Unprecedented Elimination Reactions of Cyclic Aldols: A New Biosynthetic Pathway toward the Taiwaniaquinoid Skeleton
Abstract
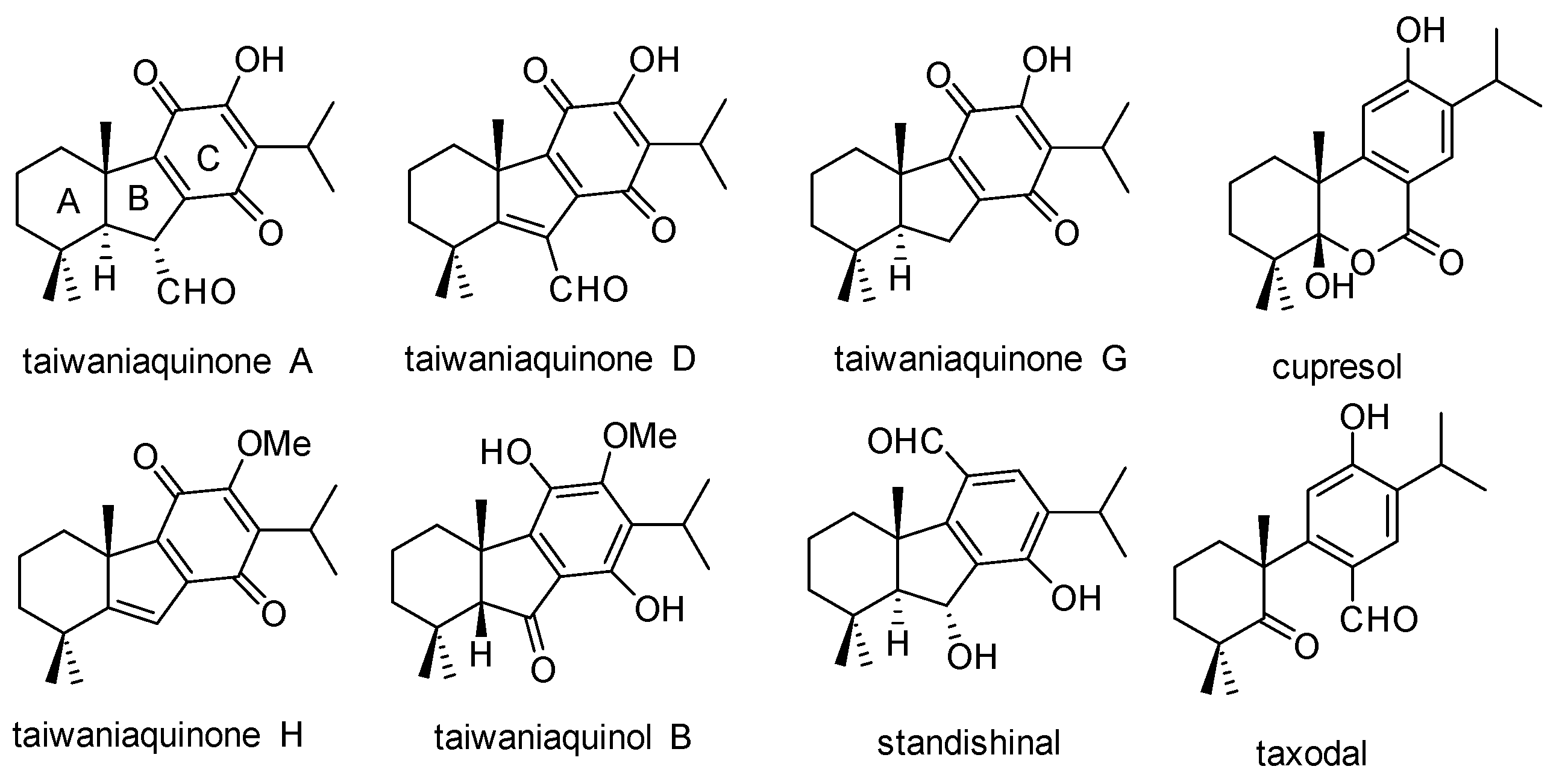
1. Introduction
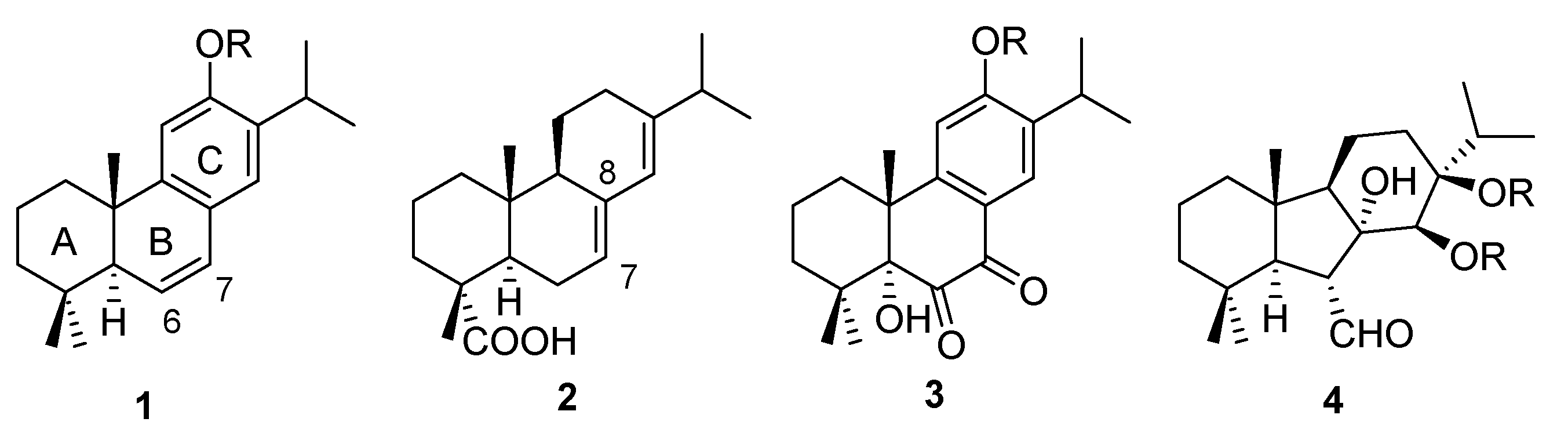
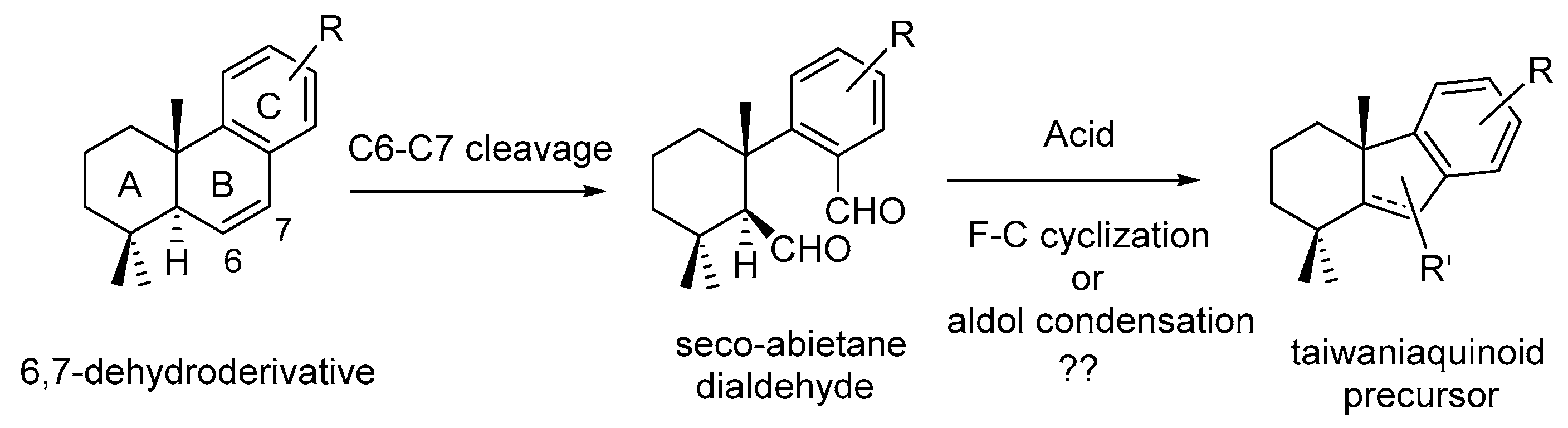
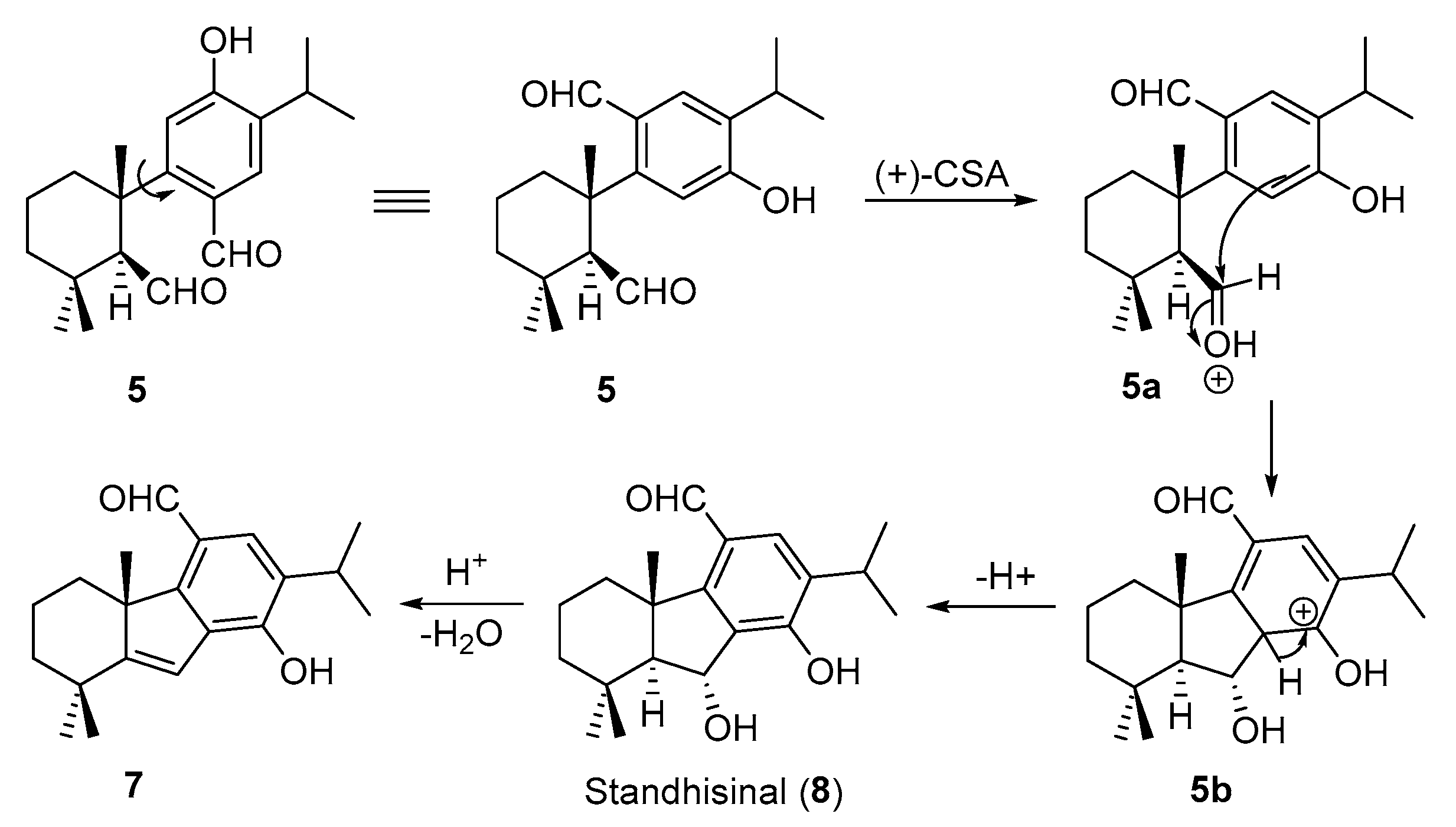
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. General Procedure for the Obtention of Seco-Abietane Dialdehydes
3.2.2. General Procedure for the Reaction of Seco-Abietane Dialdehydes with (+)-CSA, Amberlyst A-15, Bi(OTf)3, Sc(OTf)3, or Gd(OTf)3
3.2.3. General Procedure for the Reaction of Seco-Abietane Dialdehydes with H2SO4, CF3CO2H, HCO2H, or BF3·Et2O
3.2.4. Experimental Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Majetich, G.; Shimkus, J. The Taiwaniaquinoids: A Review. J. Nat. Prod. 2010, 73, 284–298. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ohtsu, H.; Tokuda, H.; Nishino, H.; Matsunaga, S.; Tanaka, R. Anti-tumor Promoting Diterpenes from the Stem Bark of Thuja standishii (Cupressaceae). Bioorg. Med. Chem. 2001, 9, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Iwamoto, M.; Ohtsu, H.; Ohishi, H.; Tanaka, R.; Yoshitake, A. Aromatase Inhibitory Activities of Standishinal and the Diterpenoids from the Bark of Thuja standishii. Planta Med. 2002, 68, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J. Diterpenoids. Nat. Prod. Rep. 2004, 21, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-I.; Chang, J.-Y.; Kuo, C.-C.; Pan, W.-Y.; Kuo, Y.-H. Four New 6-Nor-5(6→7)abeo-abietane Type Diterpenes and Antitumoral Cytotoxic Diterpene Constituents from the Bark of Taiwania cryptomerioides. Planta Med. 2005, 71, 72–76. [Google Scholar] [CrossRef]
- Ramírez-Macías, I.; Marín, C.; Es-Samti, H.; Fernández, A.; Guardia, J.J.; Zentar, H.; Agil, A.; Chahboun, R.; Alvarez-Manzaneda, E.; Sánchez-Moreno, M. Taiwaniaquinoid and Abietane Quinone Derivatives with Trypanocidal Activity against T. cruzi and Leishmania spp. Parasitol. Int. 2012, 61, 405–413. [Google Scholar] [CrossRef]
- Aránega Jiménez, A.; Alvarez-Manzaneda, E.; Chaboun, R.; Rodríguez Serrano, F.; Prados Salazar, J.; Melguizo Alonso, C.; Tapia Martín, R.; Es-Samti, H.; Guardia Monteagudo, J.J.; Vázquez Vázquez, M.I.; et al. Antitumour Activity of Taiwaniaquinoids and Related Compounds. WO2013156659A1, 24 October 2013. [Google Scholar]
- Joland, S.D.; Hoffmann, J.J.; Schram, K.H.; Cole, J.R. A New Diterpene from Cupressus goveniana var. Abramasiana: 5 3-Hydroxy-6-Oxasugiol (Cupresol). J. Nat. Prod. 1984, 47, 983–987. [Google Scholar]
- Kusumoto, N.; Murayama, T.; Kawai, Y.; Ashitani, T.; Ogiyama, K.; Takahashi, K. Taxodal, A Novel Irregular Abietane-Type Diterpene from the Cones of Taxodium distichum. Tetrahedron Lett. 2008, 49, 4845–4847. [Google Scholar] [CrossRef]
- Banerjee, M.; Mukhopadhyay, R.; Achari, B.; Banerjee, A.K. First Total Synthesis of the 4a-Methyltetrahydrofluorene Diterpenoids (±)-Dichroanal B and (±)-Dichroanone. Org. Lett. 2003, 5, 3931–3933. [Google Scholar] [CrossRef]
- Banerjee, M.; Mukhopadhyay, R.; Achari, B.; Banerjee, A.K. General Route to 4a-Methylhydrofluorene Diterpenoids: Total Syntheses of (±)-Taiwaniaquinones D and H, (±)-Taiwaniaquinol B, (±)-Dichroanal B, and (±)-Dichroanone. J. Org. Chem. 2006, 71, 2787–2796. [Google Scholar] [CrossRef]
- Fillion, E.; Fishlock, D. Total Synthesis of (±)-Taiwaniaquinol B via a Domino Intramolecular Friedel-Crafts Acylation/Carbonyl α-tert-Alkylation Reaction. J. Am. Chem. Soc. 2005, 127, 13144–13145. [Google Scholar] [CrossRef] [PubMed]
- Planas, L.; Mogi, M.; Takita, H.; Ajimoto, T.; Node, M. Efficient Route to 4a-Methyltetrahydrofluorenes: A Total Synthesis of (±)-Dichroanal B via Intramolecular Heck Reaction. J. Org. Chem. 2006, 71, 2896–2898. [Google Scholar] [CrossRef]
- Liang, G.; Xu, Y.; Sciple, I.B.; Trauner, D. Synthesis of Taiwaniaquinoids via Nazarov Triflation. J. Am. Chem. Soc. 2006, 126, 11022–11023. [Google Scholar] [CrossRef]
- Li, S.; Chiu, P. Acid-Promoted Sequential Cationic Cyclizations for the Synthesis of (±)-Taiwaniaquinol, B. Tetrahedron Lett. 2008, 49, 1741–1744. [Google Scholar] [CrossRef]
- Tang, S.; Xu, Y.; He, J.; He, Y.; Zheng, J.; Pan, X.; She, X. Application of a Domino Friedel-Crafts Acylation/Alkylation Reaction to the Formal Syntheses of (±)-Taiwaniaquinol B and (±)-Dichroanone. Org. Lett. 2008, 10, 1855–1858. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, C.; Meng, Y.; Wu, J.; Song, C.; Chang, J. Synthesis of 5-epi-Taiwaniaquinone, G. J. Org. Chem. 2014, 79, 6354–6359. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Manzaneda, E.; Chahboun, R.; Cabrera, E.; Alvarez, E.; Alvarez-Manzaneda, R.; Meneses, R.; Es-Samti, H.; Fernández, A. A Very Efficient Route toward the 4a-Methyltetrahydrofluorene Skeleton: Short Synthesis of (±)-Dichroanone and (±)-Taiwaniaquinone, H. J. Org. Chem. 2009, 74, 3384–3388. [Google Scholar] [CrossRef]
- Majetich, G.; Shimkus, J.H. Concise Syntheses of (±)-Dichroanone, (±)-Dichroanal B, (±)-Taiwaniaquinol B, and (±)-Taiwaniaquinone, D. Tetrahedron Lett. 2009, 50, 3311–3313. [Google Scholar] [CrossRef]
- Kakde, B.N.; Kumari, P.; Bisai, A. Total Synthesis of (±)-Taiwaniaquinol F and Related Taiwaniaquinoids. J. Org. Chem. 2015, 80, 9889–9899. [Google Scholar] [CrossRef]
- Yan, X.; Hu, X. Protecting-Group-Free Synthesis of Taiwaniaquinone H Using a One-Pot Thermal Ring Expansion/4π-Electrocyclization Strategy. J. Org. Chem. 2014, 79, 5282–5286. [Google Scholar] [CrossRef]
- McFadden, R.M.; Stoltz, B.M. The Catalytic Enantioselective, Protecting Group-Free Total Synthesis of (+)-Dichroanone. J. Am. Chem. Soc. 2006, 128, 7738–7739. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Manzaneda, E.; Chahboun, R.; Cabrera, E.; Alvarez, E.; Haidour, A.; Ramos, J.M.; Alvarez-Manzaneda, R.; Hmammouchi, M.; Es-Samti, H. A Thermal 6π Electrocyclization Strategy towards Taiwaniaquinoids. First Enantiospecific Synthesis of (−)-Taiwaniaquinone, G. Chem. Commun. 2009, 5, 592–594. [Google Scholar] [CrossRef]
- Alvarez-Manzaneda, E.; Chahboun, R.; Cabrera, E.; Alvarez, E.; Haidour, A.; Ramos, J.M.; Alvarez-Manzaneda, R.; Charrah, Y.; Es-Samti, H. An Enantiospecific Route towards Taiwaniaquinoids. First Synthesis of (−)-Taiwaniaquinone H and (−)-Dichroanone. Org. Biomol. Chem. 2009, 7, 5146–5155. [Google Scholar] [CrossRef] [PubMed]
- Node, M.; Ozeki, M.; Planas, L.; Nakano, M.; Takita, H.; Mori, D.; Tamatani, S.; Kajimoto, T. Efficient Asymmetric Synthesis of abeo-Abietane-Type Diterpenoids by Using the Intramolecular Heck Reaction. J. Org. Chem. 2010, 75, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Satake, M.; Toizume, T.; Fukutome, S.; Arimitsu, K.; Hosoi, S.; Kajimoto, T.; Iwasaki, H.; Kojima, N.; Node, M.; et al. First Asymmetric Total Synthesis of (+)-Taiwaniaquinol D and (−)-Taiwaniaquinone D by Using Intramolecular Heck Reaction. Tetrahedron 2013, 69, 3841–3846. [Google Scholar] [CrossRef]
- Liao, X.; Stanley, L.M.; Hartwig, J.F. Enantioselective Total Syntheses of (−)-Taiwaniaquinone H and (−)-Taiwaniaquinol B by Iridium-Catalyzed Borylation and Palladium-Catalyzed Asymmetric α-Arylation. J. Am. Chem. Soc. 2011, 133, 2088–2091. [Google Scholar] [CrossRef]
- Shockley, S.E.; Holder, J.C.; Stoltz, B.M. A Catalytic, Enantioselective Formal Synthesis of (+)-Dichroanone and (+)-Taiwaniaquinone, H. Org. Lett. 2014, 16, 6362–6365. [Google Scholar] [CrossRef]
- Lin, W.-H.; Fang, J.-M.; Cheng, Y.-S. Uncommon Diterpenes with the Skeleton of Six-Five-Six Fused-Rings from Taiwania cryptomerioides. Phytochemistry 1995, 40, 871–873. [Google Scholar] [CrossRef]
- Tapia, R.; Guardia, J.J.; Alvarez, E.; Haidöur, A.; Ramos, J.M.; Alvarez-Manzaneda, R.; Chahboun, R.; Alvarez-Manzaneda, E. General Access to Taiwaniaquinoids Based on a Hypothetical Abietane C7–C8 Cleavage Biogenetic Pathway. J. Org. Chem. 2012, 77, 573–584. [Google Scholar] [CrossRef]
- Alvarez-Manzaneda, E.; Chahboun, R.; Alvarez, E.; Tapia, R.; Alvarez-Manzaneda, R. Enantioselective Total Synthesis of Cytotoxic Taiwaniaquinones A and F. Chem. Commun. 2010, 46, 9244–9246. [Google Scholar] [CrossRef]
- Katoh, T.; Akagi, T.; Noguchi, C.; Kajimoto, T.; Node, M.; Tanaka, R.; Nishizawa, M.; Ohtsu, H.; Suzuki, N.; Saito, K. Synthesis of dl-Standishinal and Its Related Compounds for the Studies on Structure-Activity Relationship of Inhibitory Activity against Aromatase. Bioorg. Med. Chem. 2007, 15, 2736–2748. [Google Scholar] [CrossRef] [PubMed]
- Jana, C.K.; Scopelliti, R.; Gademann, K. A Synthetic Entry into the Taiwaniaquinoids Based on a Biogenetic Hypothesis: Total Synthesis of (−)-Taiwaniaquinone, H. Chem. Eur. J. 2010, 16, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Thommen, C.; Jana, C.K.; Neuburger, M.; Gademann, K. Syntheses of Taiwaniaquinone F and Taiwaniaquinol A via an Unusual Remote C-H Functionalization. Org. Lett. 2013, 15, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, R.; Luo, Y.; Li, J.; Zhou, S.; Li, Y.; Hu, J.; Li, A. Divergent Total Synthesis of Taiwaniaquinones A and F and Taiwaniaquinols B and D. Org. Lett. 2013, 15, 2022–2025. [Google Scholar] [CrossRef]
- Guardia, J.J. Seco-Abietane Dialdehydes were Obtained by Reductive Ozonolysis of the Corresponding 6,7-Dehydro-8,11,13-Abietatriene Derivatives. Ph.D. Thesis, University of Granada, Granada, Spain, 2016. [Google Scholar]
- Martin-Escolano, R.; Guardia, J.J.; Martin-Escolano, J.; Cirauqui, N.; Fernández, A.; Rosales, M.J.; Chahboun, R.; Sanchez-Moreno, M.; Alvarez-Manzaneda, E.; Marín, C. In Vivo Biological Evaluation of a Synthetic Royleanone Derivative as a Promising Fast-Acting Trypanocidal Agent by Inducing Mitochondrial-Dependent Necrosis. J. Nat. Prod. 2020, 83, 3571–3583. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Yao, S.; Zhao, J.; Tang, C.; Xu, W.; Ke, C.; Xi, C. Preparation of Abietane-Type Diterpene Compounds with Lipid-Lowering Activity. WO2016192631A1, 8 December 2016. [Google Scholar]
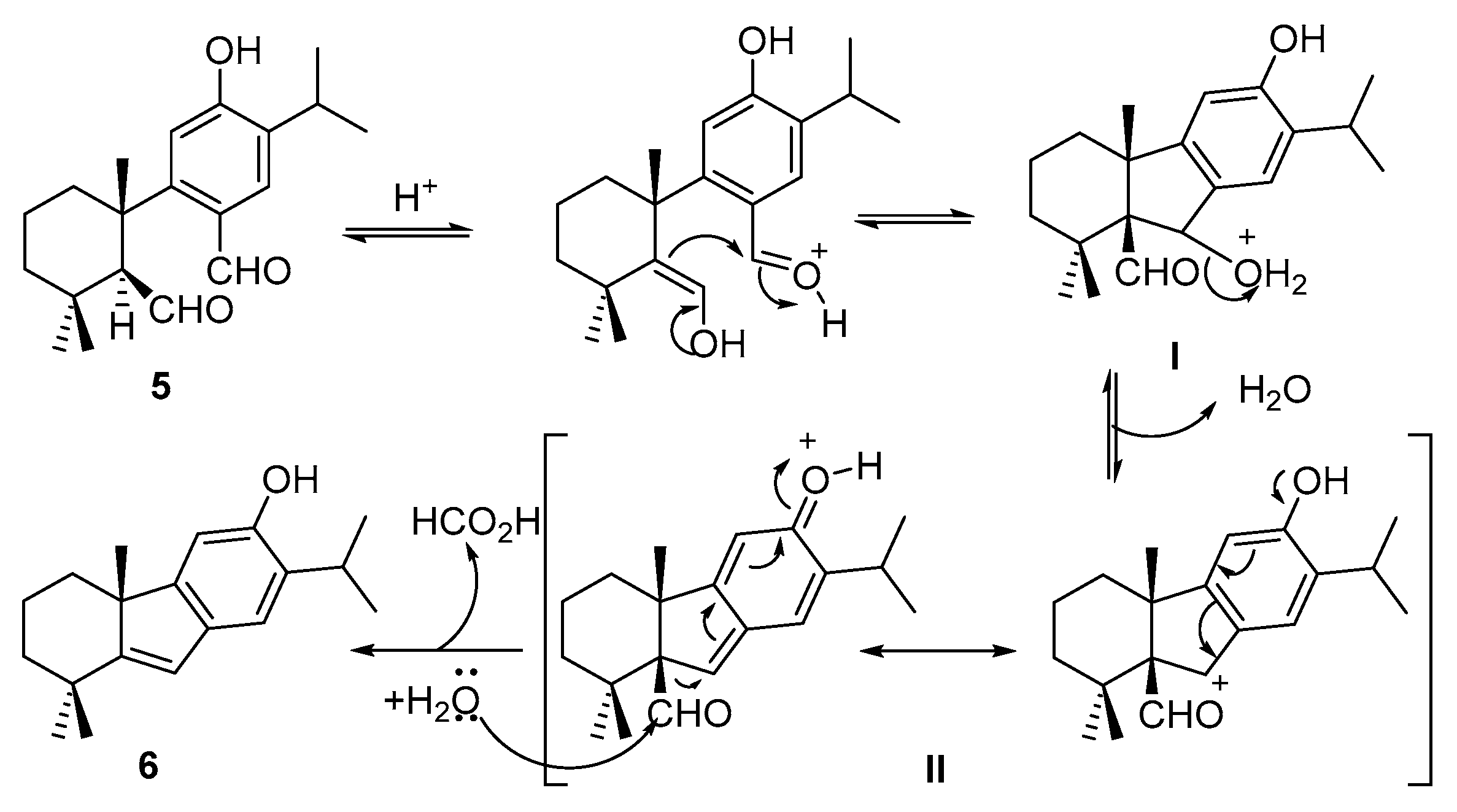

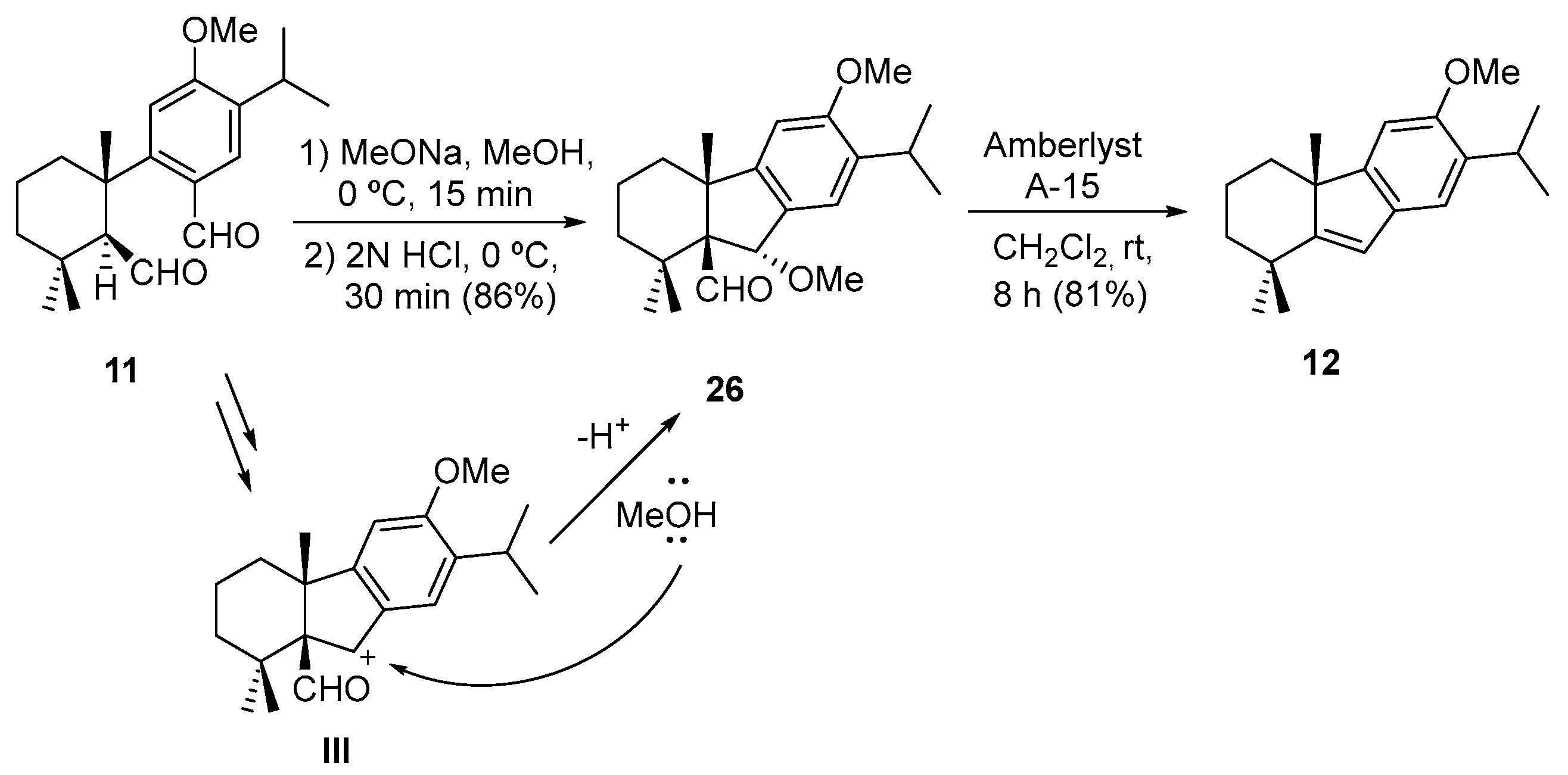
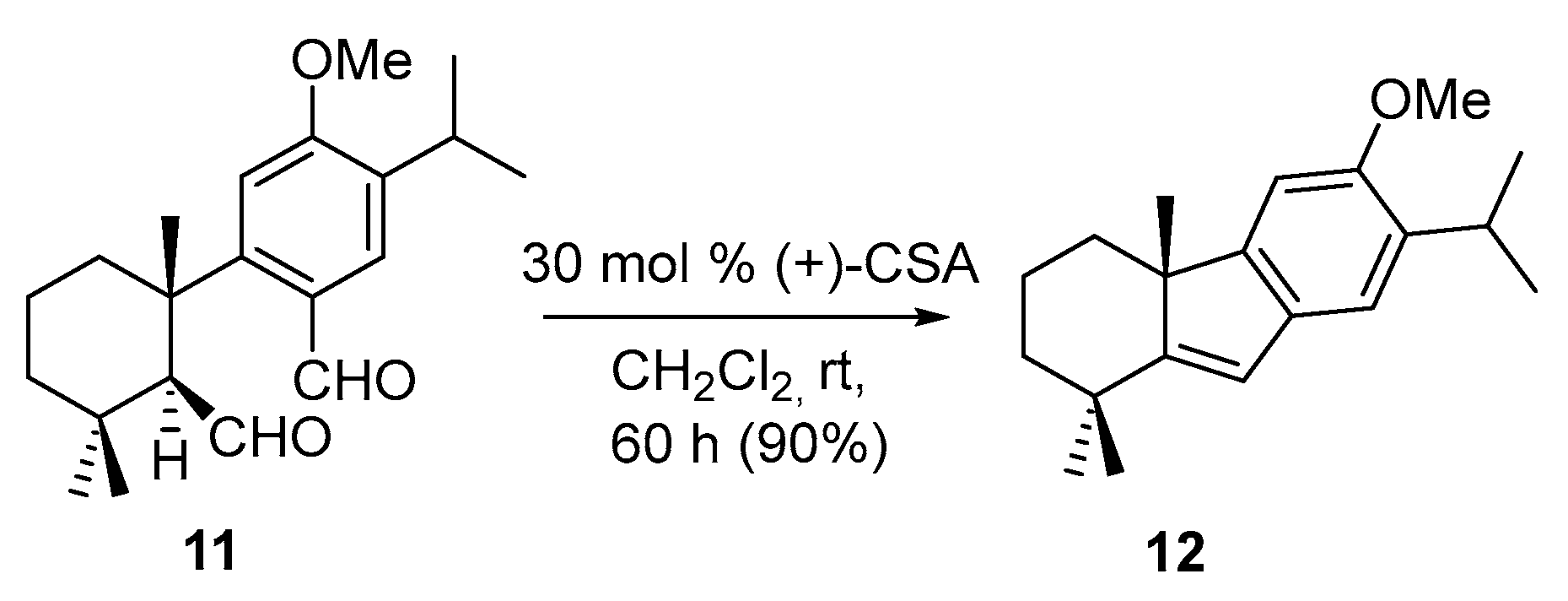

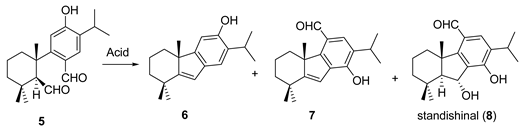
| Entry | Conditions | Products (%) |
|---|---|---|
| 1 | 1.1 eq. (+)-CSA, CH2Cl2, rt., 14 h | 6 (80), 7 (7), 8 (5) |
| 2 | 1.1 eq. CF3CO2H, 0 °C, 6 h | 6 (22), 7 (71) |
| 3 | Amberlyst A-15, CH2Cl2, rt, 16 h | 6 (88) |
| 4 | 1.1 eq. HCO2H, CH2Cl2, rt, 20 h | 6 (84) |
| 5 | 1.1 eq. BF3·Et2O, CH2Cl2, 0 °C, 4 h | 6 (39), 7 (40) |
| 6 | 1.1 eq. Bi(OTf)3, CH2Cl2, rt, 14 h | 6 (38), 7 (46) |
| 7 | 1.1 eq. Sc(OTf)3, CH2Cl2, reflux, 24 h | 6 (36), 7 (48) |
| 8 | 1.1 eq. Gd(OTf)3, CH2Cl2, reflux, 40 h | 6 (30), 7 (58) |
| Entry | Dialdehyde | Conditions | Products (%) |
|---|---|---|---|
| 1 | 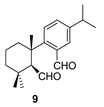 | (+)-CSA 1 CH2Cl2, reflux, 6 d | 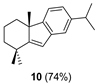 |
| 2 | 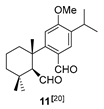 | (+)-CSA 1 CH2Cl2, rt, 36 h | 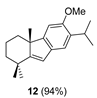 |
| 3 | 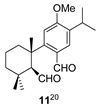 | H2SO4 2 CH2Cl2, 0 °C, 30 m | 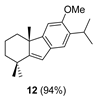 |
| 4 | 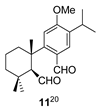 | Resin 3 CH2Cl2, rt, 20 h |  |
| 5 |  | (+)-CSA 1 CH2Cl2, rt, 75 h | 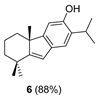 |
| 6 |  | (+)-CSA 1 CH2Cl2, rt, 60 h | 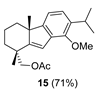 |
| 7 | 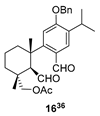 | Resin 3 CH2Cl2, rt, 18 h | 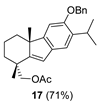 |
| 8 |  | (+)-CSA 1 CH2Cl2, rt, 14 h |  |
| 9 | 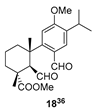 | Resin 3 CH2Cl2, rt, 19 h | 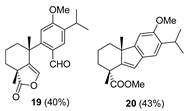 |
| 10 | 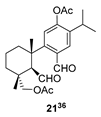 | (+)-CSA 1 CH2Cl2, reflux, 8 d | No reaction |
| 11 | 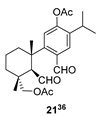 | H2SO4 2 CH2Cl2, 0 °C, 40 m | 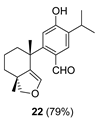 |
| 12 | 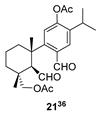 | Resin 3 CH2Cl2, rt, 19 h | 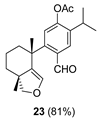 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardia, J.J.; Fernández, A.; Justicia, J.; Zentar, H.; Alvarez-Manzaneda, R.; Alvarez-Manzaneda, E.; Chahboun, R. Unprecedented Elimination Reactions of Cyclic Aldols: A New Biosynthetic Pathway toward the Taiwaniaquinoid Skeleton. Molecules 2023, 28, 1524. https://doi.org/10.3390/molecules28041524
Guardia JJ, Fernández A, Justicia J, Zentar H, Alvarez-Manzaneda R, Alvarez-Manzaneda E, Chahboun R. Unprecedented Elimination Reactions of Cyclic Aldols: A New Biosynthetic Pathway toward the Taiwaniaquinoid Skeleton. Molecules. 2023; 28(4):1524. https://doi.org/10.3390/molecules28041524
Chicago/Turabian StyleGuardia, Juan J., Antonio Fernández, José Justicia, Houda Zentar, Ramón Alvarez-Manzaneda, Enrique Alvarez-Manzaneda, and Rachid Chahboun. 2023. "Unprecedented Elimination Reactions of Cyclic Aldols: A New Biosynthetic Pathway toward the Taiwaniaquinoid Skeleton" Molecules 28, no. 4: 1524. https://doi.org/10.3390/molecules28041524
APA StyleGuardia, J. J., Fernández, A., Justicia, J., Zentar, H., Alvarez-Manzaneda, R., Alvarez-Manzaneda, E., & Chahboun, R. (2023). Unprecedented Elimination Reactions of Cyclic Aldols: A New Biosynthetic Pathway toward the Taiwaniaquinoid Skeleton. Molecules, 28(4), 1524. https://doi.org/10.3390/molecules28041524








