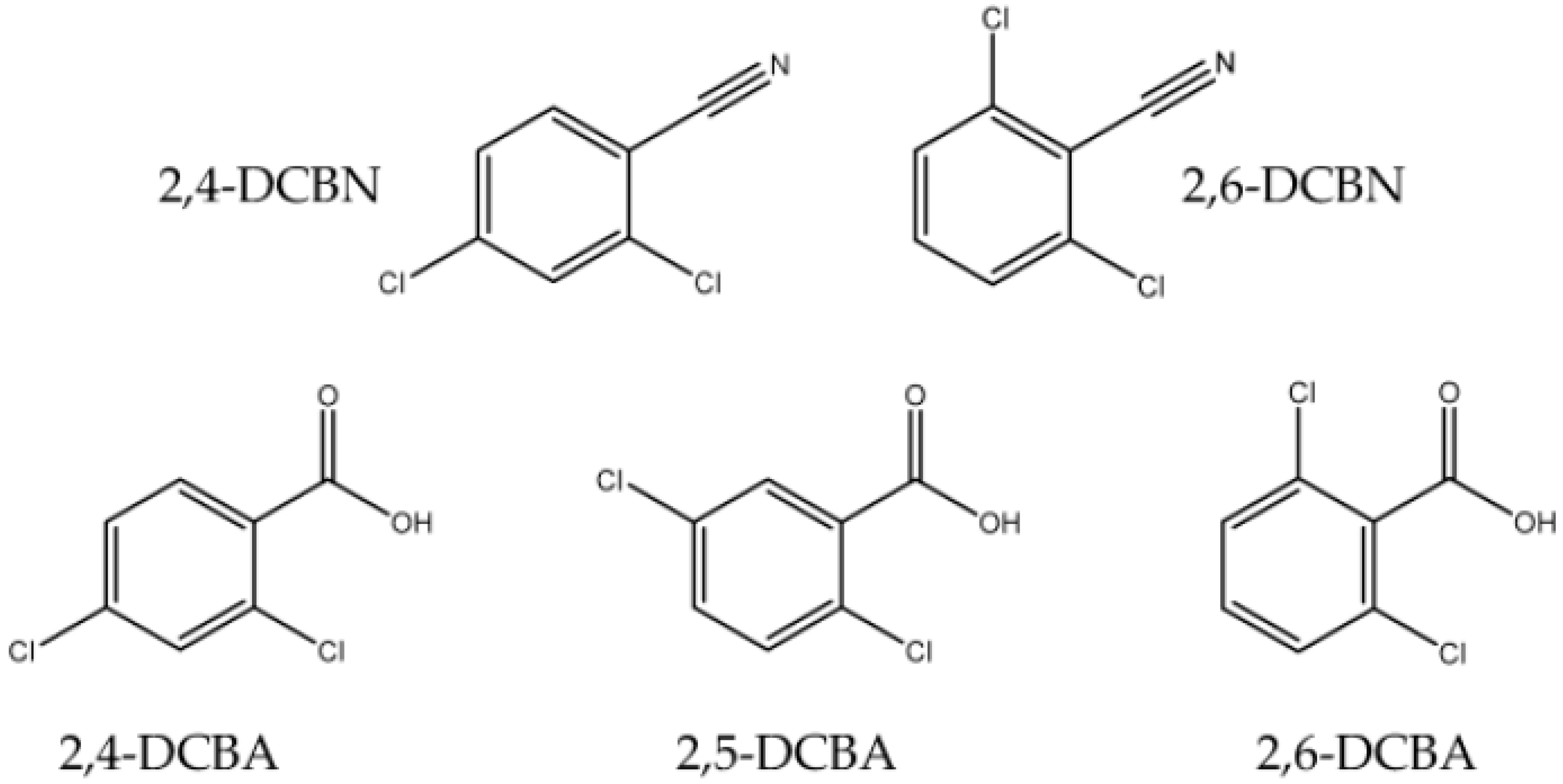
Phase Transitions Equilibria of Five Dichlorinated Substituted Benzenes
Abstract
:1. Introduction
- -
- The conversion of the gaseous phase enthalpies of formation into the condensed phases, or vice-versa;
- -
- -
- Understanding the behavior of moldy/musty contaminants in cork and improving processes for their removal [4];
- -
2. Results and Discussion
2.1. Thermodynamic Properties of Sublimation and Vaporization
Isobaric Heat Capacities
| Method | Compound | ||||
|---|---|---|---|---|---|
| 2,4-DCBA | 2,5-DCBA | 2,6-DCBA | 2,4-DCBN | 2,6-DCBN | |
| /J·K−1·mol−1 | |||||
| G3(MP2)B3LYP a,b | 160.0 ± 4.8 | 160.0 ± 4.8 | 161.4 ± 4.8 | 141.6 ± 4.2 | 141.6 ± 4.2 |
| Domalski and Hearing [47] c | 158.7 ± 4.0 d | 158.7 ± 4.0 d | 158.7 ± 4.0 d | 140.5 ± 4.0 | 140.5 ± 4.0 |
| /J·K−1·mol−1 | |||||
| DSC (this work) | 180.9 ± 2.0 | 179.5 ± 2.2 | 182.8 ± 1.8 | 168.9 ± 2.5 | 167.8 ± 2.4 |
| Domalski and Hearing [47] c | 173.0 ± 4.0 | 173.0 ± 4.0 | 173.0 ± 4.0 | 161.1± 4.0 e | 161.1± 4.0 e |
| Acree Jr. and Chickos [48] f | 171.5 ± 17.0 | 171.5 ± 17.0 | 171.5 ± 17.0 | 160.7 ± 17.0 | 160.7 ± 17.0 |
2.2. Thermodynamic Properties of Fusion
2.3. Volatility Evaluation
2.4. Estimation of Sublimation Properties of Substituted Benzenes
3. Experiment
3.1. Materials and Purity Control
3.2. Thermal Analysis
3.2.1. Differential Scanning Calorimetry
Fusion Properties
Crystalline Heat Capacities
3.3. Vapor Pressure Measurements
3.3.1. Knudsen Mass-Loss Effusion Method
3.3.2. Static Method Based on Capacitance Diaphragm Manometers
4. Conclusions
- -
- The temperatures and molar enthalpies of fusion of the five compounds studied and their crystalline isobaric molar heat capacities were determined using DSC.
- -
- The enthalpies, entropies, and Gibbs energies of sublimation of all the compounds and of the vaporization of 2,4-DCBN were derived through vapor pressure measurements (at different temperatures) and the phase diagram representation of the (p,T) results of the latter compound, including its triple point coordinates, were reported.
- -
- The evaluation of the enthalpic and entropic contributions to the volatility of the compounds studied was discussed.
- -
- The contributions of -COOH, -CN, and -Cl substituents to the sublimation properties of the substituted benzenes studied were confirmed accordingly to our estimation model.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kabo, G.J.; Blokhin, A.V.; Paulechka, E.; Roganov, G.N.; Frenkel, M.; Yursha, I.A.; Diky, V.; Zaitsau, D.; Bazyleva, A.; Simirsky, V.V.; et al. Thermodynamic properties of organic substances: Experiment, modeling, and technological applications. J. Chem. Thermodyn. 2019, 131, 225–246. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Dohrn, R.; Economou, I.G.; de Hemptinne, J.-C.; ten Kate, A.; Kuitunen, S.; Mooijer, M.; Žilnik, L.F.; Vesovic, V. Industrial requirements for thermodynamic and transport properties: 2020. Ind. Eng. Chem. Res. 2021, 60, 4987–5013. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Kahwaji, S.; Freitas, V.L.S.; Siewert, R.; Weatherby, J.A.; Ribeiro da Silva, M.D.M.C.; Verevkin, S.P.; Johnson, E.R.; Zwanziger, J.W. The Relative Thermodynamic stability of diamond and graphite. Angew. Chem. Int. Ed. 2021, 60, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.R.P.; Pinheiro, B.D.A.; Lima, C.F.R.A.C.; Santos, A.F.L.O.M.; Ferreira, A.C.S.; Paz, F.A.A.; Monte, M.J.S. Thermodynamic properties of moldy-musty contaminants of wine. J. Chem. Eng. Data 2019, 64, 4741–4753. [Google Scholar] [CrossRef]
- Barbeiro, L.B.; Falleiro, R.M.M.; Meirelles, A.J.A. Vapour pressure and VLE data of fatty compounds. J. Chem. Thermodyn. 2021, 159, 106469. [Google Scholar] [CrossRef]
- Rordorf, B.F.; Geoffroy, A.; Szelagiewicz, M.; Marti, E. Vapor Pressure methods for industrial applications. In Thermal Analysis: Volume 1: Theory Instrumentation Applied Sciences Industrial Applications; Wiedemann, H.G., Ed.; Birkhäuser: Basel, Switzerland, 1980. [Google Scholar]
- Dauber, T.E. Strengths and weaknesses of predictive methods for estimating thermophysical properties. J. Chem. Eng. Data 1996, 41, 5345–5350. [Google Scholar]
- Mathias, P.M.; Schiller, M. Analysis of vapor pressures using family variations: A case study of hexadecanol isomers. Ind. Eng. Chem. Res. 2022, 61, 12239–12248. [Google Scholar] [CrossRef]
- Fonseca, J.M.S.; Gushterov, N.; Dohrna, R. Vapour pressures of selected organic compounds down to 1mPa, using mass-loss Knudsen effusion method. J. Chem. Thermodyn. 2014, 73, 148–155. [Google Scholar] [CrossRef]
- Martin Tischer, M.; Roitzsch, M. Estimating inhalation exposure resulting from evaporation of volatile multicomponent mixtures using different modelling approaches. Int. J. Environ. Res. Public Health 2022, 19, 1957. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Azevedo, J.; Araújo, J.P.; Santos, L.M.N.B.F.; Mendes, A. High purity and crystalline thin films of methylammonium lead iodide perovskites by a vapor deposition approach. Thin Solid Film. 2018, 664, 12–18. [Google Scholar] [CrossRef]
- Kunte, G.V.; Ail, U.; Ajikumar, P.K.; Tyagi, A.K.; Shivashankar, S.A.; Umarji, A.M. Estimation of vapour pressure and partial pressure of subliming compounds by low-pressure thermogravimetry. Bull. Mater. Sci. 2011, 34, 1633–1637. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. Vapor pressures of four methyl esters of substituted benzoic acids. The intermolecular hydrogen bond OH···O. J. Chem. Eng. Data 2016, 61, 1012–1020. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. Vapour pressures of 1-methyl derivatives of benzimidazole, pyrazole and indole. The energy of the intermolecular hydrogen bond NH⋯N. J. Chem. Thermodyn. 2014, 77, 46–53. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. Thermodynamic study of benzamide, N-methylbenzamide, and N,N-dimethylbenzamide: Vapor pressures, phase diagrams, and hydrogen bond enthalpy. J. Chem. Eng. Data 2010, 55, 3507–3512. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Cunha, A.F.G.; Matos, M.A.R.; Morais, V.M.F.; Monte, M.J.S. Thermodynamic properties of the methyl esters of p-hydroxy and p-methoxy benzoic acids. J. Chem. Thermodyn. 2014, 78, 43–57. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Freitas, V.L.S.; Campos, J.I.S.; Ribeiro da Silva, M.D.M.C.; Monte, M.J.S. Volatility and thermodynamic stability of vanillin. J. Chem. Thermodyn. 2019, 128, 45–54. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Pinheiro, B.D.A.; Lobo Ferreira, A.I.M.C.; Monte, M.J.S. Thermodynamic stability of Fenclorim and Clopyralid. Molecules 2022, 27, 39. [Google Scholar] [CrossRef]
- Available online: http://ec.europa.eu/enterprise/sectors/chemicals/reach/index_en.htm (accessed on 18 October 2022).
- Monte, M.J.S.; Almeida, A.R.R.P. Estimations of the thermodynamic properties of halogenated benzenes as they relate to their environment mobility. Chemosphere 2017, 189, 590–598. [Google Scholar] [CrossRef]
- Holtze, M.S.; Sørensen, S.R.; Sørensen, J.; Aamand, J. Microbial degradation of the benzonitrile herbicides dichlobenil, bromoxynil and ioxynil in soil and subsurface environments-insights into degradation pathways, persistent metabolites and involved degrader organisms. Environ. Pollut. 2008, 154, 155–168. [Google Scholar] [CrossRef]
- Frková, Z.; Badawi, N.; Johansen, A.; Schultz-Jensen, N.; Bester, K.; Reinhold Sørensen, S.; Karlson, U.G. Degradation of three benzonitrile herbicides by Aminobacter MSH1 versus soil microbial communities: Pathways and kinetics. Pest. Manag. Sci. 2014, 70, 1291–1298. [Google Scholar] [CrossRef]
- Li, W.-K.; Zhang, H.-X.; Shi, Y.-P. Simultaneous determination of bifenox, dichlobenil and diclofop methyl by hollow carbon nanospheres enhanced magnetic carboxylic multi-walled carbon nanotubes. Anal. Chim. Acta 2018, 1011, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, B.J.; Gottschal, J.C.; Prins, R.A. Degradation of 2,5-dichlorobenzoic acid by Pseudomonas aeruginosa JB2 at low oxygen tensions. Biodegradation 1995, 6, 39–46. [Google Scholar] [CrossRef]
- Klamertha, N.; Gernjakb, W.; Malato, S.; Aguërac, A.; Lendla, B. Photo-Fenton decomposition of chlorfenvinphos: Determination of reaction pathway. Water Res. 2009, 43, 441–449. [Google Scholar] [CrossRef] [PubMed]
- United States Environment Protection Agency (USEPA). Prevention, pesticides and toxic substances. In Registration Eligibility Decision (RED) for Propiconazole: Case No. 3125; EPA 738R-06-027; United States Environment Protection Agency (USEPA): Washington, DC, USA, 2006. [Google Scholar]
- Babar, T.M.; Qadeer, G.; Rama, N.H.; Ruzickand, A.; Padelkova, Z. Methyl 2,5-dichlorobenzoate. Acta Cryst. 2008, E64, o1970. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on pesticide peer reviev: Conclusion regarding the peer review of the pesticide risk assessment of the active substance 2,5-dichlorobenzoic acid methylester. Eur. Food Saf. Auth. Sci. Rep. 2008, 180, 1–50. [Google Scholar]
- Available online: https://www.lookchem.com/2-5-Dichlorobenzoic-acid/ (accessed on 21 November 2022).
- Pinkus, A.G.; Kautz, J.A.; Vajjula, P.A. Crystal structures of 2,6- and 3,5-dichlorobenzoic acids: Nonbonded Cl⋯Cl contacts. J. Chem. Crystallogr. 2003, 33, 181–186. [Google Scholar] [CrossRef]
- Fava, L.; Orrù, M.A.; Crobe, A.; Caracciolo, A.B.; Bottonia, P.; Funaria, E. Pesticide metabolites as contaminants of groundwater resources: Assessment of the leaching potential of endosulfan sulfate, 2,6-dichlorobenzoic acid, 3,4-dichloroaniline, 2,4-dichlorophenol and 4-chloro-2-methylphenol. Microchem. J. 2005, 79, 207–211. [Google Scholar] [CrossRef]
- Ambrose, D. The correlation and estimation of vapour pressures IV. Observations on Wagner’s method of fitting equations to vapour pressures. J. Chem. Thermodyn. 1986, 18, 45–51. [Google Scholar] [CrossRef]
- An, H.; Yang, W.M. A new generalized correlation for accurate vapor pressure prediction. Chem. Phys. Lett. 2012, 543, 188–192. [Google Scholar] [CrossRef]
- Godavarthy, S.S.; Robinson, R.L.; Gasem, K.A.M. SVRC–QSPR model for predicting saturated vapor pressures of pure fluids. Fluid Phase Equilibria 2006, 246, 39–51. [Google Scholar] [CrossRef]
- Yaffe, D.; Cohen, Y. Neural network-based temperature-dependent quantitative structure property relations (QSPRs) for predicting vapor pressure of hydrocarbons. J. Chem. Inf. Model. 2001, 41, 463–477. [Google Scholar]
- Ruzicka, V. Estimation of vapor pressures by a group-contribution method. Ind. Eng. Chem. Fundam. 1983, 22, 266–267. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hesse, D.G.; Liebman, J.F. A group additivity approach for the estimation of heat capacities of organic liquids and solids at 298 K. Struct. Chem. 1993, 4, 261–269. [Google Scholar] [CrossRef]
- Pankow, J.F.; Asher, W.E. SIMPOL.1: A simple group contribution method for predicting vapor pressures and enthalpies of vaporization of multifunctional organic compounds. Atmos. Chem. Phys. 2008, 8, 2773–2796. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Acree, W.E.; Mohammadi, A.H.; Ramjugernat, D. Improved prediction of vapor pressure for pure liquids and solids from the PR+COSMOSAC equation of state. Ind. Eng. Chem. Res. 2015, 54, 10115–10125. [Google Scholar]
- Monte, M.J.S.; Almeida, A.R.R.P. A new approach for the estimation of sublimation enthalpies and vapor pressures of crystalline benzene derivatives. Struct. Chem. 2013, 24, 2001–2016. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Sousa, C.A.D.; Santos, L.M.N.B.F.; Monte, M.J.S. Thermodynamic properties of sublimation of the ortho and meta isomers of acetoxy and acetamido benzoic acids. J. Chem. Thermodyn. 2015, 86, 6–12. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. Vapour pressures and phase transition properties of four substituted acetophenones. J. Chem. Thermodyn. 2017, 107, 42–50. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. The influence of the halogen atoms and acetyl group on vapour pressures and related properties of the p-haloacetophenones. J. Chem. Thermodyn. 2016, 92, 118–125. [Google Scholar] [CrossRef]
- Clarke, E.C.W.; Glew, D.N. Evaluation of thermodynamic functions from equilibrium constants. Trans. Faraday Soc. 1966, 62, 539–547. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision, C.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- NIST Computational Chemistry Comparison and Benchmark Database. NIST Standard Reference Database Number 101. Release 16a; Johnson, R.D., III, Ed.; NIST Chemistry Web Book, 2013; SRD 69. Available online: http://cccbdb.nist.gov/vibscalejust.asp (accessed on 2 December 2022).
- Domalski, E.S.; Hearing, E.D. Estimation of the thermodynamic properties of C-H-N-O-S-halogen compounds at 298.15 K. J. Phys. Chem. Ref. Data 1993, 22, 805–1159. [Google Scholar] [CrossRef]
- Acree, W., Jr.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds. Sublimation, vaporization and fusion enthalpies from 1880 to 2015. Part 1. C1–C10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat capacity corrections to a standard state: A comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 271–278. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Almeida, A.R.R.P.; Matos, M.A.R. Thermodynamic study on the sublimation of five aminomethoxybenzoic acids. J. Chem. Eng. Data 2010, 55, 419–423. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Santos, L.M.N.B.F.; Fonseca, J.M.S.; Sousa, C.A.D. Vapour pressures, enthalpies and entropies of sublimation of para substituted benzoic acids. J. Therm. Anal. Calorim. 2010, 100, 465–474. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Rocha, I.M.; Cimas, Á. Energetic study applied to the knowledge of the structural and electronic properties of monofluorobenzonitriles. J. Org. Chem. 2012, 77, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Altau, K.; Beasley, J.G.; Pine, H.J.; Crawford, R.H.; Tucker, W.T.; Brown, A.D.; Capps, J.D. Some derivatives of ethylbenzene. J. Chem. Eng. Data 1963, 8, 122. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C50793&Units=SI&Mask=4#Thermo-Phase (accessed on 21 November 2022). [CrossRef]
- Stork, G.; White, W.N. The stereochemistry of the SN2’ Reaction. II1. J. Am. Chem. Soc. 1956, 78, 4609. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C50306&Units=SI&Mask=4#ref-1 (accessed on 21 November 2022). [CrossRef]
- Plato, C. Differential scanning calorimetry as a general method for determining purity and heat of fusion of high-purity organic chemicals. Application to 64 compounds. Anal. Chem. 1972, 44, 1531–1534. [Google Scholar] [CrossRef]
- Donnelly, J.R.; Drewes, L.A.; Johnson, R.L.; Munslow, W.D.; Knapp, K.K.; Sovocool, G.W. Purity and heat of fusion data for environmental standards as determined by differential scanning calorimetry. Thermochim. Acta 1990, 167, 155–187. [Google Scholar] [CrossRef]
- Acree, W.E., Jr. Thermodynamic properties of organic compounds: Enthalpy of fusion and melting point temperature compilation. Thermochim. Acta 1991, 189, 37–56. [Google Scholar] [CrossRef]
- Rodante, F.; Vecchio, S.; Catalani, G.; Guidotti, M. Thermal analysis and non-isothermal kinetic study of some pesticides. Part II. Chlorinate derivatives. J. Therm. Anal. Calorim. 2000, 60, 605–622. [Google Scholar] [CrossRef]
- Kumar, G.N.A.; Hathwar, V.R. Quantitative investigation of halogen and hydrogen bonding in 2-chloro, 4-X-benzoic acids. ChemistrySelect 2022, 7, e202104338. [Google Scholar]
- Britton, D.; Noland, W.E.; Pinnow, M.J. Isomorphism and pseudosymmetry in 2,6-dichloro- and 2,6-dibromobenzonitrile. Acta Cryst. 2000, B56, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Britton, D. o-Chloro- and o-bromobenzonitrile: Pseudosymmetry and pseudo-isostructural packing. Acta Cryst. 2007, C63, o14–o16. [Google Scholar] [CrossRef]
- Pink, M.; Britton, D.; Noland, W.E.; Pinnow, M.J. 2,4,6-Trichlorophenylisonitrile and 2,4,6-trichlorobenzonitrile. Acta Cryst. 2000, C56, 1271–1273. [Google Scholar] [CrossRef]
- NETZSCH Analyzing & Testing DSC 204 F1 Phoenix. Calibration Set. Selb, Germany, 2022. Available online: https://analyzing-testing.netzsch.com/_Resources/Persistent/3/f/6/2/3f62fce9e4fd19c3cb5d75f1514a90ef444f9c23/DSC_204_F1_Phoenix_en_web.pdf (accessed on 14 December 2022).
- Sabbah, R.; El Watik, L. New reference materials for the calibration (temperature and energy) of differential thermal analysers and scanning calorimeters. J. Therm. Anal. 1992, 38, 855–863. [Google Scholar] [CrossRef]
- Sabbah, R.; Xu-Wu, A.; Chickos, J.S.; Planas Leitão, M.L.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Della Gatta, G.; Richarson, M.J.; Sarge, S.M.; Stølen, S. Standards, calibration, and guidelines in microcalorimetry part 2. Calibration standards for differential scanning calorimetry. Pure Appl. Chem. 2006, 78, 1455–1476. [Google Scholar] [CrossRef]
- Roux, M.V.; Temprado, M.; Chickos, J.S.; Nagano, Y.J. Critically evaluated thermos chemical properties of polycyclic aromatic hydrocarbons. Phys. Chem. Ref. Data 2008, 37, 1855–1996. [Google Scholar] [CrossRef]
- Chang, S.S.; Bestul, A.B. Heat capacity and thermodynamic properties of o-terphenyl crystal, glass, and liquid. J. Chem. Phys. 1972, 56, 503–516. [Google Scholar] [CrossRef]
- Furukawa, G.T.; McCoskey, R.E.; King, G.J. Calorimetric properties of benzoic acid from 0o to 410o K. J. Res. Natl. Inst. 1951, 47, 256–261. [Google Scholar]
- Ribeiro da Silva, M.A.V.; Monte, M.J.S.; Santos, L.M.N.B.F. The design, construction and testing of a new Knudsen effusion apparatus. J. Chem. Thermodyn. 2006, 38, 778–787. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Oliveira, J.A.S.A.; Monte, M.J.S. Thermodynamic study of nicotinamide, N-methylnicotinamide and N,N-dimethylnicotinamide: Vapour pressures, phase diagrams, and hydrogen bonds. J. Chem. Thermodyn. 2015, 82, 108–115. [Google Scholar] [CrossRef]
- Mohr, P.J.; Newell, D.B.; Taylor, B.N. CODATA recommended values of the fundamental physical constants: 2014. Rev. Mod. Phys. 2016, 88, 1–73. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Santos, L.M.N.B.F.; Fulem, M.; Fonseca, J.M.S.; Sousa, C.A.D. New static apparatus and vapor pressure of reference materials: Naphthalene, benzoic acid, benzophenone and ferrocene. J. Chem. Eng. Data 2006, 51, 757–766. [Google Scholar] [CrossRef]
- Freitas, V.L.S.; Monte, M.J.S.; Gomes, J.R.B.; Santos, L.M.N.B.F.; Ribeiro da Silva, M.D.M.C. Energetic studies and phase diagram of thioxanthene. J. Phys. Chem. A 2009, 113, 12988–12994. [Google Scholar] [CrossRef] [PubMed]





| ΔT | θ | a | pb | a | c | R2 | a | σrd |
|---|---|---|---|---|---|---|---|---|
| K | K | kJ·mol−1 | Pa | kJ·mol−1 | J·K−1.mol−1 | J·K−1·mol−1 | ||
| 2,4-DCBA | ||||||||
| Crystalline phase (Knudsen effusion method) | ||||||||
| 335.2 to 357.2 | 298.15 | 46.42 ± 0.17 | 7.37 × 10−4 | 111.4 ± 1.2 | 217.9 ± 4.1 | 0.9997 | 20.9 ± 5.2 e | 0.0143 |
| 346.19 f | 36.03 ± 0.02 | 0.366 | 110.4 ± 1.2 | |||||
| 2,5-DCBA | ||||||||
| Crystalline phase (Knudsen effusion method) | ||||||||
| 332.1 to 354.3 | 298.15 | 45.49 ± 0.10 | 1.07 × 10−3 | 109.6 ± 0.8 | 215.0 ± 2.7 | 0.9999 | 19.5 ± 5.3 e | 0.0093 |
| 343.20 f | 35.86 ± 0.02 | 0.348 | 108.7 ± 0.8 | |||||
| 2,6-DCBA | ||||||||
| Crystalline phase (Knudsen effusion method) | ||||||||
| 321.1 to 343.3 | 298.15 | 41.56 ± 0.07 | 5.24 × 10−3 | 99.6 ± 0.7 | 194.7 ± 2.4 | 0.9999 | 21.4 ± 5.1 e | 0.0088 |
| 332.20 f | 34.97 ± 0.01 | 0.317 | 98.8 ± 0.7 | |||||
| 2,6-DCBN | ||||||||
| Crystalline phase (Knudsen effusion method) | ||||||||
| 295.3 to 319.2 | 298.15 | 33.73 ± 0.02 | 1.23 × 10−1 | 88.3 ± 0.6 | 183.0 ± 2.0 | 0.9999 | 26.4 ± 5.5 g | 0.0095 |
| 307.27 f | 32.06 ± 0.01 | 0.355 | 88.0 ± 0.6 | |||||
| Crystalline phase (static method) | ||||||||
| 328.7 to 391.8 | 298.15 | 33.51 ± 0.06 | 1.35 × 10−1 | 87.3 ± 0.7 | 180.4 ± 2.4 | 1.0000 | 26.4 ± 5.5 h | 0.0046 |
| 360.24 f | 22.47 ± 0.01 | 55.2 | 85.7 ± 0.1 | |||||
| 2,4-DCBN | ||||||||
| Crystalline phase (static method) | ||||||||
| 303.0 to 329.0 | 298.15 | 27.00 ± 0.04 | 1.86 | 79.9 ± 0.6 | 177.4 ± 2.0 | 0.9998 | 27.3 ± 4.9 e | 0.0110 |
| 315.99 f | 23.85 ± 0.02 | 11.4 | 79.4 ± 0.6 | |||||
| 331.83 i | 21.07 ± 0.04 | 48.2 | 79.0 ± 0.6 | 174.6 ± 0.9 | ||||
| Liquid phase (static method) | ||||||||
| 333.3 to 380.8 | 298.15 | 25.29 ± 0.04 | 3.71 | 63.7 ± 0.5 | 128.8 ± 1.7 | 1.0000 | 65.8 ± 8.5 h | 0.0036 |
| 357.08 f | 18.05 ± 0.01 | 229 | 59.8 ± 0.1 | |||||
| 331.83 i | 21.07 ± 0.03 | 48.2 | 61.5 ± 0.2 | 121.8 ± 0.6 | ||||
| Ttp/K | Tfus/K a | b/kJ·mol−1 | b,c/J·K−1·mol−1 | Method/Ref. |
|---|---|---|---|---|
| 2,4-DCBA | ||||
| 435.20 ± 0.89 | 28.19 ± 0.44 a | 64.8 ± 1.0 | DSC/this work | |
| 2,5-DCBA | ||||
| 426.87 ± 0.89 | 27.54 ± 0.50 a | 64.5 ± 1.2 | DSC/this work | |
| 426.65 | [53] | |||
| 427.65 | [53] | |||
| 2,6-DCBA | ||||
| 414.18 ± 0.89 | 14.13 ± 0.26 a | 34.1 ± 0.6 | DSC/this work | |
| 415 ± 2 | [54] | |||
| 2,6-DCBN | ||||
| 416.46 ± 0.89 | 26.19 ± 0.34 a | 62.9 ± 0.8 | DSC/this work | |
| 416.7 | 25.94 | 62.3 | DSC/[55] | |
| 417.2 | 26.17 | 62.7 | DSC/[56,57] | |
| 421.2 | 24.56 | 58.3 | DSC/[58] | |
| 2,4-DCBN | ||||
| 331.46 ± 0.89 | 17.61 ± 0.26 a | 53.1 ± 0.8 | DSC/this work | |
| 331.8 | 17.5 ± 0.4 | 52.8 ± 1.1 | static/this work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.R.R.P.; Pinheiro, B.D.A.; Monte, M.J.S. Phase Transitions Equilibria of Five Dichlorinated Substituted Benzenes. Molecules 2023, 28, 1590. https://doi.org/10.3390/molecules28041590
Almeida ARRP, Pinheiro BDA, Monte MJS. Phase Transitions Equilibria of Five Dichlorinated Substituted Benzenes. Molecules. 2023; 28(4):1590. https://doi.org/10.3390/molecules28041590
Chicago/Turabian StyleAlmeida, Ana R. R. P., Bruno D. A. Pinheiro, and Manuel J. S. Monte. 2023. "Phase Transitions Equilibria of Five Dichlorinated Substituted Benzenes" Molecules 28, no. 4: 1590. https://doi.org/10.3390/molecules28041590
APA StyleAlmeida, A. R. R. P., Pinheiro, B. D. A., & Monte, M. J. S. (2023). Phase Transitions Equilibria of Five Dichlorinated Substituted Benzenes. Molecules, 28(4), 1590. https://doi.org/10.3390/molecules28041590








