A Zn(II)–Metal–Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline
Abstract
1. Introduction
2. Results and Discussion
2.1. The Crystal Structure of [Zn3(BMP)2L2(H2O)4]·2H2O
2.2. Morphological Properties and Thermal Stability of the Zn-MOF
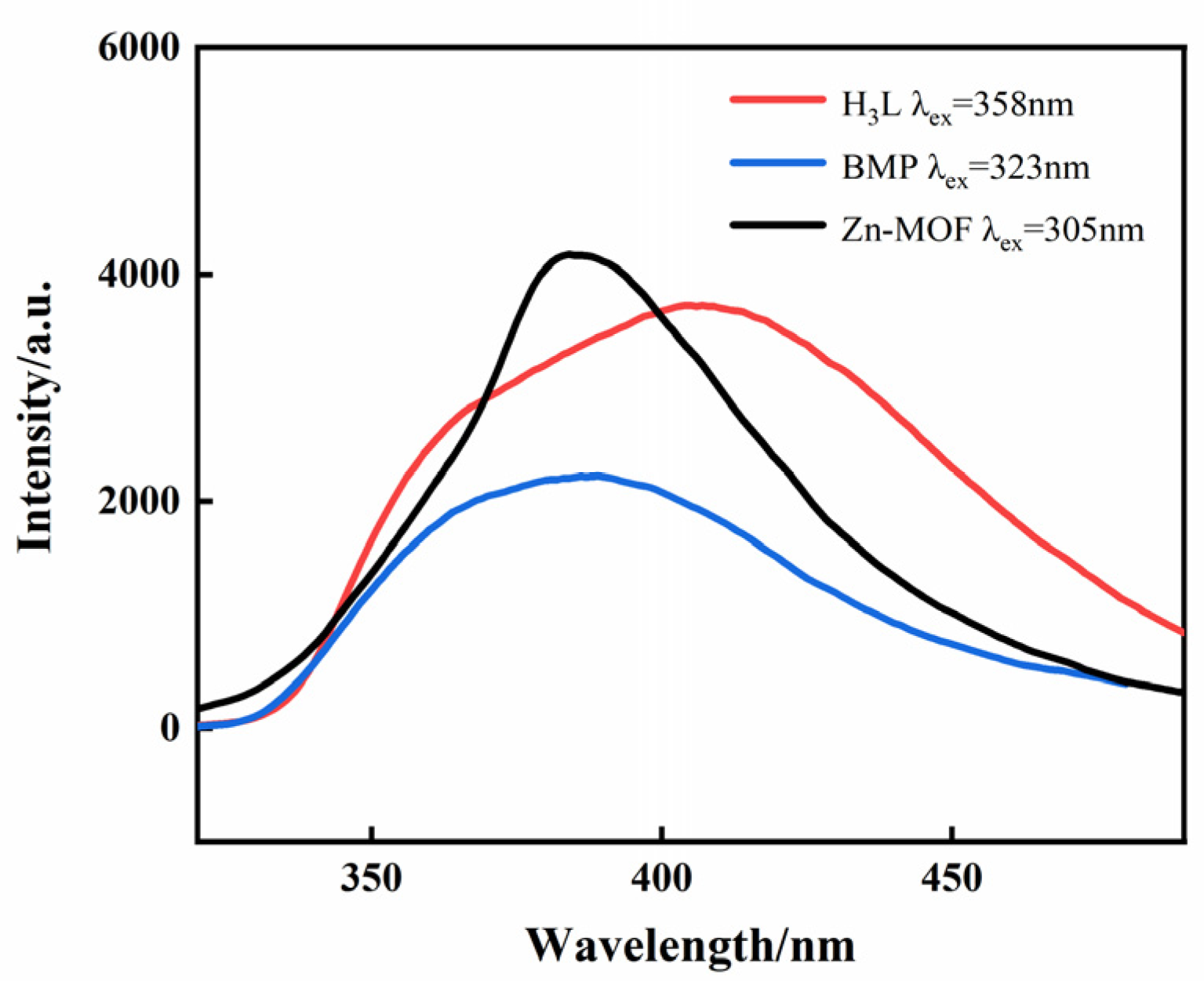
2.3. The Solid-State Photoluminescence of the Zn-MOF
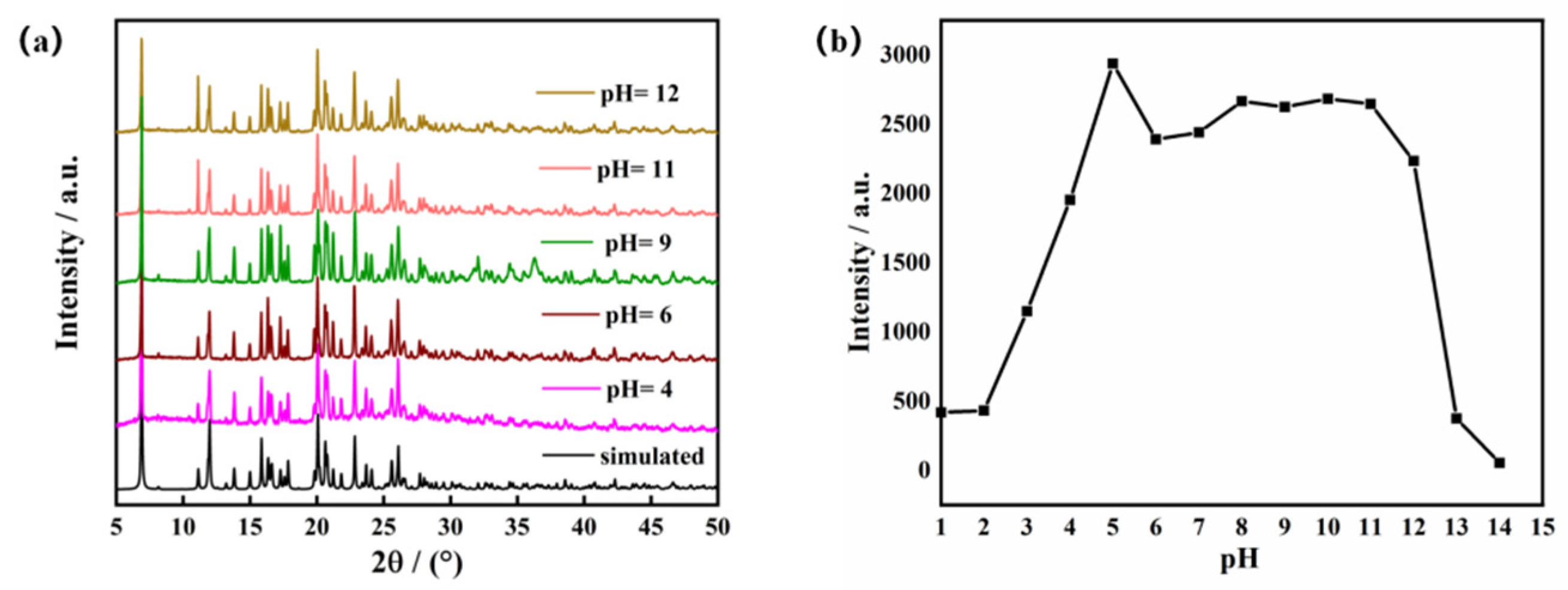
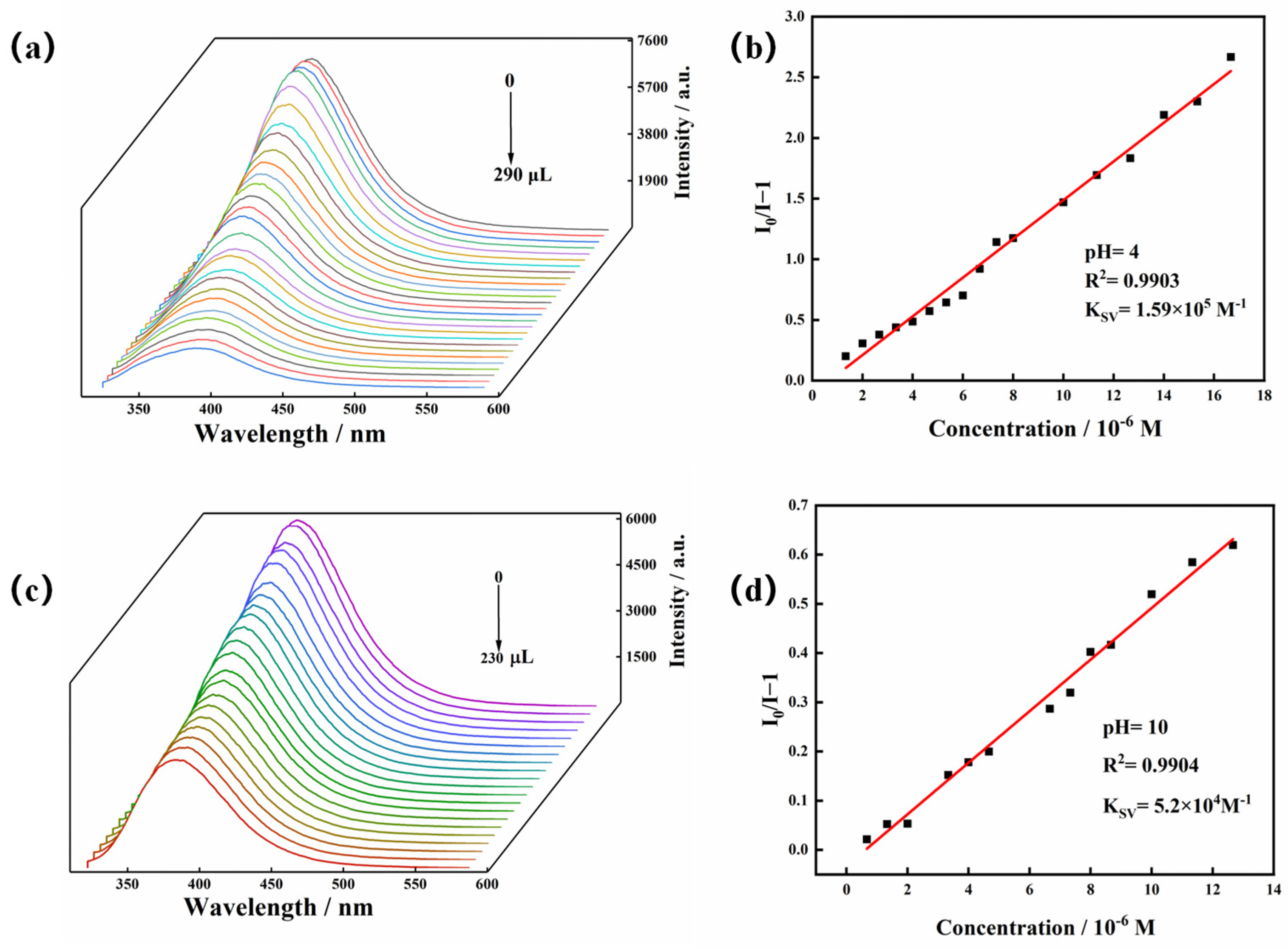
2.4. Luminescence of the Zn-MOF in Water with Different pH
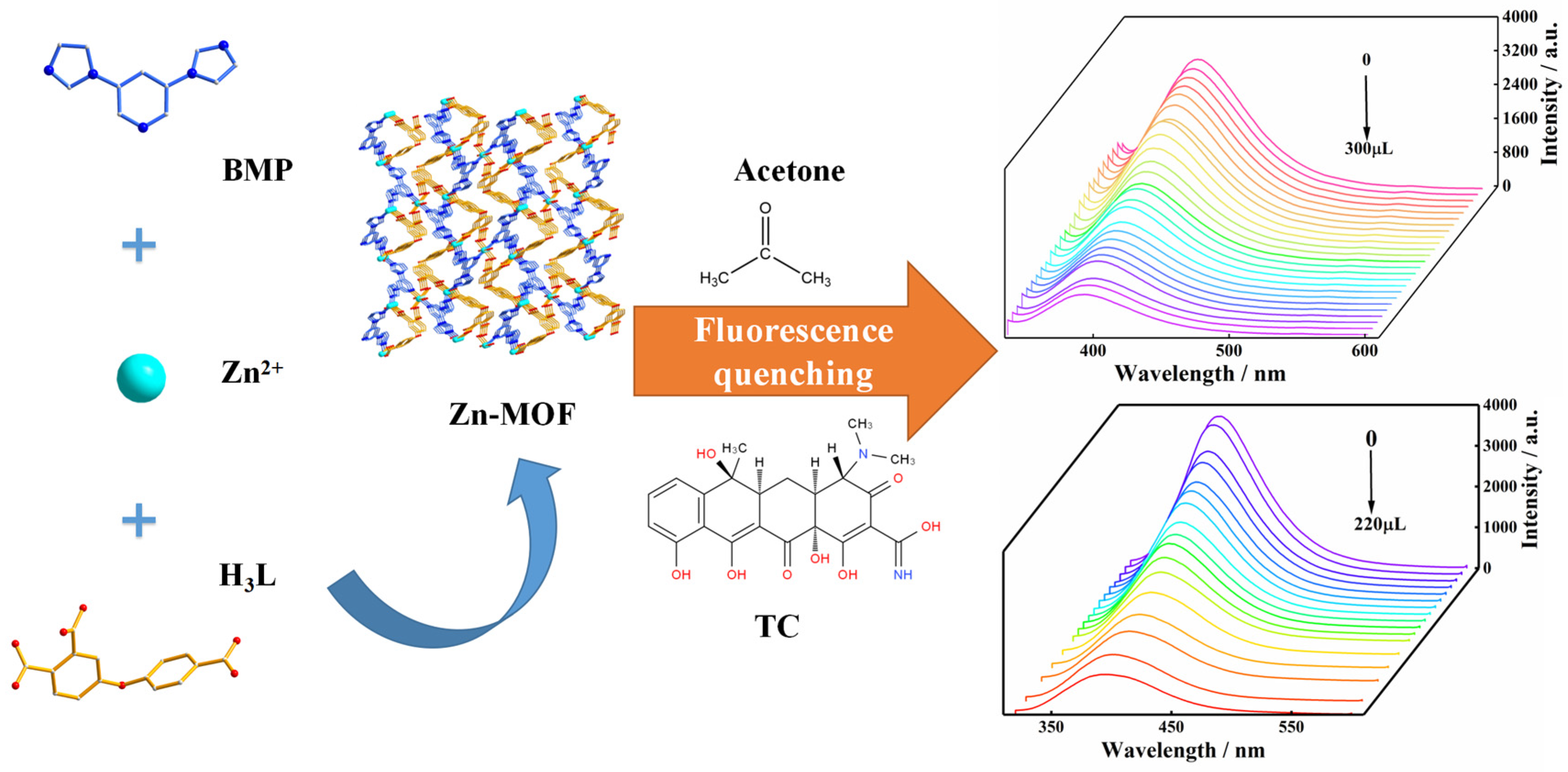
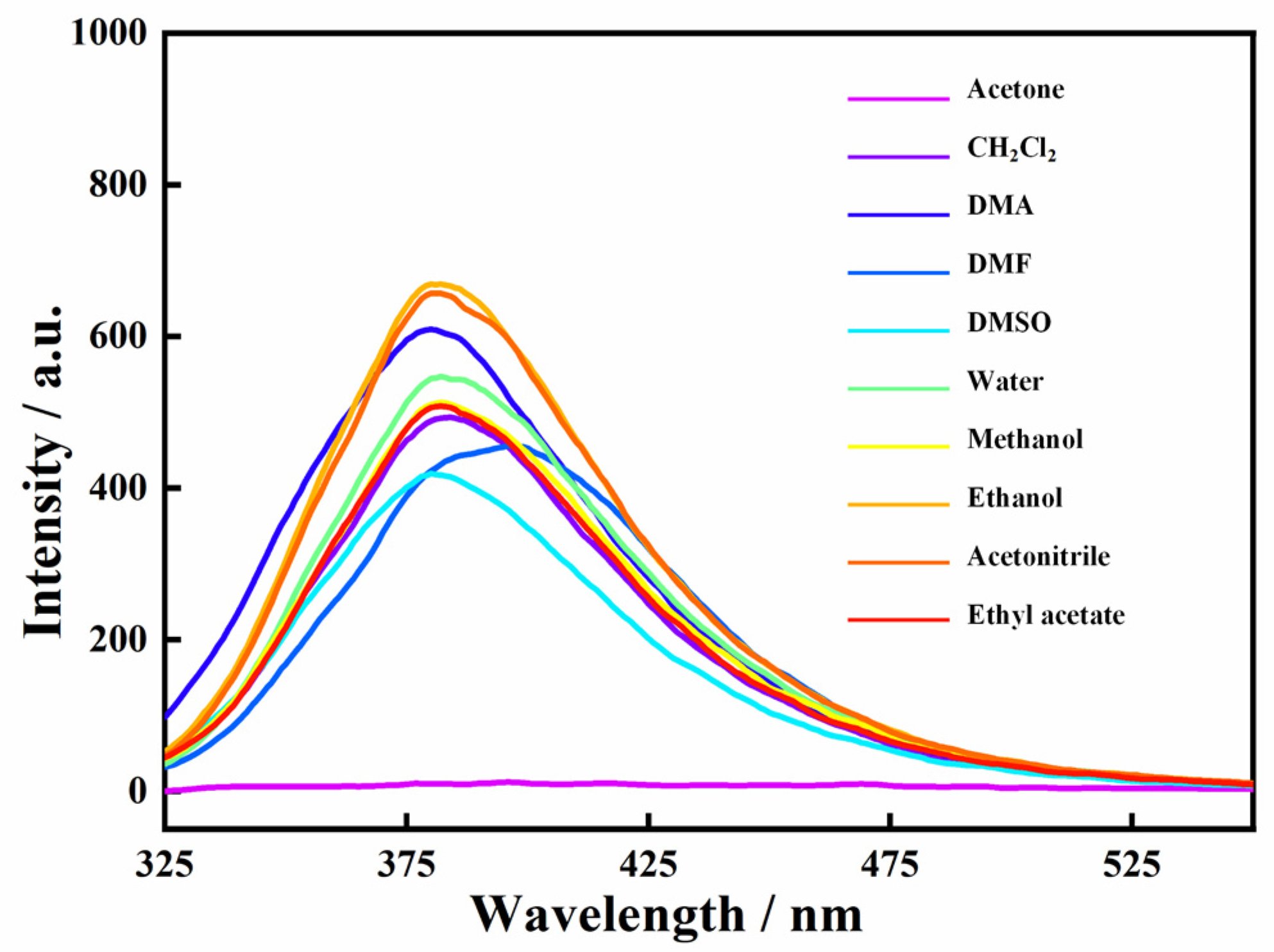
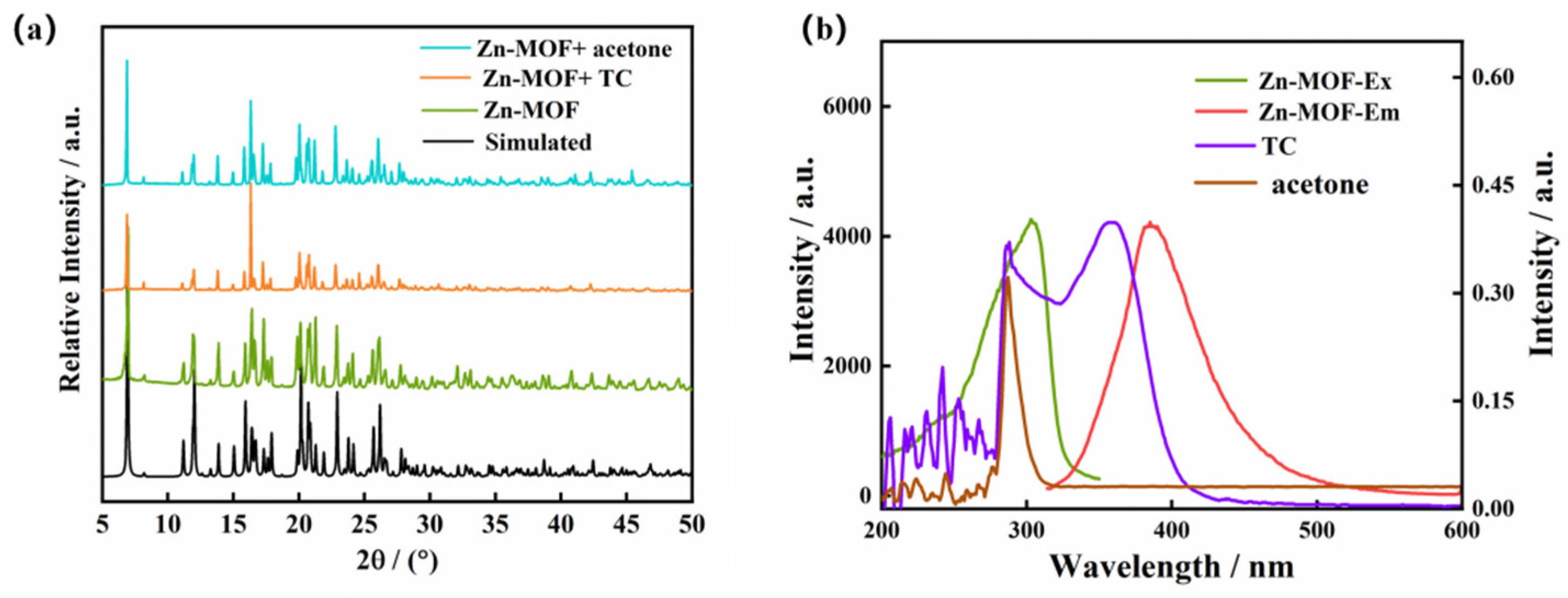
2.5. The Luminescence Properties of the Zn-MOF in Solvents and Sensing for Acetone
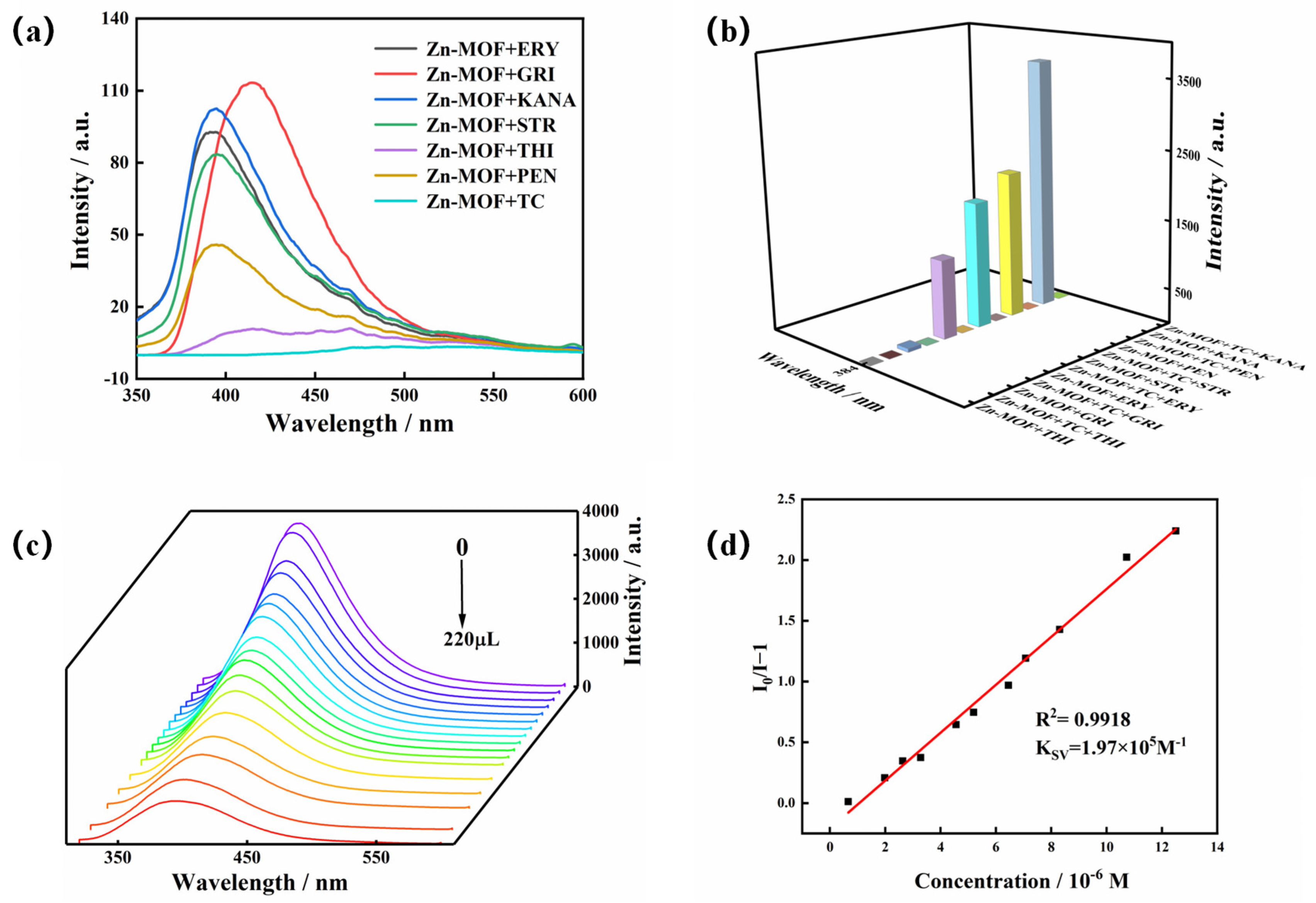
2.6. Luminescence Sensing of the Zn-MOF to TC
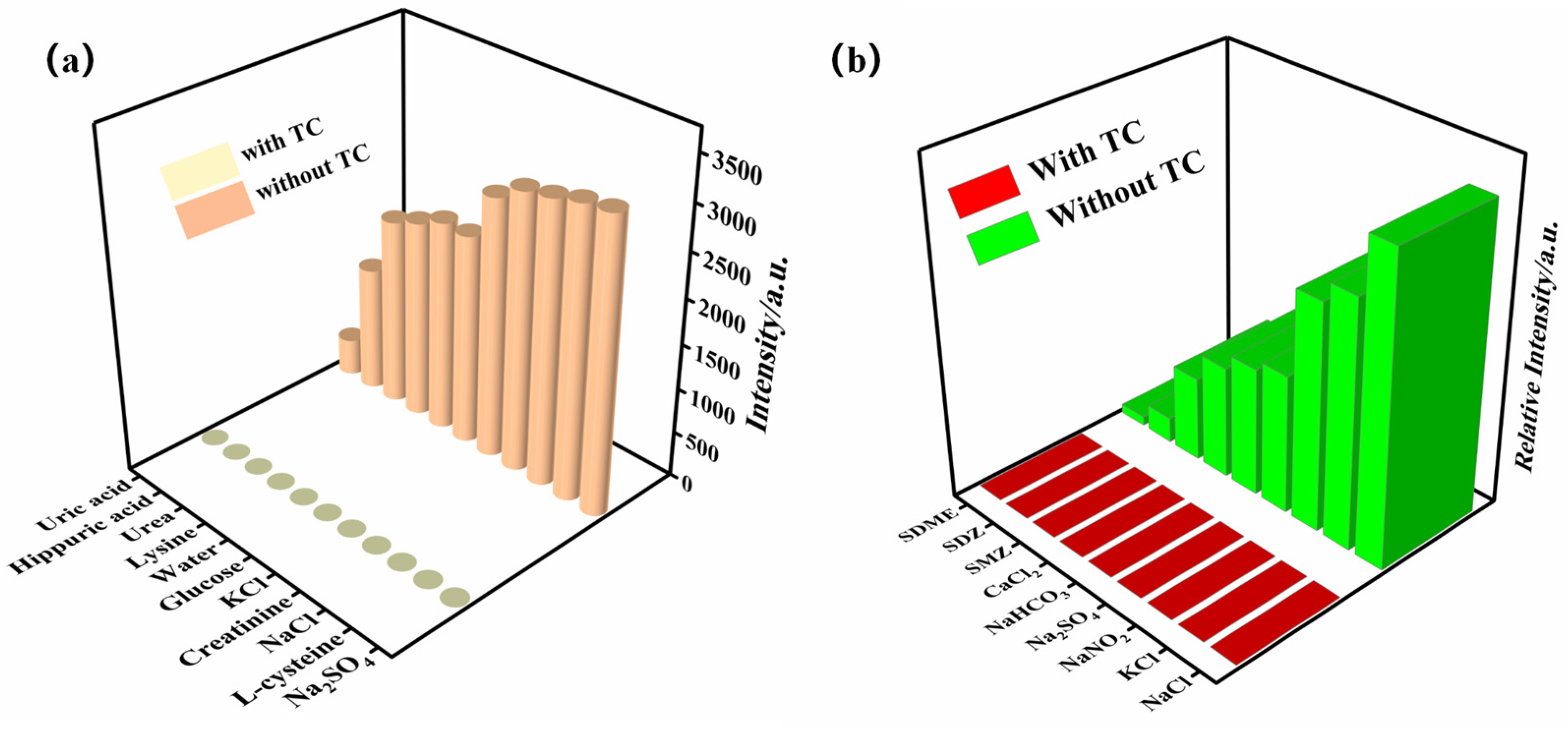
2.7. Luminescence Sensing of the Zn-MOF for TC in Urine and Aquaculture Wastewater Systems
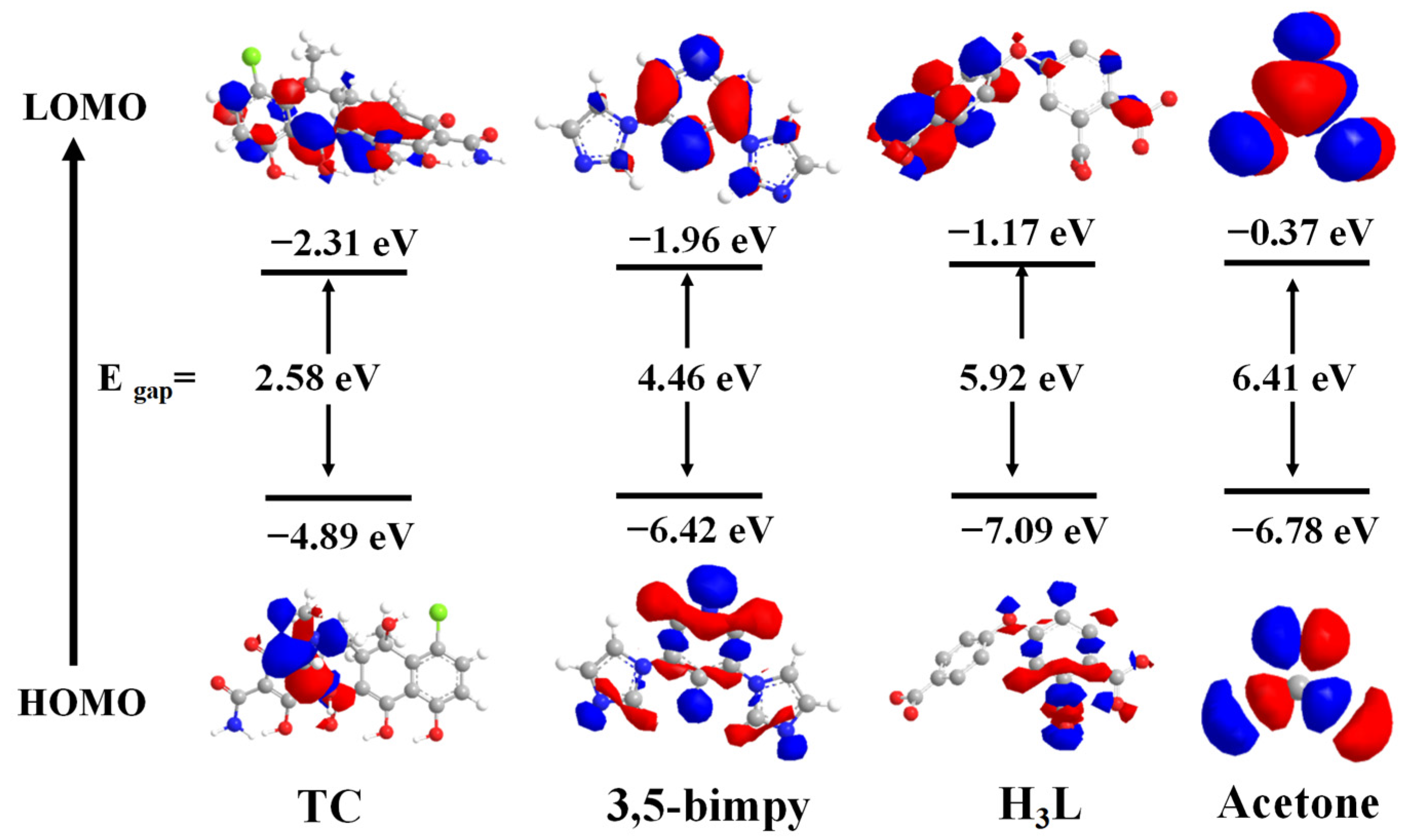
2.8. The Luminescence Sensing Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Zn-MOF
3.3. X-ray Crystallographic Study
3.4. Luminescence Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
References
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Zhang, Z.; Shen, Y.; Li, Y.; Ma, T.; Zhang, Q.; Ying, Y.; Fu, Y. Portable and durable sensor based on porous MOFs hybrid sponge for fluorescent-visual detection of organophosphorus pesticide. Biosens Bioelectron 2022, 216, 114659. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Q.; Lv, J.; Li, Y.; Wang, K.; Li, J.R. Stable Metal-Organic Frameworks for Fluorescent Detection of Tetracycline Antibiotics. Inorg. Chem. 2022, 61, 8015–8021. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, S.; Jia, L.; Zhu, T.; Chen, X.; Zhao, T. A smartphone-integrated method for visual detection of tetracycline. Chem. Eng. J. 2021, 416, 127741. [Google Scholar] [CrossRef]
- Li, K.; Li, J.J.; Zhao, N.; Ma, Y.; Di, B. Removal of Tetracycline in Sewage and Dairy Products with High-Stable MOF. Molecules 2020, 25, 1312. [Google Scholar] [CrossRef]
- Lyu, L.; Xie, Q.; Yang, Y.; Wang, R.; Cen, W.; Luo, S.; Yang, W.; Gao, Y.; Xiao, Q.; Zou, P.; et al. A novel CeO2 Hollow-Shell sensor constructed for high sensitivity of acetone gas detection. Appl. Surf. Sci. 2022, 571, 151337. [Google Scholar] [CrossRef]
- Joshi, S.; Tonde, S.; Wakhure, U.; Bornare, D.; Chatterjee, A.; Syed, K.; Sunkara, M.V. Hierarchical CaTiO3 microspheres for acetone sensing. Sens. Actuators B Chem. 2022, 359, 131621. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Chen, Q.; Liu, K.; Zhao, F.-Y.; Ruan, W.-J.; Chang, Z. Two coordination polymers with enhanced ligand-centered luminescence and assembly imparted sensing ability for acetone. J. Mater. Chem. A 2014, 2, 9469–9473. [Google Scholar] [CrossRef]
- Xu, H.; Mi, H.Y.; Guan, M.M.; Shan, H.Y.; Fei, Q.; Huan, Y.F.; Zhang, Z.Q.; Feng, G.D. Residue analysis of tetracyclines in milk by HPLC coupled with hollow fiber membranes-based dynamic liquid-liquid micro-extraction. Food Chem. 2017, 232, 198–202. [Google Scholar] [CrossRef]
- Bu, T.; Jia, P.; Sun, X.; Liu, Y.; Wang, Q.; Wang, L. Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples. Sens. Actuators B Chem. 2020, 320, 128440. [Google Scholar] [CrossRef]
- Deng, B.; Xu, Q.; Lu, H.; Ye, L.; Wang, Y. Pharmacokinetics and residues of tetracycline in crucian carp muscle using capillary electrophoresis on-line coupled with electrochemiluminescence detection. Food Chem. 2012, 134, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, H.; Wang, J.; Gong, P.; Cai, C.; Dai, X.; Wang, P. Pretreatment of spiramycin fermentation residue by thermally activated peroxydisulfate for improving biodegradability: Insights into matrix disintegration and antibiotics degradation. Chemical. Eng. J. 2022, 427, 130973. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Shariati, J. Synthesis of a nanostructured pillar MOF with high adsorption capacity towards antibiotics pollutants from aqueous solution. J. Hazard. Mater. 2019, 366, 439–451. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B: Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Orr, A.A.; Makam, P.; Redko, B.; Haimov, E.; Wang, Y.; Shimon, L.J.W.; Rencus-Lazar, S.; Ju, M.; et al. Self-Assembled Peptide Nano-Superstructure towards Enzyme Mimicking Hydrolysis. Angew. Chem. Int. Ed. Engl. 2021, 60, 17164–17170. [Google Scholar] [CrossRef]
- Chen, Y.; Guerin, S.; Yuan, H.; O’Donnell, J.; Xue, B.; Cazade, P.A.; Haq, E.U.; Shimon, L.J.W.; Rencus-Lazar, S.; Tofail, S.A.M.; et al. Guest Molecule-Mediated Energy Harvesting in a Conformationally Sensitive Peptide-Metal Organic Framework. J. Am. Chem. Soc. 2022, 144, 3468–3476. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Jiang, W.J.; Cai, Z.H.; Li, D.L.; Liu, Y.L.; Chen, Z.Z. Recent Progress in Metal-Organic Framework Based Fluorescent Sensors for Hazardous Materials Detection. Molecules 2022, 27, 2226. [Google Scholar] [CrossRef]
- Li, P.Z.; Wang, X.J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A Triazole-Containing Metal-Organic Framework as a Highly Effective and Substrate Size-Dependent Catalyst for CO2 Conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal-Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, Z.; Cao, R. Palladium nanoparticles encapsulated in a metal-organic framework as efficient heterogeneous catalysts for direct C2 arylation of indoles. Chemistry 2011, 17, 12706–12712. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent advances in Ti-based MOFs in biomedical applications. Dalton. Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef] [PubMed]
- Minguez Espallargas, G.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef]
- Coronado, E.; Minguez Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Wei, M.; Qi, W.; Li, X.; Niu, Y. A stable and highly luminescent 3D Eu(III)-organic framework for the detection of colchicine in aqueous environment. Environ. Res. 2022, 208, 112652. [Google Scholar] [CrossRef]
- Liu, D.; Dong, G.; Wang, X.; Nie, F.; Li, X. A luminescent Eu coordination polymer with near-visible excitation for sensing and its homologues constructed from 1,4-benzenedicarboxylate and 1H-imidazo[4,5-f][1,10]-phenanthroline. CrystEngComm 2020, 22, 7877–7887. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.L.; Feng, D.; Xie, L.H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.R.; Zhou, H.C. Highly Stable Zr(IV)-Based Metal-Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Guo, C.; Sun, Y.; Xie, L.H.; Li, J.R. Stable Zr(IV)-Based Metal-Organic Frameworks with Predesigned Functionalized Ligands for Highly Selective Detection of Fe(III) Ions in Water. ACS Appl. Mater. Interfaces 2017, 9, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Che, J.; Jiang, X.; Fan, Y.; Gao, D.; Bi, J.; Ning, Z. A Novel Turn-On Fluorescence Probe Based on Cu(II) Functionalized Metal-Organic Frameworks for Visual Detection of Uric Acid. Molecules 2022, 27, 4803. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Aminabhavi, T.M.; Arjmand, M. Simultaneous detection and removal of fluoride from water using smart metal-organic framework-based adsorbents. Coord. Chem. Rev. 2021, 445, 214037. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Wang, X.; Li, H.; Tan, X.; Chen, Y.; Wang, W.; Cheng, Q.; Yi, M.; Han, G.; et al. Rational in situ construction of Fe-modified MXene-derived MOFs as high-performance acetone sensor. Chem. Eng. J. 2022, 444, 136526. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal–organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Zhang, K.; Wang, Y.; Long, W.W.; Sa, R.J.; Liu, T.F.; Lu, J. Fluorescent Metal-Organic Framework (MOF) as a Highly Sensitive and Quickly Responsive Chemical Sensor for the Detection of Antibiotics in Simulated Wastewater. Inorg. Chem. 2018, 57, 1060–1065. [Google Scholar] [CrossRef]
- Gai, S.; Zhang, J.; Fan, R.; Xing, K.; Chen, W.; Zhu, K.; Zheng, X.; Wang, P.; Fang, X.; Yang, Y. Highly Stable Zinc-Based Metal-Organic Frameworks and Corresponding Flexible Composites for Removal and Detection of Antibiotics in Water. ACS Appl. Mater. Interfaces 2020, 12, 8650–8662. [Google Scholar] [CrossRef]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef]
- Xian, G.; Wang, L.; Wan, X.; Yan, H.; Cheng, J.; Chen, Y.; Lu, J.; Li, Y.; Li, D.; Dou, J.; et al. Two Multiresponsive Luminescent Zn-MOFs for the Detection of Different Chemicals in Simulated Urine and Antibiotics/Cations/Anions in Aqueous Media. Inorg. Chem. 2022, 61, 7238–7250. [Google Scholar] [CrossRef]
- Mandal, A.; Adhikary, A.; Sarkar, A.; Das, D. Naked Eye Cd(2+) Ion Detection and Reversible Iodine Uptake by a Three-Dimensional Pillared-Layered Zn-MOF. Inorg. Chem. 2020, 59, 17758–17765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.S.; Gao, Q.; Chang, Z.; Liu, X.T.; Zhao, B.; Xuan, Z.H.; Hu, T.L.; Zhang, Y.H.; Zhu, J.; Bu, X.H. Rational Construction of Highly Tunable Donor-Acceptor Materials Based on a Crystalline Host-Guest Platform. Adv. Mater. 2018, 30, e1804715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Q.; Jiang, F.-L.; Wu, M.-Y.; Ma, J.; Bu, Y.; Hong, M.-C. Assembly of Discrete One-, Two-, and Three-Dimensional Zn(II) Complexes Containing Semirigid V-Shaped Tricarboxylate Ligands. Cryst. Growth Des. 2012, 12, 1452–1463. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Jiang, F.-L.; Bu, Y.; Wu, M.-Y.; Ma, J.; Shan, X.-C.; Xiong, K.-C.; Hong, M.-C. Two dual-emissive Zn(ii) coordination polymers with tunable photoluminescence properties. CrystEngComm 2012, 14, 6394–6396. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, L.; Han, Y.; Song, G.; Jing, H.; Guo, G.; Wang, Z.; Li, J. Metal-organic frameworks constructed from tetradentate carboxylic acids: Structural diversity, Fluorescence (Fe3+ detection) and Dye adsorption properties. J. Mol. Struct. 2022, 1270, 133925. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, C.-M.; Zhou, C.-S.; Qiao, C.-F.; Lu, L.; Ma, A.-Q.; Singh, A.; Kumar, A. Syntheses and photocatalytic properties of three new d10-based coordination polymers: Effects of metal centres and ancillary ligands. CrystEngComm 2019, 21, 6558–6565. [Google Scholar] [CrossRef]
- Yi, K.; Zhang, L. Designed Eu(III)-functionalized nanoscale MOF probe based on fluorescence resonance energy transfer for the reversible sensing of trace Malachite green. Food Chem. 2021, 354, 129584. [Google Scholar] [CrossRef]
- Wang, J.X.; Yin, J.; Shekhah, O.; Bakr, O.M.; Eddaoudi, M.; Mohammed, O.F. Energy Transfer in Metal-Organic Frameworks for Fluorescence Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9970–9986. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Li, S.; Li, Z.; Gong, Y.; Li, X. A Zn(II)–Metal–Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline. Molecules 2023, 28, 999. https://doi.org/10.3390/molecules28030999
Wang N, Li S, Li Z, Gong Y, Li X. A Zn(II)–Metal–Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline. Molecules. 2023; 28(3):999. https://doi.org/10.3390/molecules28030999
Chicago/Turabian StyleWang, Nairong, Shanshan Li, Zhenhua Li, Yuanyuan Gong, and Xia Li. 2023. "A Zn(II)–Metal–Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline" Molecules 28, no. 3: 999. https://doi.org/10.3390/molecules28030999
APA StyleWang, N., Li, S., Li, Z., Gong, Y., & Li, X. (2023). A Zn(II)–Metal–Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline. Molecules, 28(3), 999. https://doi.org/10.3390/molecules28030999




