Searching for the Best Values of NMR Shielding and Spin-Spin Coupling Parameters: CH4-nFn Series of Molecules as the Example
Abstract
:1. Introduction
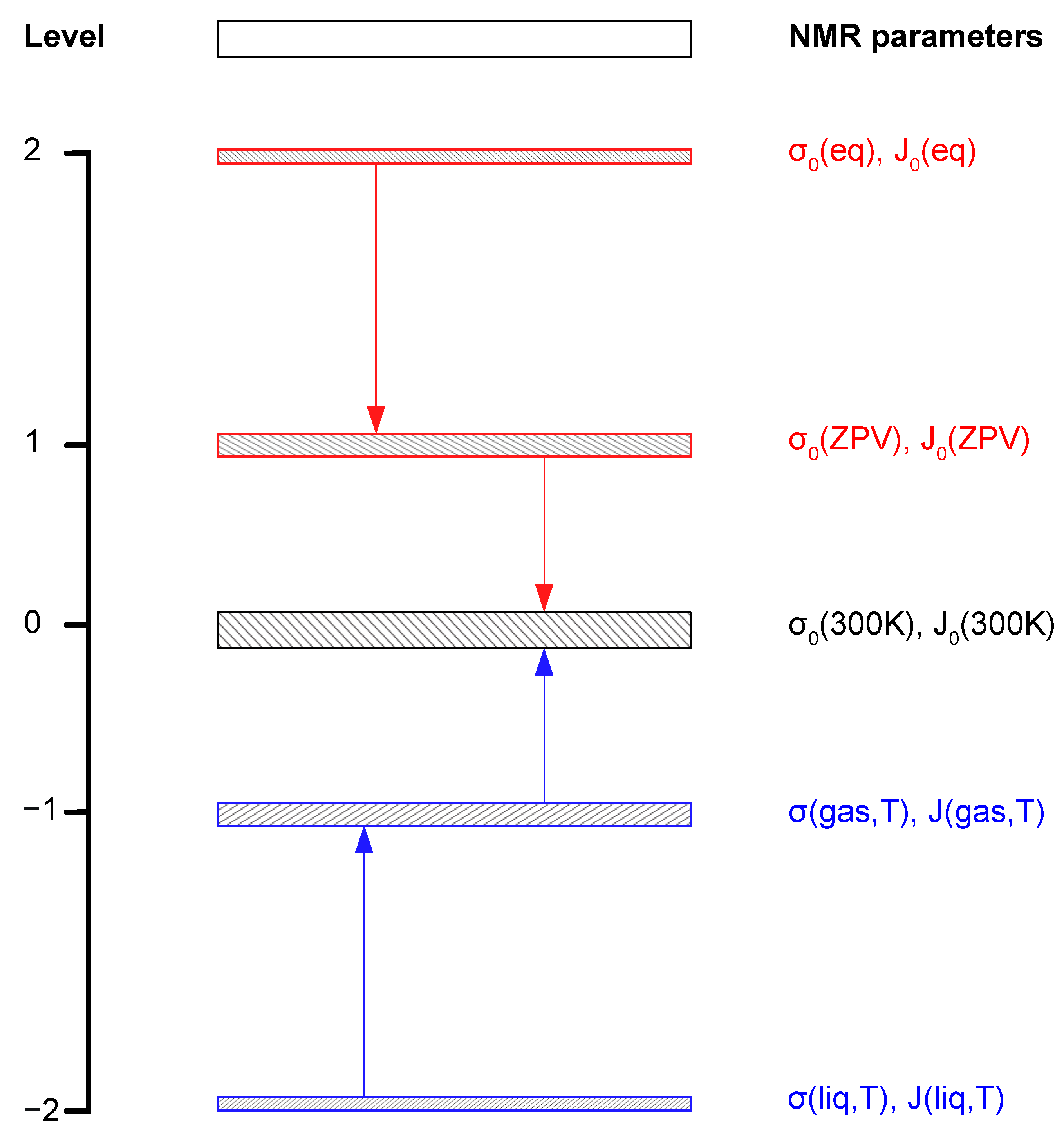
2. NMR Spectral Parameters of Isolated Molecules
2.1. Chemical Shifts and Magnetic Shielding
2.2. Referencing of Shielding
2.3. Indirect Spin-Spin Coupling
3. Experimental vs. Calculated NMR Spectral Parameters
3.1. Chemical Shifts
3.2. Nuclear Magnetic Shielding and Indirect Spin-Spin Coupling
4. Experimental and Calculated Spectral Parameters for Isolated CH4-nFn Molecules
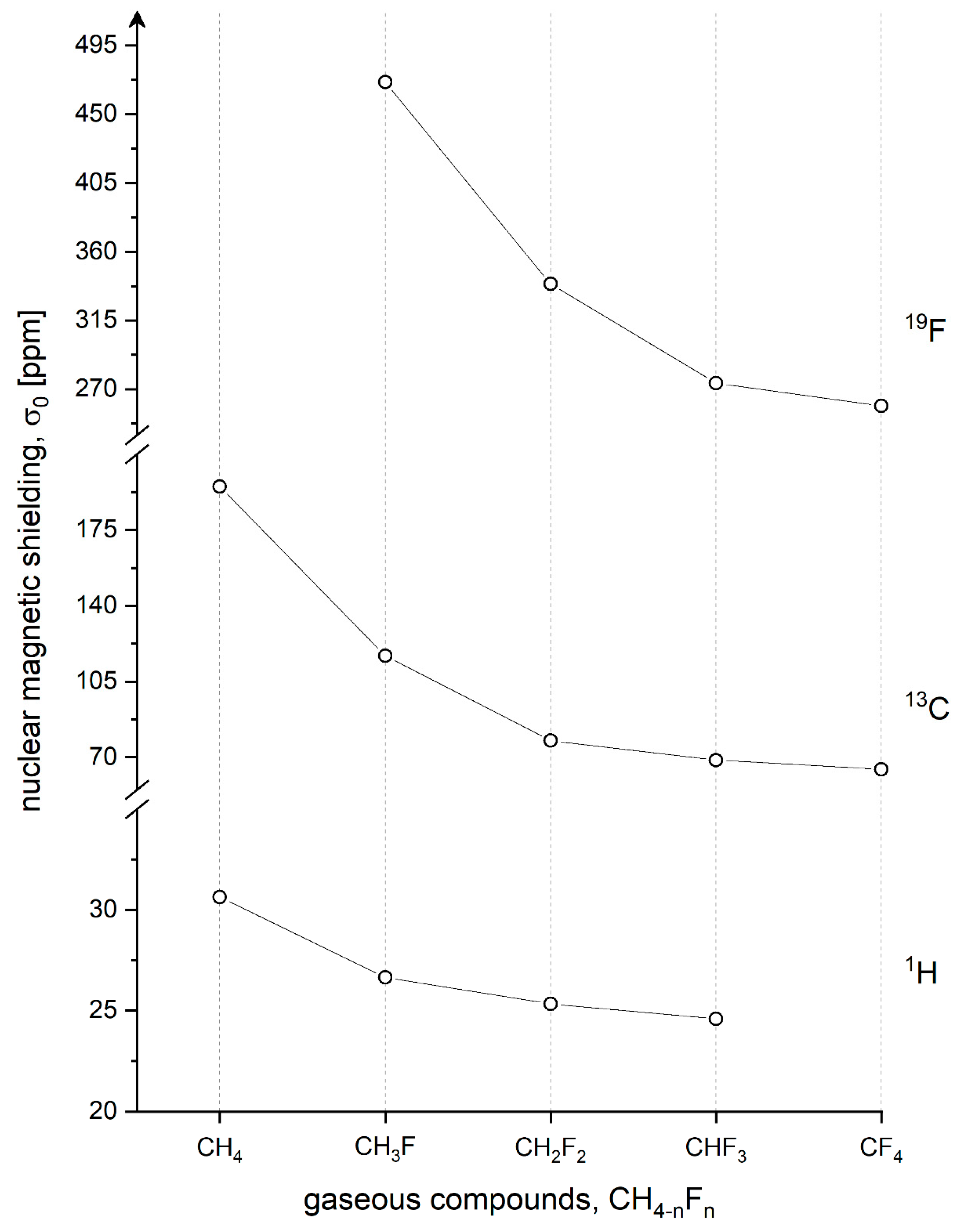
4.1. 1H, 13C, and 19F Nuclear Magnetic Shielding
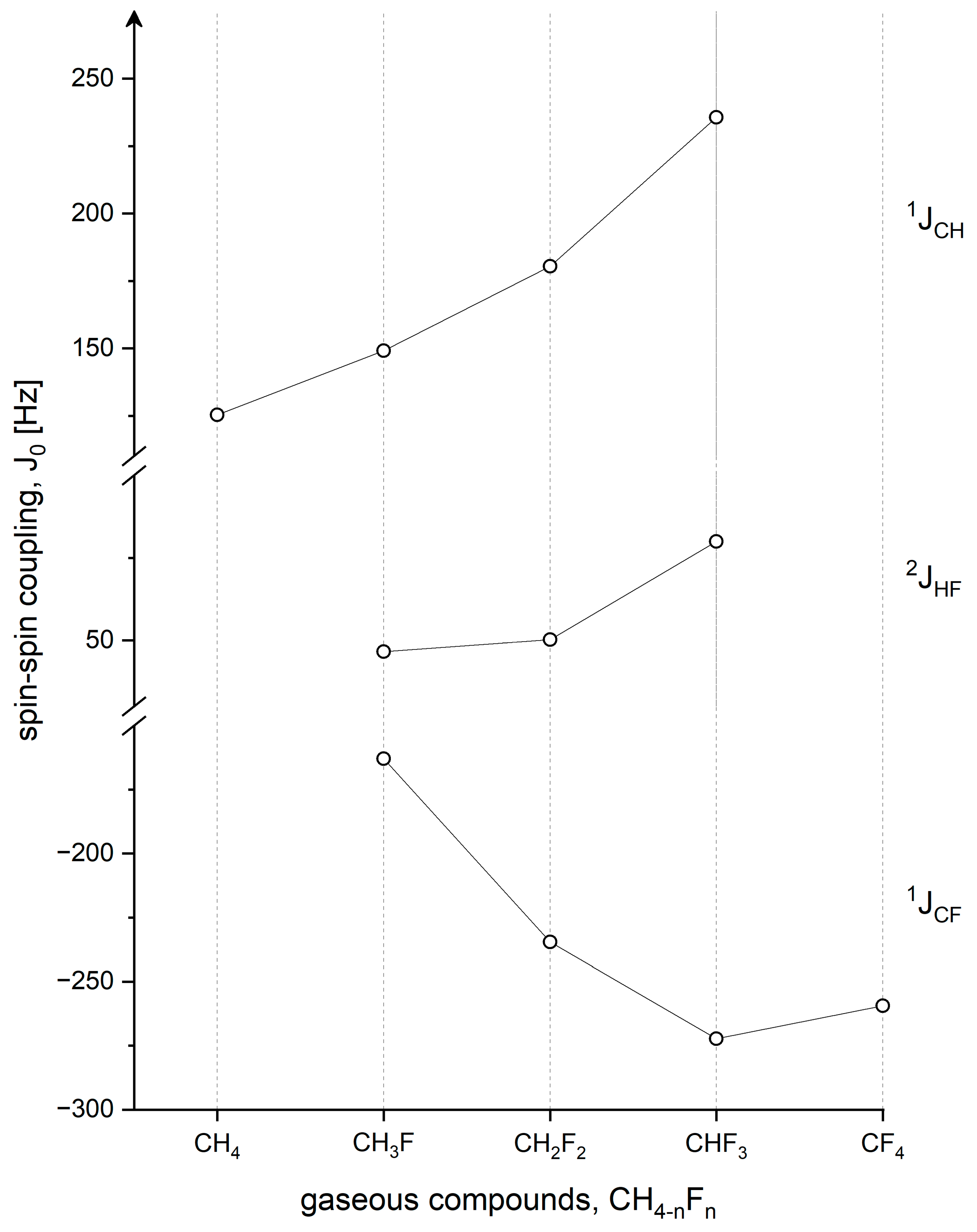
4.2. 1JCH, 1JCF, and 2JFH Indirect Spin-Spin Coupling Constants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabi, I.I. Space Quantization in a Gyrating Magnetic Field. Phys. Rev. 1937, 51, 652–654. [Google Scholar] [CrossRef]
- Rabi, I.I.; Millman, S.; Kusch, P.; Zacharias, J.R. A New Method of Measuring Nuclear Magnetic Moment. Phys. Rev. 1938, 53, 318. [Google Scholar] [CrossRef]
- Purcell, E.M.; Torrey, H.C.; Pound, R.V. Resonance Absorption by Nuclear Magnetic Moments in a Solid. Phys. Rev. 1946, 69, 37–38. [Google Scholar] [CrossRef]
- Bloch, F.; Hansen, W.W.; Packard, M.E. Nuclear Induction. Phys. Rev. 1946, 69, 127. [Google Scholar] [CrossRef]
- Ramsey, N.F. The Internal Diamagnetic Field Correction in Measurements of the Proton Magnetic Moment. Phys. Rev. 1950, 77, 567. [Google Scholar] [CrossRef]
- Proctor, W.G.; Yu, F.C. The Dependence of a Nuclear Magnetic Resonance Frequency upon Chemical Compound. Phys. Rev. 1950, 77, 717. [Google Scholar] [CrossRef]
- Dickinson, W.C. Dependence of the F19 Nuclear Resonance Position on Chemical Compound. Phys. Rev. 1950, 77, 736–737. [Google Scholar] [CrossRef]
- Ramsey, N.F. Magnetic Shielding of Nuclei in Molecules. Phys. Rev. 1950, 78, 699–703. [Google Scholar] [CrossRef]
- Ramsey, N.F. Dependence of Magnetic Shielding of Nuclei upon Molecular Orientation. Phys. Rev. 1951, 83, 540–541. [Google Scholar] [CrossRef]
- Ramsey, N.F. Chemical Effects in Nuclear Magnetic Resonance and in Diamagnetic Susceptibility. Phys. Rev. 1952, 86, 243–246. [Google Scholar] [CrossRef]
- Proctor, W.G.; Yu, F.C. On the Nuclear Magnetic Moments of Several Stable Isotopes. Phys. Rev. 1951, 81, 20–30. [Google Scholar] [CrossRef]
- Gutowski, H.S.; McCall, D.W. Nuclear Magnetic Resonance Fine Structure in Liquids. Phys. Rev. 1951, 82, 748–749. [Google Scholar] [CrossRef]
- Hahn, E.L.; Maxwell, D.E. Chemical Shift and Field Independent Frequency Modulation of the Spin Echo Envelope. Phys. Rev. 1951, 84, 1246–1247. [Google Scholar] [CrossRef]
- Pople, J.A.; Schneider, W.G.; Bernstein, H.J. High-Resolution Nuclear Magnetic Resonance; McGraw-Hill: New York, NY, USA, 1959. [Google Scholar]
- Dickinson, W.C. The Time Average Magnetic Field at the Nucleus in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1951, 81, 717–731. [Google Scholar] [CrossRef]
- Bothner-By, A.A.; Glick, R.E. Specific Medium Effects in Nuclear Magnetic Resonance Spectra of Liquids. J. Am. Chem. Soc. 1956, 78, 1071–1072. [Google Scholar] [CrossRef]
- Buckingham, A.D.; Schaefer, T.; Schneider, W.G. Solvent Effects in Nuclear Magnetic Resonance Spectra. J. Chem. Phys. 1960, 32, 1227–1233. [Google Scholar] [CrossRef]
- Bothner-By, A.A. Medium Effects in the NMR Spectra of Liquids. J. Mol. Spectrosc. 1960, 5, 52–61. [Google Scholar] [CrossRef]
- Abraham, R.J. A Proton Magnetic Resonance Investigation of Some Weak Interactions in Solutions. Mol. Phys. 1961, 4, 369–383. [Google Scholar] [CrossRef]
- Buckingham, A.D.; Schaefer, T.; Schneider, W.G. Solvent Effects in Nuclear Magnetic Resonance. J. Chem. Phys. 1961, 34, 1064–1065. [Google Scholar] [CrossRef]
- Rummens, F.H.A. Van der Waals Forces in NMR Intermolecular Shielding Effects. In NMR Basic Principles and Progress; Diehl, P., Fluck, E., Kosfeld, R., Eds.; Springer: New York, NY, USA, 1975; Volume 10. [Google Scholar]
- Lauterbur, P.C. C13 Nuclear Magnetic Resonance Spectra. J. Chem. Phys. 1957, 26, 217–218. [Google Scholar] [CrossRef]
- Ernst, R.R.; Anderson, W.A. Application of Fourier Transform Spectroscopy to Magnetic Resonance. Rev. Sci. Instrum. 1966, 37, 93–102. [Google Scholar] [CrossRef]
- Jackowski, K.; Raynes, W.T. Carbon-13 Magnetic Shielding in the Methyl Halides. J. Chem. Res. (S) 1977, 66–67. [Google Scholar]
- Tiers, G.D.V. Proton Nuclear Resonance Spectroscopy: I. Reliable Shielding Values by Internal Referencing with Tetramethylsilane, J. Phys. Chem. 1958, 62, 1151–1152. [Google Scholar]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818, Erratum in Magn. Reson. Chem. 2002, 40, 489–405.. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Granger, P.; Hoffman, R.E.; Zilm, K.W. Further Conventions for NMR Shielding and Chemical Shifts (IUPAC Recommendations). Pure Appl. Chem. 2008, 80, 59–84, Erratum in Magn. Reson. Chem. 2008, 46, 582–598.. [Google Scholar] [CrossRef]
- Emsley, J.W.; Feeney, J.; Sutcliffe, L.H. High Resolution Nuclear Magnetic Resonance Spectroscopy; Appendix B; Pergamon Press: Oxford, UK, 1966; Volume 2, pp. 1115–1129. [Google Scholar]
- Antušek, A.; Jaszuński, M. Accurate Non-Relativistic Calculations of NMR Shielding Constants. In New Developments in NMR; Chapter 6 in Gas Phase, N.M.R., Price, W.S., Jackowski, K., Jaszuński, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 186–217. [Google Scholar]
- Faber, R.; Kaminsky, J.; Sauer, S.P.A. Rovibrational and Temperature Effects in Theoretical Studies of NMR Parameters. In New Developments in NMR; Chapter 7 in Gas Phase, N.M.R., Price, W.S., Jackowski, K., Jaszuński, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 218–266. [Google Scholar]
- Repisky, M.; Komorovsky, S.; Bast, R.; Ruud, K. Relativistic Calculations of Nuclear Magnetic Resonance Parameters. In New Developments in NMR; Chapter 8 in Gas Phase, N.M.R., Price, W.S., Jackowski, K., Jaszuński, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 267–303. [Google Scholar]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics; Edition 2012–2013; Taylor and Francis Group: Boca Raton, NJ, USA, 2012; Volume 93, pp. 9–59. [Google Scholar]
- Yonker, C.R.; Palmer, B.J. Investigation of CO2/Fluorine Interactions through the Intermolecular Effects on the 1H and 19F Shielding of CH3F and CHF3 at Various Temperatures and Pressures. J. Phys. Chem. A 2001, 105, 308–314. [Google Scholar] [CrossRef]
- Blanco, S.; López, J.C.; Lesarri, A.; Alonso, J.L. A Molecular-Beam Fourier Transform Microwave Study of Difluoromethane Dimer. J. Mol. Struct. 2002, 612, 255–260. [Google Scholar] [CrossRef]
- Tsuzaki, S.; Uchimaru, T.; Mikami, M.; Urata, S. Ab Initio Calculations of Intermolecular Interaction of CHF3 Dimer: Origin of Attraction and Magnitude of CH/F Interaction. J. Phys. Chem. A 2003, 107, 7962–7968. [Google Scholar] [CrossRef]
- Jameson, A.K.; Reger, J.P. A Gas-Phase Density-Dependent Directly Bonded Coupling Constant. J. Phys. Chem. 1971, 78, 437–439. [Google Scholar] [CrossRef]
- Jackowski, K.; Wilczek, M.; Makulski, W.; Koźmiński, W. Effects of intermolecular interactions on 33S magnetic shielding in gaseous SF6. J. Phys. Chem. A 2002, 106, 2829–2832. [Google Scholar] [CrossRef]
- Marshall, T.W.; Pople, J.A. Nuclear Magnetic Shielding of a Hydrogen Atom in an Electric Field. Mol. Phys. 1958, 1, 199–202. [Google Scholar] [CrossRef]
- Stephen, M.J. The Effect of Molecular Interaction on Magnetic Shielding Constants. Mol. Phys. 1958, 1, 223–232. [Google Scholar] [CrossRef]
- Buckingham, A.D. Chemical Shifts in the Nuclear Magnetic Resonance Spectra of Molecules Containing Polar Groups. Can. J. Chem. 1960, 38, 300–307. [Google Scholar] [CrossRef]
- Bothner-By, A.A. Medium Effects in the Nuclear Magnetic Resonance of Liquids. 4. Nature of the Effects. J. Mol. Spectrosc. 1960, 5, 52–61. [Google Scholar] [CrossRef]
- Raynes, W.T.; Buckingham, A.D.; Bernstein, H.J. Medium Effects in Proton Magnetic Resonance. I. Gases. J. Chem. Phys. 1962, 36, 3481–3488. [Google Scholar] [CrossRef]
- Petrakis, L.; Bernstein, H.J. Medium Effects in NMR. III. Fluorine Resonance in Gases. J. Chem. Phys. 1963, 38, 1562–1568. [Google Scholar] [CrossRef]
- Jameson, C.J. Fundamental Intramolecular and Intermolecular Information from NMR in the Gas Phase. In Gas Phase NMR—New Developments in NMR; Price, W.S., Jackowski, K., Jaszuński, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–51, Chapter 1. [Google Scholar]
- Makulski, W.; Jackowski, K. 1H, 13C and 29Si Magnetic Shielding in Gaseous and Liquid Tetramethylsilane. J. Magn. Reson. 2020, 313, 106716. [Google Scholar] [CrossRef]
- Jackowski, K.; Jaszuński, M.; Kamieński, B.; Wilczek, M. NMR Frequency and Magnetic Dipole Moment of 3He Nucleus. J. Magn. Reson. 2008, 193, 147–149. [Google Scholar] [CrossRef]
- Rudziński, A.; Puchalski, M.; Pachucki, K. Relativistic, QED, and Nuclear Mass Effects in the Magnetic Shielding of 3He. J. Chem. Phys. 2009, 130, 244102. [Google Scholar] [CrossRef]
- Antušek, A.; Jackowski, K.; Jaszuński, M.; Makulski, W.; Wilczek, M. Nuclear Magnetic Dipole Moments from NMR Spectra. Chem. Phys. Lett. 2005, 411, 111–116. [Google Scholar] [CrossRef]
- Jackowski, K.; Jaszuński, M. Nuclear Magnetic Moments from NMR Spectra—Experimental Gas Phase Studies and Nuclear Shielding Calculations. Concepts Magn. Reson. A 2007, 30, 246–260. [Google Scholar] [CrossRef]
- Jackowski, K.; Jaszuński, M.; Wilczek, M. Alternative Approach to the Standardization of NMR Spectra. Direct Measurements of Nuclear Magnetic Shielding in Molecules. J. Phys. Chem. A 2010, 114, 2471–2475. [Google Scholar] [CrossRef]
- Jaszuński, M.; Antušek, A.; Garbacz, P.; Jackowski, K.; Makulski, W. The Determination of Accurate Nuclear Magnetic Dipole Moments and Direct Measurements of NMR Shielding Constants. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 49–63. [Google Scholar] [CrossRef]
- Garbacz, P.; Jackowski, K. Referencing of 1H and 13C NMR Shielding Measurements. Chem. Phys. Lett. 2019, 728, 148–152. [Google Scholar] [CrossRef]
- Barfield, M.; Johnson, M.D., Jr. Solvent Dependence of Nuclear Spin-Spin Coupling Constants. Chem. Rev. 1973, 12, 53–73. [Google Scholar] [CrossRef]
- Jackowski, K. Gas–Phase Studies of Spin-Spin Coupling Constants. Int. J. Mol. Sci. 2003, 4, 135–142. [Google Scholar] [CrossRef]
- Aucar, G.A.; Aucar, A.I. Recent Developments in Absolute Shielding Scales for NMR Spectroscopy. Annu. Rep. NMR Spectrosc. 2019, 96, 77–141. [Google Scholar] [CrossRef]
- Melo, J.I.; Ruiz de Azua, M.C.; Giribet, C.G.; Aucar, G.A.; Romero, R.H. Relativistic Effects on the Nuclear Magnetic Shielding Tensor. J. Chem. Phys. 2003, 118, 471–486. [Google Scholar] [CrossRef]
- Gauss, J.; Stanton, J.F. Coupled-Cluster Calculations of Nuclear Magnetic Resonance Chemical Shifts. J. Chem. Phys. 1995, 103, 3561–3577. [Google Scholar] [CrossRef]
- Gauss, J.; Stanton, J.F. Perturbative Treatment of Triple Excitations in Coupled-Cluster Calculations of Nuclear Magnetic Shielding Constants. J. Chem. Phys. 1996, 104, 2574–2583. [Google Scholar] [CrossRef]
- Krivdin, L.B. Computational Protocols for Calculating 13C NMR Chemical Shifts. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 112–113, 103–156. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F. Segmented Contracted Basis Sets Optimized for Nuclear Magnetic Shielding. J. Chem. Theory Comput. 2015, 11, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F. The Optimum Contraction of Basis Sets for Calculating Spin-Spin Coupling Constants. Theor. Chem. Acc. 2010, 126, 371–382. [Google Scholar] [CrossRef]
- Picard, C.J.; Mauri, F. All-Electron Magnetic Response with Pseudopotentials: MNR Chemical Shifts. Phys. Rev. B 2001, 63, 245101. [Google Scholar] [CrossRef]
- Helgaker, T.; Jaszuński, M.; Pecul, M. The Quantum-Chemical Calculation of NMR Indirect Spin-Spin Coupling Constants. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 53, 249–268. [Google Scholar] [CrossRef]
- Fukuya, H.; Ono, T. DFT-GIAO Calculations of 19F NMR Chemical Shifts for Perfluoro Compounds. J. Compt. Chem. 2003, 25, 51–60. [Google Scholar] [CrossRef]
- Shaghaghi, H.; Ebrahimi, H.; Tafazzoli, M.; Jalali-Heravi, M. A survey of wave function effects on theoretical calculation of gas phase 19F NMR chemical shifts using factorial design. J. Fluor. Chem. 2010, 131, 47–52. [Google Scholar] [CrossRef]
- Hindermann, D.K.; Cornwell, C.D. Vibrational Corrections to the Nuclear-Magnetic Shielding and Spin–Rotation Constants for Hydrogen Fluoride. Shielding Scale for 19F. J. Chem. Phys. 1968, 48, 4154–4161. [Google Scholar] [CrossRef]
- Kutzelnigg, W.; Fleischer, U.; Schindler, M. The IGLO-Method. Ab-Initio Calculation and Interpretation of NMR Chemical Shifts and Magnetic Susceptibilities. In NMR Basic Principles and Progress; Diehl, P., Fluck, E., Kosfeld, R., Eds.; Springer: New York, NY, USA, 1991; Volume 23, pp. 165–262. [Google Scholar]
- Jameson, C.J. Chemical Shift Scales on an Absolute Basis. In Encyclopedia of Nuclear Magnetic Resonance; Grant, D.M., Harris, R.K., Eds.; John Wiley: London, UK, 1996; pp. 1273–1281. [Google Scholar]
- Gryff-Keller, A. Theoretical Modeling of 13C NMR Chemical Shifts—How to Use the Calculation Results. Concepts Magn. Reson. A 2011, 38, 289–307. [Google Scholar] [CrossRef]
- Fedorov, S.V.; Rusakov, J.J.; Krivdin, L.B. Quantum-Chemical Calculations of NMR Chemical Shifts of Organic Molecules: XII. Calculation of the 13C NMR Chemical Shifts of Fluoromethanes at the DFT Level. Russ. J. Org. Chem. 2014, 50, 160–164. [Google Scholar] [CrossRef]
- Samultsev, D.O.; Rusakov, Y.Y.; Krivdin, L.B. Normal Halogen Dependence of 13C NMR Chemical Shifts of Halomethanes Revisited at the Four-Component Relativistic Level. Magn. Reson. Chem. 2016, 54, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Krivdin, L.B. Computational Aspects of 19F NMR. Russ. Chem. Rev. 2020, 89, 1040–1073. [Google Scholar] [CrossRef]
- Kupka, T. Theory and Computation of Nuclear Shielding. Nucl. Magn. Reson. 2021, 46, 1–33. [Google Scholar] [CrossRef]
- Kupka, T. Theory and Computation of Nuclear Shielding. Nucl. Magn. Reson. 2022, 48, 1–15. [Google Scholar] [CrossRef]
- Muller, N.; Carr, D.T. Carbon-13 Splittings in Fluorine Nuclear Magnetic Resonance Spectra. J. Phys. Chem. 1963, 67, 112–115. [Google Scholar] [CrossRef]
- Kaupp, M.; Bühl, M.; Malkin, V.G. Calculation of NMR and EPR Parameters—Theory and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Krivdin, L.B. Theoretical Calculations of Carbon-Hydrogen Spin-Spin Coupling Constants. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 108, 17–73. [Google Scholar] [CrossRef]
- Heisenberg, W. Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik. Z. Phys. 1927, 43, 172–198. (In German) [Google Scholar]
- Jackowski, K.; Raynes, W.T. Density-Dependent Magnetic Shielding in Gas Phase 13C N.M.R. Mol. Phys. 1977, 34, 465–475. [Google Scholar] [CrossRef]
- Garbacz, P.; Jackowski, K.; Makulski, W.; Wasylishen, R.E. Nuclear Magnetic Shielding for Hydrogen in Selected Isolated Molecules. J. Phys. Chem. A 2012, 118, 11896–11904. [Google Scholar] [CrossRef]
- Åstrand, P.O.; Mikkelsen, K.V.; Ruud, K.; Helgaker, T. Magnetizabilities and Nuclear Shielding Constants of the Fluoromethanes in the Gas Phase and Solution. J. Phys. Chem. 1996, 100, 19771–19782. [Google Scholar] [CrossRef]
- Makulski, W.; Szyprowska, A.; Jackowski, K. Precise Determination of the 13C Nuclear Magnetic Moment from 13C, 3He and 1H NMR Measurements in the Gas Phase. Chem. Phys. Lett. 2011, 511, 224–228. [Google Scholar] [CrossRef]
- Auer, A.A.; Gauss, J. Quantitative Prediction of Gas-Phase 13C Nuclear Magnetic Shielding Constants. J. Chem. Phys. 2003, 118, 10407–10417. [Google Scholar] [CrossRef]
- Raynes, W. Theoretical and Physical Aspects of Nuclear Shielding. Spec. Period. Rep. Nucl. Magn. Reson. 1977, 7, 1–25. [Google Scholar]
- Jameson, A.K.; Jameson, C.J. Gas-Phase 13C Chemical Shifts in the Zero-Pressure Limit: Refinements to the Absolute Shielding Scale for 13C. Chem. Phys. Lett. 1987, 134, 461–466. [Google Scholar] [CrossRef]
- Raynes, W.T.; Fowler, P.W.; Lazzeretti, P.; Zanasi, R.; Grayson, M. The Effects of Rotation and Vibration on the Carbon-13 Shielding, Magnetizabilities and Geometrical Parameters of some Methane Isotopomers. Mol. Phys. 1988, 64, 143–162. [Google Scholar] [CrossRef]
- Dračinsky, M.; Kaminsky, J.; Bouř, P. Relative Importance of First and Second Derivatives of Nuclear Magnetic Resonance Chemical Shifts and Spin-Spin Coupling Constants for Vibrational Averaging. J. Chem. Phys. 2009, 130, 094106. [Google Scholar] [CrossRef] [PubMed]
- Kantola, A.M.; Lantto, P.; Vaara, J.; Jokisaari, J. Carbon and Proton Shielding Tensors in Methyl Halides. Phys. Chem. Chem. Phys. 2010, 12, 2679–2692. [Google Scholar] [CrossRef]
- Jackowski, K.; Kubiszewski, M.; Makulski, W. 13C and 19F Nuclear Magnetic Shielding and Spin-Spin Coupling in Gaseous Fluoromethane-d3. J. Mol. Struct. 2002, 614, 267–272. [Google Scholar] [CrossRef]
- Harding, M.E.; Lenhart, M.; Auer, A.A.; Gauss, J. Quantitative Prediction of Gas-Phase 19F Nuclear Magnetic Shielding Constants. J. Chem. Phys. 2008, 128, 244111. [Google Scholar] [CrossRef]
- Kubiszewski, M. Wpływ Oddziaływań Międzymolekularnych w Fazie Gazowej na Stałe Sprzężenia Spinowo-spinowego i Przesunięcia Chemiczne Fluorowych Pochodnych Metanu. Ph.D.Thesis, University of Warsaw, Warszawa, Poland, 2005. (In Polish). [Google Scholar]
- Jameson, C.J.; Jameson, A.K.; Burrell, P.M. 19F Nuclear Magnetic Shielding Scales from Gas Phase Studies. J. Chem. Phys. 1980, 73, 6013–6020. [Google Scholar] [CrossRef]
- Åstrand, P.O.; Ruud, K. Zero-Point Vibrational Contribution to Fluorine Shieldings in Organic Molecules. Phys. Chem. Chem. Phys. 2003, 5, 5015–5020. [Google Scholar] [CrossRef]
- Kubiszewski, M.; Makulski, W.; Jackowski, K. Intermolecular Effects on Spin-Spin Coupling and Magnetic Shielding Constants in Gaseous Difluoromethane. J. Mol. Struct. 2004, 704, 211–214. [Google Scholar] [CrossRef]
- Jameson, C.J.; Jameson, A.K.; Honarbakhsh, J. 19F Nuclear Magnetic Shielding Scale from Gas Phase Studies. II. J. Chem. Phys. 1984, 81, 5266–5267. [Google Scholar] [CrossRef]
- Kubiszewski, M.; Makulski, W.; Jackowski, K. 1H, 13C and 19F Nuclear Magnetic Shielding and Spin-Spin Coupling in Gaseous Trifluoromethane. J. Mol. Struct. 2005, 737, 7–10. [Google Scholar] [CrossRef]
- Zuschneid, T.; Fischer, H.; Handel, T.; Albert, K.; Häfelinger, G. Experimental Gas Phase 1H NMR Spectra and Basis Set Dependence of ab initio GIAO MO Calculations of 1H and 13C NMR Absolute Shieldings and Chemical Shifts of Small Hydrocarbons. Z. Naturforsch. 2004, 59b, 1153–1176. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Ab Initio Calculations of Absolute Nuclear Shieldings for Representative Compounds Containing 1(2)H, 6(7)Li, 11B, 13C, 14(15)N, 17O, 19F, 29Si, 31P, 33S, and 35Cl Nuclei. Struct. Chem. 1998, 9, 187–202. [Google Scholar] [CrossRef]
- Bennett, B.; Raynes, W.T.; Anderson, C.W. Temperature Dependences of J(C,H) and J(C,D) in 13CH4 and Some of its Deuterated Isotopomers. Spectrochim. Acta 1989, 45A, 821–827. [Google Scholar] [CrossRef]
- Antušek, A.; Kędziera, D.; Jackowski, K.; Jaszuński, M.; Makulski, M. Indirect Spin-Spin Coupling Constants in CH4, SiH4 and GeH4—Gas-phase NMR Experiment and ab initio Calculations. Chem. Phys. 2008, 352, 320–326. [Google Scholar] [CrossRef]
- Kjær, H.; Sauer, S.P.A.; Kongsted, J. Benchmarking NMR Indirect Nuclear Spin-Spin Coupling Constants: SOPPA, SOPPA(CC2), and SOPPA(CCSD) versus CCSD. J. Chem. Phys. 2010, 133, 144106. [Google Scholar] [CrossRef]
- Jokisaari, J.; Hiltunen, Y.; Lounila, J. Methyl Fluoride-13C in Nematic Liquid Crystals: Anisotropy of the Indirect 13C-19F Spin-Spin Coupling and of the 1H, 13C, and 19F Chemical Shieldings. J. Chem. Phys. 1986, 85, 3198–3202. [Google Scholar] [CrossRef]
- Lantto, P.; Kaski, J.; Vaara, J.; Jokisaari, J. Spin-Spin Coupling Tensors in Fluoromethanes. Chem. Eur. J. 2000, 6, 1395–1406. [Google Scholar] [CrossRef]
- Tiers, G.V.D.; Fluorine, N.M.R. Spectroscopy. XII. Proof of Opposite Signs for the “Direct” Carbon-13 Coupling Constants to Hydrogen and to Fluorine. J. Am. Chem. Soc. 1962, 84, 3972–3973. [Google Scholar] [CrossRef]
- Jackowski, K.; Raynes, W.T.; Sadlej, A.J. Intermolecular Effects on the 1H and 13C Magnetic Shielding of Methane. Chem. Phys. Lett. 1978, 54, 128–131. [Google Scholar] [CrossRef]
- Riley, J.P.; Hillier, I.H.; Raynes, W.T. An Ab Initio Coupled Hartree-Fock Calculation of the Nuclear Shielding in the H2/He Interacting System. Mol. Phys. 1978, 38, 353–365. [Google Scholar] [CrossRef]
- Hapka, M.; Jaszuński, M. The Effect of Weak Intermolecular Interactions on the Nuclear Magnetic Resonance Shielding Constant in N2. Magn. Reson. Chem. 2020, 58, 245–248. [Google Scholar] [CrossRef] [PubMed]
| Molecule | 6-31G(d,p) | 6-31G++(d,p) | Experiment |
|---|---|---|---|
| CH3F | −278.22 ppm | −279.16 ppm | 276.3 ppm |
| CHF3 | −93.91 ppm | −87.13 ppm | −84 ppm |
| CF4 | −80.71 ppm | −70.43 ppm | −69 ppm |
| CFCl3 | 179.41 ppm | 179.16 ppm | 188.7 ppm |
| Parameter | 1H Shielding (ppm) | 13C Shielding (ppm) | 19F Shielding (ppm) | |||
|---|---|---|---|---|---|---|
| Molecule | σ0H (exp) | σ0H (calc) | σ0C (exp) | σ0C (calc) | σ0F (exp) | σ0F (calc) |
| CH4 | 30.633(6) c 30.611(24) g | 31.41 d 31.6 q 31.4 w 31.93 z | 195.01 e 195.1 h 195.15 i | 195.2 j 196.2 f 193.6 d 197.52 z | ||
| CH3F | 26.635(8) c 26.62 l | 26.59 k 26.90 l 27.52 d | 116.83 l 116.69 o 116.8 h 116.3 s | 118.30 k 118.8 f 122.0 d 120.3 l | 470.98(1) m 471.0 p | 472.9 n 475.85 r 482.0 d |
| CH2F2 | 25.331(3) t | 25.24 k 26.58 d | 77.726(4) t 77.6 s | 78.87 k 89.6 d | 338.935(2) t 339.1 u | 340.7 n 358.40 r 363.7 d |
| CHF3 | 24.583(10) c | 24.55 k 25.80 d | 68.738(8) v 68.5 s | 69.28 k 82.6 d | 273.988(4) v 274.1 p | 275.2 n 298.03 r 302.0 d |
| CF4 | 64.5 h 64.5 s | 63.9 l 79.1 d | 258.80 o 259.0 u | 259.5 n 278.0 d 281.22 r | ||
| Parameter | 1J(13C−1H) Coupling (Hz) | 1J(13C−19F) Coupling (Hz) | 2J(19F−1H) Coupling (Hz) | |||
|---|---|---|---|---|---|---|
| Molecule | 1J0(exp) | 1J0(calc) | 1J0(exp) | 1J0(calc) | 2J0(exp) | 2J0(calc) |
| CH4 | 125.304 c 125.31 d | 125.65 e 124.26 f 120.61 g | ||||
| CH3F | 147.37(5) h 149.15 i | 148.09 g 145.62 f 141.5 k | −163.10(5) h −163.00(2) k −160.2 l | −160.8 f −156.6 k | 46.64(5) h | 46.81 f 46.3 k |
| CH2F2 | 180.42(5) m 180.38(4) k | 179.85 f 175.7 k | −234.55(5) m −233.91(11) k −232.7l | −224.52 f −220.7 k | 50.24(5) m | 50.25 f 51.9 k |
| CHF3 | 235.63(5) n 235.26(9) k | 235.63 f 236.8 k | −272.29(5) n −272.18(7) k −272.4 l | −238.28 f −242.1 k | 79.92(5) n | 79.18 f 79.3 k |
| CF4 | −258.32(9) h −259.4 l | −272.84 g | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackowski, K.; Słowiński, M.A. Searching for the Best Values of NMR Shielding and Spin-Spin Coupling Parameters: CH4-nFn Series of Molecules as the Example. Molecules 2023, 28, 1499. https://doi.org/10.3390/molecules28031499
Jackowski K, Słowiński MA. Searching for the Best Values of NMR Shielding and Spin-Spin Coupling Parameters: CH4-nFn Series of Molecules as the Example. Molecules. 2023; 28(3):1499. https://doi.org/10.3390/molecules28031499
Chicago/Turabian StyleJackowski, Karol, and Mateusz A. Słowiński. 2023. "Searching for the Best Values of NMR Shielding and Spin-Spin Coupling Parameters: CH4-nFn Series of Molecules as the Example" Molecules 28, no. 3: 1499. https://doi.org/10.3390/molecules28031499
APA StyleJackowski, K., & Słowiński, M. A. (2023). Searching for the Best Values of NMR Shielding and Spin-Spin Coupling Parameters: CH4-nFn Series of Molecules as the Example. Molecules, 28(3), 1499. https://doi.org/10.3390/molecules28031499






