Fabrication, Evaluation, and Antioxidant Properties of Carrier-Free Curcumin Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
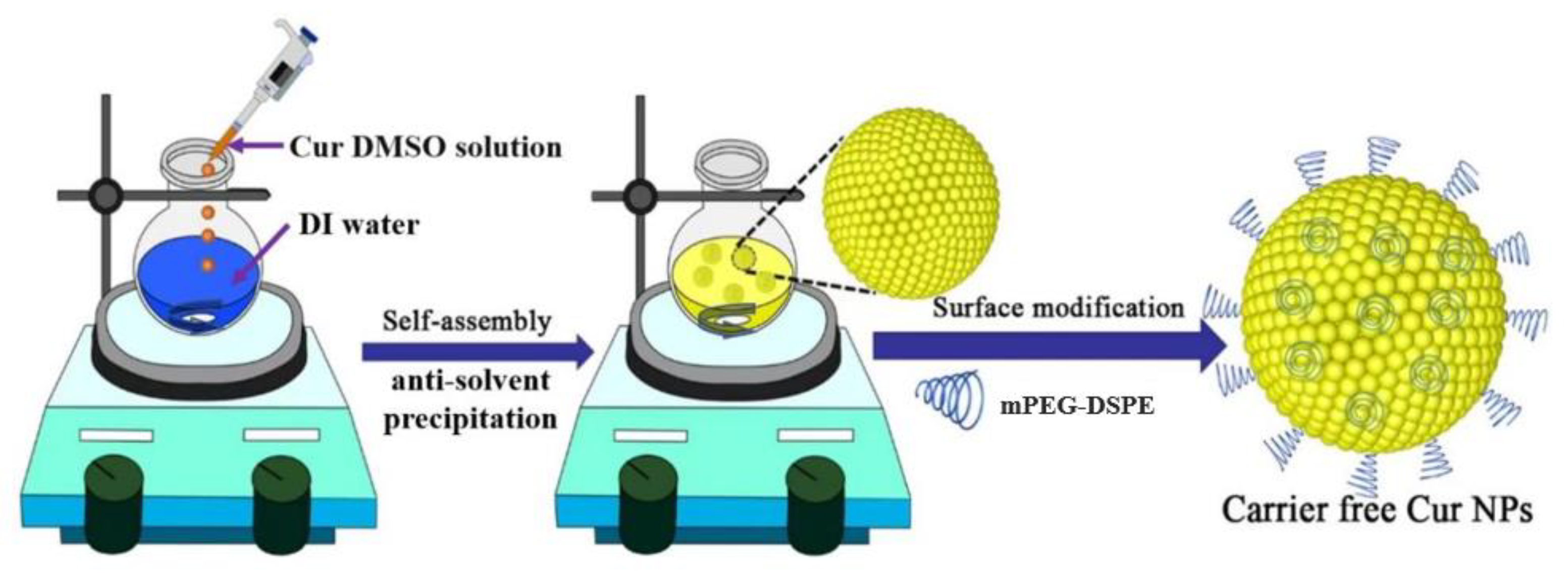
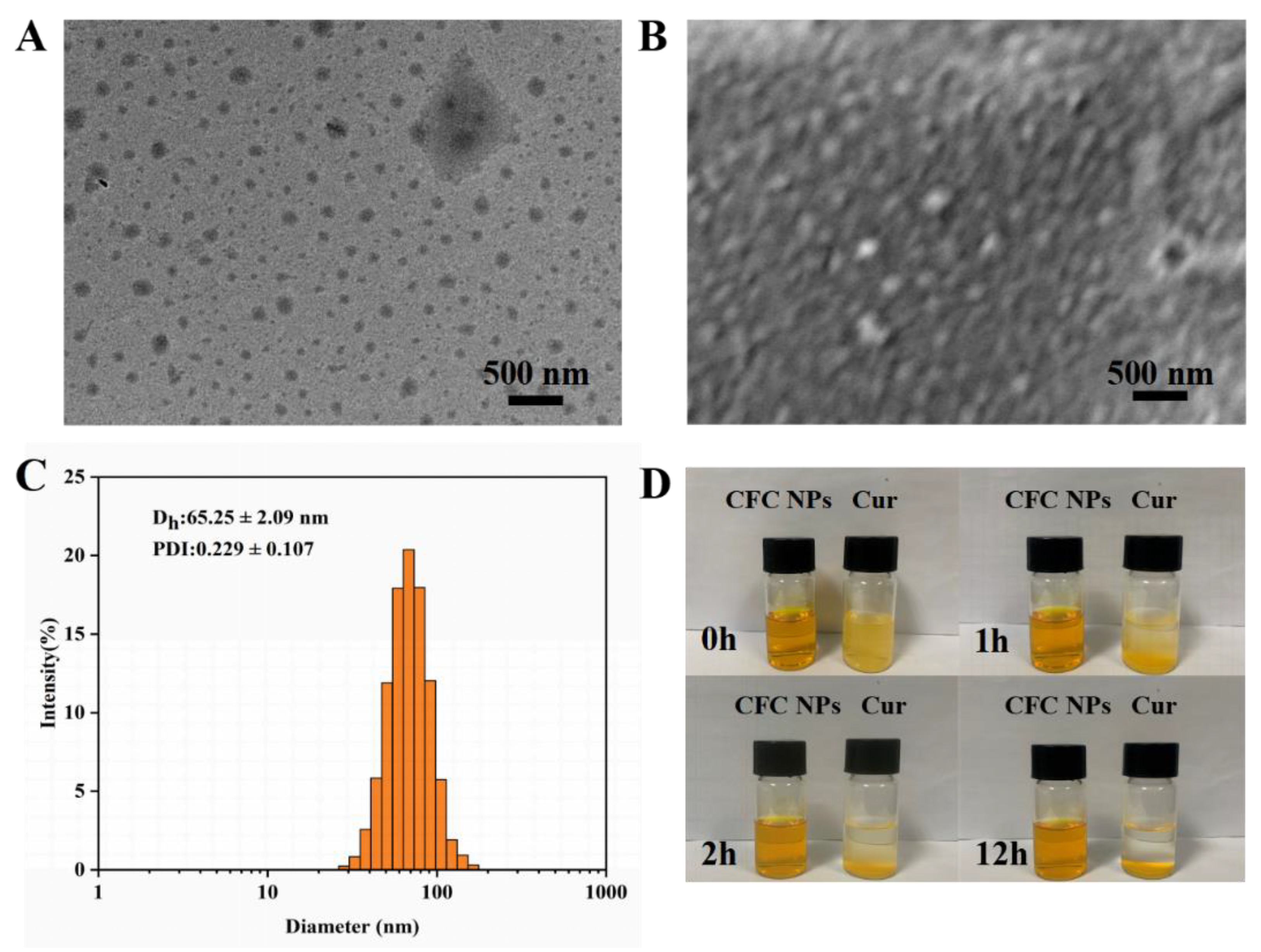
2.1. Preparation and Characterization of CFC NPs
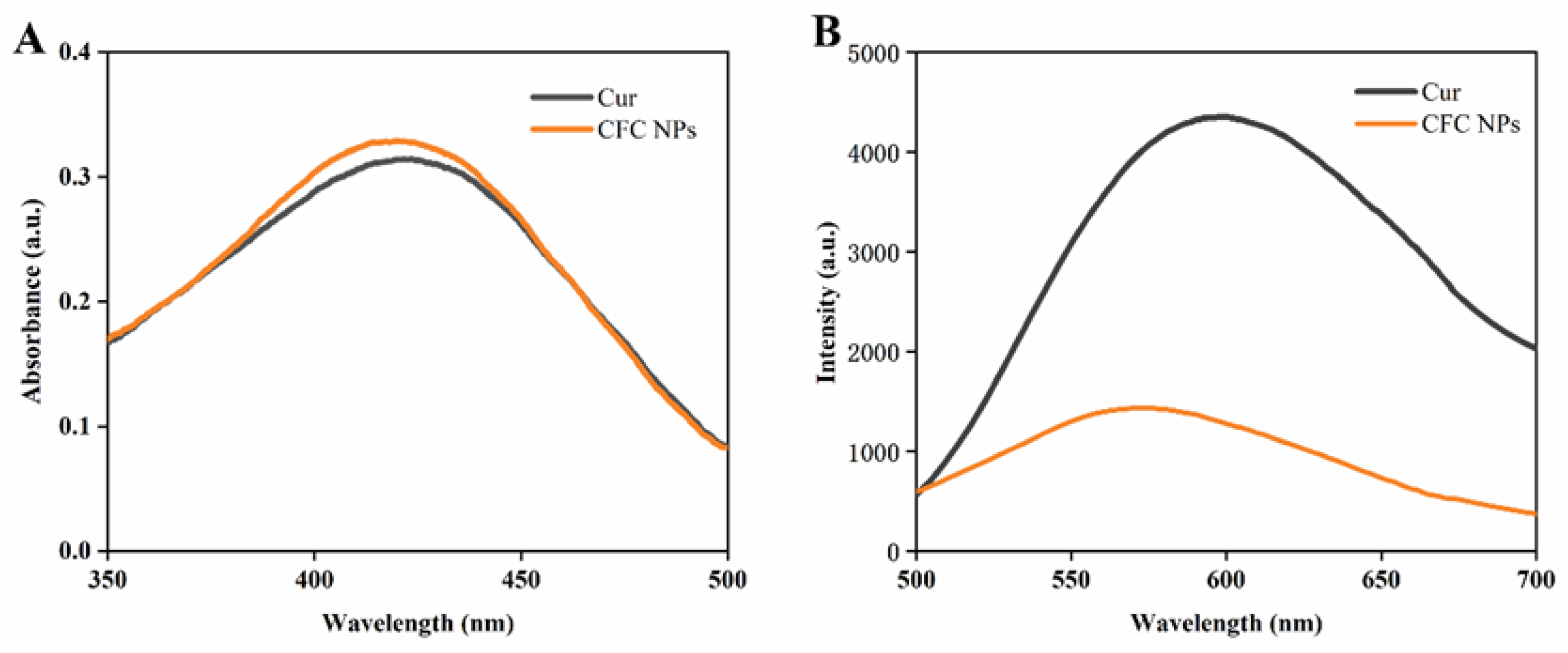
2.2. UV-Vis and Fluorescence Spectroscopy Analysis
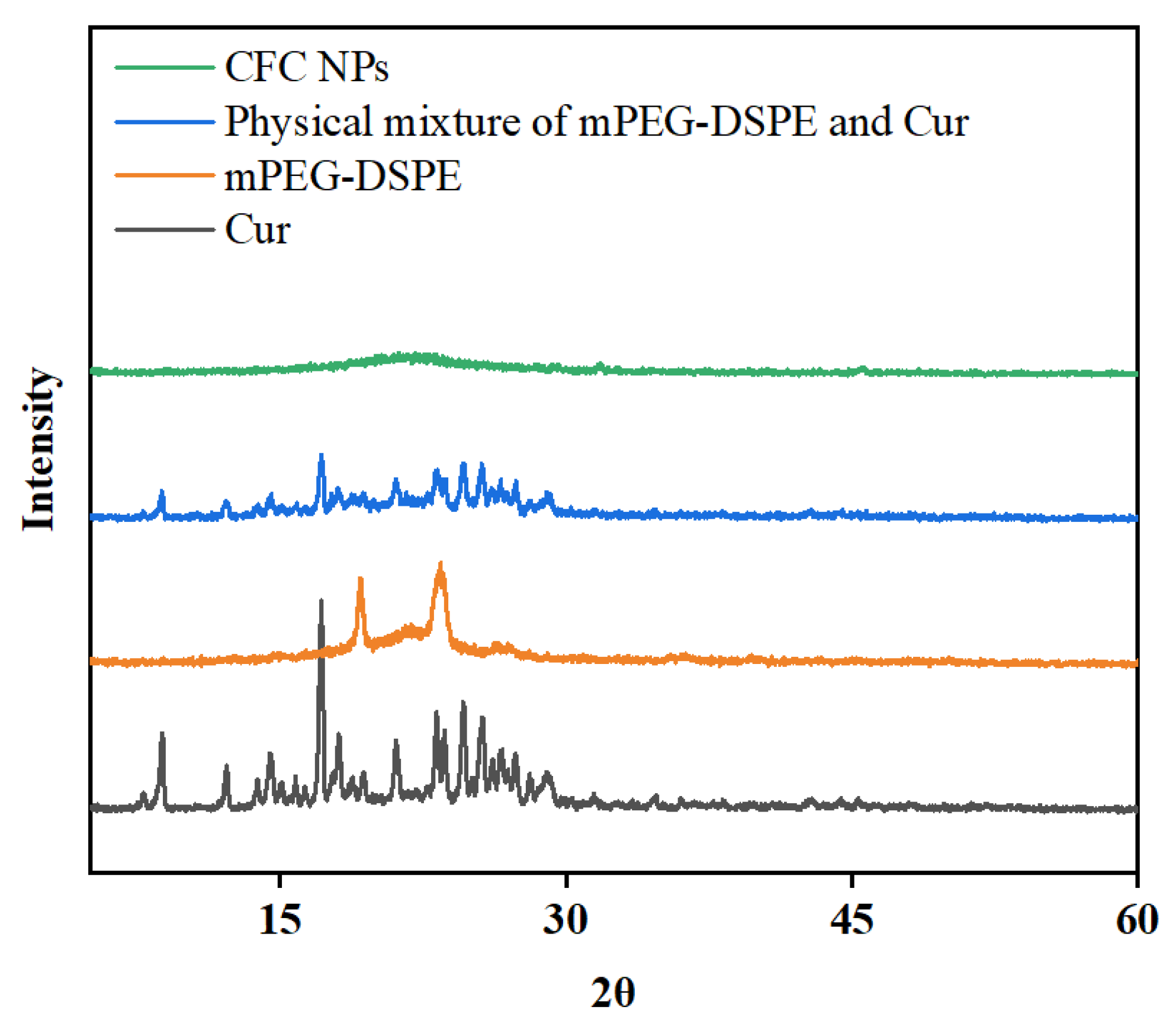
2.3. XRD Analysis
2.4. Photostability Assay
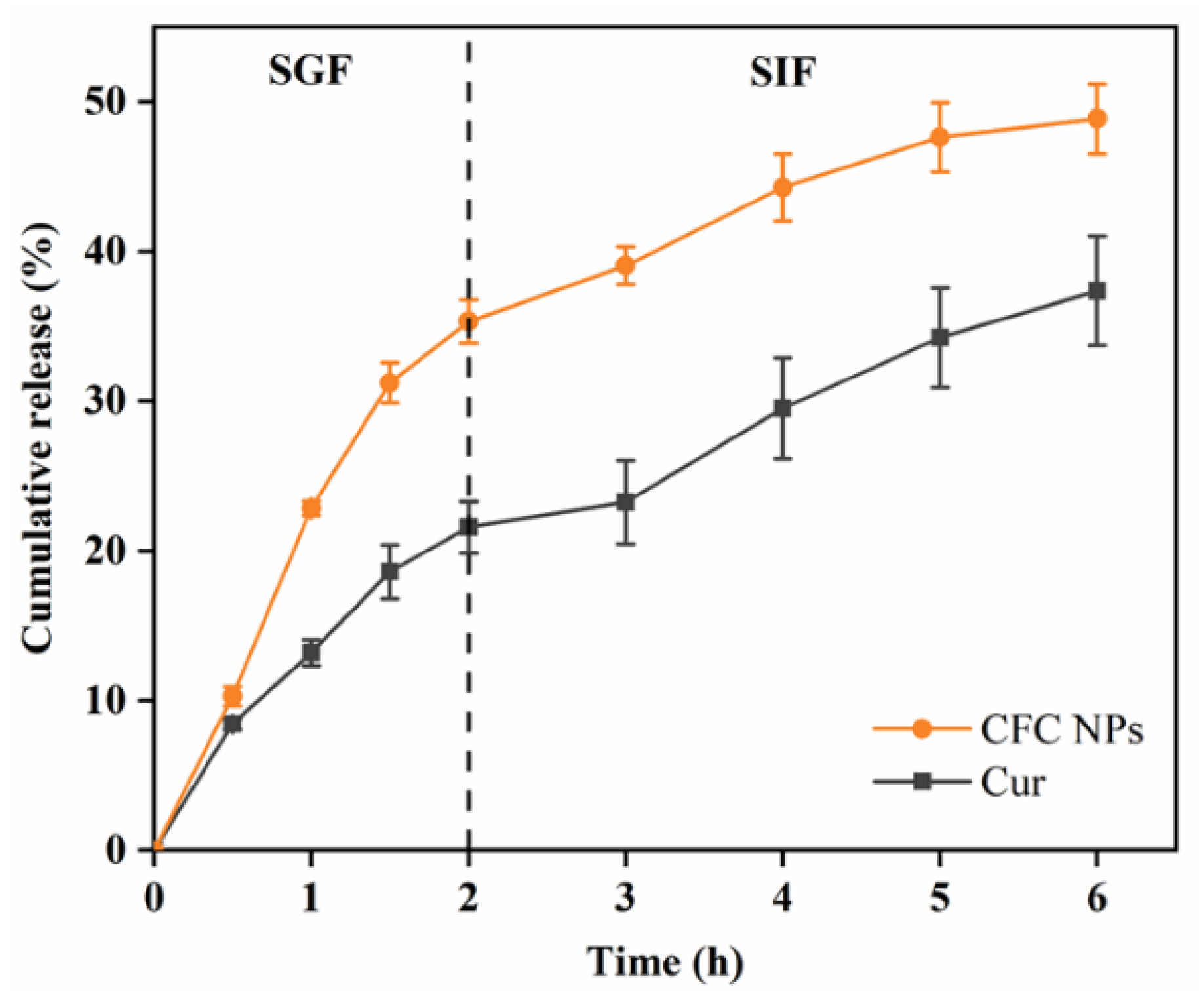
2.5. In Vitro Release Study
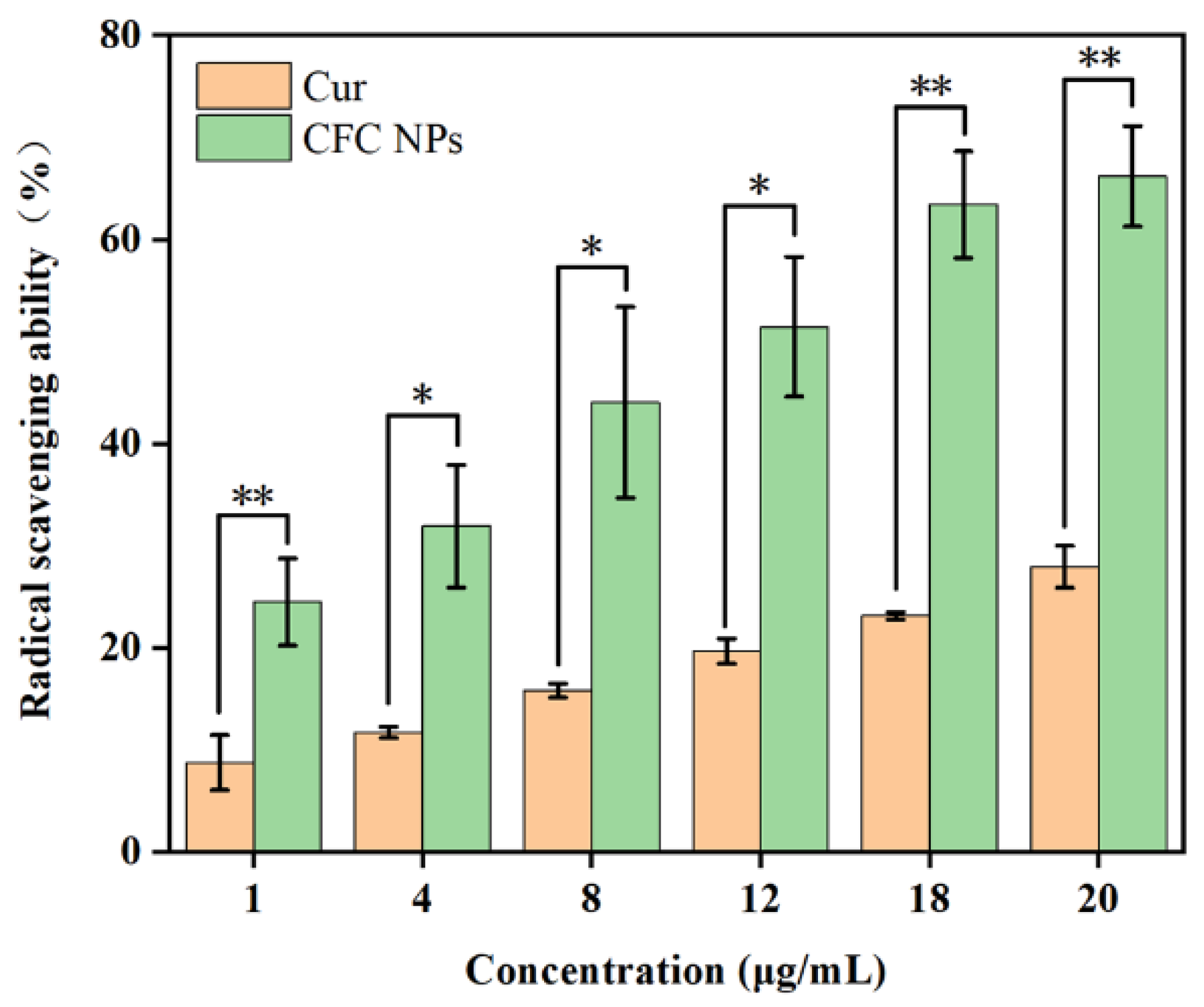
2.6. Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of CFC NPs
3.3. Characterization of CFC NPs
3.4. Loading Capacity (LC)
3.5. Spectroscopic Techniques
3.6. XRD Patterns
3.7. Photostability Assay
3.8. In Vitro Simulated Gastrointestinal Studies
3.9. Antioxidant Activity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, S.; Zhou, M.; Shi, B.; Zhang, X.; Williams, G.R.; Chen, X. Carrier-free nanodrugs for safe and effective cancer treatment. J. Control Release 2021, 329, 805–832. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, K.; Shen, Z.; Zheng, G.; Fan, L.; Zhao, R.; Shao, J. A Small Molecule Nanodrug by Self-Assembly of Dual Anticancer Drugs and Photosensitizer for Synergistic Near-Infrared Cancer Theranostics. ACS Appl. Mater. Interfaces 2017, 9, 43508–43519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Chen, Y.; Zhang, Y.; Wang, Y.; Zhang, Y.; Song, L.; Jiang, B.; Su, G.; Li, Y.; et al. Glutathione-responsive self-delivery nanoparticles assembled by curcumin dimer for enhanced intracellular drug delivery. Int. J. Pharm. 2018, 549, 230–238. [Google Scholar] [CrossRef]
- Etter, E.L.; Mei, K.C.; Nguyen, J. Delivering more for less: Nanosized, minimal-carrier and pharmacoactive drug delivery systems. Adv. Drug Deliv. Rev. 2021, 179, 113994. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, J.; Zhang, P.; Ma, M.; Wang, D.; Xu, Y. Development of pH-driven zein/tea saponin composite nanoparticles for encapsulation and oral delivery of curcumin. Food Chem. 2021, 364, 130401. [Google Scholar] [CrossRef]
- Chang, C.; Meikle, T.G.; Su, Y.; Wang, X.; Dekiwadia, C.; Drummond, C.J.; Conn, C.E.; Yang, Y. Encapsulation in egg white protein nanoparticles protects anti-oxidant activity of curcumin. Food Chem. 2019, 280, 65–72. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Ma, Q.; Zhang, H.; Liu, Y.; Hong, J.; Ding, Z.; Liu, M.; Han, J. Novel carrier-free nanoparticles composed of 7-ethyl-10-hydroxycamptothecin and chlorin e6: Self-assembly mechanism investigation and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2020, 188, 110722. [Google Scholar] [CrossRef]
- Zhi, K.; Wang, R.; Wei, J.; Shan, Z.; Shi, C.; Xia, X. Self-assembled micelles of dual-modified starch via hydroxypropylation and subsequent debranching with improved solubility and stability of curcumin. Food Hydrocoll. 2021, 118, 106809. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, B.; Xu, A.; Shen, Z.; Guo, Y.; Zhao, R.; Yao, H.; Shao, J.W. Carrier-Free, Pure Nanodrug Formed by the Self-Assembly of an Anticancer Drug for Cancer Immune Therapy. Mol. Pharm. 2018, 15, 2466–2478. [Google Scholar] [CrossRef]
- Deka, S.R.; Yadav, S.; Kumar, D.; Garg, S.; Mahato, M.; Sharma, A.K. Self-assembled dehydropeptide nano carriers for delivery of ornidazole and curcumin. Colloids Surf. B Biointerfaces 2017, 155, 332–340. [Google Scholar] [CrossRef]
- Feng, B.; Niu, Z.; Hou, B.; Zhou, L.; Li, Y.; Yu, H. Enhancing Triple Negative Breast Cancer Immunotherapy by ICG-Templated Self-Assembly of Paclitaxel Nanoparticles. Adv. Funct. Mater. 2020, 30, 1906605. [Google Scholar] [CrossRef]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Khan, S.; Karim, A.; Usman, M.; Hussain, A.; et al. Improving the oxidative stability of fish oil nanoemulsions by co-encapsulation with curcumin and resveratrol. Colloids Surf. B Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef] [PubMed]
- Urmi, W.T.; Rahman, M.M.; Kadirgama, K.; Ramasamy, D.; Maleque, M.A. An overview on synthesis, stability, opportunities and challenges of nanofluids. Mater. Today Proc. 2021, 41, 30–37. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H.J.F. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W. High storage capacity of curcumin loaded onto hollow mesoporous silica nanoparticles prepared via improved hard-templating method optimized by Taguchi DoE. Eng. Sci. Technol. Int. J. 2022, 33, 101070. [Google Scholar] [CrossRef]
- Kumar Tyagi, P.; Sharma, S.; Tyagi, S.; Mishra, A.; Gola, D. Toxicity assessment of silica nanoparticles, and their conjugates with curcumin on Drosophila melanogaster. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100616. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Periyathambi, P.; Hemalatha, T. Development of water-soluble curcumin grafted magnetic nanoparticles for enhancing bioavailability, fluorescence, and magnetic resonance imaging activity. Mater. Lett. 2021, 294, 129763. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Xiao, J.; Nian, S.; Huang, Q. Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin. Food Hydrocoll. 2015, 51, 166–175. [Google Scholar] [CrossRef]
- Slavova-Kazakova, A.; Janiak, M.A.; Sulewska, K.; Kancheva, V.D.; Karamac, M. Synergistic, additive, and antagonistic antioxidant effects in the mixtures of curcumin with (−)-epicatechin and with a green tea fraction containing (−)-epicatechin. Food Chem. 2021, 360, 129994. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-waterhouse, D.; Cui, C.; Wang, W.; Dong, K. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Rao, C.; Li, M.; Sun, X.; Li, M.; Lian, X.; Wang, H.; Lan, J.; Niu, B.; Li, W. Preparation and characterization of phosphate-stabilized amorphous calcium carbonate nanoparticles and their application in curcumin delivery. Mater. Chem. Phys. 2020, 255, 123552. [Google Scholar] [CrossRef]
- Shen, W.; Yan, M.; Wu, S.; Ge, X.; Liu, S.; Du, Y.; Zheng, Y.; Wu, L.; Zhang, Y.; Mao, Y. Chitosan nanoparticles embedded with curcumin and its application in pork antioxidant edible coating. Int. J. Biol. Macromol. 2022, 204, 410–418. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.T.; Cheng, J.S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef]
- Niu, F.; Hu, D.; Gu, F.; Du, Y.; Zhang, B.; Ma, S.; Pan, W. Preparation of ultra-long stable ovalbumin/sodium carboxymethylcellulose nanoparticle and loading properties of curcumin. Carbohydr. Polym. 2021, 271, 118451. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Chen, J.; Wei, Z.; Zhu, P.; Li, B.; Qing, Q.; Chen, H.; Lin, W.; Lin, J.; Hong, X.; et al. Fabrication, Evaluation, and Antioxidant Properties of Carrier-Free Curcumin Nanoparticles. Molecules 2023, 28, 1298. https://doi.org/10.3390/molecules28031298
Wu J, Chen J, Wei Z, Zhu P, Li B, Qing Q, Chen H, Lin W, Lin J, Hong X, et al. Fabrication, Evaluation, and Antioxidant Properties of Carrier-Free Curcumin Nanoparticles. Molecules. 2023; 28(3):1298. https://doi.org/10.3390/molecules28031298
Chicago/Turabian StyleWu, Jinwei, Jiaxin Chen, Zizhan Wei, Pingchuan Zhu, Bangda Li, Qing Qing, Huimin Chen, Weiying Lin, Jianyan Lin, Xuehui Hong, and et al. 2023. "Fabrication, Evaluation, and Antioxidant Properties of Carrier-Free Curcumin Nanoparticles" Molecules 28, no. 3: 1298. https://doi.org/10.3390/molecules28031298
APA StyleWu, J., Chen, J., Wei, Z., Zhu, P., Li, B., Qing, Q., Chen, H., Lin, W., Lin, J., Hong, X., Yu, F., & Chen, X. (2023). Fabrication, Evaluation, and Antioxidant Properties of Carrier-Free Curcumin Nanoparticles. Molecules, 28(3), 1298. https://doi.org/10.3390/molecules28031298




