Supercapacitor Performance of Magnetite Nanoparticles Enhanced by a Catecholate Dispersant: Experiment and Theory
Abstract
:1. Introduction
2. Results and Discussion
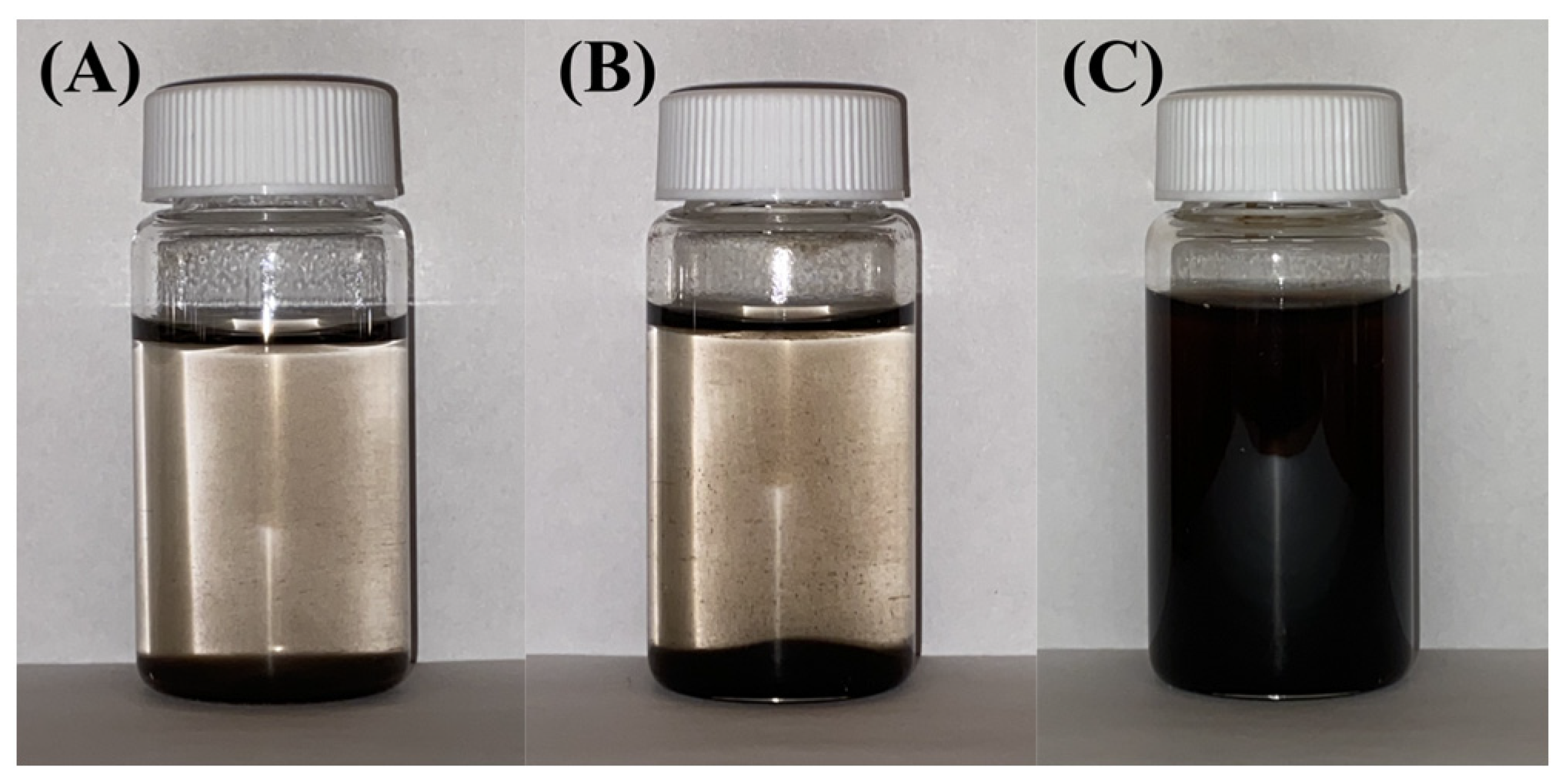
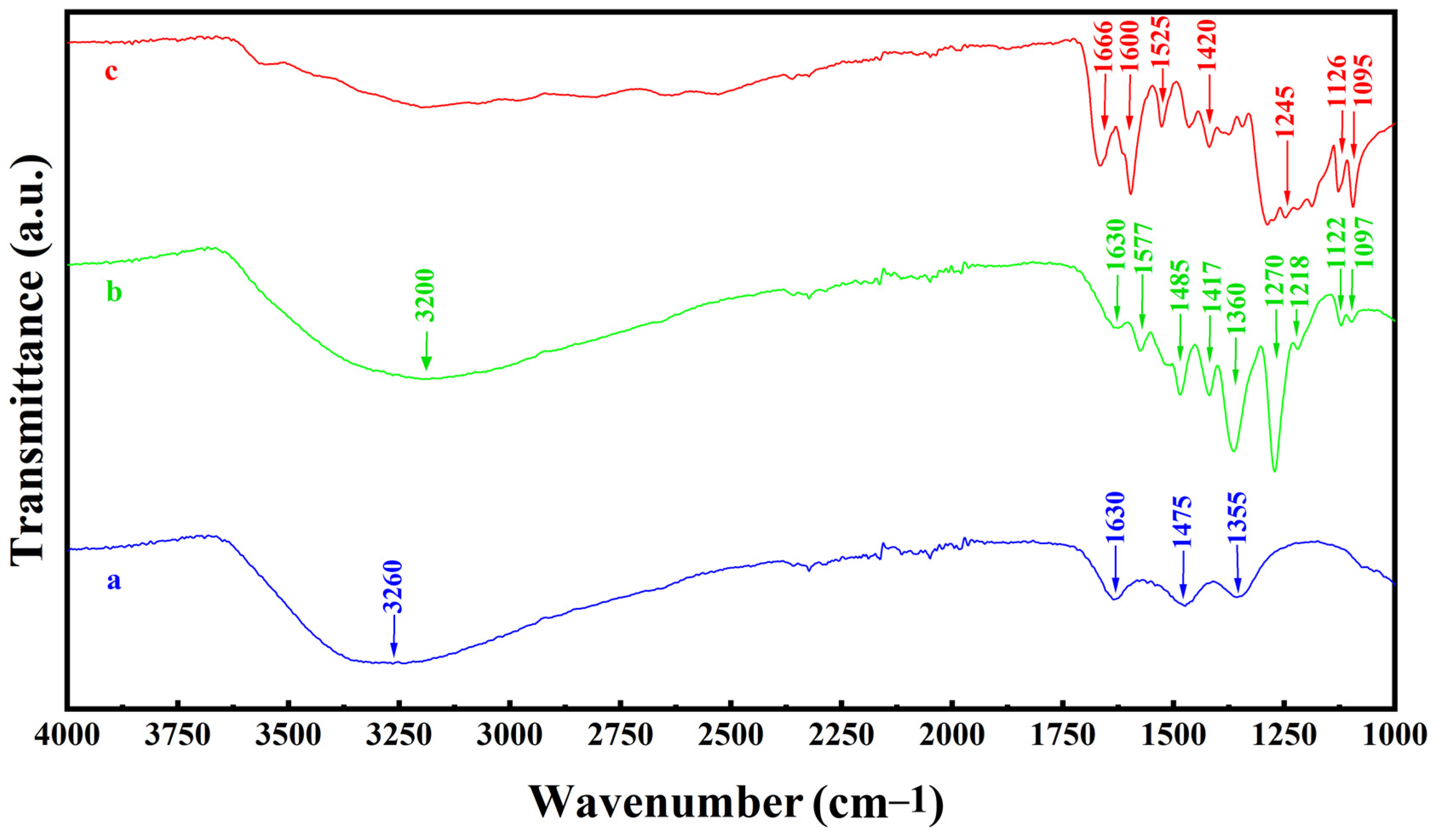
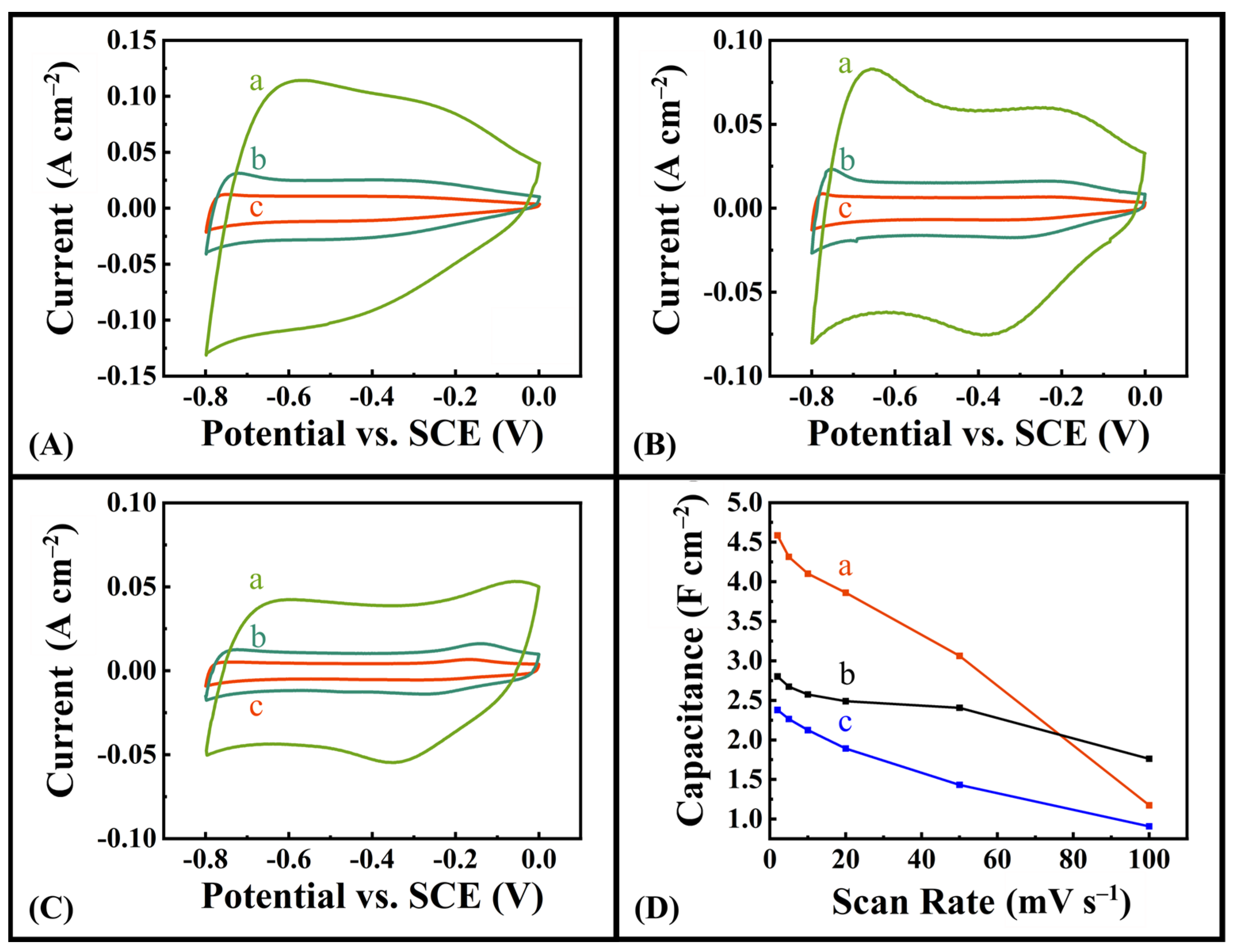
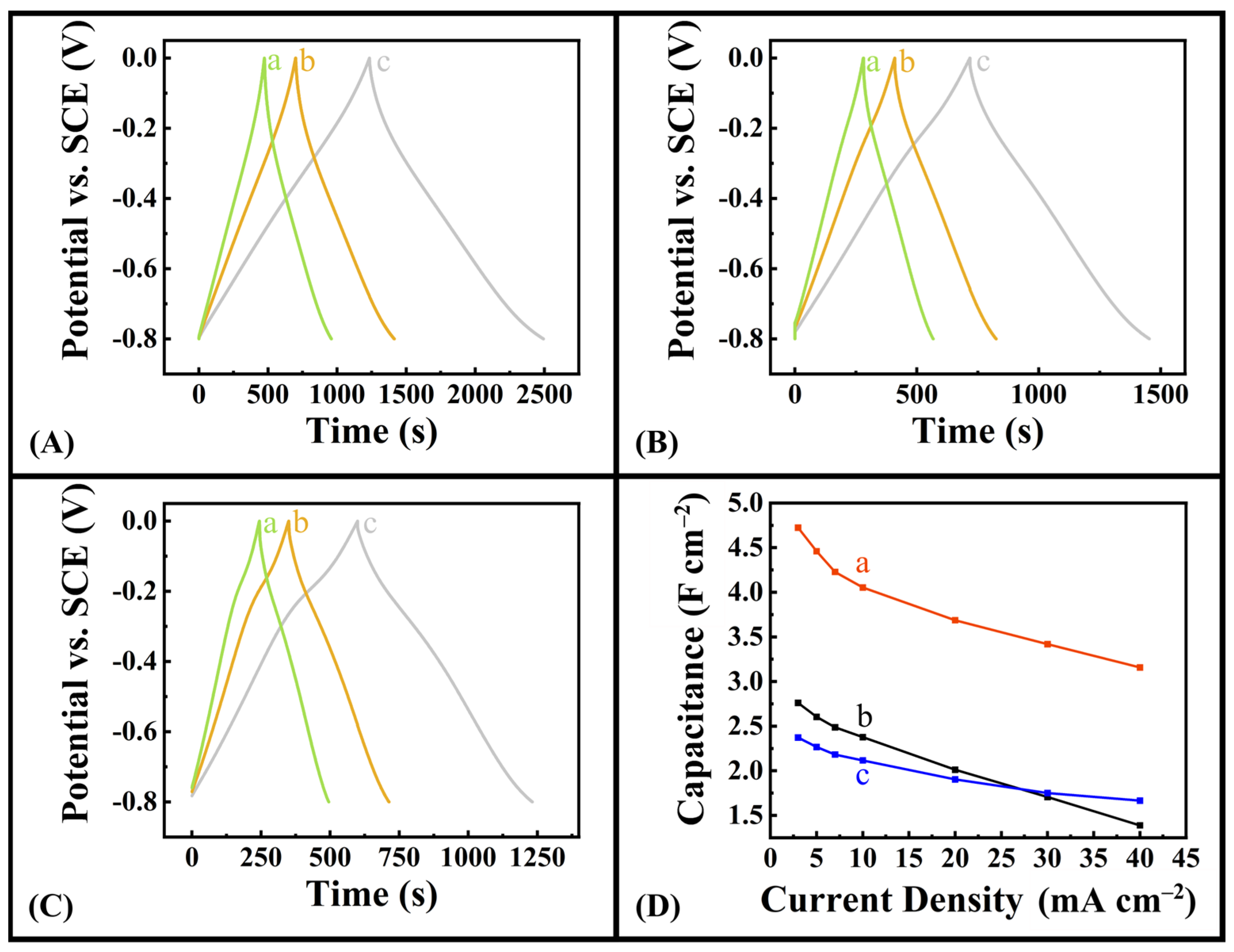
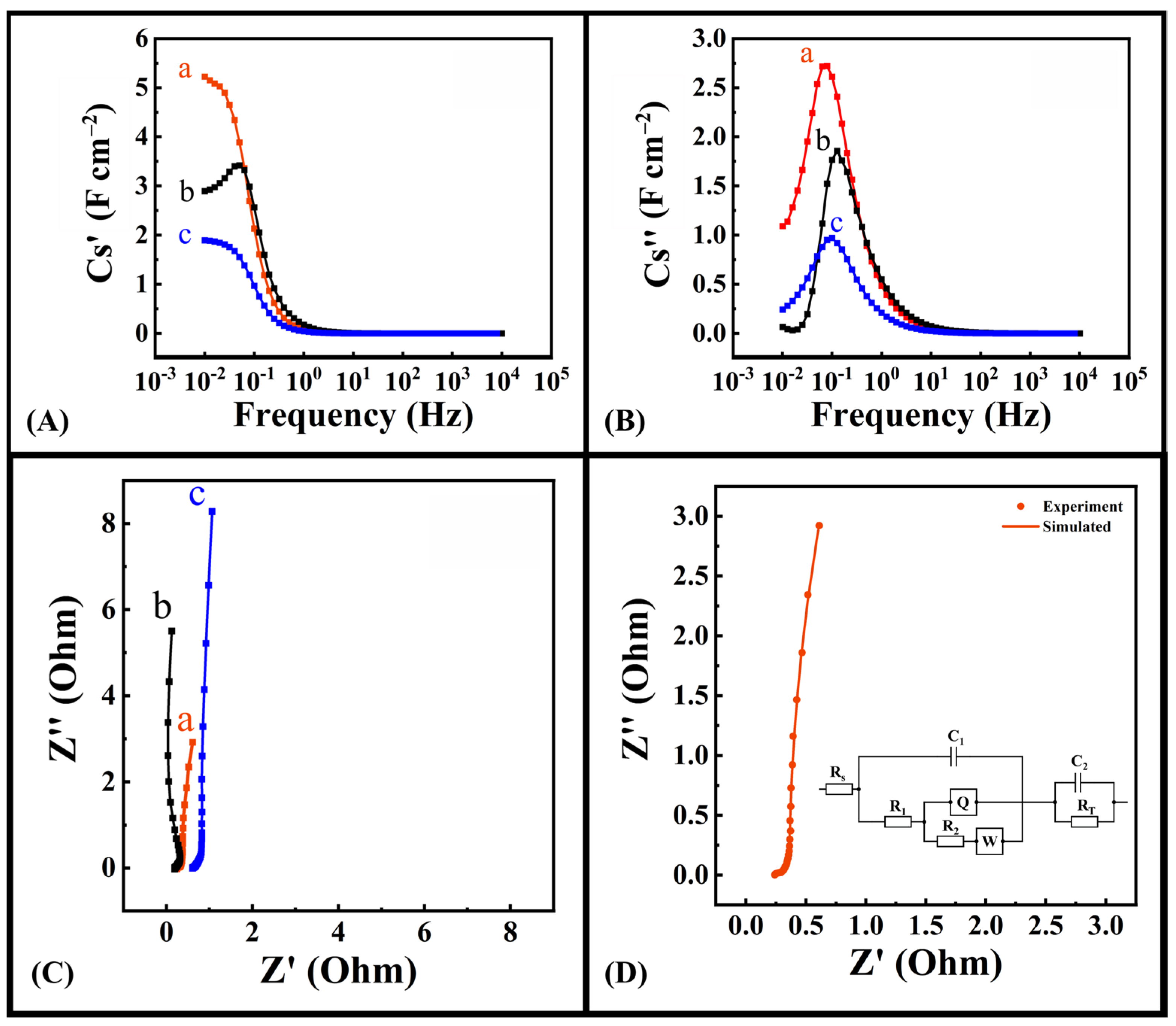
2.1. Experimental Results
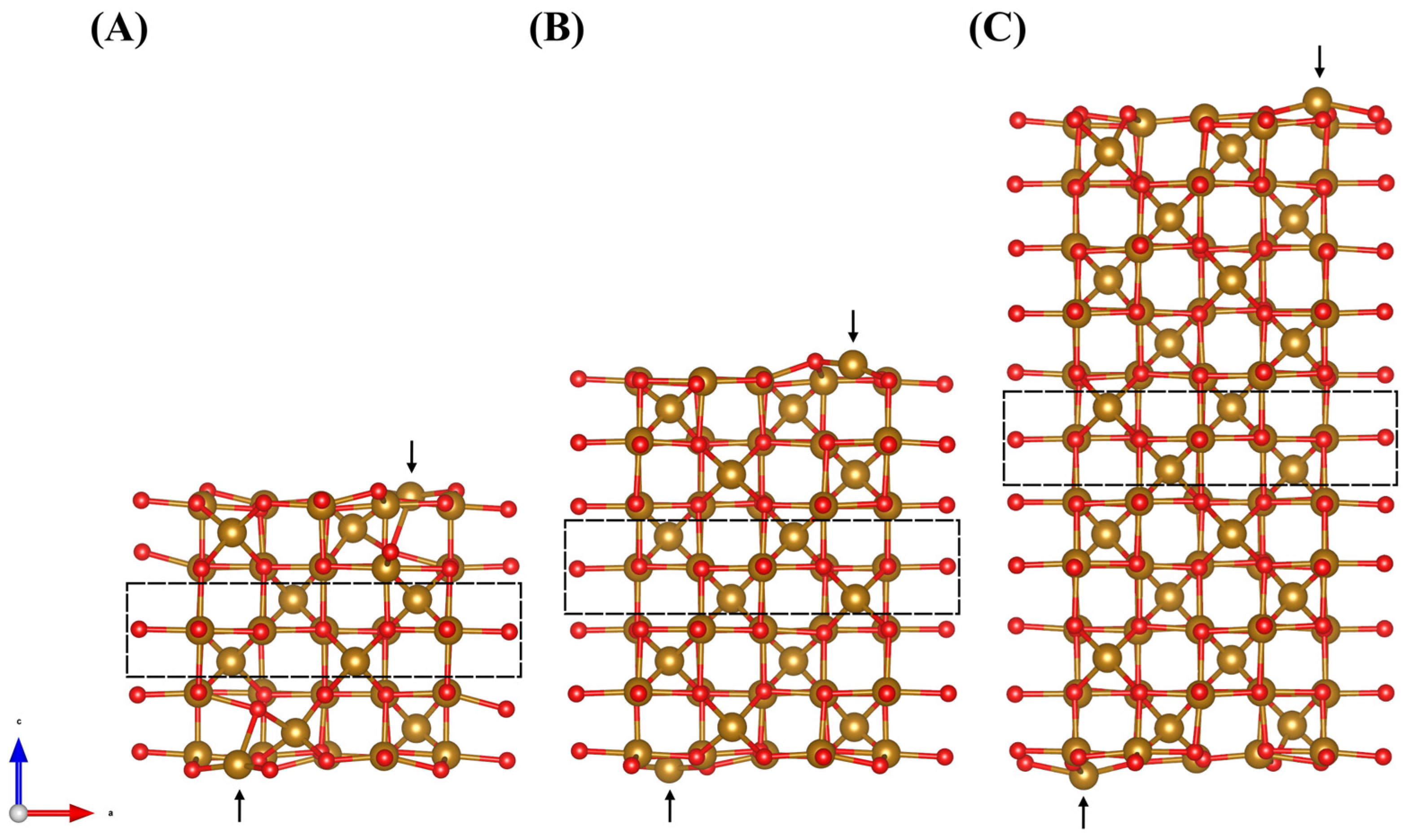
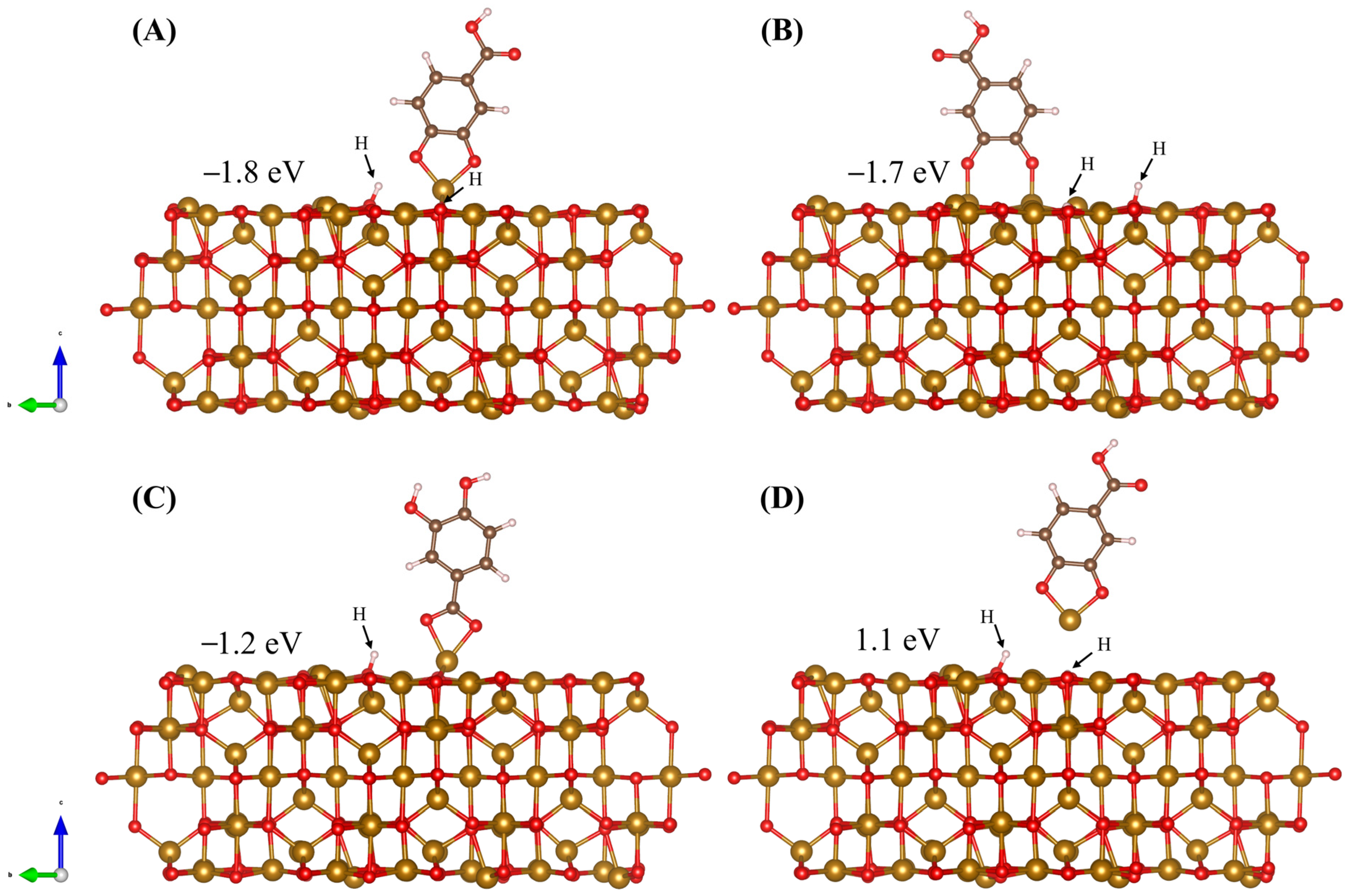
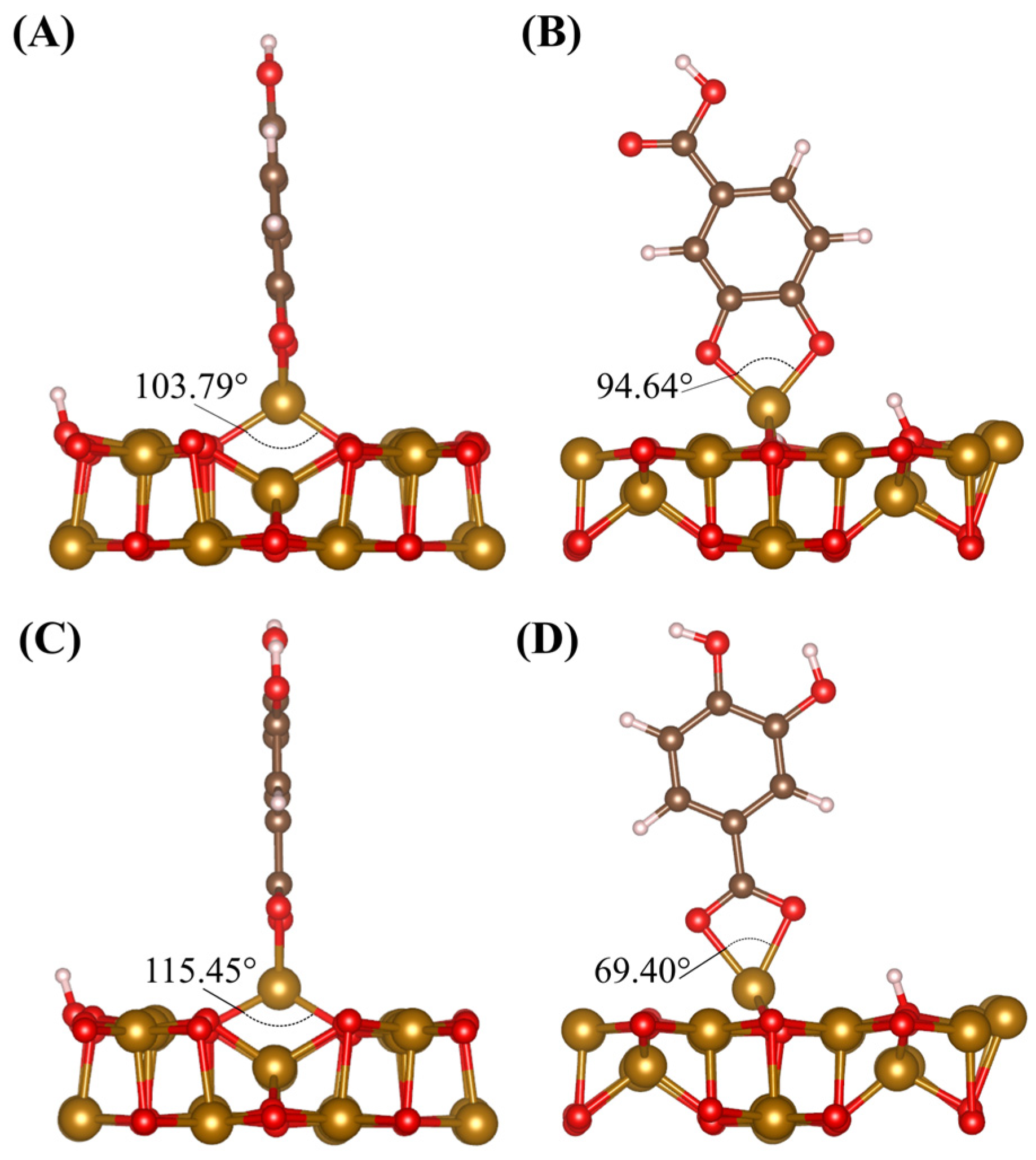
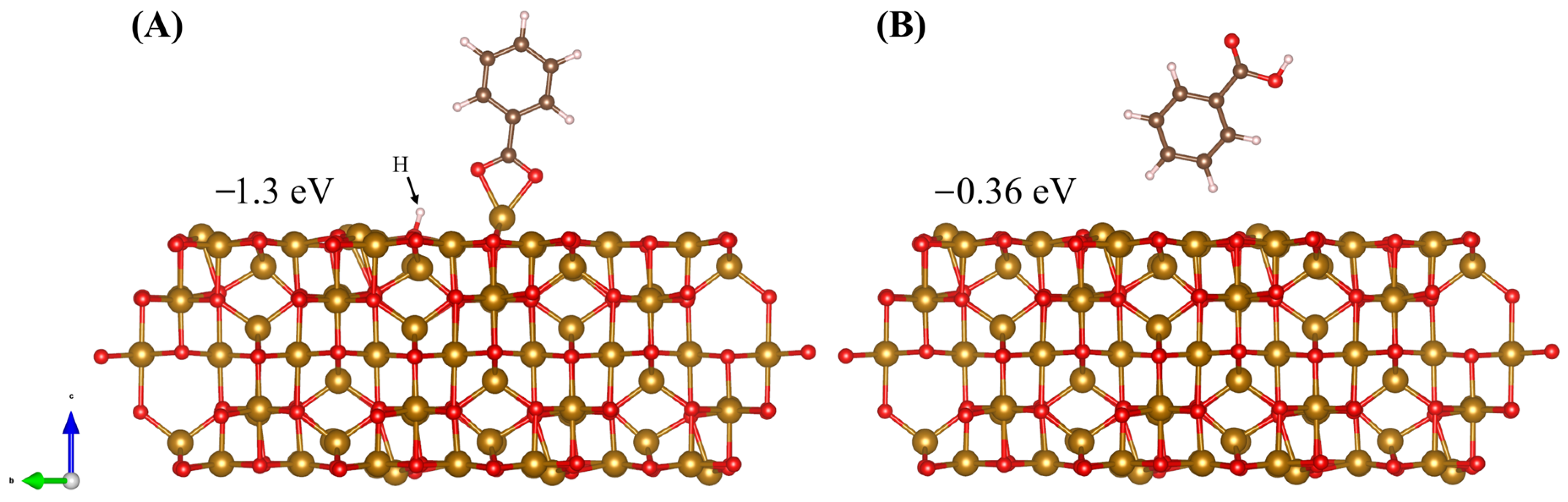
2.2. DFT Modeling of Adsorption on Fe3O4 (001) Surface
2.3. DFT Analysis of DHBA and BA Adsorption at the (001) Surface
3. Materials and Methods
3.1. Materials and Experimental Methods
3.2. Computational
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, T.; Wojtal, P.; Rubel, O.; Zhitomirsky, I. Density functional theory and experimental studies of caffeic acid adsorption on zinc oxide and titanium dioxide nanoparticles. R. Soc. Chem. Adv. 2015, 5, 106877–106885. [Google Scholar] [CrossRef]
- Melrose, J. High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired. Molecules 2022, 27, 8982. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Hennig, K.; Meyer, W. Synthesis and Characterization of Catechol-Containing Polyacrylamides with Adhesive Properties. Molecules 2022, 27, 4027. [Google Scholar] [CrossRef]
- Ata, M.S.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. R. Soc. Chem. Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef]
- Jankovic, I.A. Surface Modification of Colloidal TiO2 Nanoparticles with Bidentate Benzene Derivatives. J. Phys. Chem. C 2009, 113, 12645–12652. [Google Scholar] [CrossRef]
- Cornard, J.P. Theoretical and Spectroscopic Investigations of a Complex of Al(III) with Caffeic Acid. J. Phys. Chem. A 2004, 108, 4470–4478. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Clifford, A.; Pang, X.; Zhitomirsky, I. Biomimetically modified chitosan for electrophoretic deposition of composites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 28–34. [Google Scholar] [CrossRef]
- Clifford, A.; Lee, B.; Grandfield, K.; Zhitomirsky, I. Biomimetic modification of poly-L-lysine and electrodeposition of nanocomposite coatings for orthopaedic applications. Colloids Surf. B Biointerfaces 2019, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, Y.; Zhitomirsky, I. Electrophoretic deposition of TiO2 and composite TiO2–MnO2 films using benzoic acid and phenolic molecules as charging additives. J. Colloid Interface Sci. 2010, 352, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhitomirsky, I. Biomimetic strategy for electrophoretic deposition of composite ferroelectric poly(vinylidene, fluoride-co-hexafluoropropylene)—Ferrimagnetic NiFe2O4 films. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129743. [Google Scholar] [CrossRef]
- Silva, R.M.E.; Poon, R.; Milne, J.; Syed, A.; Zhitomirsky, I. New developments in liquid-liquid extraction, surface modification and agglomerate-free processing of inorganic particles. Adv. Colloid Interface Sci. 2018, 261, 15–27. [Google Scholar] [CrossRef]
- Milne, J.; Marques Silva, R.; Zhitomirsky, I. Surface modification and dispersion of ceramic particles using liquid-liquid extraction method for application in supercapacitor electrodes. J. Eur. Ceram. Soc. 2019, 39, 3450–3455. [Google Scholar] [CrossRef]
- Mowbray, D.J.; Migani, A. Optical Absorption Spectra and Excitons of Dye-Substrate Interfaces: Catechol on TiO2(110). J. Chem. Theory Comput. 2016, 12, 2843–2852. [Google Scholar] [CrossRef]
- Sakib, S.; Pandey, R.; Soleymani, L.; Zhitomirsky, I. Surface modification of TiO2 for photoelectrochemical DNA biosensors. Med. Devices Sens. 2020, 3, e10066. [Google Scholar] [CrossRef]
- Savin, R.; Blanck, C.; Benzaamia, N.-O.; Boulmedais, F. Optimization of Nanohybrid Biosensors Based on Electro-Crosslinked Tannic Acid Capped Nanoparticles/Enzyme. Molecules 2022, 27, 3309. [Google Scholar] [CrossRef]
- Pinto, A.L.; Cruz, L.; Gomes, V.; Cruz, H.; Calogero, G.; de Freitas, V.; Pina, F.; Parola, A.J.; Carlos Lima, J. Catechol versus carboxyl linkage impact on DSSC performance of synthetic pyranoflavylium salts. Dye. Pigment. 2019, 170, 107577. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Guan, Y.; Bhokisham, N.; Tsao, C.Y.; Kim, E.; Shi, X.W.; Wang, Q.; Bentley, W.E.; Payne, G.F. Catechol-chitosan redox capacitor for added amplification in electrochemical immunoanalysis. Colloids Surf. B Biointerfaces 2018, 169, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kim, E.; Li, J.; Bentley, W.E.; Shi, X.-W.; Payne, G.F. Catechol-based capacitor for redox-linked bioelectronics. J. Am. Chem. Soc. Appl. Electron. Mater. 2019, 1, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Segoviano-Garfias, J.J.N.; Zanor, G.A.; Ávila-Ramos, F.; Bivián-Castro, E.Y. Equilibrium Studies of Iron (III) Complexes with Either Pyrazine, Quinoxaline, or Phenazine and Their Catecholase Activity in Methanol. Molecules 2022, 27, 3257. [Google Scholar] [CrossRef] [PubMed]
- Touzé, E.; Gohier, F.; Daffos, B.; Taberna, P.-L.; Cougnon, C. Improvement of electrochemical performances of catechol-based supercapacitor electrodes by tuning the redox potential via different-sized O-protected catechol diazonium salts. Electrochim. Acta 2018, 265, 121–130. [Google Scholar] [CrossRef]
- Jokar, E.; Shahrokhian, S. Electrochemical functionalization of graphene nanosheets with catechol derivatives as an effective method for preparation of highly performance supercapacitors. Electrochim. Acta 2014, 147, 136–142. [Google Scholar] [CrossRef]
- Malka, D.; Giladi, S.; Hanna, O.; Weitman, M.; Cohen, R.; Elias, Y.; Attias, R.; Brousse, T.; Frimer, A.A.; Aurbach, D. Catechol-Modified Carbon Cloth as Hybrid Electrode for Energy Storage Devices. J. Electrochem. Soc. 2019, 166, A1147–A1153. [Google Scholar] [CrossRef]
- Pourghobadi, R.; Nematollahi, D.; Baezzat, M.R.; Alizadeh, S.; Goljani, H. Electropolymerization of catechol on wireless graphite electrode. Unusual cathodic polycatechol formation. J. Electroanal. Chem. 2020, 866, 114180. [Google Scholar] [CrossRef]
- Beiginejad, H.; Nematollahi, D.; Bayat, M.; Varmaghani, F.; Nazaripour, A. Experimental and Theoretical Analysis of the Electrochemical Oxidation of Catechol and Hydroquinone Derivatives in the Presence of Various Nucleophiles. J. Electrochem. Soc. 2013, 160, H693–H698. [Google Scholar] [CrossRef]
- Sadaba, N.; Salsamendi, M.; Casado, N.; Zuza, E.; Munoz, J.; Sarasua, J.R.; Mecerreyes, D.; Mantione, D.; Detrembleur, C.; Sardon, H. Catechol End-Functionalized Polylactide by Organocatalyzed Ring-Opening Polymerization. Polymers 2018, 10, 155. [Google Scholar] [CrossRef]
- Barham, A.S.; Kennedy, B.M.; Cunnane, V.J.; Daous, M.A. The Electrochemical polymerisation of 1,2 dihydroxybenzene and 2-hydroxybenzyl alcohol prepared in different solutions media. Electrochim. Acta 2014, 147, 19–24. [Google Scholar] [CrossRef]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef] [PubMed]
- Razaviamri, S.; Wang, K.; Liu, B.; Lee, B.P. Catechol-Based Antimicrobial Polymers. Molecules 2021, 26, 559. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Nawwar, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. Pseudocapacitive behavior of ferrimagnetic NiFe2O4-carbon nanotube electrodes prepared with a multifunctional dispersing agent. Open Ceram. 2021, 6, 100127. [Google Scholar] [CrossRef]
- Tallman, D.; Vang, C.; Wallace, G.; Bierwagen, G. Direct electrodeposition of polypyrrole on aluminum and aluminum alloy by electron transfer mediation. J. Electrochem. Soc. 2002, 149, C173. [Google Scholar] [CrossRef]
- Shi, C.; Zhitomirsky, I. Electrodeposition of composite polypyrrole–carbon nanotube films. Surf. Eng. 2011, 27, 655–661. [Google Scholar] [CrossRef]
- Shi, C.; Zhitomirsky, I. Electrodeposition and Capacitive Behavior of Films for Electrodes of Electrochemical Supercapacitors. Nanoscale Res. Lett. 2010, 5, 518. [Google Scholar] [CrossRef]
- Nagesha, D.K.; Plouffe, B.D.; Phan, M.; Lewis, L.H.; Sridhar, S.; Murthy, S.K. Functionalization-induced improvement in magnetic properties of Fe3O4 nanoparticles for biomedical applications. J. Appl. Phys. 2009, 105, 07B317. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, P.; Wei, C.; Zhuang, D.; Shi, J. Low-temperature one-step synthesis of covalently chelated ZnO/dopamine hybrid nanoparticles and their optical properties. J. Mater. Res. 2008, 23, 1946–1952. [Google Scholar] [CrossRef]
- Bloemen, M.; Debruyne, D.; Demeyer, P.-J.; Clays, K.; Gils, A.; Geukens, N.; Bartic, C.; Verbiest, T. Catechols as ligands for CdSe–ZnS quantum dots. R. Soc. Chem. Adv. 2014, 4, 10208–10211. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. Fe3O4 spinel-Mn3O4 spinel supercapacitor prepared using Celestine blue as a dispersant, capping agent and charge transfer mediator. Ceram. Int. 2020, 46, 18851–18858. [Google Scholar] [CrossRef]
- Togashi, T.; Takami, S.; Kawakami, K.; Yamamoto, H.; Naka, T.; Sato, K.; Abe, K.; Adschiri, T. Continuous hydrothermal synthesis of 3,4-dihydroxyhydrocinnamic acid-modified magnetite nanoparticles with stealth-functionality against immunological response. J. Mater. Chem. 2012, 22, 9041–9045. [Google Scholar] [CrossRef]
- Sugimoto, T.; Itoh, H.; Mochida, T. Shape control of monodisperse hematite particles by organic additives in the gel–sol system. J. Colloid Interface Sci. 1998, 205, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Pagnoux, C.; Baumard, J.-F.; Nogami, M. Preparation of gold nanoparticles (GNP) aqueous suspensions by a new method involving Tiron. J. Mater. Sci. 2007, 42, 80–86. [Google Scholar] [CrossRef]
- Huang, X.; Pang, Y.; Liu, Y.; Zhou, Y.; Wang, Z.; Hu, Q. Green synthesis of silver nanoparticles with high antimicrobial activity and low cytotoxicity using catechol-conjugated chitosan. R. Soc. Chem. Adv. 2016, 6, 64357–64363. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Yan, X. Applications of magnetic field for electrochemical energy storage. Appl. Phys. Rev. 2022, 9, 031307. [Google Scholar] [CrossRef]
- Venevtsev, Y.N.; Gagulin, V.; Zhitomirsky, I. Material science aspects of seignette-magnetism problem. Ferroelectrics 1987, 73, 221–248. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-layered double hydroxide and α-Fe2O3 nanorods on hollow porous carbon nanofibers: A free-standing electrode for solid-state symmetric supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Malaie, K.; Ganjali, M.R. Spinel nano-ferrites for aqueous supercapacitors; linking abundant resources and low-cost processes for sustainable energy storage. J. Energy Storage 2021, 33, 102097. [Google Scholar] [CrossRef]
- Ghasemi, S.; Ahmadi, F. Effect of surfactant on the electrochemical performance of graphene/iron oxide electrode for supercapacitor. J. Power Sources 2015, 289, 129–137. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; He, G. Fe3O4 doped double-shelled hollow carbon spheres with hierarchical pore network for durable high-performance supercapacitor. Carbon 2016, 99, 514–522. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D. A hybrid Fe3O4 MnO2 capacitor in mild aqueous electrolyte. Electrochem. Solid-State Lett. 2003, 6, A244. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Consequences of electrodeposition parameters on the microstructure and electrochemical behavior of electrolytic manganese dioxide (EMD) for supercapacitor. Ceram. Int. 2022, 48, 19913–19924. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Wang, L.; Ji, H.; Wang, S.; Kong, L.; Jiang, X.; Yang, G. Preparation of Fe3O4 with high specific surface area and improved capacitance as a supercapacitor. Nanoscale 2013, 5, 3793–3799. [Google Scholar] [CrossRef]
- Saikia, N.; Sarma, J.; Borah, J.M.; Mahiuddin, S. Adsorption of 3,4-dihydroxybenzoic acid onto hematite surface in aqueous medium: Importance of position of phenolic -OH groups and understanding of the same using catechol as an auxiliary model. J. Colloid Interface Sci. 2013, 398, 227–233. [Google Scholar] [CrossRef]
- Borah, J.M.; Sarma, J.; Mahiuddin, S. Adsorption comparison at the α-alumina/water interface: 3,4-Dihydroxybenzoic acid vs. catechol. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 50–56. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Kamakshi, T.; Sundari, G.S.; Erothu, H.; Singh, R.S. Effect of nickel dopant on structural morphological and optical characteristics of Fe3O4 nanoparticles. Rasayan J. Chem 2019, 12, 531–536. [Google Scholar] [CrossRef]
- Dobson, K.D.; McQuillan, A.J. In situ infrared spectroscopic analysis of the adsorption of aromatic carboxylic acids to TiO2, ZrO2, Al2O3, and Ta2O5 from aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Gulley-Stahl, H.; Hogan, P.A.; Schmidt, W.L.; Wall, S.J.; Buhrlage, A.; Bullen, H.A. Surface complexation of catechol to metal oxides: An ATR-FTIR, adsorption, and dissolution study. Environ. Sci. Technol. 2010, 44, 4116–4121. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yang, M.; Kim, S.-K. Pseudocapacitive organic catechol derivative-functionalized three-dimensional graphene aerogel hybrid electrodes for high-performance supercapacitors. Appl. Surf. Sci. 2017, 422, 316–320. [Google Scholar] [CrossRef]
- Golabi, S.; Nematollahi, D. Electrochemical study of 3,4-dihydroxybenzoic acid and 4-tert-butylcatechol in the presence of 4-hydroxycoumarin application to the electro-organic synthesis of coumestan derivatives. J. Electroanal. Chem. 1997, 430, 141–146. [Google Scholar] [CrossRef]
- Litos, C.; Aletras, V.; Hatzipanayioti, D.; Kamariotaki, M.; Lymberopoulou-Karaliota, A. CV and NMR study on the reaction of Mo (VI) with 3, 4-dihydroxybenzoic acid and ascorbic acid in aqueous solution. Inorg. Chim. Acta 2007, 360, 2321–2330. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.R.; Ramakrishan, S.; Logeshwaran, N.; Ramasamy, S.K.; Kim, H.J.; Yoo, D.J. Integrating the essence of metal organic framework-derived ZnCoTe–N–C/MoS2 cathode and ZnCo-NPS-N-CNT as anode for high-energy density hybrid supercapacitors. Compos. Part B Eng. 2022, 247, 110339. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface modification of MnO2 and carbon nanotubes using organic dyes for nanotechnology of electrochemical supercapacitors. J. Mater. Chem. A 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef]
- Noh, J.; Osman, O.I.; Aziz, S.G.; Winget, P.; Bredas, J.L. A density functional theory investigation of the electronic structure and spin moments of magnetite. Sci. Technol. Adv. Mater. 2014, 15, 044202. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.N.; Ustinov, A.B. Magnetic properties of Fe3O4 surface. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2010, 4, 395–400. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Xing, Y.; Song, S.; Yu, S.; Shi, W.; Guo, X.; Yang, J.; Lei, Y.; Cao, F. Morphology-Controlled Synthesis of Magnetites with Nanoporous Structures and Excellent Magnetic Properties. Chem. Mater. 2007, 20, 198–204. [Google Scholar] [CrossRef]
- Bliem, R.; McDermott, E.; Ferstl, P.; Setvin, M.; Gamba, O.; Pavelec, J.; Schneider, M.A.; Schmid, M.; Diebold, U.; Blaha, P.; et al. Subsurface cation vacancy stabilization of the magnetite (001) surface. Science 2014, 346, 1215–1218. [Google Scholar] [CrossRef]
- Gargallo-Caballero, R.; Martin-Garcia, L.; Quesada, A.; Granados-Miralles, C.; Foerster, M.; Aballe, L.; Bliem, R.; Parkinson, G.S.; Blaha, P.; Marco, J.F.; et al. Co on Fe3O4(001): Towards precise control of surface properties. J. Chem. Phys. 2016, 144, 094704. [Google Scholar] [CrossRef]
- Santos-Carballal, D.; Roldan, A.; Grau-Crespo, R.; de Leeuw, N.H. A DFT study of the structures, stabilities and redox behaviour of the major surfaces of magnetite Fe3O4. R. Soc. Chem. Phys. Chem. Chem. Phys. 2014, 16, 21082–21097. [Google Scholar] [CrossRef]
- Yang, T.; Wen, X.-d.; Ren, J.; Li, Y.-w.; Wang, J.-g.; Huo, C.-f. Surface structures of Fe3O4 (111), (110), and (001): A density functional theory study. J. Fuel Chem. Technol. 2010, 38, 121–128. [Google Scholar] [CrossRef]
- Bliem, R.; Pavelec, J.; Gamba, O.; McDermott, E.; Wang, Z.; Gerhold, S.; Wagner, M.; Osiecki, J.; Schulte, K.; Schmid, M.; et al. Adsorption and incorporation of transition metals at the magnetite Fe3O4(001) surface. Phys. Rev. B 2015, 92, 075440. [Google Scholar] [CrossRef]
- Mulakaluri, N. Coverage-Dependent Adsorption Mode of Water on Fe3O4(001): Insights from First Principles Calculations. J. Phys. Chem. C 2010, 114, 11148–11156. [Google Scholar] [CrossRef]
- Mulakaluri, N.; Pentcheva, R. Hydrogen Adsorption and Site-Selective Reduction of the Fe3O4(001) Surface: Insights from First Principles. J. Phys. Chem. C 2012, 116, 16447–16453. [Google Scholar] [CrossRef]
- Albert, M.; Clifford, A.; Zhitomirsky, I.; Rubel, O. Adsorption of Maleic Acid Monomer on the Surface of Hydroxyapatite and TiO2: A Pathway toward Biomaterial Composites. J. Am. Chem. Soc. Appl. Mater. Interfaces 2018, 10, 24382–24391. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhitomirsky, I. Electrophoretic nanotechnology of graphene-carbon nanotube and graphene-polypyrrole nanofiber composites for electrochemical supercapacitors. J. Colloid Interface Sci. 2013, 407, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Zhuo, R.; Wu, Z.; Geng, B.; Wang, J.; Ren, P.; Yan, P.; Geng, Z. Hydrothermal synthesis and electrochemical properties of hexagonal hydrohausmannite plates as supercapacitor electrode material. Mater. Lett. 2014, 117, 62–65. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Dudarev, S.L. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Chiter, F.; Nguyen, V.B.; Tarrat, N.; Benoit, M.; Tang, H.; Lacaze-Dufaure, C. Effect of van der Waals corrections on DFT-computed metallic surface properties. Mater. Res. Express 2016, 3, 046501. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
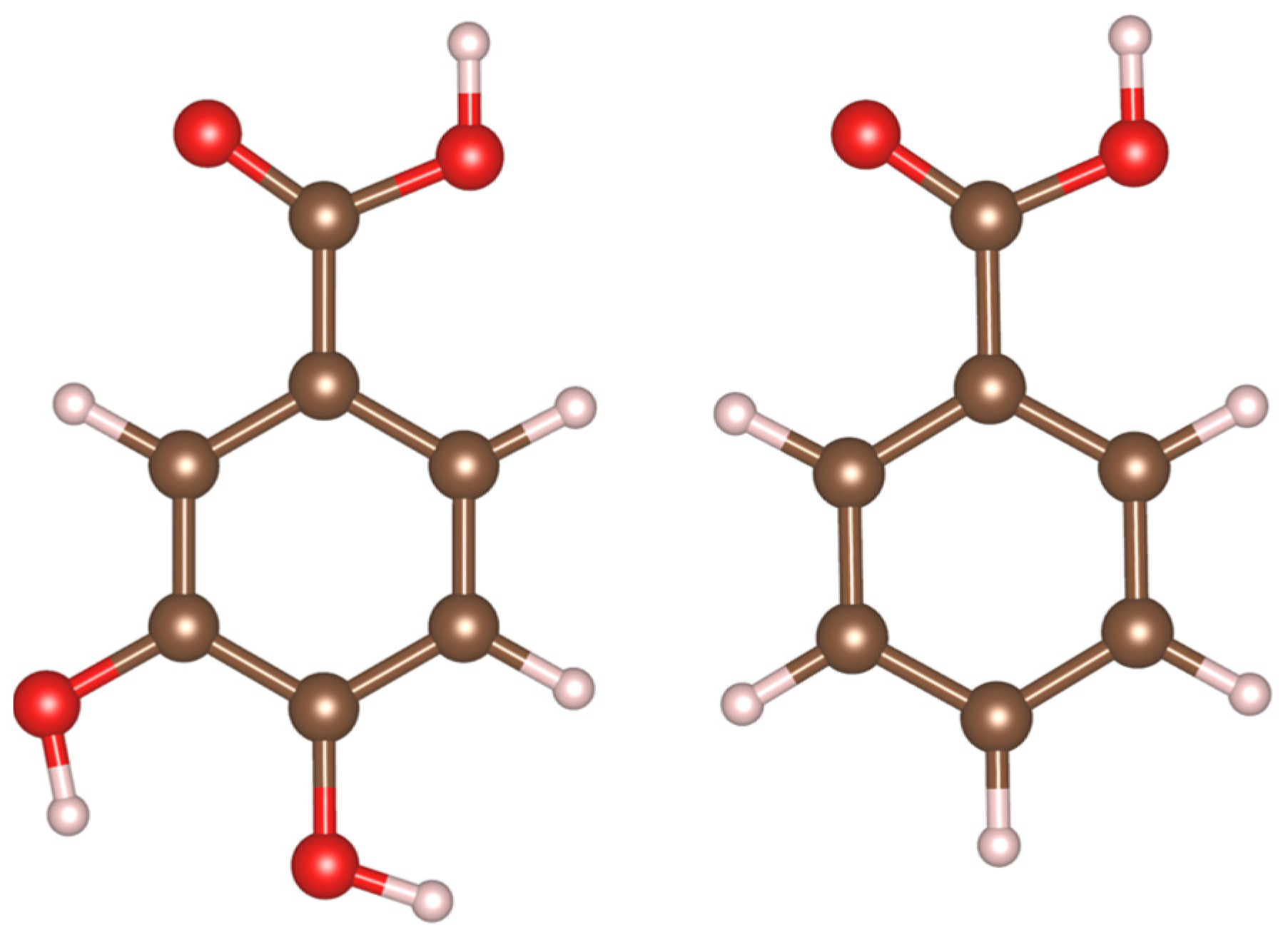
| Parameter | Literature PBE+U [71] | This Work PBE+U+D3 | Experimental |
|---|---|---|---|
| Lattice parameter (Å) | 8.488 | 8.453 | 8.396 [72] |
| Bond length (oct) (Å) | 2.09 | 2.07 | 2.07 [73] |
| Bond length (tet) (Å) | 1.90 | 1.91 | 1.88 [73] |
| Magnetic moment (Feoct) (μB) | 3.96 | 3.92 | - |
| Magnetic moment (Fetet) (μB) | 4.09 | 4.02 | - |
| Magnetic moment (O) (μB) | 0.030 | 0.045 | - |
| Total magnetic moment (μB /f.u.) | 4.0 | 4.0 | 4.1 [72] |
| Surfaces (PBE+U+D3) | Surface Energy (J m−2) | Surface Fe-O Bond Length (Å) |
|---|---|---|
| 23 layers | 0.48 | 1.92 |
| 15 layers | 0.73 | 1.81 |
| 9 layers | 0.90 | 1.90 |
| Literature, 9 layers (PBE+U) | 0.96 [77] | 1.89 [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boucher, C.; Rubel, O.; Zhitomirsky, I. Supercapacitor Performance of Magnetite Nanoparticles Enhanced by a Catecholate Dispersant: Experiment and Theory. Molecules 2023, 28, 1562. https://doi.org/10.3390/molecules28041562
Boucher C, Rubel O, Zhitomirsky I. Supercapacitor Performance of Magnetite Nanoparticles Enhanced by a Catecholate Dispersant: Experiment and Theory. Molecules. 2023; 28(4):1562. https://doi.org/10.3390/molecules28041562
Chicago/Turabian StyleBoucher, Coulton, Oleg Rubel, and Igor Zhitomirsky. 2023. "Supercapacitor Performance of Magnetite Nanoparticles Enhanced by a Catecholate Dispersant: Experiment and Theory" Molecules 28, no. 4: 1562. https://doi.org/10.3390/molecules28041562
APA StyleBoucher, C., Rubel, O., & Zhitomirsky, I. (2023). Supercapacitor Performance of Magnetite Nanoparticles Enhanced by a Catecholate Dispersant: Experiment and Theory. Molecules, 28(4), 1562. https://doi.org/10.3390/molecules28041562






