A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer
Abstract
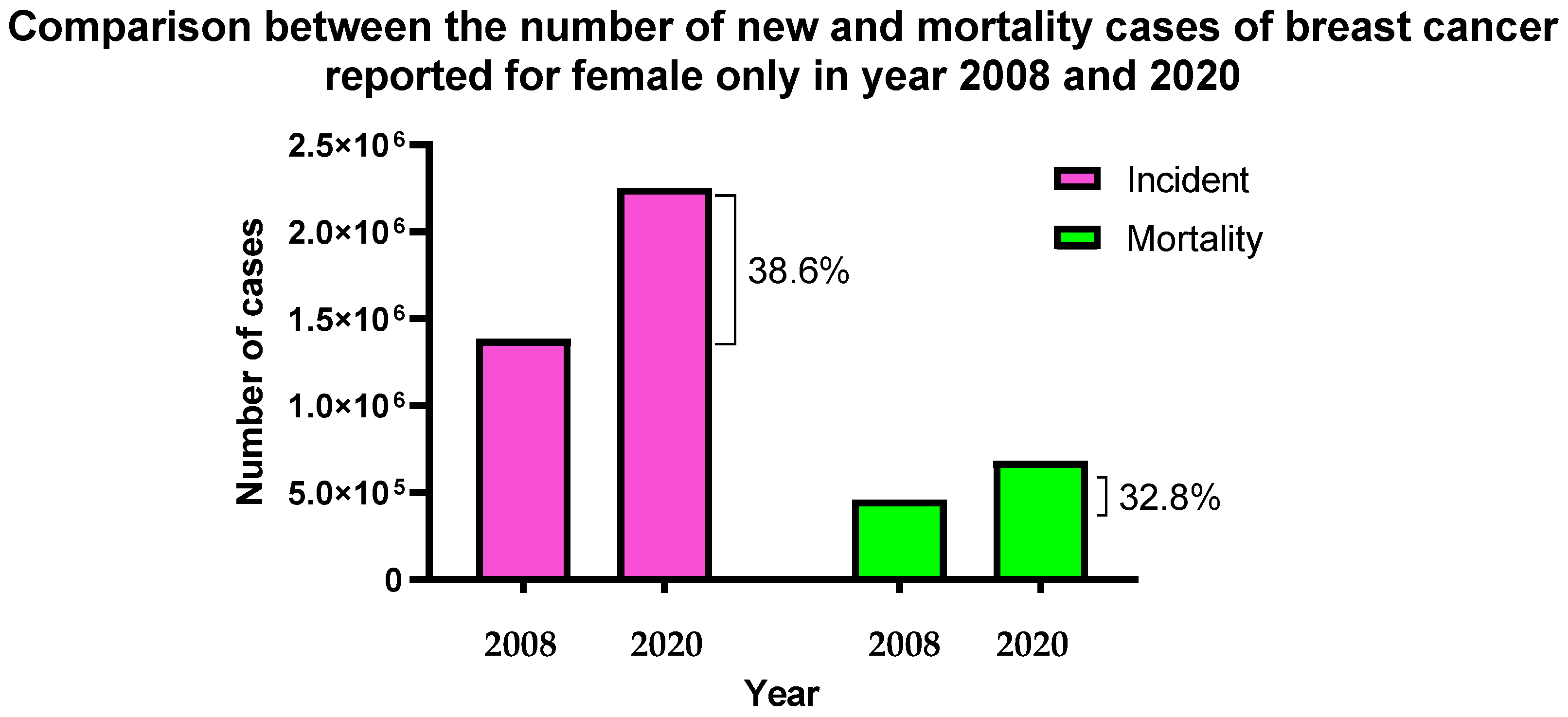
1. Introduction
2. Gene Therapy
3. Liposomes
4. Cationic Liposomes
4.1. Stability of Cationic Liposomes
4.2. Cytotoxicity of Cationic Liposomes
4.3. Cellular Uptake of Cationic Liposome
4.4. Transfection Ability of Cationic Liposomes
4.5. Disadvantages of Cationic Liposomes
4.6. Cationic Liposome Formulations for Breast Cancer Therapy Strategy
5. Anionic Liposomes
5.1. Stability of Anionic Liposomes
5.2. Cytotoxicity of Anionic Liposomes
5.3. Cellular Uptake of Anionic Liposomes
5.4. Transfection Ability of Anionic Liposomes
5.5. Disadvantages of Anionic Liposomes
5.6. Anionic Liposome Formulations for Breast Cancer Therapy Strategy
6. Neutral Liposomes
6.1. Stability of Neutral Liposomes
6.2. Cytotoxicity of Neutral Liposomes
6.3. Cellular Uptake of Neutral Liposomes
6.4. Transfection Ability of Neutral Liposomes
6.5. Neutral Liposome Formulations for Breast Cancer Therapy Strategy
7. Future Perspective
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key Steps for Effective Breast Cancer Prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. J. Am. Med. Assoc. 2019, 22, 288–300. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A.I. Current Status of Nanomedicine in the Chemotherapy of Breast Cancer. Cancer Chemother. Pharmacol. 2019, 84, 689–706. [Google Scholar] [CrossRef]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 Ligand-Binding Domain Mutations in Hormone-Resistant Breast Cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef]
- Greenlee, H.; Kwan, M.L.; Ergas, I.J.; Sherman, K.J.; Krathwohl, S.E.; Bonnell, C.; Lee, M.M.; Kushi, L.H. Complementary and Alternative Therapy Use before and after Breast Cancer Diagnosis: The Pathways Study. Breast Cancer Res. Treat. 2009, 117, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Anguela, X.M.; High, K.A. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Badran, A.H.; Liu, D.R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 2017, 168, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B.; Ali, M.H.; Wilkhu, J.; Kirby, D.J.; Mohammed, A.R.; Zheng, Q.; Perrie, Y. The Application of Monolayer Studies in the Understanding of Liposomal Formulations. Int. J. Pharm. 2011, 417, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Keles, E.; Song, Y.; Du, D.; Dong, W.J.; Lin, Y. Recent Progress in Nanomaterials for Gene Delivery Applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Hacein-Bey, S.; de Saint Basile, G.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.L.; et al. Gene Therapy of Human Severe Combined Immunodeficiency (SCID)-X1 Disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef]
- Hollon, T. Researchers and Regulators Reflect on First Gene Therapy Death. Nat. Med. 2000, 6, 6. [Google Scholar] [CrossRef]
- Aiuti, A.; Cattaneo, F.; Galimberti, S.; Benninghoff, U.; Cassani, B.; Callegaro, L.; Scaramuzza, S.; Andolfi, G.; Mirolo, M.; Brigida, I.; et al. Gene Therapy for Immunodeficiency Due to Adenosine Deaminase Deficiency. N. Engl. J. Med. 2009, 360, 447–458. [Google Scholar] [CrossRef]
- Notarangelo, L.; Casanova, J.L.; Fischer, A.; Puck, J.; Rosen, F.; Seger, R.; Geha, R. Primary Immunodeficiency Diseases: An Update. J. Allergy Clin. Immunol. 2004, 114, 677–687. [Google Scholar] [CrossRef]
- Patil, S.; Gao, Y.G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.J.; Jiang, S.F.; Qadir, A.; Qian, A.R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef]
- Belete, T.M. The Current Status of Gene Therapy for the Treatment of Cancer. Biologics 2021, 15, 67–77. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Srivastava, A.; Mallela, K.M.G.; Deorkar, N.; Brophy, G. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J. Pharm. Sci. 2021, 110, 2609–2624. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Hill, M.W.; Miller, N.G.A. Preparation and Use of Liposomes as Models of Biological Membranes. In Methods in Membrane Biology; Springer: Boston, MA, USA, 1974; pp. 1–68. [Google Scholar] [CrossRef]
- Balazs, D.A.; Godbey, W.T. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Liposomes in Drug Delivery: How It All Happened. Pharmaceutics 2016, 8, 19. [Google Scholar] [CrossRef]
- Tavares Luiz, M.; Aparecida, J.; Dutra, P.; Bueno Tofani, L.; Cavalcante De Araújo, J.T.; Delello, L.; Filippo, D.; Marchetti, J.M.; Chorilli, M. Targeted Liposomes: A Nonviral Gene Delivery System for Cancer Therapy. Pharmaceutics 2022, 14, 821. [Google Scholar] [CrossRef]
- Schepelmann, S.; Springer, C.J. Gene Therapy for Cancer. Br. J. Cancer 2008, 98, 674–675. [Google Scholar] [CrossRef]
- Yang, B.; Song, B.P.; Shankar, S.; Guller, A.; Deng, W. Recent Advances in Liposome Formulations for Breast Cancer Therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243. [Google Scholar] [CrossRef]
- Dastjerd, N.T.; Valibeik, A.; Rahimi Monfared, S.; Goodarzi, G.; Moradi Sarabi, M.; Hajabdollahi, F.; Maniati, M.; Amri, J.; Samavarchi Tehrani, S. Gene Therapy: A Promising Approach for Breast Cancer Treatment. Cell Biochem. Funct. 2022, 40, 28–48. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection Types, Methods and Strategies: A Technical Review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Eberwine, J.H. Mammalian Cell Transfection: The Present and the Future. Anal. Bioanal. Chem. 2010, 397, 3173. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; O’Banion, C.P.; Welfare, J.G.; Lawrence, D.S. Cellular Cyborgs: On the Precipice of a Drug Delivery Revolution. Cell Chem. Biol. 2018, 25, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A Review on Cationic Lipids with Different Linkers for Gene Delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef]
- Frolov, V.A.; Shnyrova, A.v.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef]
- Hafez, I.M.; Cullis, P.R. Roles of Lipid Polymorphism in Intracellular Delivery. Adv. Drug Deliv. Rev. 2001, 47, 139–148. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Sahebkar, A. Aptamer-Functionalized Liposomes for Targeted Cancer Therapy. Cancer Lett. 2019, 448, 144–154. [Google Scholar] [CrossRef]
- Key, J.; Palange, A.L.; Gentile, F.; Aryal, S.; Stigliano, C.; di Mascolo, D.; de Rosa, E.; Cho, M.; Lee, Y.; Singh, J.; et al. Soft Discoidal Polymeric Nanoconstructs Resist Macrophage Uptake and Enhance Vascular Targeting in Tumors. ACS Nano 2015, 9, 11628–11641. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; Shimoni, O.; Städler, B.; Caruso, F. Hydrogels: Advanced Subcompartmentalized Microreactors: Polymer Hydrogel Carriers Encapsulating Polymer Capsules and Liposomes (Small 21/2013). Small 2013, 9, 3572. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine Review: Clinical Developments in Liposomal Applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Krause, R.W.M. Encapsulation of Isoniazid-Conjugated Phthalocyanine-In-Cyclodextrin-In-Liposomes Using Heating Method. Sci. Rep. 2019, 9, 11485. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.K. Current Demands for Food-Approved Liposome Nanoparticles in Food and Safety Sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent Advancements in Liposome Technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of Safety and Toxicity of Liposomal Doxorubicin vs. Conventional Anthracyclines: A Meta-Analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-M.; Jeong, H.-J. Liposomes: Biomedical Applications. Chonnam Med. J. 2021, 57, 27. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. Anionic Liposomal Delivery System for DNA Transfection. AAPS J. 2004, 6, 13–22. [Google Scholar] [CrossRef]
- Giulimondi, F.; Digiacomo, L.; Pozzi, D.; Palchetti, S.; Vulpis, E.; Capriotti, A.L.; Chiozzi, R.Z.; Laganà, A.; Amenitsch, H.; Masuelli, L.; et al. Interplay of Protein Corona and Immune Cells Controls Blood Residency of Liposomes. Nat. Commun. 2019, 10, 3686. [Google Scholar] [CrossRef] [PubMed]
- Chemotherapy Combined with Gene Therapy in Treating Patients Who Have Stage III or Stage IV Breast Cancer—Full Text View—ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00044993?term=liposome+gene+therapy&cond=breast+cancer&draw=2&rank=1 (accessed on 23 September 2022).
- SGT-53, Carboplatin, and Pembrolizumab for the Treatment of Metastatic Triple Negative Inflammatory Breast Cancer—Full Text View—ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05093387?term=liposome+gene+therapy&cond=breast+cancer&draw=2&rank=2 (accessed on 23 September 2022).
- Wonder, E.; Simón-Gracia, L.; Scodeller, P.; Majzoub, R.N.; Kotamraju, V.R.; Ewert, K.K.; Teesalu, T.; Safinya, C.R. Competition of Charge-Mediated and Specific Binding by Peptide-Tagged Cationic Liposome-DNA Nanoparticles in Vitro and in Vivo. Biomaterials 2018, 166, 52–63. [Google Scholar] [CrossRef]
- Simões, S.; Filipe, A.; Faneca, H.; Mano, M.; Penacho, N.; Düzgünes, N.; de Lima, M.P. Cationic Liposomes for Gene Delivery. Expert Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef]
- Kapoor, M.; Burgess, D.J. Efficient and Safe Delivery of SiRNA Using Anionic Lipids: Formulation Optimization Studies. Int. J. Pharm. 2012, 432, 80–90. [Google Scholar] [CrossRef]
- Pisani, M.; Mobbili, G.; Bruni, P. Neutral Liposomes and DNA Transfection. In Non-Viral Gene Therapy; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Hattori, Y.; Hara, E.; Shingu, Y.; Minamiguchi, D.; Nakamura, A.; Arai, S.; Ohno, H.; Kawano, K.; Fujii, N.; Yonemochi, E. SiRNA Delivery into Tumor Cells by Cationic Cholesterol Derivative-Based Nanoparticles and Liposomes. Biol. Pharm. Bull. 2015, 38, 30–38. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the Cytotoxic Effects of Cationic Lipids with Their Headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, S.; Wang, B.; Zhao, Y.; Yang, B.; Yu, S. Transfection Efficiency of Cationic Lipids with Different Hydrophobic Domains in Gene Delivery. Bioconjug. Chem. 2010, 21, 563–577. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, Y.; Zhao, Y.; Zhu, J.; Xu, H.; Zhang, E.; Shi, L.; Du, L.; Liu, G.; Zhang, C.; et al. Toxicological Exploration of Peptide-Based Cationic Liposomes in SiRNA Delivery. Colloids Surf. B Biointerfaces 2019, 179, 66–76. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; de Smedt, S.C.; Remaut, K.; Braeckmans, K. The Proton Sponge Hypothesis: Fable or Fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition Design and Medical Application of Liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Forouzanfar, T.; Helder, M.N.; Zandieh-Doulabi, B. Preparation of PEGylated Cationic Nanoliposome-SiRNA Complexes for Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 684–692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yue, D.; Nie, Y.; Xu, X.; He, Y.; Zhang, S.; Wagner, E.; Gu, Z. Specially-Made Lipid-Based Assemblies for Improving Transmembrane Gene Delivery: Comparison of Basic Amino Acid Residue Rich Periphery. Mol. Pharm. 2016, 13, 1809–1821. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Ni, H.; Zhang, J.; Ju, S.; Ding, W. Transfection Efficiency Evaluation and Endocytosis Exploration of Different Polymer Condensed Agents. DNA Cell. Biol. 2019, 38, 1048–1055. [Google Scholar] [CrossRef]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the Development of Lipopolyplexes as Efficient Non-Viral Gene Delivery Systems. J. Control. Release 2016, 236, 1–14. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/ Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Lv, Y.; Xin, X.; Qin, C.; Han, X.; Yang, L.; He, W.; Yin, L. Cytosolic Co-Delivery of MiRNA-34a and Docetaxel with Core-Shell Nanocarriers via Caveolae-Mediated Pathway for the Treatment of Metastatic Breast Cancer. Sci. Rep. 2017, 7, 46186. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, R.; Chaudhuri, A. Cationic Transfection Lipids in Gene Therapy: Successes, Set-Backs, Challenges and Promises. Curr. Med. Chem. 2003, 10, 1297–1306. [Google Scholar] [CrossRef]
- Li, T.; He, J.; Horvath, G.; Próchnicki, T.; Latz, E.; Takeoka, S. Lysine-Containing Cationic Liposomes Activate the NLRP3 Inflammasome: Effect of a Spacer between the Head Group and the Hydrophobic Moieties of the Lipids. Nanomedicine 2018, 14, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tomori, Y.; Iijima, N.; Hinuma, S.; Ishii, H.; Takumi, K.; Takai, S.; Ozawa, H. Morphological Analysis of Trafficking and Processing of Anionic and Cationic Liposomes in Cultured Cells. Acta Histochem. Cytochem. 2018, 51, 81. [Google Scholar] [CrossRef] [PubMed]
- Audouy, S.; Hoekstra, D. Cationic Lipid-Mediated Transfection in Vitro and in Vivo (Review). Mol. Membr. Biol. 2001, 18, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Dokka, S.; Toledo, D.; Shi, X.; Castranova, V.; Rojanasakul, Y. Oxygen Radical-Mediated Pulmonary Toxicity Induced by Some Cationic Liposomes. Pharm. Res. 2000, 17, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.F.; Duan, J.; Voelker, A.; Khanal, A.; McNally, L.; Steinbach-Rankins, J.; Ceresa, B.P. Preparation and Optimization of Anionic Liposomes for Delivery of Small Peptides and CDNA to Human Corneal Epithelial Cells. J. Microencapsul. 2016, 33, 391. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.S.; Nikkhah, M.; Hosseinkhani, S. Cholesterol-Rich Lipid-Mediated Nanoparticles Boost of Transfection Efficiency, Utilized for Gene Editing by CRISPR-Cas9. Int. J. Nanomed. 2019, 14, 4353–4366. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Kommineni, N.; Bryszewska, M.; Ionov, M.; Khan, W. Cationic Liposomes for Co-Delivery of Paclitaxel and Anti-Plk1 SiRNA to Achieve Enhanced Efficacy in Breast Cancer. J. Drug Deliv. Sci. Technol. 2018, 48, 253–265. [Google Scholar] [CrossRef]
- Swami, R.; Kumar, Y.; Chaudhari, D.; Katiyar, S.S.; Kuche, K.; Katare, P.B.; Banerjee, S.K.; Jain, S. PH Sensitive Liposomes Assisted Specific and Improved Breast Cancer Therapy Using Co-Delivery of SIRT1 ShRNA and Docetaxel. Mater. Sci. Eng. C 2021, 120, 111664. [Google Scholar] [CrossRef]
- Wan, Y.; Dai, W.; Nevagi, R.J.; Toth, I.; Moyle, P.M. Multifunctional Peptide-Lipid Nanocomplexes for Efficient Targeted Delivery of DNA and SiRNA into Breast Cancer Cells. Acta Biomater. 2017, 59, 257–268. [Google Scholar] [CrossRef]
- Akhtar, S.; Basu, S.; Wickstrom, E.; Juliano, R.L. Interactions of Antisense DNA Oligonucleotide Analogs with Phospholipid Membranes (Liposomes). Nucleic Acids Res. 1991, 19, 5551. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Dubinsky, J.M.; Low, W.C.; Rahman, Y.E. Neurons Are Protected from Excitotoxic Death by P53 Antisense Oligonucleotides Delivered in Anionic Liposomes. J. Biol. Chem. 2001, 276, 32000–32007. [Google Scholar] [CrossRef]
- Forssen, E.A.; Tökès, Z.A. Use of Anionic Liposomes for the Reduction of Chronic Doxorubicin-Induced Cardiotoxicity. Proc. Natl. Acad. Sci. USA 1981, 78, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Feng, C.-L.; Zheng, W.-S.; Huang, S.; Zhang, W.-X.; Wu, H.-N.; Zhan, Y.; Han, Y.-X.; Wu, S.; Jiang, J.-D. Tumor-Selective Lipopolyplex Encapsulated Small Active RNA Hampers Colorectal Cancer Growth in Vitro and in Orthotopic Murine. Biomaterials 2017, 141, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Place, R.F.; Jia, Z.J.; Pookot, D.; Dahiya, R.; Li, L.C. Antitumor Effect of DsRNA-Induced P21(WAF1/CIP1) Gene Activation in Human Bladder Cancer Cells. Mol. Cancer Ther. 2008, 7, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int J Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Lungwitz, U.; Breunig, M.; Blunk, T.; Göpferich, A. Polyethylenimine-Based Non-Viral Gene Delivery Systems. Eur. J. Pharm. Biopharm. 2005, 60, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Ewe, A.; Schaper, A.; Barnert, S.; Schubert, R.; Temme, A.; Bakowsky, U.; Aigner, A. Storage Stability of Optimal Liposome-Polyethylenimine Complexes (Lipopolyplexes) for DNA or SiRNA Delivery. Acta Biomater. 2014, 10, 2663–2673. [Google Scholar] [CrossRef]
- Jerzykiewicz, J.; Czogalla, A. Polyethyleneimine-Based Lipopolyplexes as Carriers in Anticancer Gene Therapies. Materials 2021, 15, 179. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kawakami, S.; Higuchi, Y.; Suzuki, R.; Maruyama, K.; Sasaki, H.; Yamashita, F.; Hashida, M. Development of Anionic Bubble Lipopolyplexes for Efficient and Safe Gene Transfection with Ultrasound Exposure in Mice. J. Control. Release 2014, 176, 24–34. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, Z.; Qu, X.; Tang, Y.; Long, Q.; Feng, X. Biocompatible Anionic Polyelectrolyte for Improved Liposome Based Gene Transfection. Int. J. Pharm. 2015, 490, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nchinda, G.; Überla, K.; Zschörnig, O. Characterization of Cationic Lipid DNA Transfection Complexes Differing in Susceptability to Serum Inhibition. BMC Biotechnol. 2002, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for Delivery of Antioxidants in Cosmeceuticals: Challenges and Development Strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Challenges in Development of Targeted Liposomal Therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bajoria, R.; Fisk, N.M.; Contractor, S.F. Liposomal Thyroxine: A Noninvasive Model for Transplacental Fetal Therapy. J. Clin. Endocrinol. Metab. 1997, 82, 3271–3277. [Google Scholar] [CrossRef]
- Figueiredo, S.; Cabral, R.; Luís, D.; Fernandes, A.R.; Baptista, P.V. Conjugation of Gold Nanoparticles and Liposomes for Combined Vehicles of Drug Delivery in Cancer. Nanomedicine 2014, 48, 48–69. [Google Scholar]
- Ibaraki, H.; Takeda, A.; Arima, N.; Hatakeyama, N.; Takashima, Y.; Seta, Y.; Kanazawa, T. In Vivo Fluorescence Imaging of Passive Inflammation Site Accumulation of Liposomes via Intravenous Administration Focused on Their Surface Charge and Peg Modification. Pharmaceutics 2021, 13, 104. [Google Scholar] [CrossRef]
- Nie, Y.; Ji, L.; Ding, H.; Xie, L.; Li, L.; He, B.; Wu, Y.; Gu, Z. Cholesterol Derivatives Based Charged Liposomes for Doxorubicin Delivery: Preparation, in Vitro and in Vivo Characterization. Theranostics 2012, 2, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Liposomes in Gene Delivery; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Srinivasan, C.; Burgess, D.J. Optimization and Characterization of Anionic Lipoplexes for Gene Delivery. J. Control. Release 2009, 136, 62–70. [Google Scholar] [CrossRef]
- Lee, R.J.; Huang, L. Folate-Targeted, Anionic Liposome-Entrapped Polylysine-Condensed DNA for Tumor Cell-Specific Gene Transfer. J. Biol. Chem. 1996, 271, 8481–8487. [Google Scholar] [CrossRef]
- Krasnici, S.; Werner, A.; Eichhorn, M.E.; Schmitt-Sody, M.; Pahernik, S.A.; Sauer, B.; Schulze, B.; Teifel, M.; Michaelis, U.; Naujoks, K.; et al. Effect of the Surface Charge of Liposomes on Their Uptake by Angiogenic Tumor Vessels. Int. J. Cancer 2003, 105, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, L.; Pozzi, D.; Palchetti, S.; Zingoni, A.; Caracciolo, G. Impact of the Protein Corona on Nanomaterial Immune Response and Targeting Ability. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1615. [Google Scholar] [CrossRef] [PubMed]
- Channarong, S.; Chaicumpa, W.; Sinchaipanid, N.; Mitrevej, A. Development and Evaluation of Chitosan-Coated Liposomes for Oral DNA Vaccine: The Improvement of Peyer’s Patch Targeting Using a Polyplex-Loaded Liposomes. AAPS PharmSciTech 2011, 12, 192–200. [Google Scholar] [CrossRef]
- Chang, K.; Chang, F.H.; Chen, M.H. Developing a Novel Cholesterol-Based Nanocarrier with High Transfection Efficiency and Serum Compatibility for Gene Therapy. J. Formos. Med. Assoc. 2019, 118, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Current Methods for Attaching Targeting Ligands to Liposomes and Nanoparticles. J. Pharm. Sci. 2004, 93, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ Entry into the Cell: Relevance to Drug Delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Montizaan, D.; Yang, K.; Reker-Smit, C.; Salvati, A. Comparison of the Uptake Mechanisms of Zwitterionic and Negatively Charged Liposomes by HeLa Cells. Nanomedicine 2020, 30, 102300. [Google Scholar] [CrossRef]
- Bruni, P.; Pisani, M.; Amici, A.; Marchini, C.; Montani, M.; Francescangeli, O. Self-Assembled Ternary Complexes of Neutral Liposomes, Deoxyribonucleic Acid, and Bivalent Metal Cations. Promising Vectors for Gene Transfer? Appl. Phys. Lett. 2006, 88, 073901. [Google Scholar] [CrossRef]
- Caracciolo, G.; Pozzi, D.; Caminiti, R.; Marchini, C.; Montani, M.; Amici, A.; Amenitsch, H. Transfection Efficiency Boost by Designer Multicomponent Lipoplexes. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2280–2292. [Google Scholar] [CrossRef]
- Lange, Y.; Ye, J.; Steck, T.L. Essentially All Excess Fibroblast Cholesterol Moves from Plasma Membranes to Intracellular Compartments. PLoS ONE 2014, 9, e98482. [Google Scholar] [CrossRef]
- Hayward, S.L.; Francis, D.M.; Kholmatov, P.; Kidambi, S. Targeted Delivery of MicroRNA125a-5p by Engineered Lipid Nanoparticles for the Treatment of HER2 Positive Metastatic Breast Cancer. J. Biomed. Nanotechnol. 2016, 12, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Mura, S.; Deloménie, C.; Sauvage, F.; Ismail, S.; Fattal, E. Aptamer-Guided SiRNA-Loaded Nanomedicines for Systemic Gene Silencing in CD-44 Expressing Murine Triple-Negative Breast Cancer Model. J. Control. Release 2018, 271, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Asai, T.; Hirai, Y.; Shimizu, K.; Koide, H.; Minamino, T.; Oku, N. Systemic Administration of SiRNA with Anti-HB-EGF Antibody-Modified Lipid Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2018, 15, 1495–1504. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.H.; Mao, C.Q.; Dou, S.; Shen, S.; Tan, Z.b.; Wang, J. Triple Negative Breast Cancer Therapy with CDK1 SiRNA Delivered by Cationic Lipid Assisted PEG-PLA Nanoparticles. J. Control. Release 2014, 192. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
| Factors | Examples |
|---|---|
| Heredity and genetic factors | Family history and breast cancer-causing gene mutation, pathogenic mutations in cancer-predisposition genes, and common single-nucleotide polymorphisms linked to breast cancer |
| Menstruation-related | Age of menarche and later age of menopause |
| Reproduction-related | Nulliparous, postponement of having firstborn, low rate of reproduction, and low rate of breastfeeding |
| Exogenous hormone consumption | Oral contraceptive medication, menopausal hormone therapy, and hormone replacement therapy |
| Nutrition | Alcohol consumption, high trans-fat content food consumption, and smoking habit |
| Anthropometry | Obesity, high body mass index (BMI), high weight gain, and body fat distribution |
| Physical inactivity | Lack of routine exercise or bodywork |
| History of breast pathologies | Atypical hyperplasia, lobular carcinoma in situ, and high mammographic density |
| Exposure to therapeutic radiation | Therapeutic chest radiation for the treatment of Hodgkin’s disease |
| Name | Type of Therapy/Vector | Function | Approval from | Year of Approval |
|---|---|---|---|---|
| GlyberaR | Adeno-associated virus based | Familial lipoprotein lipase deficiency | European Medicines Agency (EMA) | 2012 |
| IMLYGICR | Genetically modified herpes simplex virus type 1 | Local treatment of unresectable lesions in patients with melanoma, | US Food and Drug Administration (FDA) | 2015 |
| StrimvelisR | γ-retrovirus-based therapy | Treatment of severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID) | European Medicines Agency (EMA) | 2016 |
| KYMRIAHR | CD19-directed genetically modified autologous CAR T cell immunotherapies | 1—Treatment of non-Hodgkin lymphoma 2—Treatment of acute lymphoblastic leukaemia. | US Food and Drug Administration (FDA) | 2017 |
| YESCARTAR | CD19-directed genetically modified autologous CAR T cell immunotherapies | Treatment of non-Hodgkin lymphoma | US Food and Drug Administration (FDA) | 2017 |
| LUXTURNAR | AAV-based gene therapy | Treatment of biallelic RPE65 mutation-associated retinal dystrophy. | US Food and Drug Administration (FDA) | 2017 |
| Use of Liposomes in Breast Cancer Delivery System [48,49] | ||||
|---|---|---|---|---|
| Name | Year Approved | Content within | Applications | Lipid Composition |
| Doxil | 1995 | Doxorubicin | Ovarian, breast cancer, and Kaposi’s sarcoma | HSPC:Chol:PEG 2000-DSPE |
| Myocet | 2000 | Doxorubicin | Metastatic breast cancer | EPC:Chol |
| Lipo-dox | 2012 | Doxorubicin | Breast and ovarian cancer | DSPC:Chol:PEG 2000-DSPE |
| Title of Clinical Trial | Status | Phase of Study | Strategy | Target Patients | Investigators | References |
|---|---|---|---|---|---|---|
| Phase II, Single Arm, Single Institution Clinical Trial of Docetaxel and Doxorubicin in Combination with Local Administration of INGN 201 (Ad5CMV-p53) in Locally Advanced Breast Cancer | Completed, 2004 | Phase II | Combining liposomal chemotherapy drugs (docetaxel and doxorubicin hydrochloride) with gene (Ad5CMV-p53) in treating patients who have stage III or stage IV breast cancer | 18 Years and older (male/female) Stage III and IV breast cancer patient | Jill Van Warthood (Introgen Therapeutics) United States, Texas | [52] |
| A Pilot Study of SGT-53 With Carboplatin and Pembrolizumab in Metastatic Triple Negative Inflammatory Breast Cancer | Starting on 30 October 2021 | Phase I | Transferrin Receptor-Targeted Liposomal p53 cDNA, pembrolizumab, and carboplatin may help control the disease in patients with triple-negative inflammatory breast cancer. | 18 Years and older (female) inflammatory breast cancer patient | Massimo Cristofanilli, FACP (Northwestern University) United States, Illinois | [53] |
| Structure | Abbreviation | Name |
|---|---|---|
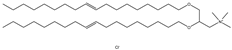 | DOTMA | 1,2-di-O-octadecenyl-3-trimethylammonium propane |
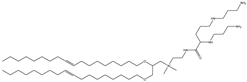 | DOSPA (derived from DOTMA) | (+)-N,N-dimethyl-N-[2-(spermine car-boxamido) ethyl]-2,3-bis(dioleyloxy)-1-propaniminium pentahy- drochloride |
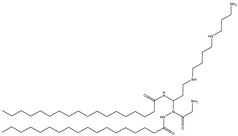 | DOGS (derived from DOSPA) | Dioctadecylamidoglycyl spermine |
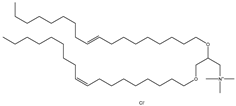 | DOTAP | N-[1-(2,3-Dioleoyloxy) propyl]-N, N, N-trimethylammonium chloride |
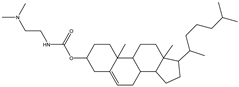 | DC-Chol | 3ß-[N-(N′, N′-dimethylaminoethane)-carbamoyl] cholesterol hydrochloride |
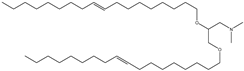 | DODMA | 1,2-dioleyloxy-3-dimethylaminopropane |
 | DDAB | Dimethyldioctadecylammonium (Bromide Salt) |
 | Octadecylamine | Stearylamine, 1-Aminooctadecane |
| Structure | Abbreviation | Name |
|---|---|---|
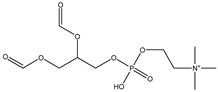 | PC | Phosphatidyl-choline |
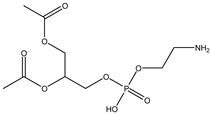 | PE | Phosphatidyl-ethanolamine |
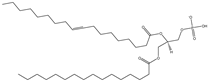 | PA | L-α-phosphatidic acid (Egg, Chicken) (sodium salt) |
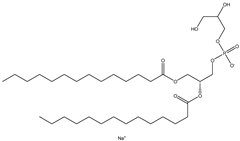 | PG | 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) |
 | PS | 1,2-dimyristoyl-sn-glycero-3-phospho-L-serine (sodium salt) |
| Structure | Abbreviation | Name |
|---|---|---|
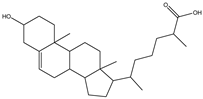 | Cholesterol | 3β-Hydroxy-5-cholestene, 5-Cholesten-3β-ol, Cholesterol |
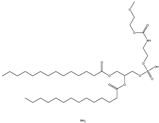 | DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
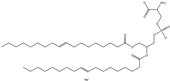 | EPC (Cl salt) | 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine (chloride salt) |
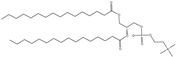 | DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
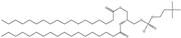 | DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
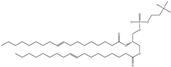 | DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| Lipid Nanomaterials | Type of Liposome | Type of Study | Cargo | Outcome | Cell Line | Year | Reference |
|---|---|---|---|---|---|---|---|
| DOTAP and cholesterol | Cationic | In Vitro | Paclitaxel and Protein-coding gene siRNA (si-Plk1) | Combined treatment that eliminates breast cancer cells better by 35–60%. | MCF-7 and MDA-MB-231 | 2018 | [82] |
| DOPE, DOTAP, PC, and cholesterol | Cationic | In Vitro and In Vivo | Docetaxel and silencing gene shRNA (Sirtuin 1) | More efficient in inducing cancer cell apoptosis and size tumour reduced | MCF-7 and MDA-MB-231 | 2021 | [83] |
| DOPE, DOTAP and well-defined synthetic multifunctional peptide, DEN-K(GALA)-TAT-K(STR)-CTP | Cationic | In Vitro | siRNA B-cell lymphoma 2 (BCL2) | Efficient cell internalisation and higher levels of gene expression | MCF-7 | 2017 | [84] |
| L-α-phosphatidylcholine (PC), palmitoyl-snGlycero-3-Phopshoethanolamine (DPPE) and cholesterol (CHOL) with HA | Neutral | In Vitro | microRNA tumour suppressor (miR-125a-5p) | Significantly inhibited HER2 expression as well as cell proliferation and migration in the 21MT-1 cell line | 21MT-1 | 2016 | [116] |
| DOPG/DOPE and calcium ion | Anionic | In Vitro | siRNA (anti-eGFP siRNA) | Maximum silencing, low cytotoxic, stable and high efficiency in serum, efficient intracellular uptake and endosomal escape. | MDA-MB-231 | 2012 | [58] |
| Poly (ethylene glycol)-block-poly (D, L-lactide) (PEG5K-PLA11K) and the cationic lipid N, N-bis(2-hydroxyethyl)-N-methyl-N-(2- cholesteryoxycarbonyl-aminoethyl) ammonium bromide (BHEM-Chol) | Cationic | In Vitro and In Vivo | siRNA, cyclin-dependent kinase 1 (CDK1) | Synthetic lethality in TNBC cells with high cMyc expression in mouse xenograft | SUM149 | 2014 | [119] |
| Anti-CD44 aptamer conjugate (Liposomes) DPPC, cholesterol, and DSPE-PEG | Cationic | In Vitro and In Vivo | siRNA Firefly luciferase with protamine | Functionalised with anti-CD44 aptamer in the TNBC model exhibits gene silencing. | (MDAMB-231) | 2017 | [117] |
| DOPE, cholesterol and DMPG liposome conjugated with Fab’ antibody against heparin-binding EGF-like growth factor | Anionic | In Vitro and In Vivo | siRNA, polo-like kinase 1 (PLK 1) | Suppression of polo-like kinase 1 expression; tumour growth reduction | (MDA-MB-231) | 2018 | [118] |
| Types of Liposomes | Advantages | Disadvantages |
|---|---|---|
| Cationic | Naturally occurring electrostatic interaction with negatively charged nucleic acid Cell binding ability with anionic endosomal membranes Better transfection efficiency Stable when administered into the bloodstream Better cellular uptake | High toxicity Low serum stability Low circulation time |
| Anionic | Low cytotoxicity Stable in the presence of serum Better transfection efficiency | Low cellular uptake Low nucleic acid encapsulation efficiency Less stable when administered into the bloodstream Low circulation time |
| Neutral | Stable when administered into the bloodstream Long circulation time Low cytotoxicity | Low cellular uptake Low nucleic acid encapsulation efficiency Low serum stability Low transfection ability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseu, G.Y.W.; Kamaruzaman, K.A. A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer. Molecules 2023, 28, 1498. https://doi.org/10.3390/molecules28031498
Tseu GYW, Kamaruzaman KA. A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer. Molecules. 2023; 28(3):1498. https://doi.org/10.3390/molecules28031498
Chicago/Turabian StyleTseu, Gloria Yi Wei, and Khairul Azfar Kamaruzaman. 2023. "A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer" Molecules 28, no. 3: 1498. https://doi.org/10.3390/molecules28031498
APA StyleTseu, G. Y. W., & Kamaruzaman, K. A. (2023). A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer. Molecules, 28(3), 1498. https://doi.org/10.3390/molecules28031498






