Abstract
Two novel 1D heterobimetallic compounds {[MnIII(SB2+)MIII(CN)6]·4H2O}n (SB2+ = N,N′-ethylenebis(5-trimethylammoniomethylsalicylideneiminate) based on orbitally degenerate cyanidometallates [OsIII(CN)6]3− (1) and [RuIII(CN)6]3− (2) and MnIII Schiff base complex were synthesized and characterized structurally and magnetically. Their crystal structures consist of electrically neutral, well-isolated chains composed of alternating [MIII(CN)6]3− anions and square planar [MnIII(SB2+)]3+ cations bridged by cyanide groups. These -ion magnetic anisotropy of MnIII centers. These results indicate that the presence of compounds exhibit single-chain magnet (SCM) behavior with the energy barriers of Δτ1/kB = 73 K, Δτ2/kB = 41.5 K (1) and Δτ1/kB = 51 K, Δτ2 = 27 K (2). Blocking temperatures of TB = 2.8, 2.1 K and magnetic hysteresis with coercive fields (at 1.8 K) of 8000, 1600 Oe were found for 1 and 2, respectively. Theoretical analysis of the magnetic data reveals that their single-chain magnet behavior is a product of a complicated interplay of extremely anisotropic triaxial exchange interactions in MIII(4d/5d)–CN–MnIII fragments: −JxSMxSMnx−JySMySMny−JzSMzSMnz, with opposite sign of exchange parameters Jx = −22, Jy = +28, Jz = −26 cm−1 and Jx = −18, Jy = +20, Jz = −18 cm−1 in 1 and 2, respectively) and single orbitally degenerate [OsIII(CN)6]3− and [RuIII(CN)6]3− spin units with unquenched orbital angular momentum in the chain compounds 1 and 2 leads to a peculiar regime of slow magnetic relaxation, which is beyond the scope of the conventional Glaubers’s 1D Ising model and anisotropic Heisenberg model.
1. Introduction
Over the past three decades, low-dimensional (LD), magnetically bistable coordination compounds featuring slow magnetic relaxation and blocking of magnetization at the molecular level [1,2,3,4,5,6,7,8] have attracted immense research interest inspired by their unique properties relevant to important future applications [9,10,11,12,13,14]. Depending on the dimensionality of the compounds, the magnetic systems are categorized into single-molecule magnets (SMMs, 0D compounds) [15,16,17,18,19,20,21] and single-chain magnets (SCMs, 1D compounds) [21,22,23,24,25,26,27]. Temperature-dependent magnetic dynamics of SMMs and SCMs is defined by the energy barrier for magnetization reversal, Ueff, and blocking temperature Tb, below which the magnetization is blocked and retained for a long period of time [16,23,26,28,29,30]. However, the mechanism underlying the slow relaxation and the origin of the energy barrier in 0D and 1D magnetic systems is different. In SMMs, negative uniaxial magnetic anisotropy (D < 0), together with high-spin ground state (S), is responsible for a double-well potential with the energy barrier Ueff = Δ = |D|S2 for integer S and |D|(S2−1/4) for half-integer S [1,3,28]. In SCMs, the barrier contains an additional energy term, Δξ, resulting from exchange coupling (J) between the magnetic ions, Ueff = kΔξ + Δ, where k = 1 for finite or 2 for infinite chain [24,26,27,31,32]. In fact, Δξ corresponds to the creation energy of the domain wall in the spin chain; it refers to a correlation energy. In the original Glauber’s model published back in 1963, for ferromagnetic Ising spin chain (S), the correlation energy is Δξ = 4|J|S2 [33]. In the alternative model, SCM is described by a Heisenberg chain of spins with uniaxial single-ion magnetic anisotropy D and Δξ = 4|J|S2 in the Ising limit (at |D/J| > 4/3) or Δξ = 4S2(|JD|)1/2 in the Heisenberg limit (|D| << |J|) [34,35,36,37,38]. Basically, SCMs can be regarded as the 1D analogs of SMMs, which are often thought to be more promising for rising the energy barrier as follows from an additional term Δξ in Ueff [22,26,39].
Since the discovery of the first SCM in 2001 [22], a huge variety of SCMs with diverse architecture and magnetic properties have been synthesized using various spin carriers and bridging ligands [40,41,42,43,44,45]. However, most of the SCMs exhibit rather low spin reversal barriers (typically, Ueff/kB < 100 K) and blocking temperatures Tb within a few Kelvin. The record barrier of ca. 400 K and blocking temperature of 14 K are held by Co(II)-radical SCMs [25,46]. Among the cyanido-bridged 1D systems, the largest barrier of 253 K has been reported for a CoII-[WV(CN)8]3− bimetallic double-chain compounds [47]. To achieve high values of Ueff and Tb, the building spin units in the chains must provide high magnetic anisotropy, strong intrachain magnetic coupling, and weak interchain interactions. Hence, for the rational molecular design of high-performance SCMs, the proper choice of magnetically anisotropic building units and tuning their magnetic interactions with spin carriers are crucial.
It is well recognized now that maximum magnetic anisotropy is ensured by molecular synthons with unquenched first-order orbital angular momentum: lanthanide (4f) [17,18,19] and actinide (5f) ions [48] as well as the special orbitally degenerate transition metal complexes [42,43,49,50,51,52,53,54,55,56,57]. In this regard, lanthanide ions (Ln) are especially appealing due to their large unquenched orbital angular momentum (L) and strong spin–orbit coupling (SOC), which—in concert with the ligand field splitting of 4f-electrons—produce very strong single-ion magnetic anisotropy [17,18,19,48]. Currently, lanthanide ions (especially heavy TbIII, DyIII, and HoIII cations) are widely used for designing advanced SMMs [58,59,60,61], including recently reported DyIII-based complexes with record barriers (>1000 cm−1) [62,63] and blocking temperatures (up to 80 K) [30]. A number of Ln-based 1D coordination compounds with slow magnetic relaxation were also reported in recent years [64,65,66,67,68,69]. However, the use of the Ln3+ ions for the SCMs design has so far led to less impressive results compared to SMMs mainly due to the absence of strong magnetic coupling of Ln3+ ions with other spin carriers caused by the core-like nature of 4f electrons. Several synthetic strategies have been employed to develop improved Ln-SCMs [17,18,19,58,59,60,61], among which the bridging radical strategy seems to be the most promising. Its main idea is that the enhanced intrachain Ln-radical spin coupling combined with the strong single-ion anisotropy of the Ln3+ ions (especially when it has a uniaxial Ising-type character) should ultimately lead to high-performance SCMs [70,71].
Among 3d metal anisotropic building blocks, orbitally degenerate six-coordinated CoII complexes are particularly popular for assembling magnetic chains [50,51,53,54,56,57]. Several CoII complexes with less common coordination environments, such as pentagonal–bipyramidal complexes [72,73], were also used in the SCMs [54]. Remarkably, in low-dimensional (LD) transition metal complexes, both record barriers were achieved for the CoII-based compounds, Ueff = 594 K (413 cm−1) for SMMs [74] and 400 K for SCMs [25,46].
Octahedral hexacyanidoferrate(III) anion has been employed in many heterometallic chain compounds as a molecular linker between various spin carries [75,76,77,78,79,80]. First-order unquenched orbital momentum of the [FeIII(CN)6]3− complex results in strong magnetic anisotropy of spin chains, which is imposed by sufficiently high anisotropic exchange interactions in the FeIII-CN-M-CN linkages, the latter having been explored in detail both experimentally [81,82] and theoretically [78,79,80]. Several magnetic chain compounds containing related orbitally degenerate metalloligands [MnIII(CN)6]3− featuring slow magnetic relaxation were reported [83,84,85]. The physical mechanism of their SCM behavior is more complicated due to the nonmagnetic character of the singlet ground state of [MnIII(CN)6]3− resulting from antiparallel coupling of the orbital (L = 1) and spin (S = 1) angular momentums, which cancel each other [85].
In recent years, efficient strategies to incorporate highly anisotropic 4d and 5d complexes into SMMs/SCMs have been actively developed [52,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. Heavy transition metals offer exciting opportunities to rise the Ueff and Tb parameters since the high energy and diffuse 4d and 5d orbitals provide stronger spin coupling as compared to 3d orbitals of the first-raw transition metal complexes [86]. Another important advantage is in considerably stronger spin-orbit spin coupling of 4d and 5d electrons favoring enhanced magnetic anisotropy [86]. Numerous 0D and 1D molecular magnetic systems involving 4d and 5d metals were reported in the past decade [52,86,87,88,89,93,94,95,96,97,98,99,100,101]. However, in most cases, these SMMs and SCMs contain spin-only (nondegenerate) 4d and 5d complexes with enhanced second-order magnetic anisotropy. In particular, many heterometallic coordination compounds were based on NbIV, MoV, and WV octacyanidometallates [90,91,93,94]. A number of high-spin (S = 3/2) 4d and 5d building units with strong zero-field splitting (ZFS) were employed in SMMs and SCMs: [MoIII(CN)6]4− [95,96], [ReIVCl4(CN)2]2− [97,98,99], [ReIV(CN)6]3− [101] and [ReIVF6]2− [100]. Low-spin complexes [RuIII(acac)2(CN)2]− [87], [MIII(salen)(CN)2]− (M = Ru [88], Os [89]) and other related systems are also popular in assembling SMMs and SCMs.
Given that magnetic anisotropy correlates with the value of L, orbitally degenerate 4d and 5d complexes with unquenched L (first-order compared to the ZFS of 3d) are expected to be the most beneficial in reaching maximal magnetic anisotropy. Currently, they are mainly presented by the pentagonal–bipyramidal heptacyanidometallates [MoIII(CN)7]4− [102,103] and [ReIV(CN)7]3− [101,104,105] and octahedral hexacyanidometalles [RuIII(CN)6]3− [106,107] and [OsIII(CN)6]3− [107,108,109]. Previous theoretical calculations showed that these low-spin orbitally degenerate (S = 1/2) complexes exhibit highly anisotropic spin coupling with connected spin carriers [110,111]. This is potentially helpful in the development of high-performance SMMs [112,113,114]. Incorporation of these molecular synthons into heterometallic coordination compounds has already led to obtaining of SMMs [115,116,117,118,119,120,121], SCMs [122,123,124] and extended polymer structures with unusual magnetic behavior [49,92,125,126,127].
In this context, a synthesis of the first heterobimetallic magnetic chain compound involving orbitally degenerate [OsIII(CN)6]3− complex and high-spin MnIII Schiff-base complex [128], which exhibits distinct SCM behavior with enhanced Ueff and Tb parameters, is worth mentioning. This system is of particular interest for understanding the underlying physical mechanism of slow magnetic relaxation in a magnetic chain with highly anisotropic non-Ising spin coupling. Theoretical analysis for the discrete [OsIII(CN)6]3−-based trinuclear clusters with similar local cyanide-bridging topology [119,121] showed that the spin coupling in the OsIII–CN–MnIII fragments is described by an extremely anisotropic triaxial spin Hamiltonian −JxSMxSMnx−JySMySMny−JzSMzSMnz with opposite signs of the exchange parameters, such as Jx = −18, Jy = +35, Jz = −33 cm−1 in the MnIII2OsIII cluster [119]. This points at a special regime of magnetic relaxation in the {NC–OsIII–CN–MnIII–NC–}n chains resulting from a complicated interplay of highly anisotropic non-Ising exchange interactions and single-ion ZFS anisotropy of MnIII ions, which is even more sophisticated due to the noncollinear orientation of the local magnetic axes. Obviously, such a scenario is the subject of new magnetic physics, which can be considered neither within the existing Ising theory nor within the anisotropic Heisenberg model.
Based on these considerations, we prepared new heterometallic chain compounds involving [MIII(CN)6]3− metalloligand and MnIII Schiff base cation (Scheme 1). In this study, we report on the synthesis, structure and magnetic properties of two novel isostructural bimetallic cyano-bridged chain compounds, {[MnIII(SB2+)MIII(CN)6]·4H2O}n based on orbitally degenerate [OsIII(CN)6]3− (1) or [RuIII(CN)6]3− (2) hexacyanide and MnIII Schiff base complex. Their crystal structure is built up of neutral, well-isolated chains composed of alternating [(RuIII/OsIII)CN)6]3− anionic complexes and square planar [MnIII(SB2+)]3+ cationic complexes. We present the results of static and dynamic magnetic measurements and a detailed theoretical interpretation based on the anisotropic spin coupling model. The presence of orbitally degenerate magnetic units with unquenched L is shown to lead to a peculiar regime of magnetic relaxation in the chain compounds 1 and 2 that goes far beyond the usual Ising and anisotropic Heisenberg models.
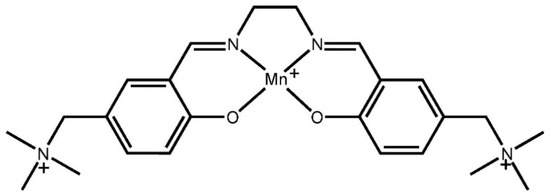
Scheme 1.
Molecular structure of triply charged salen-type [Mn(SB2+)]3+, SB2+ = N,N′-ethylenebis(5-trimethylammoniomethylsalicylideneiminate.
2. Results and Discussion
2.1. Synthetic Approach
Describing an approach chosen for the preparation of the new SCMs based on hexacyanidometallates as metalloligands, we would like point out that no SCM containing [RuIII(CN)6]3− synthon has been obtained so far. The reason for this lies in the instability of hexacyanidoruthenate(III) anion in solution during slow-diffusion crystallization of heterobimetallic assemblies, unlike its iron and osmium congeners. With the latter, the anionic 1D polymers [MnIIIacacen(FeIII/OsIII)(CN)6]2− exhibiting SCMs properties were successfully obtained and studied [78,128], whereas for [FeIII(CN)5NO]2− and [ReIV(CN)7]3− 0D÷3D, assemblies with Mn(III) complexes were obtained depending on synthetic conditions [49,92,101,127,129,130]. In such cases, the only way to obtain low-dimensional heterometallic complexes is to create conditions in which electroneutrality is a driving force of self-assembly. In order to guarantee a 1:1 stoichiometry in a chain or binuclear compound, identically charged counterions must be used as precursors. For cyanidometallates, this approach has been successfully developed and applied to the synthesis of neutral SCMs {[MnIII(SB2+)MIII(CN)6]·4H2O}n (MIII = Fe, Mn, Cr) [77] and {[Mn(SB2+)W(CN)8]·8H2O·MeCN}n (3) [131] using the previously studied tricationic Schiff base complex [Mn(SB2+)(H2O)2]3+ [132]. In addition, the binuclear species [MnIII(SB2+)WV(CN)8]·4H2O·MeCN (4) and [MnIII(MeSB2+)MIII(CN)6]·7H2O·MeCN (MIII = Fe, Mn, Cr) were obtained [131,133].
Owing to the low stability of the [Ru(CN)6]3− anion in solution [106,107,134] compound 2 was obtained through rapid precipitation of the coordination polymer by mixing solutions, a procedure earlier used for the preparation of 3 [131]. Chain 1 was synthesized using a process similar to one described in Reference [77]. A layering of H2O:MeCN solutions containing [Mn(SB2+)(H2O)2](ClO4)3·H2O and (Ph4P)3[Os(CN)6] in a 1:1 ratio after a few days gave fern-like dark crystals slightly powdered by a white precipitation of Ph4PClO4, which was removed via washing in a few milliliters of acetonitrile. The data of IR, CHN analysis, and powder XRD confirmed the good quality and purity of the samples.
2.2. Crystal Structure
For 1, we were able to grow crystals suitable for single-crystal X-ray structure analysis. This study has shown that Os-Mn polymer is isostructural to its earlier studied congener [Mn(SB2+)FeIII(CN)6]·4H2O [77], and the crystal unit cell parameters of 1 determined at 110 K are a = 11.2510(1), b = 16.7747(2), and c = 18.6078(2) Å β = 96.53(6)°, space group P2/c (#15) (Table S1, see Supplementary Materials)). The asymmetric unit is shown in Figure 1. The view of the 1D chain motif is presented in Figure 2. Selected geometric parameters for 1 compared to its Fe congener are listed in Table 1. More bond lengths and bond angles are presented in Table S2. The coordination environment of the Mn ion is an elongated tetragonal bipyramid because of the Jahn–Teller distortion. The 2O and 2N donor atoms of the SB2+ ligand in the basal plane of the pyramid form shorter bonds of 1.88–1.98 Å, while two N atoms of trans-disposed CN ligands form much longer Mn–NCN bonds of 2.28 Å with an NCN–Mn–NCN angle of 173.5°, which is larger than in Mn-Fe analog (Table 1). The Mn–N–C bond angles are much more acute than 180° and equal to 142.5°, being slightly less than 144.4° for the Fe-containing chain. In 1, Os ion coordinates four terminal CN groups, forming two hydrogen bonds N2···O1W of 2.94 Å and two N3···O2W of 2.88 Å. The additional contacts O1···O2W of 2.94 Å and O1W···O2W of 2.85 Å connect the neighboring chains into an H-bonded 3D structure (Figure S1).
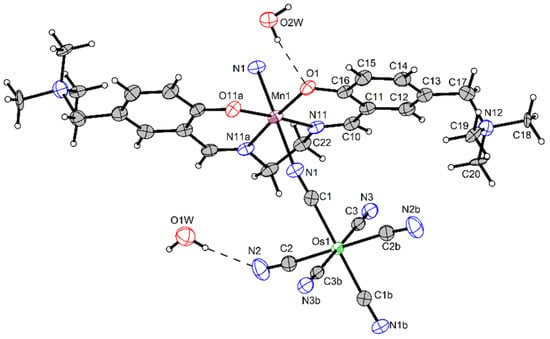
Figure 1.
ORTEP view of the structure of 1, showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
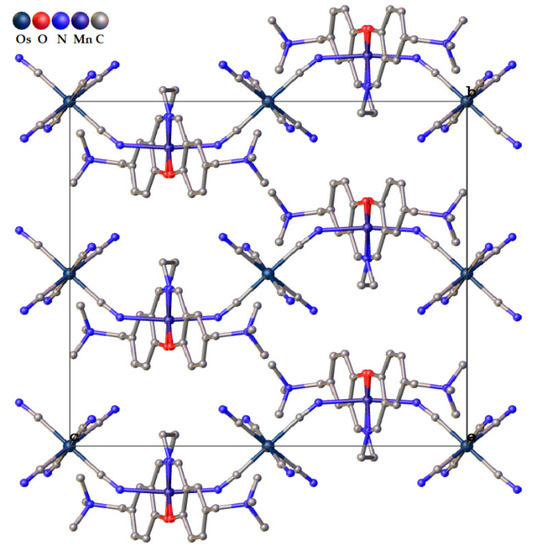
Figure 2.
View of the chain motif of 1 projected along a axis. Hydrogen atoms and interstitial water molecules have been omitted for clarity. The axes denote overlap with atoms.

Table 1.
Selected geometric parameters for [Mn(SB2+)Os(CN)6]·4H2O (1).
2.3. Powder X-ray Diffraction Investigations
The powder samples for the neutral heterobimetallic (Mn-Ru/Os) 1D polymers obtained via precipitation are crystalline, and their XRD patterns correspond well to the simulated diffractogram of the Mn-Fe chain, with the exception of one Bragg reflection (−1, 1, 2) (see Figures S2 and S3). This peak was calculated for the Mn-Fe compound significantly contributed by iron centers at 2θ = 12.867° (d = 6.8745 Å), but it is not observed due to a very low intensity. However, as the atomic weight of the central cyanidometallate atom increases, the corresponding peak emerges in the powder diffraction pattern for Ru and Os chains (Figures S2 and S3 and Table S3). The PXRD patterns confirmed that all three 1D coordination polymers containing hexacyanidometallates(III) of the iron group are isomorphic.
2.4. Magnetic Studies
2.4.1. Static Magnetic Properties and Their Theoretical Analysis
The temperature dependences of the magnetic susceptibility measured at 1 kOe are shown in Figure 3 as the χT product. At 300 K, χT = 3.32 and 3.23 cm3K/mol for 1 and 2, respectively, which agrees well with the Curie constant of 3.30 cm3K/mol expected for magnetically uncoupled MnIII spin S = 2 with g = 2.0 and (Ru/Os)III spin S = 1/2 with g = 1.8. As the temperature decreases starting from room temperature, the χT product of 2 slowly decreases, passing through a flat minimum around 100 K, and then rapidly rises at low temperatures to reach a sharp maximum of ~8 cm3K/mol at ~6 K. In contrast, χT of 1 increases monotonically, without a minimum, forming a much higher maximum of ~19 cm3 K/mol at ~8 K. It is noteworthy that the peak value of χT decreases with increasing field (Figure S4), showing saturation. A similar behavior of the χT function was observed in the closely related anionic chain compound (Ph4P)2[MnIII(acacen)OsIII(CN)6] [128]. However, the magnetization of 1 and 2 does not saturate even at 50 kOe, which is a signature of strong magnetic anisotropy. Below, in the current paper, the origin of the magnetic anisotropy of 1 and 2 is examined theoretically; our calculations reveal strong anisotropy of the χT product (Figure 4 and Figures S5 and S6).
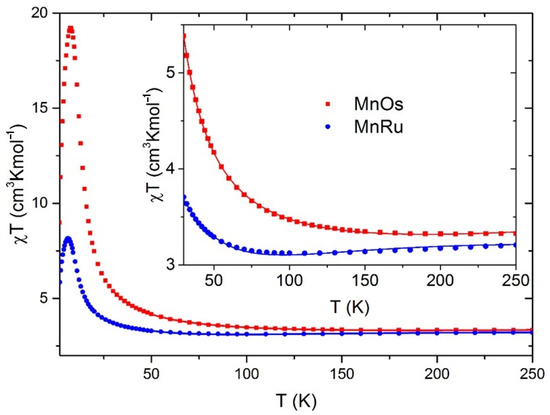
Figure 3.
Magnetic susceptibility of 1 (red) and 2 (blue) in 1 kOe; insert: lines were fitted according to the Seiden’s model.
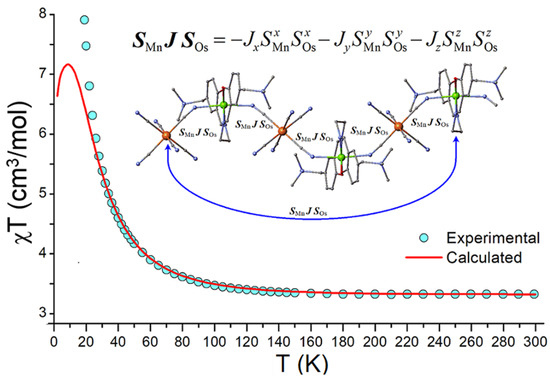
Figure 4.
Experimental (blue circles) and simulated (solid red line) magnetic susceptibility χT of 1. The χT curve was simulated with the spin Hamiltonian (2) involving anisotropic triaxial spin coupling SMnJSOs = −JxSMnxSOsx−JySMnySOsy−JzSMnzSOsz. The best fit is obtained at the set of parameters Jx = −22, Jy = +28, Jz = −26 cm−1, gMn = 2.02, gOs =1.80, DMn = −3.6 cm−1. Calculations are performed for a six-membered fragment {Mn-Os}3 of the heterometallic chain of 1 with cyclic boundary conditions for the Mn-Os spin coupling, as shown in the inset.
The magnitude of the spin coupling constant J in heterometallic chains of compounds 1 and 2 can approximately be estimated in terms of an isotropic Heisenberg model for alternating spins SOs/Ru = 1/2 and SMn = 2, which is described by the Hamiltonian (1):
A solution of this Hamiltonian, in the approximation that large spins SMn are treated classically, was obtained by Seiden as an analytical formula for susceptibility [135]. The only J value was adopted equally for all Ru-Mn or Os-Mn pairs and gRu/Os = 2 was fixed for both chains. The best fit to the magnetic data in the range of 30–300 K has resulted in and gMn = 1.76 and J/kB = +25.4 K for 1 and gMn = 2.23, J/kB = −62.8 K for 2 (inset in Figure 3). To improve the fit, an additional parameter zJ’ was added in order to take into account interchain interactions, yielding and 0.51 K for 1–1.39 K for 2. The obtained values imply antiferromagnetic interactions within the chain for 2, with weak ferromagnetic interchain coupling, while for 1, the interactions within the chain are strong ferromagnetic, with weaker antiferromagnetic coupling between the chains.
These data indicate that the Seiden’s model results in unreliable and inconsistent magnetic parameters that are difficult to expect for the isostructural and isoelectronic chain compounds 1 and 2. This is particularly apparent from the opposite sign of the exchange parameters (F in 1 and AF in 2) and the large scatter in the effective g-factor of MnIII ions. The basic reason behind these issues is that the isotropic Heisenberg model does not account for the anisotropic magnetic interactions associated with single-ion ZFS anisotropy of MnIII ions and anisotropic exchange interactions of OsIII and RuIII ions due to unquenched orbital angular momentum in the ground state. The origin of highly anisotropic exchange interactions in the OsIII-CN-MnIII and RuIII-CN-MnIII exchange-coupled pairs was examined in detail in Ref. [119] for cyanide-bridged trinuclear clusters [MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6] (M = Os, Ru) whose structures are very close to the local structure of the chains in compounds 1 and 2. The ground state of the OsIII ion in the octahedral ligand field of the [OsIII(CN)6]3− complex is an isotropic Kramers doublet Г7 resulting from the spin orbit splitting of the ground orbital triplet 2T2g(5d5). The energy separation ΔE = 3/2ζOs = 4500 cm−1 to the first excited state Г8 is determined by the spin orbit coupling constant (ζOs ≈ 3000 cm−1). The RuIII ion in complex [Ru (CN)6]3− behaves similarly but with a smaller spin orbit coupling constant (ζRu ≈ 880 cm−1). It has been shown that the spin coupling between the ground Г7 state of OsIII (corresponding to the effective spin S = 1/2) and MnIII ions (S = 2) is described by an anisotropic triaxial spin Hamiltonian, SMnJSOs = −JxSMnxSOsx−JySMnySOsy−JzSMnzSOsz, with opposite sign of exchange parameters, Jx = −18, Jy = +35, Jz = −33 cm−1 [119].
Hence, given close similarity in the molecular structure of trinuclear clusters [MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6] and chain compounds 1 and 2, the same anisotropic spin Hamiltonian can be applied to the alternating heterometallic Os-Mn and Ru-Mn chains (2):
where the sum <ij> runs over the neighboring Os(i) and Mn(j) cyanide-bridged exchange-coupled ions in the chain; the tensor of anisotropic spin coupling (J) has a three-axis structure, SMnJSOs = −JxSMnxSOsx−JySMnySOsy−JzSMnzSOsz. The ZFS Dj tensors of the MnIII(i) ions are supposed to have the axial structure (with D < 0 and E = 0). However, each Dj tensor of MnIII(i) ion is transformed by the Tj(θ) rotation matrix, Dj’ = Tj(θ)DjTj(θ)−1, specifying the noncollinear orientation (θ = ±37.5°) of these ZFS tensors in the bent structure of the {Mn-Os}n chain with respect to the local spin quantization axis z of the anisotropic exchange spin Hamiltonian SMnJSOs (see inset in Figure 4).
Calculation of magnetic susceptibility χT using Equation (2) was performed for a finite six-membered chain {OsIII-CN-MnIII-NC-}3, applying the cyclic boundary condition for the terminal OsIII and MnIII spin centers as shown in Figure 4. The best fit to the experimental data for 1 is obtained at the set of parameters Jx = −22, Jy = +28, Jz = −26 cm−1, gMn = 2.02, gOs =1.80, and DMn = −3.6 cm−1. The simulated χT curve agrees well with the experimental data in the temperature range of 20–300 K. The divergence with the experimental data at low temperatures (below 20 K) is mostly due to the long-range spin correlation effects in an infinite chain, which cannot be reproduced in a finite-size chain employed in the calculations, Figure 4. The calculated anisotropic exchange parameters Jx, Jy, Jz are well consistent with those obtained for discrete trinuclear MnIII2OsIII clusters, such as Jx = −18, Jy = +35, Jz = −33 cm−1 [119] and Jx = −23.5, Jy = +32.0, Jz = −25.9 cm−1 [121]. Similar calculations for the ruthenium chain compound 2 resulted in Jx = −18, Jy = +20, Jz = −18 cm−1, gMn= 2.00, gOs = 1.80 and DMn = −4.0 cm−1 (Figure S6). Again, the calculated anisotropic exchange parameters are reasonably consistent with those obtained for the trinuclear MnIII2RuIII complex, Jx = −20, Jy = +25, Jz = −26 cm−1 [119].
2.4.2. Magnetic Relaxation Parameters of 1 and 2 Derived from Static Magnetic Measurements
Low-field dc measurements were used to determine the ∆ξ parameter for 1 and 2. Anisotropic 1D magnetic systems have a gap in the spin excitation energy spectrum, which leads to the susceptibility dependence χT ≈ CeffExp(∆ξ/T) [26]. In this equation Ceff is an effective Curie constant, which takes into account the averaging of anisotropic magnetic susceptibility in the powder sample. ∆ξ designates an energy of the domain wall, which is the lowest excitation of the ground state in the chain of correlated spins. To estimate ∆ξ, the susceptibility data measured in 1 kOe were plotted as ln(χT) vs. T−1 (Figure 5). The linear part of the plot in the region from 10 to 30 K was used to obtain ∆ξ/kB = 20.09 K and 9.45 K, and Ceff = 2.77 and 2.71 cm3 K/mol for 1 and 2, respectively. If the linear sections of the curves at the temperatures below 8 K will be drawn, the intersection of these straight lines with the linear parts of the plots between 10 and 30 K will give the crossover temperatures. The latter determine a crossover between infinite chain regime and finite chain regime in the relaxation dynamics. In our case, they are 10 and 8 K for 1 and 2 respectively.
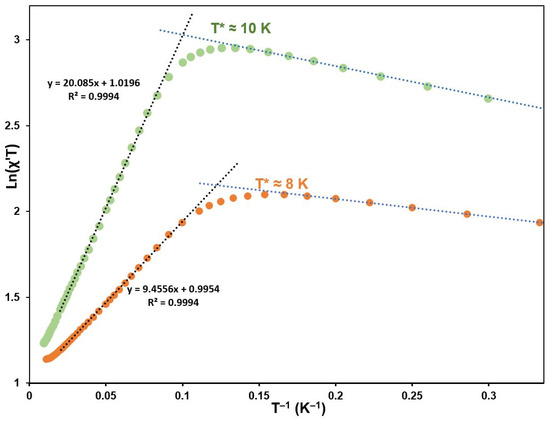
Figure 5.
Magnetic dc susceptibility measured at one kOe for 1 (green) and 2 (orange), respectively. Straight dotted lines were fitted (see text). T* represents a crossover temperature.
For the real SCMs, below a certain temperature, the χT(1/T) dependence deviates from the exponential function and saturates (Figure 5)—even for a low applied field—due to the finite chain length caused by crystal imperfection [26]. This occurs when the rising correlation length surpasses an average chain length n. For 1, it is visible below 8 K, where (χT)max = 22.1 cm3K/mol, and it is visible below 6 K for 2, for which (χT)max = 8.56 cm3K/mol (data measured at 3 and 15 Oe, respectively). The average chain length estimated using the relation (χT)max = nCeff, gives n ≥ 7.94 nm for 1 and n ≥ 3.17 nm for 2, which correspond to 16 Mn-Os and 6 Mn-Ru units, respectively (7.94 or 3.17 nm /5.166 Å). However, such an estimation of n should be treated with precaution because two other effects can also decrease the measured susceptibility in this temperature range: (a) possible antiferromagnetic inter-chain interaction and (b) demagnetization leading to a decrease in the measured χ. For this reason, the estimation given above is a lower limit of n.
2.4.3. Dynamic Magnetic Properties
To examine the magnetic dynamics of 1 and 2, the temperature-dependent and frequency-dependent ac susceptibilities were measured.
Below 6 and 5 K for 1 and 2, respectively, the ac susceptibility shows a distinction between different frequencies, indicating slow magnetization relaxation. In Figure 6, the temperature dependence of the ac susceptibility measured at zero dc field for different ac field frequencies is presented. The imaginary part of the ac susceptibility χ″(T) shows maxima below 5 and 6 K, shifting with the change of the ac drive field frequency υ, and retains the shape that is usual for a temperature induced relaxation process. The Mydosh parameter α, defined as the temperature shift of χ′(T) peak position on a decade of frequency ΔTm/[TmΔlog(υ)], remains around 0.10 in both cases. Such a value is above the range typical for spin glasses and is closer to the values for superparamagnets [136].
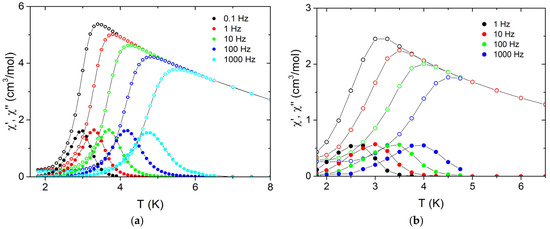
Figure 6.
Real (empty dots) and imaginary (full dots) parts of ac susceptibility for 1 (a) and 2 (b) measured at different ac frequencies using Hac = 3 Oe in zero dc field. Solid lines are to guide the eye.
For a deeper understanding of the relaxation processes, the ac susceptibility was studied over the frequency range 0.1–1000 Hz at low temperatures. These data are presented in Figure 7. The frequency dependent susceptibility measured at constant temperatures was used to determine the relaxation time at each temperature. The generalized Debye relaxation model [137] was used to fit χ′(υ) and χ″(υ) simultaneously (Equation (3)) (solid lines in Figure 7).
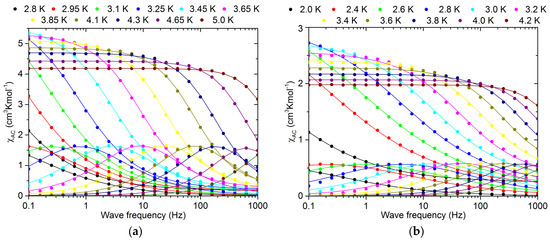
Figure 7.
ac susceptibility measured for 1 (a) and 2 (b) at selected temperatures versus ac frequency. Solid lines were fitted simultaneously to χ’(ν) and χ’’(ν) curves using a generalized Debye relaxation model.
At each temperature, the fitted parameters were χ0 and χ∞, the relaxation time τ and the parameter α, which describes the distribution of relaxation times. The values of α were in the range 0.11–0.58 for 1 and 0.09–0.47 for 2 (Tables S4 and S5), confirming the good quality of the sample and indicating the SCM nature of both compounds.
The relaxation times in the temperature range from 2.0 to 5.0 K for 1 and 1.8 to 4.2 K for 2, obtained from the ac (Figure 7) and dc (Figure S7) susceptibility analysis, are presented in Figure 8 and Figure S8. The dependence ln(τ)–(1/T) deviates from the straight line of the Arrhenius law. This is a feature of experimentally studied SCMs with finite chains, for which, below the crossover temperature T*, the probability of relaxation arising from the ends of chains becomes important, changing the relaxation barrier [138]. Above T*, where the correlation length ξ is lower than the average chain length l = na, the relaxation barrier is equal to Δτ1 = ΔA + 2Δξ, where ΔA is the anisotropy energy of a single spin unit. Below T*, the relaxation barrier is reduced and equal Δτ2 = Δτ1 + Δξ in the low temperature limit. The values of Δτ1 and Δτ2 are usually obtained from two linear regions of the ln(τ)–(1/T) dependence much above—and much below—T*, respectively. To obtain both relaxation barriers using all data points also close to T*, we used the relation derived by Luscombe et al. for the finite Ising chain [138]. The finite length l of the chain shortens the relaxation time by the factor f(l/ξ):
where f(x) = (1 + w2/x2)−1, and w is the solution of the equation,
τ = τ01 {exp(Δτ1/kB)} f(l/ξ),
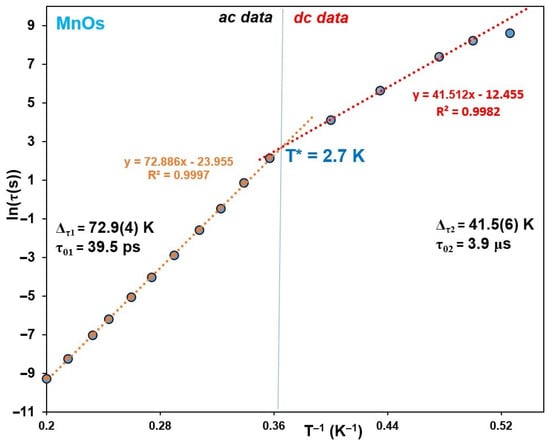
Figure 8.
Relaxation time of 1 derived from the ac data (left) and time dependent dc magnetization (right). The dotted lines correspond to the fit according to the Arrhenius law: τ = τ01·exp(Δτ1/kBT). T* represents a crossover temperature.
Together with temperature dependence of the correlation length Equation (4) allows the calculation of τ(T) as the function of parameters Δξ, Δτ1, τ01, and l/a. Equation (5) was solved numerically using the bisection method for x > 10−3, while the approximation w = (2x)1/2 was used for small values of x < 10−3.
It is worth noting that the crossover temperatures T* of 2.7 and 2.3 K for 1 and 2, respectively—which were obtained from the intersection of the linear lines in Figure 8 and Figure S8—are very close to those of 3.7 and 2.65 K, respectively, calculated from ln(χT) vs. T−1 dependencies for the susceptibility data collected at the 0 dc and 3 Oe ac fields at 1 Hz below 9 and 4.2 K for 1 and 2, respectively (Figure S9). Moreover, they are in good agreement with the magnetization blocking temperatures TB ≈ 2.8 and 2.1 K found when registering hysteresis at different temperatures see Figure S10.
The appearance of slow relaxations in 1 and 2 was also confirmed by presence of the hysteresis loops opens below 2.8 and 2.1 K respectively. Their coercive field grows with decreasing temperature. There are no additional steps on the M(H) curves (Figure S10). Such a behavior is expected for SCMs, contrary to SMMs, where the quantum tunneling leads to a faster relaxation at the specific fields. Again, this effect is typically observed for SCMs [77].
2.5. Comparison of SCM Parameters of 1, 2 and [Mn(SB2+)Fe(CN)6]·4H2O (3)
The parameters related to SCM behavior of 1 and 2 are summarized in Table 2 and compared with those of 3 [77].

Table 2.
Single chain magnet related parameters for 1, 2, and their Fe congener [77] (in K if not specified).
For the chain 1, the total energy barrier for an infinite chain is Δτ1 = ΔA + 2Δξ = 73 K at high temperature (Figure 8). However, at low temperature for a finite size chain regime, Δτ2 = ΔA + Δξ = 41.5 K, giving Δξ = 31.5 K, which is considerably larger than Δξ = 20.09 K obtained from the ln(χT) vs. T−1 plot (Figure 5). On the other hand, the intrinsic anisotropic barrier ΔA = Δτ1 − 2Δξ = 10 K obtained from the plot in Figure 8 is much smaller than ΔA = Δτ1−2Δξ = 32.82 K, calculated with Δξ = 20.09, resulting from the ln(χT) vs. T−1 dependence. This indicates that the SCM relaxation mechanism in 1 cannot be described in the frame of the traditional anisotropic Heisenberg SCM model or Glauber model. The reason lies in the interplay of two independent sources of magnetic anisotropy, i.e., single-ion ZFS anisotropy of MnIII ions and strong three-axis exchange anisotropy in the Os-CN-Mn linkages. The same also applies to the ruthenium chain of compound 2, and quantitative estimates of its SCM parameters ΔA and Δξ made from the data of Figure S8 are presented in Table 2. Similar unconventional SCM behavior was previously reported for MnII(H2Dapsc)-FeIII(CN)6 chain complex based on [FeIII(CN)6]3− units with unquenched orbital angular momentum featuring highly anisotropic spin coupling [80].
3. Conclusions
Two novel heterometallic 1D coordination polymers {[MnIII(SB2+)MIII(CN)6]·4H2O}n (SB2+ = N,N′-ethylenebis(5-trimethylammoniomethylsalicylideneiminate) based on orbitally degenerate cyanidometallates [OsIII(CN)6]3− (1) and [RuIII(CN)6]3− (2) and MnIII Schiff base complexes were synthesized and characterized structurally and magnetically. Their crystal structure consists of electrically neutral, well-isolated chains composed of alternating [MIII(CN)6]3− anions and square planar [MnIII(SB2+)]3+ cations bridged by cyanide metalloligands. dc and ac magnetic measurements reveal SCM behavior of the compounds with the energy barriers of Δτ1/kB = 73 K, Δτ2/kB = 41.5 K (1) and Δτ1/kB = 51 K, Δτ2/kB = 27 K (2). The blocking temperatures of TB = 2.8 K, 2.1, and magnetic hysteresis with a coercive field (at 1.8 K) of 8000 Oe and 1600 were found for 1 and 2, respectively. For the first time, it was shown that the SCM behavior of 1 and 2 originates from a complicated interplay of two independent sources of magnetic anisotropy in the ruthenium and osmium chains, namely, the single-ion ZFS magnetic anisotropy of MnIII ions and the anisotropic three-axis spin coupling SMnJSM = −JxSMnxSMx–JySMnySMy–JzSMnzSMz in the cyanide-bridged MIII-CN-Mn fragments. This is a result of the triple orbital degeneracy of the 2T2g(nd5) ground state of [OsIII(CN)6]3− and [RuIII(CN)6]3− complexes with unquenched orbital angular momentum. Anisotropic exchange parameters Jx, Jy, Jz obtained from our theoretical calculations (Jx = −22, Jy = +28, Jz = −26 cm−1 for osmium compound 1) are remarkably consistent with those previously reported for discrete trinuclear MnIII2OsIII clusters with similar molecular structure, such as Jx = −18, Jy = +35, Jz = −33 cm−1 [119] and Jx = −23.5, Jy = +32.0, Jz = −25.9 cm−1 [121]. Our theoretical calculations and analysis of magnetic relaxation parameters ΔA and Δξ have distinctly showed that these new 1D coordination polymers 1 and 2 are SCMs beyond the Glauber model and the anisotropic Heisenberg SCM model.
4. Materials and Methods
All chemicals were of reagent grade and were used as purchased. The complex [Mn(SB2+)(H2O)2](ClO4)3·H2O [132] and hexacyanometallates [108] (Ph4P)3[Os(CN)6], (n-Bu4N)3[Ru(CN)6] and (n-Bu4N)3[Os(CN)6] were prepared according to procedures in the literature [55,128]. Elemental analyses were performed by means of the Euro-Vector 3000 analyzer (Eurovector, Redavalle, Italy). IR spectra were recorded using a Scimitar FTS 2000 spectrophotometer (Digilab LLC, Canton, MA, USA) (KBr pellets) and Nicolet 300 FT-IR spectrometer in reflectance mode (Thermo Electron Scientific Instruments LLC, Madison, WI, USA). Powder X-ray measurements were performed with CuKα radiation (λ = 1.5418 Å) with an Expert-Pro powder diffractometer (PANalytical Inc., Almelo, The Netherlands). Magnetic measurements were performed using the QD MPMS 5XL magnetometer (Quantum Design, Inc., San Diego, CA, USA). The magnetic signal of the sample holder and the diamagnetic correction of the sample were taken into account. A check for small ferromagnetic impurities was performed at room temperature. The powder sample was restrained in cyanoacrylate glue for low-temperature ac measurements.
[Mn(SB2+)Os(CN)6]·4H2O (1). A solution of (Ph4P)3[Os(CN)6] (95 mg, 0.07 mmol) in H2O:CH3CN (1:3, 3 mL) was added dropwise to a solution of [Mn(SB2+)(H2O)2] (ClO4)3·H2O (47 mg, 0.07 mmol) in the same solvent (3 mL). A reaction mixture, permanently agitated, was heated gradually up to boiling and then cooled down. A dark olive powder was filtered off; washed a few times with the solvent, acetonitrile, and ether; and air-dried. Yield: 62.5 mg (95.3 %). Calculated for Mn(C24H34N4O2)(H2O)5.4Os(CN)6 (Ph4PClO4)0.1: C, 40.83; H, 4.95; N, 14.70; found: C, 40.7; H, 4.85; N, 14.7; IR (reflectance): = 2112, 2088 and 2049 (νC≡N), 1632 cm−1 (νC=N). The final products prepared starting from (Ph4P)3[Os(CN)6] are slightly contaminated by Ph4PClO4 because of its poor solubility in MeCN. For this reason, it is better to use (n-Bu4N)3[Os(CN)6] as a Os-precursor.
[Mn(SB2+)Ru(CN)6]·4H2O (2). This compound was synthesized analogously to [Mn(SB2+)Os(CN)6]·4H2O with the difference that (n-Bu4N)3[Ru(CN)6] was used as a starting material, and a reaction mixture was not heated during precipitation of the final product. Yield 92 %. Calculated for C28H28MnN10O2Ru·4(H2O): C, 44.33; H, 5.46; N, 17.23; found: C, 44.7; H, 4.98; N, 17.5; IR (reflectance): ῡ = 2100, 2089 and 2048 (νC≡N), 1630.8 cm−1 (νC=N).
Crystallographic Details. Single crystals of 1 were obtained via slow diffusion of the starting solutions used for a bulk powder sample.
The single crystals covered by a drop of the oil were directly placed into a stream of cold nitrogen with the precentered goniometer head with CryoMount® (Chelan County, WA, USA) and attached to the goniometer of a diffractometer. The data for 1 were collected on an Agilent Technologies Gemini diffractometer equipped with an AtlasS2 CCD detector and a CuKα microfocus source using 0.5° ω scans. The data processing was performed with the CrysAlis software package (CrysAlisPro 1.171.40.47a, Rigaku Oxford Diffraction, 2019). Empirical absorption correction was applied based on the equivalent reflections. The structure was solved by direct methods with SHELXS [139] and refined by full-matrix least squares method against F2 in anisotropic approximation using the SHELXL-2014/6 package (Shelx, Göttingen, Germany). All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms on the organic part were placed in idealized positions and refined isotropically according to the riding model. The hydrogen atoms of the water molecule were located from the electron density map and refined in a riding model (Uiso(H) = 1.5 Uiso(O)) with only one distance of 4 (O2w-H21) restrained to 0.82(2) Å. The residual electron density has to chemical meaning. Crystallographic data and further details of the diffraction experiments are given in Table S1.
Theoretical calculations details. Magnetic properties of chain compounds 1 and 2 were analyzed in terms of the anisotropic spin Hamiltonian in Equation (2), which involves contributions from the single-ion ZFS anisotropy of MnIII ions and three-axis anisotropic spin coupling SMJSMn = −JxSMxSMnx—JySMySMny—JzSMzSMnz in the MIII-CN-MnIII linkaged (M = Ru, Os). Computational details are described in Supporting Information. Specific calculations for 1 and 2 were performed with routines written by V.S. Mironov.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031516/s1, Table S1: Experimental details for 1; Table S2: Selected geometric parameters; Figure S1: Hydrogen bonding system in 1; Table S3: Some crystallographic parameters for [Mn(SB2−)M(CN)6]·4H2O; Figure S2: Simulated (red) and experimental X-ray powder pattern for neutral Mn-Os chain polymer; Figure S3: Simulated and experimental X-ray powder patterns (red) for neutral MnIII-MIII(CN)6 chain polymers (MIII = Fe, Ru, Os) with additional reflections (green rectangle) from (–1, 1, 2) plane consisted of metal atoms; Figure S4: Magnetic susceptibility times temperature vs. T at 1 kOe and lower fields for 1 in 3 Oe (left) and 2 in 15 Oe (right); Figure S5: Calculated components χx, χy, χz of magnetic susceptibility of the Mn-Os chain (1). Below 50 K, magnetic susceptibility is strongly anisotropic; Figure S6: Experimental (yellow circles) and simulated (solid blue line) magnetic susceptibility χT of 2. The χT curve was simulated with the spin Hamiltonian (Equation (2)) involving anisotropic 3-axes spin coupling SMnJSRu = −JxSMnxSRux −JySMnySRuy −JzSMnzSRuz. The best fit is obtained at the set of parameters Jx = −18, Jy = +20, Jz = −18 cm−1, gMn= 2.00, gRu =1.80, DMn = -4.0 cm−1. Calculations are performed for a six-membered fragment {Mn-Ru}3 of the heterometallic chain of 2 with cyclic boundary conditions for the Mn-Ru spin coupling, as shown in the inset; Table S4: Cole–Cole fits parameters for 1; Table S5: Cole–Cole fits parameters for 2; Figure S7: Time dependence of magnetization relaxation for 1 (left) and 2 (right) following the field change from 10 to 0 kOe at constant temperatures of 1.8 ÷ 2.5 K; Figure S8: Relaxation time of 2 derived from the ac data (left) and time dependent dc magnetization (right). The dotted lines correspond to the linear fit according to the Arrhenius law: τ = τ01Exp(Δτ1/kBT); Figure S9: Crossover temperatures T* obtained from the ln(χT) vs. T−1 dependencies for the susceptibility data collected at 0 dc and 3 Oe ac field at 1 Hz below 9 and 4.2 K for 1 (left) and 2 (right), respectively; Figure S10: Magnetization versus field for 1 (top) and 2 (bottom)—hysteresis loops. Solid lines are to guide the eye; Figure S11: Zero-field cooling/field cooling magnetic susceptibility vs. temperature for 1 and 2 in 15 Oe with a temperature sweep rate of 2 K/min; Figure S12: FTIR (ATR) spectra of 1 (top) and 2 (bottom).
Author Contributions
Conceptualization, K.E.V.; funding acquisition, K.E.V.; investigation, K.E.V., V.S.M. and E.V.P.; supervision K.E.V.; visualization, K.E.V. and V.S.M.; writing—original draft, K.E.V., V.S.M. and E.V.P.; writing—review and editing, K.E.V., V.S.M. and E.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (No. 121031700321-3) for the synthesis, chemical and structural characterization, and SCM properties explanations. This work was supported by the Ministry of Science and Higher Education within the State assignment FSRC “Crystallography and Photonics” RAS (No. 075-01025-22-00) for the static magnetic properties study and its theoretical analysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The complete crystallographic data for 1 have been deposited with the Cambridge Crystallographic Data Centre under the reference numbers CCDC 1958305. These data can be obtained, free of charge, from the CCDC via www.ccdc.cam.ac.uk/structures (accessed on 29 September 2022).
Acknowledgments
K.E.V is very grateful to Michal Rams for collecting the magnetic data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic Bistability in a Metal-Ion Cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Coronado, E.; Delhaès, P.; Gatteschi, D.; Miller, J.S. (Eds.) Molecular Magnetism: From Molecular Assemblies to the Devices; Springer: Dordrecht, The Netherlands, 1996; ISBN 978-90-481-4724-3. [Google Scholar]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef]
- Sessoli, R.; Tsai, H.L.; Schake, A.R.; Wang, S.; Vincent, J.B.; Folting, K.; Gatteschi, D.; Christou, G.; Hendrickson, D.N. High-Spin Molecules: [Mn12O12(O2CR)16(H2O)4]. J. Am. Chem. Soc. 1993, 115, 1804–1816. [Google Scholar] [CrossRef]
- Coronado, E.; Dunbar, K.R. Preface for the Forum on Molecular Magnetism: The Role of Inorganic Chemistry. Inorg. Chem. 2009, 48, 3293–3295. [Google Scholar] [CrossRef]
- Introduction to the Themed Issue on Molecular Magnets. Dalt. Trans. 2010, 39, 4671. [CrossRef]
- Dunbar, K.R. Editorial for the Virtual Issue on Quantum Molecular Magnets. Inorg. Chem. 2012, 51, 12055–12058. [Google Scholar] [CrossRef]
- Thompson, L.K. Magnetism—Molecular and Supramolecular Perspectives. Coord. Chem. Rev. 2005, 249, 2549–2730. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular Spintronics Using Single-Molecule Magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Leuenberger, M.N.; Loss, D. Quantum Computing in Molecular Magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic Memory of a Single-Molecule Quantum Magnet Wired to a Gold Surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Layfield, R.A. Organometallic Single-Molecule Magnets. Organometallics 2014, 33, 1084–1099. [Google Scholar] [CrossRef]
- Coronado, E. Molecular Magnetism: From Chemical Design to Spin Control in Molecules, Materials and Devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Barra, A.L.; Brunel, L.C.; Guillot, M. Alternating Current Susceptibility, High Field Magnetization, and Millimeter Band EPR Evidence for a Ground S = 10 State in [Mn12O12(Ch3COO)16(H2O)4]·2CH3COOH·4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006; ISBN 9780198567530. [Google Scholar]
- Habib, F.; Murugesu, M. Lessons Learned from Dinuclear Lanthanide Nano-Magnets. Chem. Soc. Rev. 2013, 42, 3278. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, P. Lanthanide Single Molecule Magnets; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-46998-9. [Google Scholar]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-Ac to Dysprosium Metallocenes, a Travel in Time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Wang, J.-H.; Li, Z.-Y.; Yamashita, M.; Bu, X.-H. Recent Progress on Cyano-Bridged Transition-Metal-Based Single-Molecule Magnets and Single-Chain Magnets. Coord. Chem. Rev. 2021, 428, 213617. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-Nitronyl Nitroxide Chains as Molecular Magnetic Nanowires. Angew. Chem. Int. Ed. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Clérac, R.; Miyasaka, H.; Yamashita, M.; Coulon, C. Evidence for Single-Chain Magnet Behavior in a MnIII−NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef]
- Bogani, L.; Vindigni, A.; Sessoli, R.; Gatteschi, D. Single Chain Magnets: Where to from Here? J. Mater. Chem. 2008, 18, 4750. [Google Scholar] [CrossRef]
- Vaz, M.G.F.; Cassaro, R.A.A.; Akpinar, H.; Schlueter, J.A.; Lahti, P.M.; Novak, M.A. A Cobalt Pyrenylnitronylnitroxide Single-Chain Magnet with High Coercivity and Record Blocking Temperature. Chem. A Eur. J. 2014, 20, 5460–5467. [Google Scholar] [CrossRef]
- Coulon, C.; Miyasaka, H.; Clérac, R. Single-Chain Magnets: Theoretical Approach and Experimental Systems BT—Single-Molecule Magnets and Related Phenomena; Winpenny, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 163–206. ISBN 978-3-540-33240-4. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic Assemblies Based on Mn(III) Salen Analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Luo, Q.-C.; Zheng, Y.-Z. Methods and Models of Theoretical Calculation for Single-Molecule Magnets. Magnetochemistry 2021, 7, 107. [Google Scholar] [CrossRef]
- Moreno-Pineda, E.; Wernsdorfer, W. Measuring Molecular Magnets for Quantum Technologies. Nat. Rev. Phys. 2021, 3, 645–659. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Ishikawa, R.; Breedlove, B.; Yamashita, M. Single-Chain Magnets: Beyond the Glauber Model. RSC Adv. 2013, 3, 3772. [Google Scholar] [CrossRef]
- Coulon, C.; Clérac, R.; Lecren, L.; Wernsdorfer, W.; Miyasaka, H. Glauber Dynamics in a Single-Chain Magnet: From Theory to Real Systems. Phys. Rev. B 2004, 69, 132408. [Google Scholar] [CrossRef]
- Glauber, R.J. Time-Dependent Statistics of the Ising Model. J. Math. Phys. 1963, 4, 294–307. [Google Scholar] [CrossRef]
- Barbara, B. Propriétés Des Parois Étroites Dans Les Substances Ferromagnétiques à Forte Anisotropie. J. Phys. 1973, 34, 1039–1046. [Google Scholar] [CrossRef]
- Barbara, B. Magnetization Processes in High Anisotropy Systems. J. Magn. Magn. Mater. 1994, 129, 79–86. [Google Scholar] [CrossRef]
- Pianet, V.; Urdampilleta, M.; Colin, T.; Clérac, R.; Coulon, C. Domain Walls in Single-Chain Magnets. Phys. Rev. B 2017, 96, 214429. [Google Scholar] [CrossRef]
- Nakamura, K.; Sasada, T. Statistical Mechanics of Classical One-Dimensional Heisenberg Ferromagnets with Single-Site Anisotropy. J. Phys. C Solid State Phys. 1978, 11, 331–343. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Vindigni, A.; Sessoli, R.; Coulon, C.; Clérac, R. Single-Chain Magnets. In Molecular Magnetic Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 131–159. [Google Scholar] [CrossRef]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-Chain Magnets and Related Systems. Mol. Nanomagnets Relat. Phenom. 2014, 143–184. [Google Scholar] [CrossRef]
- Lescouëzec, R.; Toma, L.M.; Vaissermann, J.; Verdaguer, M.; Delgado, F.S.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Design of Single Chain Magnets through Cyanide-Bearing Six-Coordinate Complexes. Coord. Chem. Rev. 2005, 249, 2691–2729. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, Z.-M.; Gao, S. Constructing Magnetic Molecular Solids by Employing Three-Atom Ligands as Bridges. Chem. Commun. 2008, 281–294. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A Toolbox of Building Blocks, Linkers and Crystallisation Methods Used to Generate Single-Chain Magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards Single-Chain Magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Ceglarska, M.; Böhme, M.; Neumann, T.; Plass, W.; Näther, C.; Rams, M. Magnetic Investigations of Monocrystalline [Co(NCS)2(L)2]N: New Insights into Single-Chain Relaxations. Phys. Chem. Chem. Phys. 2021, 23, 10281–10289. [Google Scholar] [CrossRef]
- Bretosh, K.; Béreau, V.; Duhayon, C.; Pichon, C.; Sutter, J.-P. A Ferromagnetic Ni(II)–Cr(III) Single-Chain Magnet Based on Pentagonal Bipyramidal Building Units. Inorg. Chem. Front. 2020, 7, 1503–1511. [Google Scholar] [CrossRef]
- Cassaro, R.A.A.; Reis, S.G.; Araujo, T.S.; Lahti, P.M.; Novak, M.A.; Vaz, M.G.F. A Single-Chain Magnet with a Very High Blocking Temperature and a Strong Coercive Field. Inorg. Chem. 2015, 54, 9381–9383. [Google Scholar] [CrossRef]
- Wei, R.-M.; Cao, F.; Li, J.; Yang, L.; Han, Y.; Zhang, X.-L.; Zhang, Z.; Wang, X.-Y.; Song, Y. Single-Chain Magnets Based on Octacyanotungstate with the Highest Energy Barriers for Cyanide Compounds. Sci. Rep. 2016, 6, 24372. [Google Scholar] [CrossRef]
- Layfield, R.A.; Murugesu, M. (Eds.) Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; ISBN 9783527673476. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Low-Dimensional Heterometallic Assemblies Involving Orbitally Degenerate Cyanometallate and Displaying Slow Magnetic Dynamics. J. Magn. Magn. Mater. 2018, 459, 71–77. [Google Scholar] [CrossRef]
- Juráková, J.; Šalitroš, I. Co(II) Single-Ion Magnets: Synthesis, Structure, and Magnetic Properties. Mon. Chem. Chem. Mon. 2022, 153, 1001–1036. [Google Scholar] [CrossRef]
- Murrie, M. Cobalt(Ii) Single-Molecule Magnets. Chem. Soc. Rev. 2010, 39, 1986. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-Molecule Magnet Engineering: Building-Block Approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar] [CrossRef]
- Gómez-Coca, S.; Aravena, D.; Morales, R.; Ruiz, E. Large Magnetic Anisotropy in Mononuclear Metal Complexes. Coord. Chem. Rev. 2015, 289–290, 379–392. [Google Scholar] [CrossRef]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic Anisotropy in Two- to Eight-Coordinated Transition–Metal Complexes: Recent Developments in Molecular Magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Homoleptic Osmium Cyanide Complexes: Synthesis and Perspective Application in Molecular Magnetism. In Osmium: Synthesis Characterization and Applications; Wise, G., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 43–78. ISBN 978-1-63483-517-6. Available online: https://novapublishers.com/shop/osmium-synthesis-characterization-and-applications/ (accessed on 30 December 2022).
- Craig, G.A.; Murrie, M. 3d Single-Ion Magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef]
- Frost, J.M.; Harriman, K.L.M.; Murugesu, M. The Rise of 3-d Single-Ion Magnets in Molecular Magnetism: Towards Materials from Molecules? Chem. Sci. 2016, 7, 2470–2491. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Wang, B.-W.; Gao, S. Advances in Lanthanide Single-Ion Magnets BT—Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 111–141. ISBN 978-3-662-45723-8. [Google Scholar] [CrossRef]
- Pointillart, F.; Cador, O.; Le Guennic, B.; Ouahab, L. Uncommon Lanthanide Ions in Purely 4f Single Molecule Magnets. Coord. Chem. Rev. 2017, 346, 150–175. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry Strategies for High Performance Lanthanide-Based Single-Molecule Magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular Magnetism of Lanthanide: Advances and Perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Sangregorio, C.; Sessoli, R.; Gatteschi, D. Molecular Engineering for Single-Chain-Magnet Behavior in a One-Dimensional Dysprosium-Nitronyl Nitroxide Compound. Angew. Chem. Int. Ed. 2005, 44, 5817–5821. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A Family of Rare-Earth-Based Single Chain Magnets: Playing with Anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shi, W.; Cheng, P. Rational Design and Synthesis of a Chiral Lanthanide-Radical Single-Chain Magnet. Inorg. Chem. 2018, 57, 13409–13414. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, H.-D.; Yang, M.; Sun, J.; Li, L.-C.; Sutter, J.-P. Improved Single-Chain-Magnet Behavior in a Biradical-Based Nitronyl Nitroxide-Cu–Dy Chain. Chem. Commun. 2019, 55, 3398–3401. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Rouzières, M.; Clérac, R.; Brooker, S. Discrete versus Chain Assembly: Hexacyanometallate Linkers and Macrocyclic {3d–4f} Single-Molecule Magnet Building Blocks. Inorg. Chem. 2019, 58, 5543–5554. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bag, P.; Kalita, P.; Chandrasekhar, V. Heterometallic CuII–LnIII Complexes: Single Molecule Magnets and Magnetic Refrigerants. Coord. Chem. Rev. 2021, 432, 213707. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef]
- Drahoš, B.; Herchel, R.; Trávníček, Z. Impact of Halogenido Coligands on Magnetic Anisotropy in Seven-Coordinate Co(II) Complexes. Inorg. Chem. 2017, 56, 5076–5088. [Google Scholar] [CrossRef]
- Shao, D.; Shi, L.; Zhang, S.-L.; Zhao, X.-H.; Wu, D.-Q.; Wei, X.-Q.; Wang, X.-Y. Syntheses, Structures, and Magnetic Properties of Three New Chain Compounds Based on a Pentagonal Bipyramidal Co(II) Building Block. CrystEngComm 2016, 18, 4150–4157. [Google Scholar] [CrossRef]
- Yao, X.-N.; Du, J.-Z.; Zhang, Y.-Q.; Leng, X.-B.; Yang, M.-W.; Jiang, S.-D.; Wang, Z.-X.; Ouyang, Z.-W.; Deng, L.; Wang, B.-W.; et al. Two-Coordinate Co(II) Imido Complexes as Outstanding Single-Molecule Magnets. J. Am. Chem. Soc. 2017, 139, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Re, N.; Gallo, E.; Floriani, C.; Miyasaka, H.; Matsumoto, N. Magnetic Properties of a One-Dimensional Ferromagnet Containing a Mn(III)−NC−Fe(III) Linkage: Synthesis and Crystal Structure of a Chainlike [Mn(Acacen)Fe(CN)6]n2n− Polyanion. Inorg. Chem. 1996, 35, 6004–6008. [Google Scholar] [CrossRef]
- Ferbinteanu, M.; Miyasaka, H.; Wernsdorfer, W.; Nakata, K.; Sugiura, K.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet (NEt4)[Mn2(5-MeOsalen)2Fe(CN)6] Made of MnIII−FeIII−MnIII Trinuclear Single-Molecule Magnet with an S = 9/2 Spin Ground State. J. Am. Chem. Soc. 2005, 127, 3090–3099. [Google Scholar] [CrossRef]
- Miyasaka, H.; Madanbashi, T.; Saitoh, A.; Motokawa, N.; Ishikawa, R.; Yamashita, M.; Bahr, S.; Wernsdorfer, W.; Clérac, R. Cyano-Bridged MnIII-MIII Single-Chain Magnets with MIII=CoIII, FeIII, MnIII, and CrIII. Chem. A Eur. J. 2012, 18, 3942–3954. [Google Scholar] [CrossRef]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorfer, W.; Vostrikova, K.E. Magnetic Relaxation of 1D Coordination Polymers (X)2[Mn(acacen)Fe(CN)6], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10300. [Google Scholar] [CrossRef]
- Aguilà, D.; Jeannin, O.; Fourmigué, M.; Jeon, I.-R. MnIII–FeIII Heterometallic Compounds within Hydrogen-Bonded Supramolecular Networks Promoted by an [Fe(CN)5(CNH)]2− Building Block: Structural and Magnetic Properties. Inorg. Chem. 2018, 57, 7892–7903. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Sasnovskaya, V.D.; Talantsev, A.D.; Morgunov, R.B.; Mironov, V.S.; Yagubskii, E.B. Slow Magnetic Relaxation, Antiferromagnetic Ordering, and Metamagnetism in MnII(H2Dapsc)-FeIII(CN)6 Chain Complex with Highly Anisotropic Fe-CN-Mn Spin Coupling. Chem. A Eur. J. 2019, 25, 14583–14597. [Google Scholar] [CrossRef]
- Lutz, P.; Aguilà, D.; Mondal, A.; Pinkowicz, D.; Marx, R.; Neugebauer, P.; Fåk, B.; Ollivier, J.; Clérac, R.; van Slageren, J. Elementary Excitations in Single-Chain Magnets. Phys. Rev. B 2017, 96, 094415. [Google Scholar] [CrossRef]
- Tregenna-Piggott, P.L.W.; Sheptyakov, D.; Keller, L.; Klokishner, S.I.; Ostrovsky, S.M.; Palii, A.V.; Reu, O.S.; Bendix, J.; Brock-Nannestad, T.; Pedersen, K.; et al. Single-Ion Anisotropy and Exchange Interactions in the Cyano-Bridged Trimers MnIII2MIII(CN)6 (MIII = Co, Cr, Fe) Species Incorporating [Mn(5-Brsalen)]+ Units: An Inelastic Neutron Scattering and Magnetic Susceptibility Study. Inorg. Chem. 2009, 48, 128–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Funck, E.; Dunbar, K.R. A Single-Chain Magnet Tape Based on Hexacyanomanganate(III). Angew. Chem. Int. Ed. 2015, 54, 5583–5587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Zhao, X.-H.; Wang, X.-Y. Syntheses, Structures, and Magnetic Properties of Three New Cyano-Bridged Complexes Based on the [Mn(CN)6]3− Building Block. Dalt. Trans. 2015, 44, 15189–15197. [Google Scholar] [CrossRef]
- Sasnovskaya, V.D.; Kopotkov, V.A.; Talantsev, A.D.; Morgunov, R.B.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Mironov, V.S. Synthesis, Structure, and Magnetic Properties of 1D{[MnIII(CN)6][MnII(Dapsc)]}n Coordination Polymers: Origin of Unconventional Single-Chain Magnet Behavior. Inorg. Chem. 2017, 56, 8926–8943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular Magnetic Materials Based on 4d and 5d Transition Metals. Chem. Soc. Rev. 2011, 40, 3213. [Google Scholar] [CrossRef]
- Toma, L.M.; Toma, L.D.; Delgado, F.S.; Ruiz-Perez, C.; Sletten, J.; Cano, J.; Clemenejuan-Juan, J.M.; Lloret, F.; Julve, M. Trans-Dicyanobis(Acetylacetonato)Ruthenate(III) as a Precursor to Build Novel Cyanide-Bridged RuIII–MII Bimetallic Compounds [M=Co and Ni]. Coord. Chem. Rev. 2006, 250, 2176–2193. [Google Scholar] [CrossRef]
- Yeung, W.-F.; Lau, P.-H.; Lau, T.-C.; Wei, H.-Y.; Sun, H.-L.; Gao, S.; Chen, Z.-D.; Wong, W.-T. Heterometallic MIIRuIII2 Compounds Constructed from Trans -[Ru(Salen)(CN)2]− and Trans—[Ru(acac)2(CN)2]−. Synthesis, Structures, Magnetic Properties, and Density Functional Theoretical Study. Inorg. Chem. 2005, 44, 6579–6590. [Google Scholar] [CrossRef]
- Guo, J.-F.; Yeung, W.-F.; Lau, P.-H.; Wang, X.-T.; Gao, S.; Wong, W.-T.; Chui, S.S.-Y.; Che, C.-M.; Wong, W.-Y.; Lau, T.-C. Trans-[OsIII(Salen)(CN)2]: A New Paramagnetic Building Block for the Construction of Molecule-Based Magnetic Materials. Inorg. Chem. 2010, 49, 1607–1614. [Google Scholar] [CrossRef]
- Charytanowicz, T.; Jankowski, R.; Zychowicz, M.; Chorazy, S.; Sieklucka, B. The Rationalized Pathway from Field-Induced Slow Magnetic Relaxation in CoII–WIV Chains to Single-Chain Magnetism in Isotopological CoII–WV Analogues. Inorg. Chem. Front. 2022, 9, 1152–1170. [Google Scholar] [CrossRef]
- Korzeniak, T.; Nowicka, B.; Sieklucka, B. Hybrid Organic–Inorganic Cyanide-Bridged Networks BT—Organometallic Magnets; Chandrasekhar, V., Pointillart, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–34. ISBN 978-3-030-26009-5. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Wernsdorfer, W.; Vostrikova, K.E. Slow Magnetic Relaxation in Neutral 0D and 1D Assemblies of a Mn(III) Schiff Base Complex and Heptacyanorhenate(IV). Magnetochemistry 2022, 8, 126. [Google Scholar] [CrossRef]
- Nowicka, B.; Korzeniak, T.; Stefańczyk, O.; Pinkowicz, D.; Chorąży, S.; Podgajny, R.; Sieklucka, B. The Impact of Ligands upon Topology and Functionality of Octacyanidometallate-Based Assemblies. Coord. Chem. Rev. 2012, 256, 1946–1971. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nowicka, B.; Chorazy, S.; Reczyński, M.; Sieklucka, B. Magnetic Clusters Based on Octacyanidometallates. Inorg. Chem. Front. 2015, 2, 10–27. [Google Scholar] [CrossRef]
- Freedman, D.E.; Jenkins, D.M.; Long, J.R. Strong Magnetic Exchange Coupling in the Cyano-Bridged Coordination Clusters [(PY5Me2)4V4M(CN)6]5+ (M = Cr, Mo). Chem. Commun. 2009, 4829. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Southerland, H.; Wang, X.-Y.; Dunbar, K.R. Record Antiferromagnetic Coupling for a 3d/4d Cyanide-Bridged Compound. J. Am. Chem. Soc. 2014, 136, 9922–9924. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.D.; Bennett, M.V.; Clérac, R.; Long, J.R. [ReCl4(CN)2]2−: A High Magnetic Anisotropy Building Unit Giving Rise to the Single-Chain Magnets (DMF)4MReCl4(CN)2 (M = Mn, Fe, Co, Ni). J. Am. Chem. Soc. 2010, 132, 3980–3988. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.D.; Coulon, C.; Clérac, R.; Long, J.R. Record Ferromagnetic Exchange through Cyanide and Elucidation of the Magnetic Phase Diagram for a CuIIReIV(CN)2 Chain Compound. J. Am. Chem. Soc. 2011, 133, 123–130. [Google Scholar] [CrossRef]
- Feng, X.; David Harris, T.; Long, J.R. Influence of Structure on Exchange Strength and Relaxation Barrier in a Series of FeIIReIV(CN)2 Single-Chain Magnets. Chem. Sci. 2011, 2, 1688. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Sigrist, M.; Sørensen, M.A.; Barra, A.-L.; Weyhermüller, T.; Piligkos, S.; Thuesen, C.A.; Vinum, M.G.; Mutka, H.; Weihe, H.; et al. [ReF6]2−: A Robust Module for the Design of Molecule-Based Magnetic Materials. Angew. Chem. Int. Ed. 2014, 53, 1351–1354. [Google Scholar] [CrossRef]
- Vostrikova, K.E. Application of the Heptacyanidorhenate(IV) as a Metalloligand in the Design of Molecular Magnets. Magnetochemistry 2022, 8, 189. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Malik, K.M.A.; Soares, A.M.; Gibson, J.F.; Griffith, W.P. The X-Ray Crystal Structure of NaK3[Mo(CN)7]·2H2O and the Structure of Its Anion in Aqueous Solution. Inorg. Chim. Acta 1980, 45, L81–L82. [Google Scholar] [CrossRef]
- Larionova, J.; Clérac, R.; Sanchiz, J.; Kahn, O.; Golhen, S.; Ouahab, L. Ferromagnetic Ordering, Anisotropy, and Spin Reorientation for the Cyano-Bridged Bimetallic Compound Mn2(H2O)5Mo(CN)7·4H2O (α-Phase). J. Am. Chem. Soc. 1998, 120, 13088–13095. [Google Scholar] [CrossRef]
- Bennett, M.V.V.; Long, J.R.R. New Cyanometalate Building Units: Synthesis and Characterization of [Re(CN)7]3− and [Re(CN)8]3−. J. Am. Chem. Soc. 2003, 125, 2394–2395. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Mendizábal, F.; Arratia-Pérez, R. Electronic Structure and Molecular Properties of the Heptacyanorhenate [Re(CN)7]3− and [Re(CN)7]4− Complexes. J. Phys. Chem. A 2006, 110, 1072–1077. [Google Scholar] [CrossRef]
- Bendix, J.; Steenberg, P.; Søtofte, I. Isolation and Molecular Structure of Hexacyanoruthenate(III). Inorg. Chem. 2003, 42, 4510–4512. [Google Scholar] [CrossRef] [PubMed]
- Samsonenko, D.G.; Vostrikova, K.E. Effective Preparation of a Variety of Ruthenium and Osmium Cyanides: Valuable Precursors for Molecular Nanomagnets. Eur. J. Inorg. Chem. 2016, 2016, 1369–1375. [Google Scholar] [CrossRef]
- Albores, P.; Slep, L.D.; Baraldo, L.M.; Baggio, R.; Garland, M.T.; Rentschler, E. Crystal Structure and Electronic and Magnetic Properties of Hexacyanoosmate(III). Inorg. Chem. 2006, 45, 2361–2363. [Google Scholar] [CrossRef]
- Van den Heuvel, W.; Hendrickx, M.F.A.; Ceulemans, A. A CASPT2 Study of the Electronic Spectrum of Hexacyanoosmate(III). Inorg. Chem. 2007, 46, 8032–8037. [Google Scholar] [CrossRef]
- Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. Mechanism of a Strongly Anisotropic MoIII−CN−MnII Spin−Spin Coupling in Molecular Magnets Based on the [Mo(CN)7]4− Heptacyanometalate: A New Strategy for Single-Molecule Magnets with High Blocking Temperatures. J. Am. Chem. Soc. 2003, 125, 9750–9760. [Google Scholar] [CrossRef]
- Mironov, V.S. New Approaches to the Problem of High-Temperature Single-Molecule Magnets. Dokl. Phys. Chem. 2006, 408, 130–136. [Google Scholar] [CrossRef]
- Mironov, V.S. Origin of Dissimilar Single-Molecule Magnet Behavior of Three MnII2MoIII Complexes Based on [MoIII(CN)7]4− Heptacyanomolybdate: Interplay of MoIII –CN–MnII Anisotropic Exchange Interactions. Inorg. Chem. 2015, 54, 11339–11355. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V. Molecular Engineering of High Energy Barrier in Single-Molecule Magnets Based on [MoIII(CN)7]4− and V(II) Complexes. Inorganics 2018, 6, 58. [Google Scholar] [CrossRef]
- Mironov, V.S.; Bazhenova, T.A.; Manakin, Y.V.; Yagubskii, E.B. Pentagonal-Bipyramidal 4d and 5d Complexes with Unquenched Orbital Angular Momentum as a Unique Platform for Advanced Single-Molecule Magnets: Current State and Perspectives. Dalt. Trans. 2023, 52, 509–539. [Google Scholar] [CrossRef]
- Freedman, D.E.; Jenkins, D.M.; Iavarone, A.T.; Long, J.R. A Redox-Switchable Single-Molecule Magnet Incorporating [Re(CN)7]3−. J. Am. Chem. Soc. 2008, 130, 2884–2885. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Freedman, D.E.; Jenkins, D.M.; Harris, T.D.; Iavarone, A.T.; Mathonière, C.; Clérac, R.; Long, J.R. Slow Magnetic Relaxation and Charge-Transfer in Cyano-Bridged Coordination Clusters Incorporating [Re(CN)7]3−/4−. Inorg. Chem. 2010, 49, 8886–8896. [Google Scholar] [CrossRef]
- Shi, L.; Wei, X.; Wang, X.; Wu, D. Research Progress in Molecular Magnetic Materials Based on the [Mo(CN)7]4- Unit. Sci. Sin. Chim. 2020, 50, 1637–1653. [Google Scholar] [CrossRef]
- Hilfiger, M.G.; Shatruk, M.; Prosvirin, A.; Dunbar, K.R. Hexacyanoosmate(III) Chemistry: Preparation and Magnetic Properties of a Pentanuclear Cluster and a Prussian Blue Analogue with Ni(II). Chem. Commun. 2008, 5752. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, J.; Pedersen, K.S.; Schnegg, A.; Holldack, K.; Nehrkorn, J.; Sigrist, M.; Tregenna-Piggott, P.; Mutka, H.; Weihe, H.; Mironov, V.S.; et al. Three-Axis Anisotropic Exchange Coupling in the Single-Molecule Magnets NEt4[MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6] (M=Ru, Os). Chem. A Eur. J. 2013, 19, 3693–3701. [Google Scholar] [CrossRef]
- Hoeke, V.; Stammler, A.; Bögge, H.; Schnack, J.; Glaser, T. Strong and Anisotropic Superexchange in the Single-Molecule Magnet (SMM) [MnIII6OsIII]3+: Promoting SMM Behavior through 3d–5d Transition Metal Substitution. Inorg. Chem. 2014, 53, 257–268. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Southerland, H.I.; Avendaño, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clérac, R.; Nehrkorn, J.; et al. Cyanide Single-Molecule Magnets Exhibiting Solvent Dependent Reversible “On” and “Off” Exchange Bias Behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Qian, K.; Wei, H.-Y.; Wang, X.-Y. A One-Dimensional Magnet Based on [MoIII(CN)7]4−. Inorg. Chem. 2016, 55, 5107–5109. [Google Scholar] [CrossRef]
- Wang, K.; Xia, B.; Wang, Q.-L.; Ma, Y.; Liao, D.-Z.; Tang, J. Slow Magnetic Relaxation Based on the Anisotropic Ising-Type Magnetic Coupling between the MoIII and MnII Centers. Dalt. Trans. 2017, 46, 1042–1046. [Google Scholar] [CrossRef]
- Shi, L.; Shao, D.; Wei, X.; Dunbar, K.R.; Wang, X. Enhanced Single-Chain Magnet Behavior via Anisotropic Exchange in a Cyano-Bridged MoIII–MnII Chain. Angew. Chem. Int. Ed. 2020, 59, 10379–10384. [Google Scholar] [CrossRef]
- Samsonenko, D.G.; Paulsen, C.; Lhotel, E.; Mironov, V.S.; Vostrikova, K.E. [MnIII(SchiffBase)]3[ReIV(CN)7], Highly Anisotropic 3D Coordination Framework: Synthesis, Crystal Structure, Magnetic Investigations, and Theoretical Analysis. Inorg. Chem. 2014, 53, 10217–10231. [Google Scholar] [CrossRef] [PubMed]
- Peresypkina, E.V.; Samsonenko, D.G.; Vostrikova, K.E. Heterobimetallic Coordination Polymers Involving 3d Metal Complexes and Heavier Transition Metals Cyanometallates. J. Solid State Chem. 2015, 224, 107–114. [Google Scholar] [CrossRef]
- Moreno Pineda, E.; Wernsdorfer, W.; Vostrikova, K.E. Very Anisotropic 2D Molecular Magnetic Materials Based on Pentagonal Bipyramidal Heptacyanidorhenate(IV). Materials 2022, 15, 8324. [Google Scholar] [CrossRef]
- Peresypkina, E.V.; Majcher, A.M.; Rams, M.; Vostrikova, K.E. A Single Chain Magnet Involving Hexacyanoosmate. Chem. Commun. 2014, 50, 7150–7153. [Google Scholar] [CrossRef] [PubMed]
- Peresypkina, E.V.; Vostrikova, K.E. 2[Mn(acacen)]2+ + [Fe(CN)5NO]2− Polynuclear Heterobimetallic Coordination Compounds of Different Dimensionality in the Solid State. Dalt. Trans. 2012, 41, 4100. [Google Scholar] [CrossRef]
- Sukhikh, T.; Vostrikova, K. Assembly of Mn(III) Schiff Base Complexes with Heptacyanorhenate (IV). Inorganics 2017, 5, 59. [Google Scholar] [CrossRef]
- Majcher, A.M.; Pilet, G.; Mironov, V.S.; Vostrikova, K.E. Neutral Low-Dimensional Assemblies of a Mn(III) Schiff Base Complex and Octacyanotungstate(V): Synthesis, Characterization, and Magnetic Properties. Magnetochemistry 2017, 3, 16. [Google Scholar] [CrossRef]
- Sakamoto, F.; Sumiya, T.; Fujita, M.; Tada, T.; Tan, X.S.; Suzuki, E.; Okura, I.; Fujii, Y. T-Site Selective Photocleavage of DNA by Cationic Schiff Base Complex of Manganese(III). Chem. Lett. 1998, 27, 1127–1128. [Google Scholar] [CrossRef]
- Ishikawa, R.; Nakano, M.; Breedlove, B.K.; Yamashita, M. Syntheses, Structures, and Magnetic Properties of Discrete Cyano-Bridged Heterodinuclear Complexes Composed of MnIII(Salen)-Type Complex and MIII(CN)6 Anion (MIII= Fe, Mn, and Cr). Polyhedron 2013, 64, 346–351. [Google Scholar] [CrossRef]
- Vostrikova, K.E.; Peresypkina, E.V. Facile Preparation of Paramagnetic RuIII and OsIII Hexacyanides. Eur. J. Inorg. Chem. 2011, 2011, 811–815. [Google Scholar] [CrossRef]
- Seiden, J. Propriétés Statiques d’une Chaîne Isotrope Alternée de Spins Quantiques 1/2 et de Spins Classiques. J. Phys. Lett. 1983, 44, 947–952. [Google Scholar] [CrossRef]
- Mydosh, J.A. Spin Glasses: An Experimental Introduction, 1st ed.; CRC Press: London, UK, 1993. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Luscombe, J.H.; Luban, M.; Reynolds, J.P. Finite-Size Scaling of the Glauber Model of Critical Dynamics. Phys. Rev. E 1996, 53, 5852–5860. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).