Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties
Abstract
1. Introduction
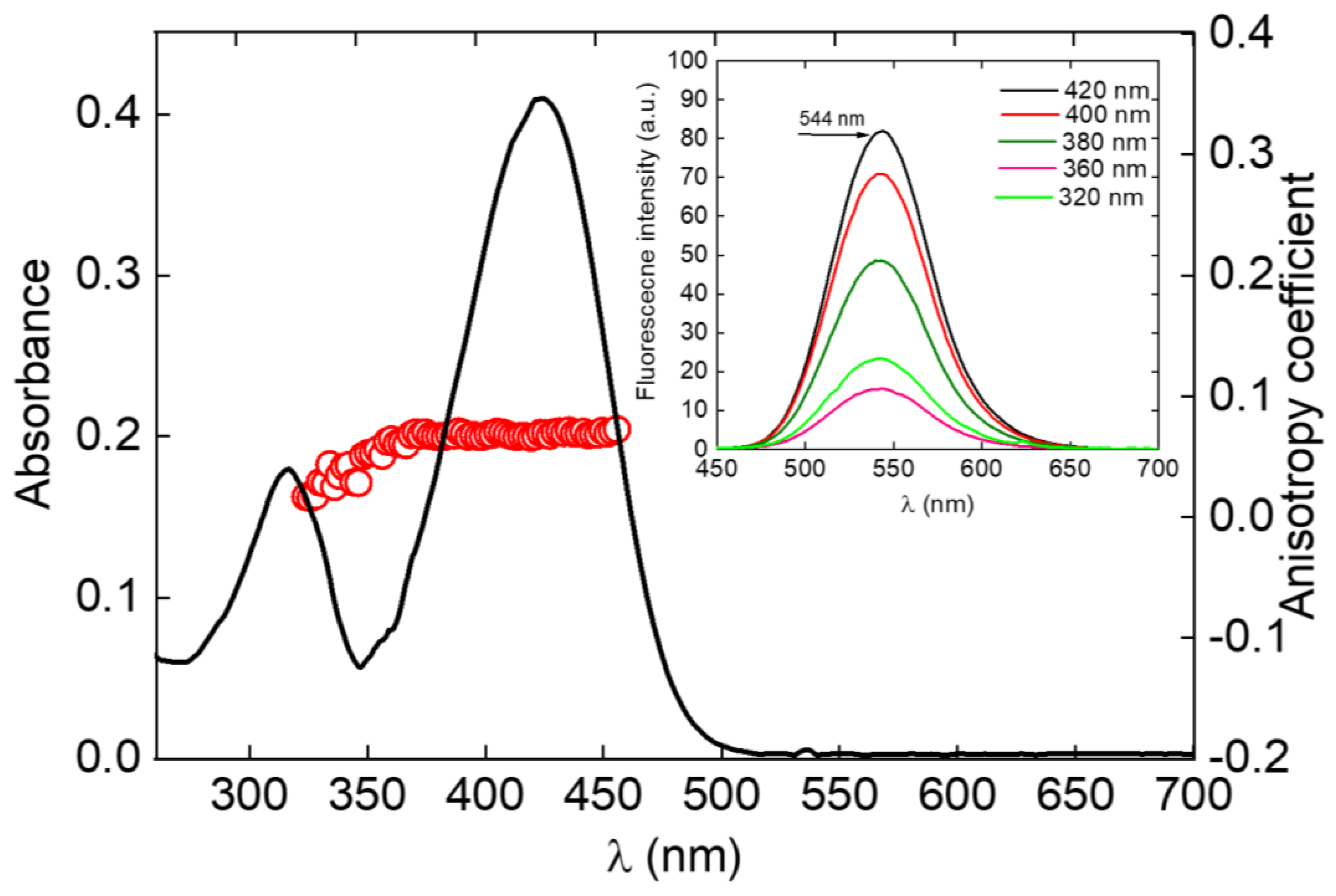
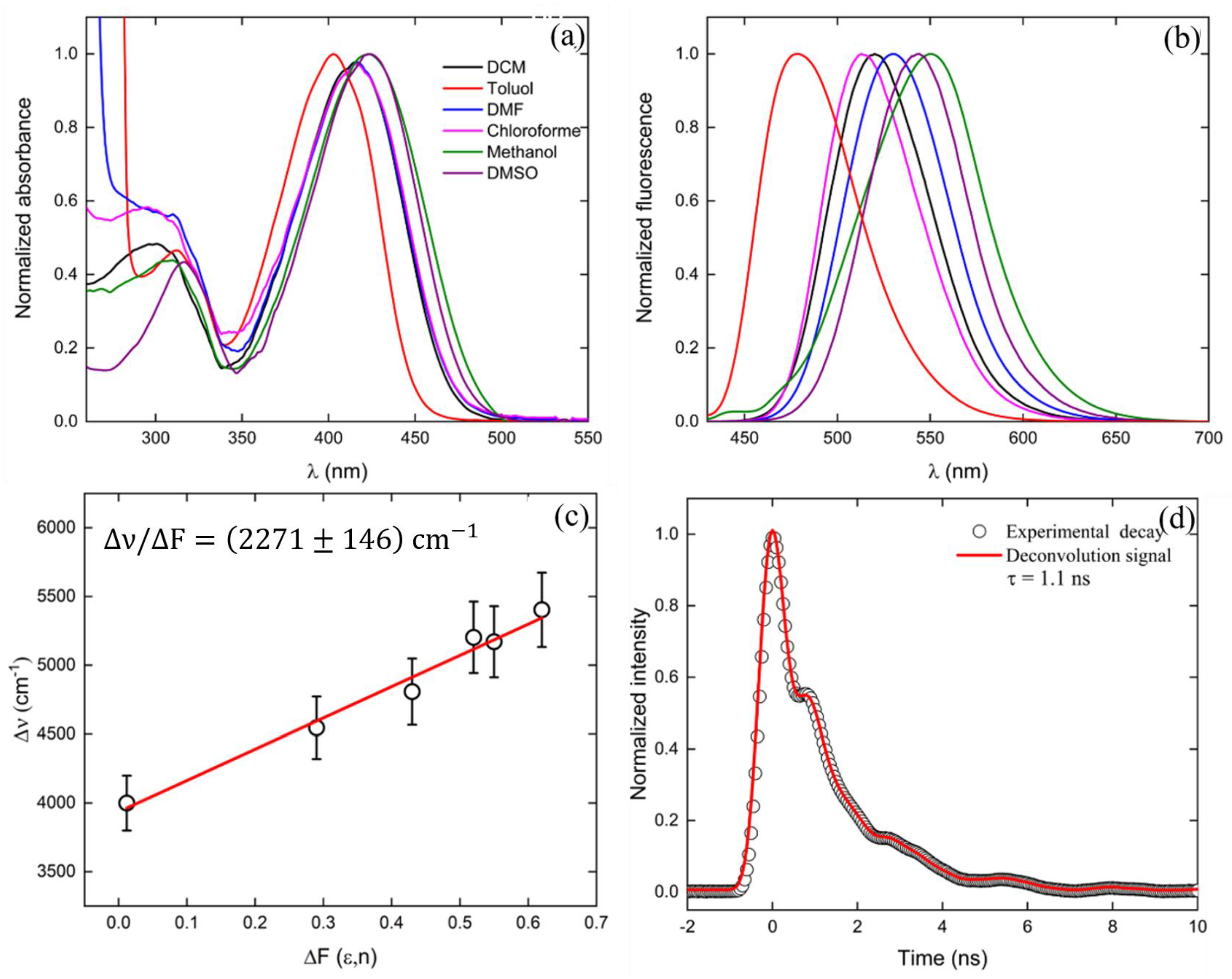
2. Results and Discussion
3. Materials and Methods
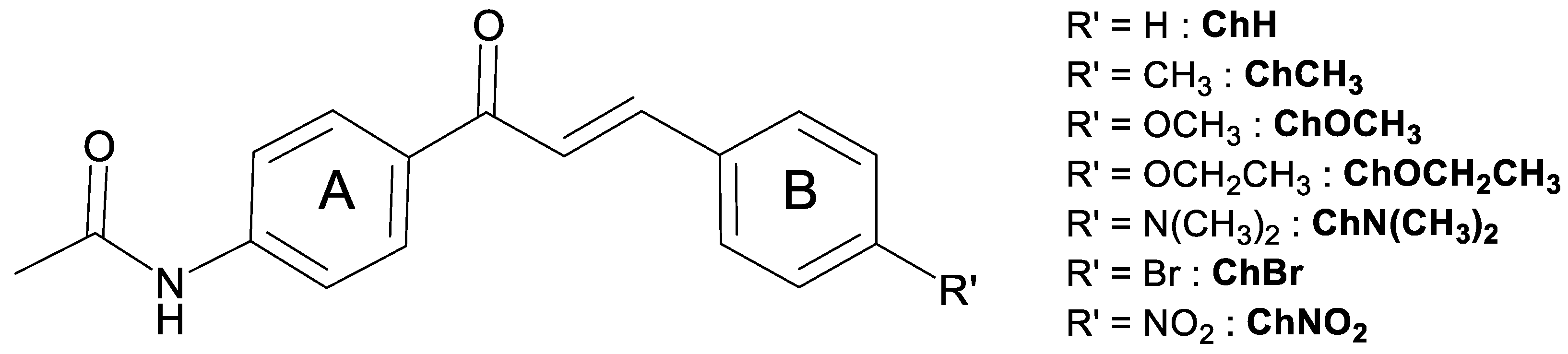
- ChH: N-[4-[(2E)-1-Oxo-3-phenyl-2-propen-1-yl]phenyl]acetamide,
- ChCH3: N-[4-[(2E)-3-(4-Methylphenyl)-1-oxo-2-propen-1-yl]phenyl]acetamide,
- ChCH2CH3: N-[4-[(2E)-3-(4-Ethylphenyl)-1-oxo-2-propen-1-yl]phenyl]acetamide,
- ChOCH3: N-[4-[(2E)-3-(4-Methoxyphenyl)-1-oxo-2-propen-1-yl]phenyl]acetamide,
- ChOCH2CH3: -[4-[(2E)-3-(4-Ethoxyphenyl)-1-oxo-2-propen-1-yl]phenyl]acetamide,
- ChN(CH3)2: N-[4-[(2E)-3-[4-(Dimethylamino)phenyl]-1-oxo-2-propen-1-yl]phenyl]acetamide,
- ChBr: N-[4-[3-(4-Bromophenyl)-1-oxo-2-propen-1-yl]phenyl]acetamide,
- ChNO2: N-[4-[(2E)-3-(4-Nitrophenyl)-1-oxo-2-propen-1-yl]phenyl]acetamide.
- Linear optical measurements
- Fluorescence Anisotropy measurements
- Time-resolved fluorescence technique
- Tunable Femtosecond Z-Scan technique
- Quantum Chemistry Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abegão, L.M.; Santos, F.A.; Fonseca, R.D.; Barreiros, A.L.; Barreiros, M.L.; Alves, P.B.; Costa, E.V.; Souza, G.B.; Alencar, M.A.; Mendonça, C.R.; et al. Chalcone-based molecules: Experimental and theoretical studies on the two-photon absorption and molecular first hyperpolarizability. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 227, 117772. [Google Scholar] [CrossRef]
- Manoel, D.S.; Pelosi, A.G.; Cocca, L.H.Z.; Almeida, G.F.; Sciuti, L.F.; Rodriguez, R.D.; Junior, L.A.; Lima, R.S.; Noda-Perez, C.; Martins, F.T.; et al. Second- and third-order nonlinear optical properties of mono-substituted terpenoid-like chalcones. J. Photochem. Photobiol. A Chem. 2022, 429, 113898. [Google Scholar] [CrossRef]
- Lemes, S.R.; Júnior, L.A.; Manoel, D.D.S.; de Sousa, M.A.M.; Fonseca, R.D.; Lima, R.S.; Noda-Perez, C.; Reis, P.R.D.M.; Cardoso, C.G.; Silveira-Lacerda, E.D.P.; et al. Optical properties and antiangiogenic activity of a chalcone derivate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 685–695. [Google Scholar] [CrossRef]
- Abegão, L.M.; Fonseca, R.D.; Santos, F.A.; Souza, G.B.; Barreiros, A.L.B.; Barreiros, M.L.; Alencar, M.; Mendonça, C.R.; Silva, D.L.; De Boni, L.; et al. Second- and third-order nonlinear optical properties of unsubstituted and mono-substituted chalcones. Chem. Phys. Lett. 2016, 648, 91–96. [Google Scholar] [CrossRef]
- da Costa, R.G.M.; Garcia, R.D.Q.; Fiuza, R.M.d.R.; Maqueira, L.; Pazini, A.; de Boni, L.; Limberger, J. Synthesis, photophysical properties and aggregation-induced enhanced emission of bischalcone-benzothiadiazole and chalcone-benzothiadiazole hybrids. J. Lumin. 2021, 239, 118367. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; D’Oliveira, G.D.C.; Gotardo, F.; Cocca, L.H.Z.; De Boni, L.; Perez, C.N.; Maia, L.J.Q.; Valverde, C.; Osório, F.A.P.; Napolitano, H.B. Chalcone as Potential Nonlinear Optical Material: A Combined Theoretical, Structural, and Spectroscopic Study. J. Phys. Chem. C 2019, 123, 5931–5941. [Google Scholar] [CrossRef]
- Alain-Rizzo, V.; Thouin, L.; Blanchard-Desce, M.; Gubler, U.; Bosshard, C.; Gunter, P.; Muller, J.; Alain, F.; Barzoukas, M. Molecular Engineering of Push–Pull Phenylpolyenes for Nonlinear Optics: Improved Solubility, Stability, and Nonlinearities. Adv. Mater. 1999, 11, 1210–1214. [Google Scholar] [CrossRef]
- Fonseca, S.; Modesto-Costa, L.; Milán-Garcés, E.; Andrade-Filho, T.; Gester, R.; da Cunha, A.R. Designing a novel organometallic chalcone with an enormous second-harmonic generation response. Mater. Today Commun. 2022, 31, 103762. [Google Scholar] [CrossRef]
- Zhang, G.J.; Kinoshita, T.; Sasaki, K.; Goto, Y.; Nakayama, M. Second-harmonic generation of a new chalcone-type crystal. Appl. Phys. Lett. 1990, 57, 221–223. [Google Scholar] [CrossRef]
- de Araújo, R.S.; de Alcântara, A.M.; Abegão, L.M.; de Souza, Y.P.; Silva, A.C.B.; Machado, R.; Rodrigues, J.J.; Pliego, J.R.; D’Errico, F.; Valle, M.S.; et al. Second harmonic generation in pyrazoline derivatives of dibenzylideneacetones and chalcone: A combined experimental and theoretical approach. J. Photochem. Photobiol. A Chem. 2020, 388, 112147. [Google Scholar] [CrossRef]
- Tao, X.T.; Watanabe, T.; Kono, K.; Deguchi, T.; Nakayama, M.; Miyata, S. Synthesis and Characterization of Poly(aryl ether chalcone)s for Second Harmonic Generation. Chem. Mater. 1996, 8, 1326–1332. [Google Scholar] [CrossRef]
- Goto, Y.; Hayashi, A.; Kimura, Y.; Nakayama, M. Second harmonic generation and crystal growth of substituted thienyl chalcone. J. Cryst. Growth 1991, 108, 688–698. [Google Scholar] [CrossRef]
- Patil, P.; Dharmaprakash, S.; Ramakrishna, K.; Fun, H.-K.; Kumar, R.S.S.; Rao, D.N. Second harmonic generation and crystal growth of new chalcone derivatives. J. Cryst. Growth 2007, 303, 520–524. [Google Scholar] [CrossRef]
- Derevyashkin, S.V.; Soboleva, E.A.; Shelkovnikov, V.V.; Spesivtsev, E.V. Holographic Recording in Micron Films Based on Polyfluorochalcones. High Energy Chem. 2019, 53, 50–57. [Google Scholar] [CrossRef]
- Mager, L.; Melzer, C.; Barzoukas, M.; Fort, A.; Méry, S.; Nicoud, J.-F. High net gain at 514 nm in a photorefractive polymer doped with a chalcone derivative. Appl. Phys. Lett. 1997, 71, 2248–2250. [Google Scholar] [CrossRef]
- Shettigar, S.; Umesh, G.; Chandrasekharan, K.; Sarojini, B.; Narayana, B. Studies on third-order nonlinear optical properties of chalcone derivatives in polymer host. Opt. Mater. 2008, 30, 1297–1303. [Google Scholar] [CrossRef]
- D’Silva, E.; Podagatlapalli, G.K.; Rao, S.V.; Dharmaprakash, S. Study on third-order nonlinear optical properties of 4-methylsulfanyl chalcone derivatives using picosecond pulses. Mater. Res. Bull. 2012, 47, 3552–3557. [Google Scholar] [CrossRef]
- Gaber, M.; El-Daly, S.; Fayed, T.; El-Sayed, Y. Photophysical properties, laser activity and photoreactivity of a heteroaryl chalcone: A model of solvatochromic fluorophore. Opt. Laser Technol. 2008, 40, 528–537. [Google Scholar] [CrossRef]
- Tomasch, M.; Schwed, J.S.; Weizel, L.; Stark, H. Novel Chalcone-Based Fluorescent Human Histamine H3 Receptor Ligands as Pharmacological Tools. Front. Syst. Neurosci. 2012, 6, 14. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, R.; Kawashima, H.; Cheng, Y.; Kimura, H.; Watanabe, H.; Haratake, M.; Saji, H.; Nakayama, M. Fluoro-pegylated Chalcones as Positron Emission Tomography Probes for in vivo Imaging of β-Amyloid Plaques in Alzheimer’s Disease. J. Med. Chem. 2009, 52, 6394–6401. [Google Scholar] [CrossRef]
- Lee, S.-C.; Kang, N.-Y.; Park, S.-J.; Yun, S.-W.; Chandran, Y.; Chang, Y.-T. Development of a fluorescent chalcone library and its application in the discovery of a mouse embryonic stem cell probe. Chem. Commun. 2012, 48, 6681–6683. [Google Scholar] [CrossRef]
- Tiwari, A.; Bendi, A.; Bhathiwal, A.S. An Overview on Synthesis and Biological Activity of Chalcone Derived Pyrazolines. ChemistrySelect 2021, 6, 12757–12795. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, P.; Lu, J.; Xing, C. Characterization of the Fluorescence Properties of 4-Dialkylaminochalcones and Investigation of the Cytotoxic Mechanism of Chalcones. Arch. Pharm. 2016, 349, 539–552. [Google Scholar] [CrossRef]
- Custodio, J.; Faria, E.; Sallum, L.; Duarte, V.; Vaz, W.F.; De Aquino, G.; Carvalho, P.S., Jr.; Napolitano, H. The Influence of Methoxy and Ethoxy Groups on Supramolecular Arrangement of Two Methoxy-chalcones. J. Braz. Chem. Soc. 2017, 28, 2180–2191. [Google Scholar] [CrossRef]
- Anderson, G.M.I.I.I.; Kollman, P.K.; Domelsmith, L.N.; Houk, K.N. Methoxy group nonplanarity in o-dimethoxybenzenes. Simple predictive models for conformations and rotational barriers in alkoxyaromatics. J. Am. Chem. Soc. 1979, 101, 2344–2352. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N. Warren, Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Fayed, T.A.; Awad, M. Dual emission of chalcone-analogue dyes emitting in the red region. Chem. Phys. 2004, 303, 317–326. [Google Scholar] [CrossRef]
- Santos, F.A.; Abegão, L.M.; Fonseca, R.D.; Alcântara, A.M.; Mendonça, C.R.; Valle, M.S.; Alencar, M.; Kamada, K.; De Boni, L.; Rodrigues, J. Bromo-and chloro-derivatives of dibenzylideneacetone: Experimental and theoretical study of the first molecular hyperpolarizability and two-photon absorption. J. Photochem. Photobiol. A Chem. 2019, 369, 70–76. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A.; Mahmood, A.; Nazar, M.F.; Yang, Z.; Pan, S. Electronic absorption spectra and nonlinear optical properties of ruthenium acetylide complexes: A DFT study toward the designing of new high NLO response compounds. Acta Chim. Slov. 2014, 61, 382–390. [Google Scholar]
- Tan, J.; Zhang, Y.; Zhang, M.; Tian, X.; Wang, Y.; Li, S.; Wang, C.; Zhou, H.; Yang, J.; Tian, Y.; et al. Small molecules of chalcone derivatives with high two-photon absorption activities in the near-IR region. J. Mater. Chem. C 2016, 4, 3256–3267. [Google Scholar] [CrossRef]
- Niu, C.-G.; Guan, A.-L.; Zeng, G.-M.; Liu, Y.-G.; Li, Z.-W. Fluorescence water sensor based on covalent immobilization of chalcone derivative. Anal. Chim. Acta 2006, 577, 264–270. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Elzupir, A.; AlSalhi, M.; Alaamer, A.S. Influence of functional groups on the photophysical properties of dimethylamino chalcones as laser dyes. Opt. Mater. 2018, 76, 216–221. [Google Scholar] [CrossRef]
- Albrecht, C. Joseph R. Lakowicz: Principles of fluorescence spectroscopy, 3rd Edition. Anal. Bioanal. Chem. 2008, 390, 1223–1224. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Lewis, G.N.; Kasha, M. Phosphorescence and the Triplet State. J. Am. Chem. Soc. 1944, 66, 2100–2116. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Rurack, K.; Dekhtyar, M.L.; Bricks, J.L.; Resch-Genger, U.; Rettig, W. Quantum Yield Switching of Fluorescence by Selectively Bridging Single and Double Bonds in Chalcones: Involvement of Two Different Types of Conical Intersections. J. Phys. Chem. A 1999, 103, 9626–9635. [Google Scholar] [CrossRef]
- Aldaghri, O. Spectral Characteristics and Molecular Structure of (E)-1-(4-Chlorophenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-en-1-One (DAP). Materials 2021, 14, 2766. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Lippert, E. Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1957, 61, 962–975. [Google Scholar] [CrossRef]
- Islam, M.A. Einstein–Smoluchowski Diffusion Equation: A Discussion. Phys. Scr. 2004, 70, 120–125. [Google Scholar] [CrossRef]
- Meath, W.J.; Power, E.A. On the importance of permanent moments in multiphoton absorption using perturbation theory. J. Phys. B At. Mol. Phys. 1984, 17, 763–781. [Google Scholar] [CrossRef]
- Tristão, T.C.; Campos-Buzzi, F.; Corrêa, R.; Cruz, R.B.; Filho, V.C.; Cruz, A.B. Antimicrobial and Cytotoxicity Potential of Acetamido, Amino and Nitrochalcones. Arzneimittelforschung 2012, 62, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.K.A.; da Silva, A.W.; Silva, F.C.O.; Holanda, C.L.A.; Barroso, S.M.; Lima, J.D.R.; Neto, A.E.V.; Campos, A.R.; Bandeira, P.N.; dos Santos, H.S.; et al. Anxiolytic-like effect of chalcone N-{(4′-[(E)-3-(4-fluorophenyl)-1-(phenyl) prop-2-en-1-one]} acetamide on adult zebrafish (Danio rerio): Involvement of the GABAergic system. Behav. Brain Res. 2019, 374, 111871. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Kadian, K.; Ojha, R.; Dhiman, R.; Garg, A.; Singh, G.; Buddhiraja, A.; Bedi, P.M.S.; Dhar, K.L. Effect of ring A and ring B substitution on the cytotoxic potential of pyrazole tethered chalcones. Med. Chem. Res. 2012, 21, 2990–2997. [Google Scholar] [CrossRef]
- Pelosi, A.G.; Cocca, L.H.Z.; Abegão, L.M.; Sciuti, L.F.; Piguel, S.; De Boni, L.; Mendonça, C.R. Influence of electron-withdrawing groups in two-photon absorption of imidazopyridines derivatives. Dye. Pigment. 2022, 198, 109972. [Google Scholar] [CrossRef]
- Cocca, L.H.Z.; Abegão, L.M.G.; Sciuti, L.F.; Vabre, R.; Siqueira, J.D.P.; Kamada, K.; Mendonça, C.R.; Piguel, S.; De Boni, L. Two-Photon Emissive Dyes Based on Push–Pull Purines Derivatives: Toward the Development of New Photoluminescence Bioprobes. J. Phys. Chem. C 2020, 124, 12617–12627. [Google Scholar] [CrossRef]
- Cocca, L.H.Z.; Pelosi, A.; Sciuti, L.F.; Abegão, L.M.G.; Kamada, K.; Piguel, S.; Mendonça, C.R.; De Boni, L. Two-photon brightness of highly fluorescent imidazopyridine derivatives: Two-photon and ultrafast transient absorption studies. J. Mol. Liq. 2022, 348, 118379. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Fox, Gaussian 09, Revision D.01, Gaussian 09, Revis. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009; pp. 1–20. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mariz, I.F.; Maçôas, E.M.; Afonso, C.A.M.; Martinho, J. Two-photon absorption properties of push–pull oxazolones derivatives. Dye. Pigment. 2012, 95, 713–722. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Schlüter, M.; Sham, L.J. Density functional theory. Phys. Today 1982, 35, 36–43. [Google Scholar] [CrossRef]
- Rebane, A.; Drobizhev, M.; Makarov, N.S.; Beuerman, E.; Haley, J.E.; Douglas, M.K.; Burke, A.R.; Flikkema, J.L.; Cooper, T.M. Relation between Two-Photon Absorption and Dipolar Properties in a Series of Fluorenyl-Based Chromophores with Electron Donating or Electron Withdrawing Substituents. J. Phys. Chem. A 2011, 115, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
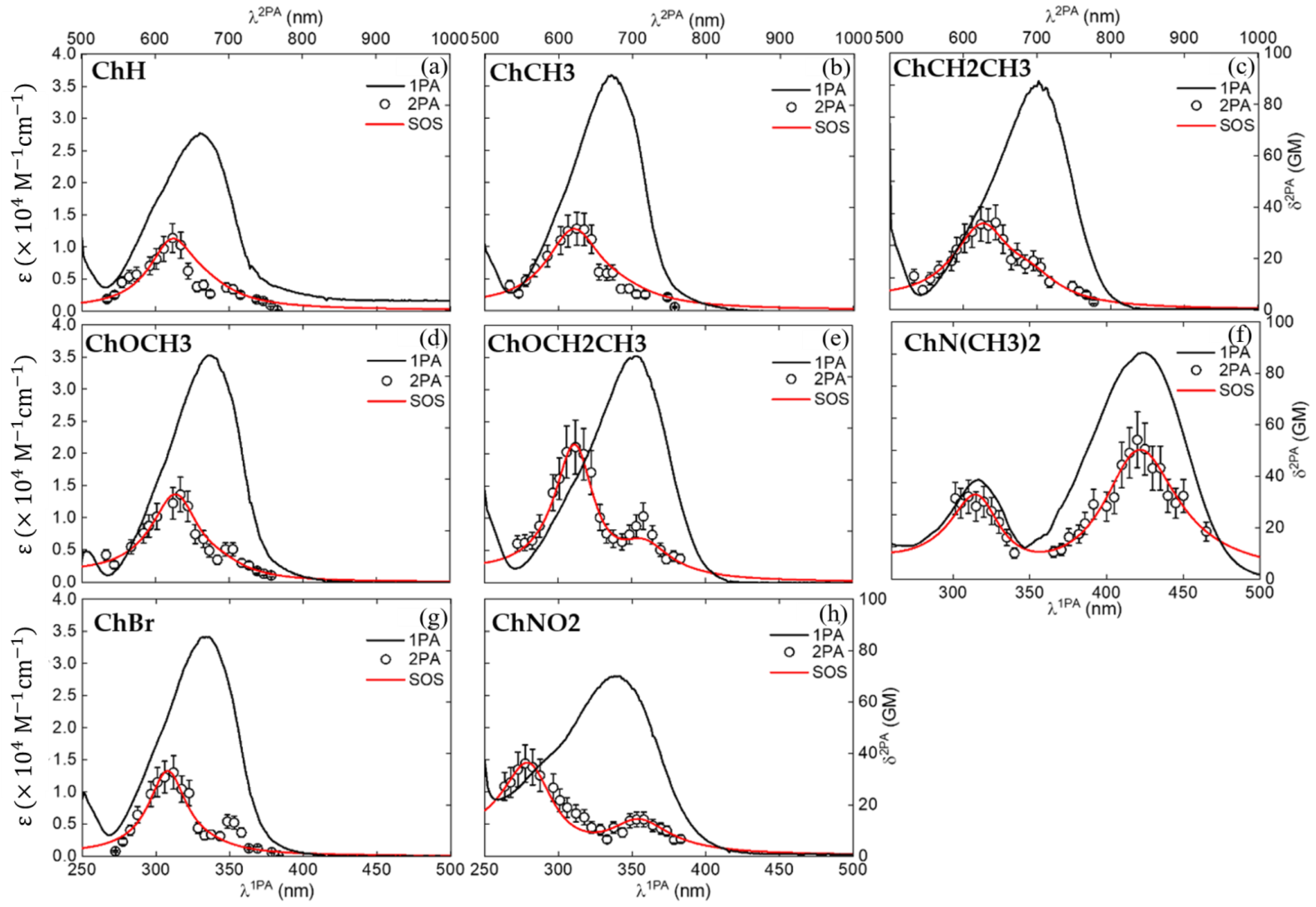
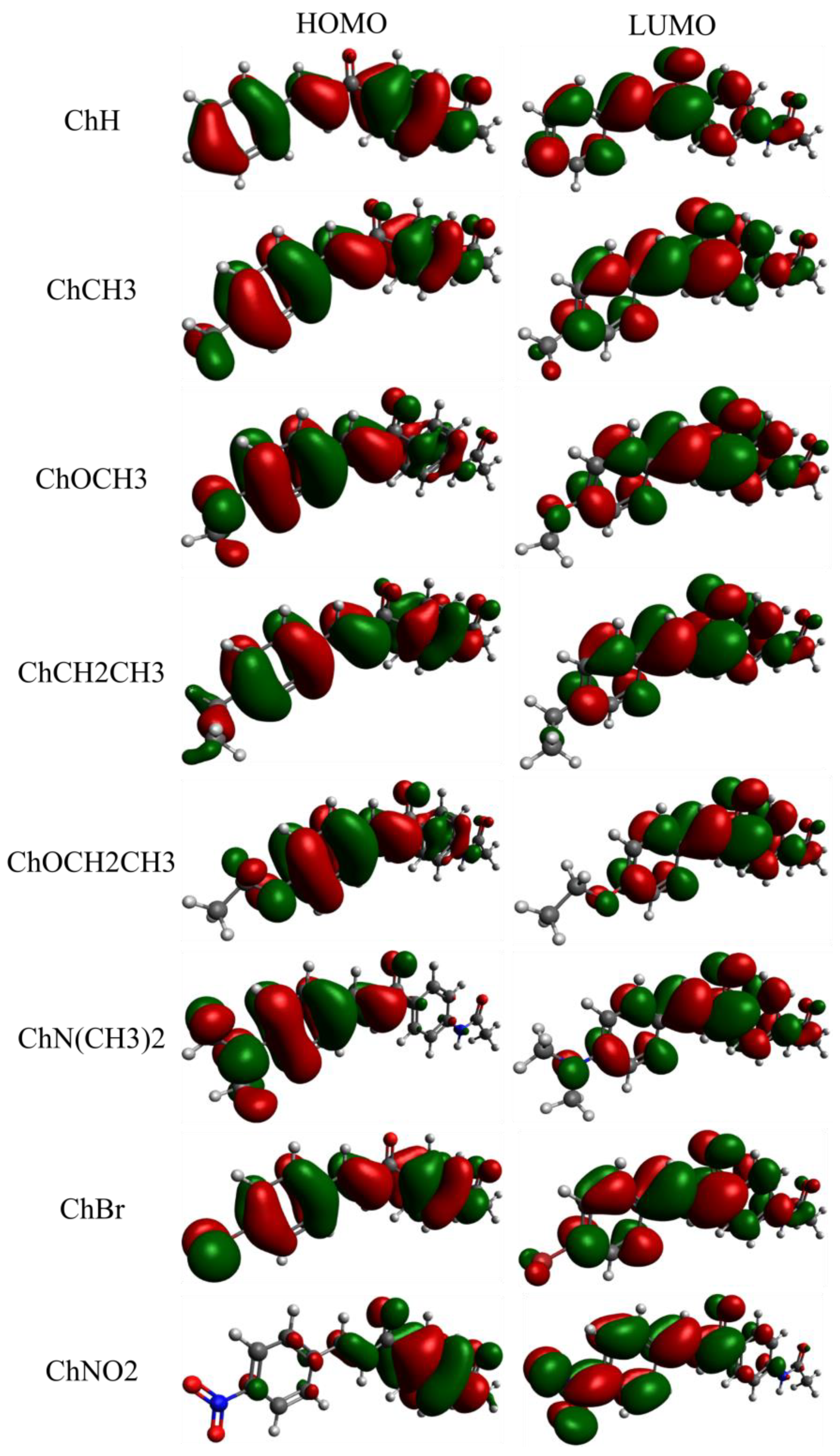


| Compound | ||||
|---|---|---|---|---|
| ChH | 27,414 336 | 10,767 314 | 9 697 | 15 624 |
| ChCH3 | 34,843 342 | 13,783 314 | 15 673 | 32 624 |
| ChOCH3 | 30,661 355 | 14,512 319 | 20 695 | 33 634 |
| ChCH2CH3 | 34,312 342 | 13,582 319 | 13 695 | 33 634 |
| ChOCH2CH3 | 33,113 355 | 13,311 319 | 18 706 | 53 624 |
| ChN(CH3)2 | 35,168 422 | 16,331 314 | 54 840 | 32 620 |
| ChBr | 34,164 336 | 14,378 309 | 8 697 | 33 624 |
| ChNO2 | 21,665 342 | 13,852 294 | 15 706 | 36 555 |
| Compound | μ01 (D) | μ02 (D) | ||
|---|---|---|---|---|
| ChH | 5.9 | 3.3 | 2.5 | 6.1 |
| ChCH3 | 6.5 | 4.2 | 4.6 | 7.7 |
| ChOCH3 | 6.3 | 4.9 | 3.1 | 9.4 |
| ChCH2CH3 | 6.4 | 3.9 | 4.8 | 8.0 |
| ChOCH2CH3 | 6.7 | 4.5 | 3.1 | 9.3 |
| ChN(CH3)2 | 7.5 | 4.7 | 11.5, 7.1 * | 10.2 |
| ChBr | 6.5 | 4.3 | 3.1 | 7.0 |
| ChNO2 | 6.3 | 5.9 | 8.2 | 8.2 |
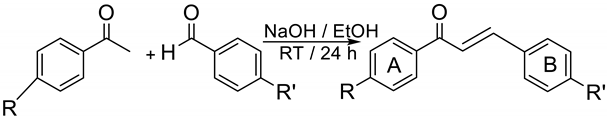 | ||||
| R- | R’- | Yield | Melting Point | Color |
| CH3COHN- | -H | 30% | 162.5–163.1 °C | Pale yellow |
| CH3COHN- | -CH3 | 72% | 197.8–199.9 °C | Bright yellow |
| CH3COHN- | -CH2CH3 | 46% | 200.3–201.6 °C | Bright yellow |
| CH3COHN- | -OCH3 | 41% | 204.2–205.7 °C | Pale yellow |
| CH3COHN- | -OCH2CH3 | 42% | 134.6–135.8 °C | Pale yellow |
| CH3COHN- | -N(CH3)2 | 30% | 150.5–152.1 °C | Orangish |
| CH3COHN- | -Br | 74% | 221.7–222.1 °C | Yellow |
| CH3COHN- | -NO2 | 27% | 234.0–237.0 °C | Bright yellow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelosi, A.G.; Silveira-Alves, E., Jr.; Cocca, L.H.Z.; Valverde, J.V.; Oliveira, G.R.; da Silva, D.L.; De Boni, L.; Gonçalves, P.J.; Mendonca, C.R. Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties. Molecules 2023, 28, 1572. https://doi.org/10.3390/molecules28041572
Pelosi AG, Silveira-Alves E Jr., Cocca LHZ, Valverde JV, Oliveira GR, da Silva DL, De Boni L, Gonçalves PJ, Mendonca CR. Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties. Molecules. 2023; 28(4):1572. https://doi.org/10.3390/molecules28041572
Chicago/Turabian StylePelosi, André Gasparotto, Eli Silveira-Alves, Jr., Leandro Henrique Zucolotto Cocca, João Victor Valverde, Guilherme Roberto Oliveira, Daniel Luiz da Silva, Leonardo De Boni, Pablo José Gonçalves, and Cleber Renato Mendonca. 2023. "Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties" Molecules 28, no. 4: 1572. https://doi.org/10.3390/molecules28041572
APA StylePelosi, A. G., Silveira-Alves, E., Jr., Cocca, L. H. Z., Valverde, J. V., Oliveira, G. R., da Silva, D. L., De Boni, L., Gonçalves, P. J., & Mendonca, C. R. (2023). Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties. Molecules, 28(4), 1572. https://doi.org/10.3390/molecules28041572









