Structure and Conformational Mobility of OLED-Relevant 1,3,5-Triazine Derivatives
Abstract
1. Introduction
2. Results and Discussion
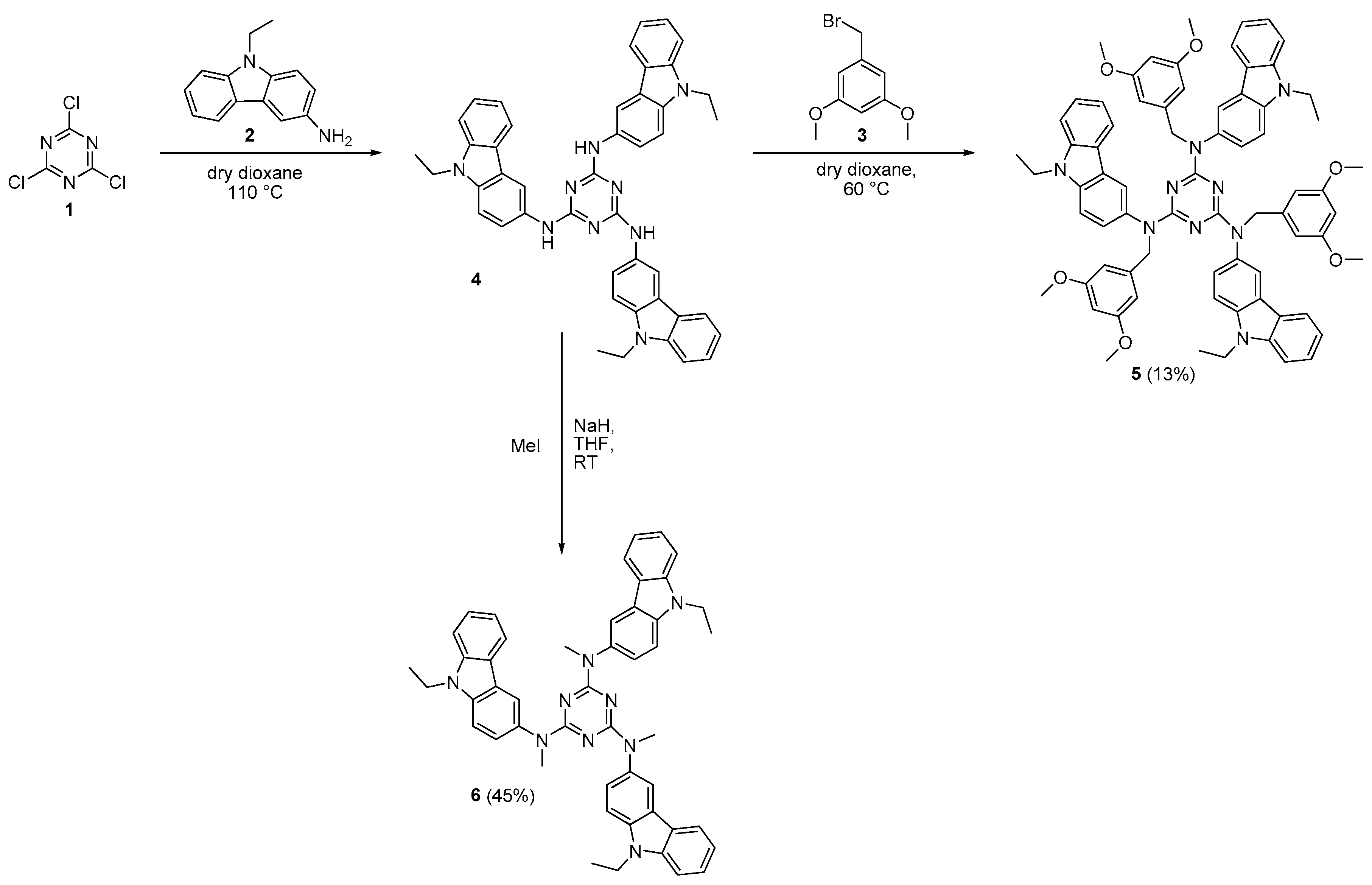
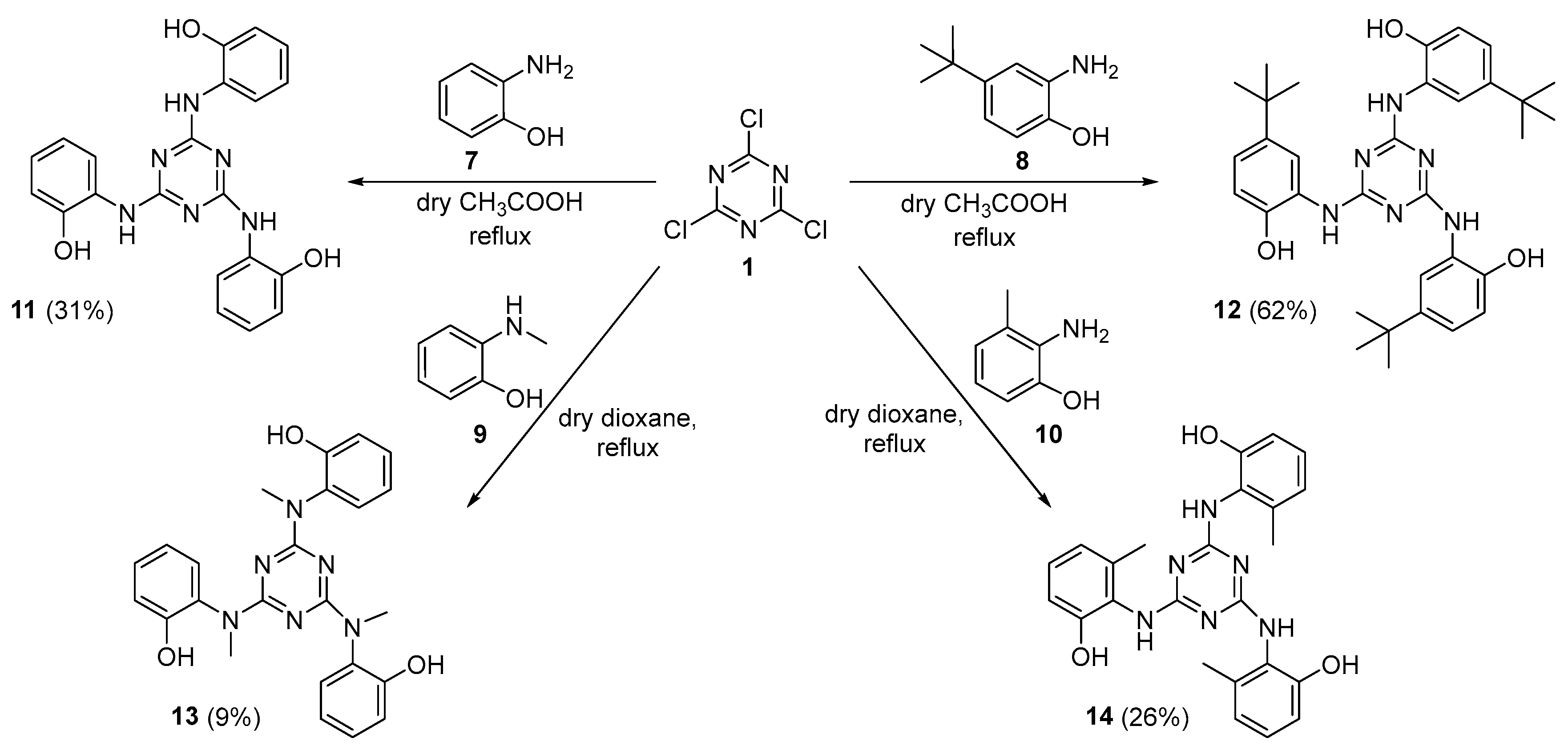
2.1. Synthesis
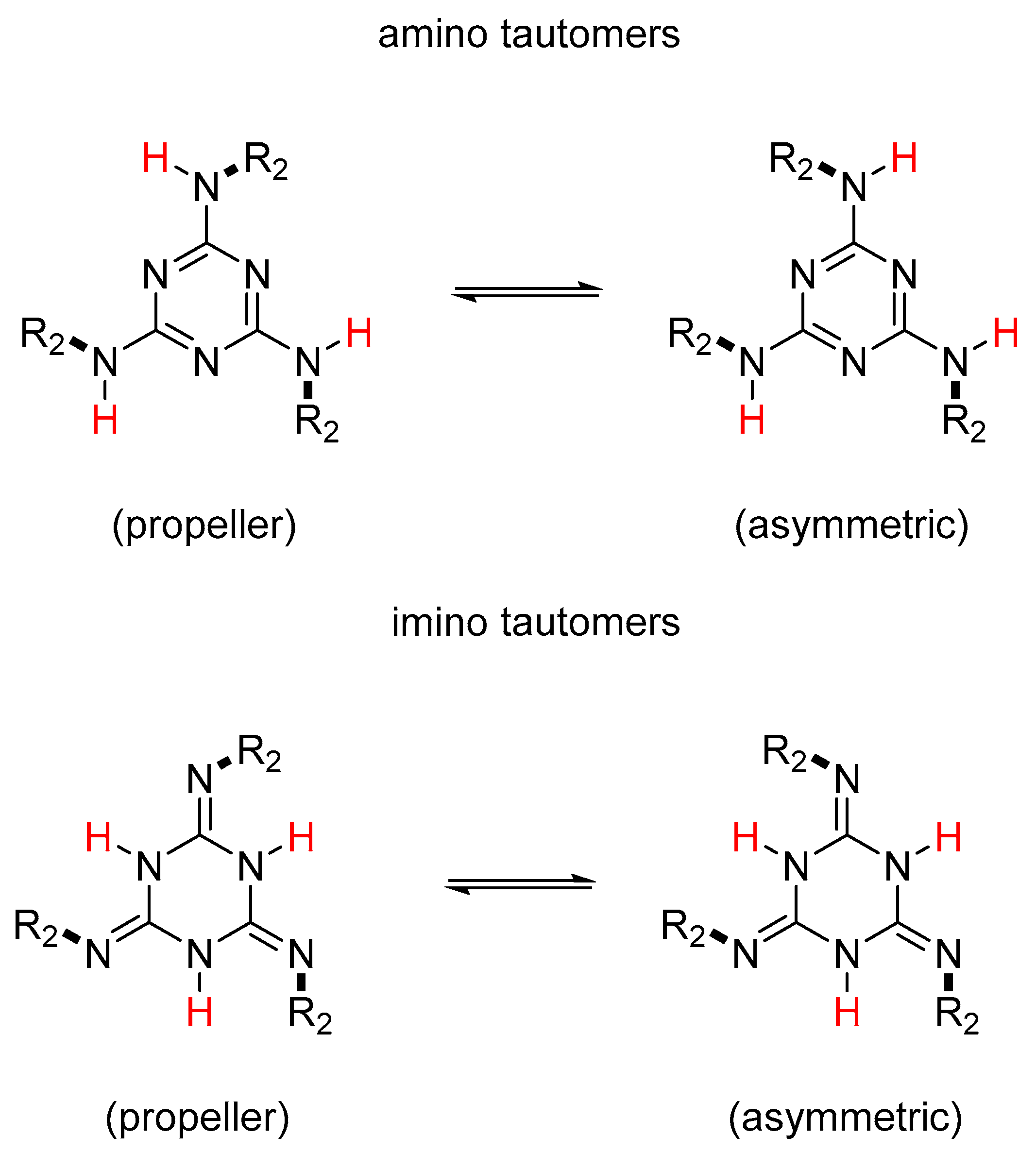
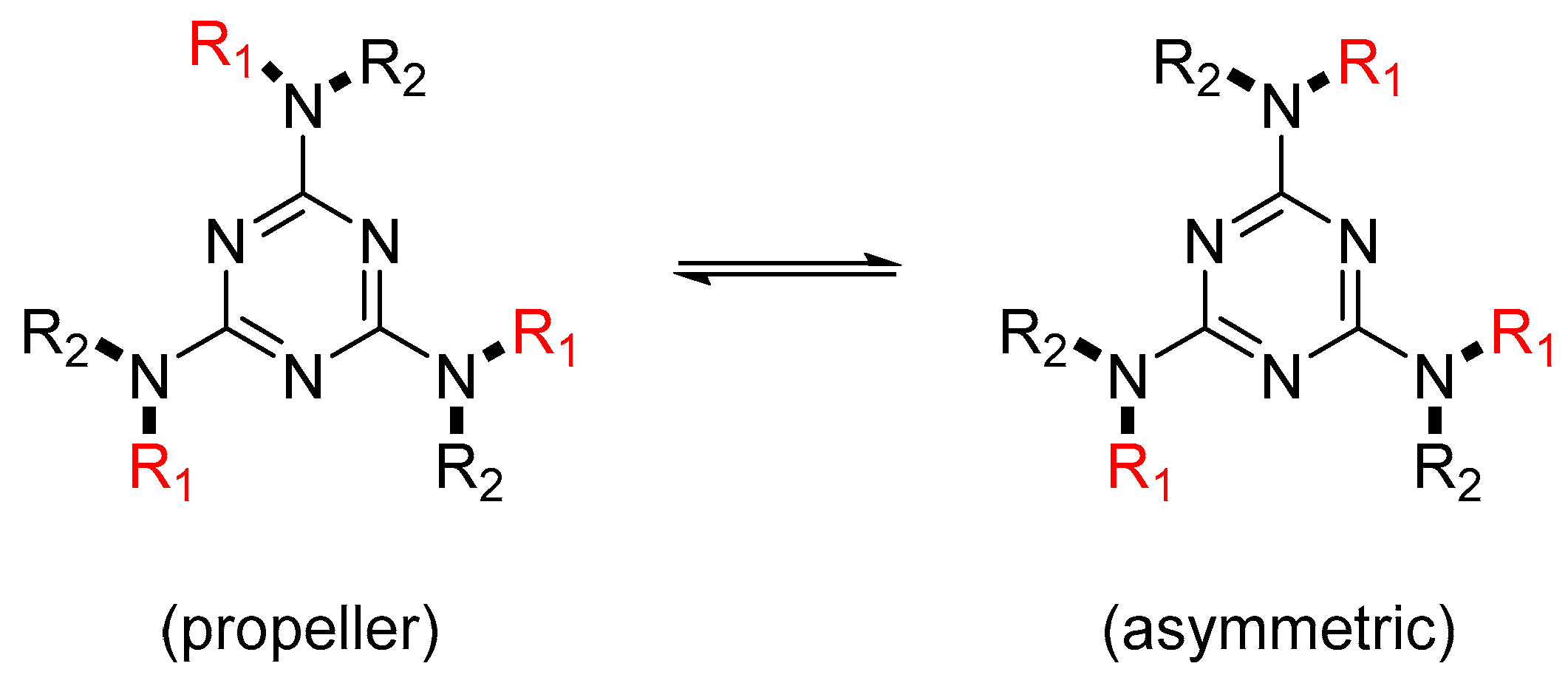
2.2. Potential Tautomerism
2.3. Dynamic NMR Spectroscopy, Conformational Analysis and Molecular Geometry
2.4. NMR Structure Determination
2.5. Dynamic NMR Studies of Compounds 4–6 and 11–14
2.6. DFT Calculations
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. UV-Vis and Fluorescence Spectra
3.4. Computational Details
3.5. Dynamic NMR Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef]
- Jarosz, T.; Lapkowski, M.; Ledwon, P. Advances in Star-Shaped π-Conjugated Systems: Properties and Applications. Macromol. Rapid Commun. 2014, 35, 1006–1032. [Google Scholar] [CrossRef]
- Kanibolotsky, A.L.; Perepichka, I.F.; Skabara, P.J. Star-shaped π-conjugated oligomers and their applications in organic electronics and photonics. Chem. Soc. Rev. 2010, 39, 2695–2728. [Google Scholar] [CrossRef]
- Herrera, H.; de Echegaray, P.; Urdanpilleta, M.; Mancheño, M.J.; Mena-Osteritz, E.; Bäuerle, P.; Segura, J.L. Linear and star-shaped naphthalimide-fused pyrazinacenes. Chem. Commun. 2013, 49, 713–715. [Google Scholar] [CrossRef]
- Skabara, P.J.; Arlin, J.-B.; Geerts, Y.H. Close Encounters of the 3D Kind—Exploiting High Dimensionality in Molecular Semiconductors. Adv. Mater. 2013, 25, 1948–1954. [Google Scholar] [CrossRef]
- Lai, W.-Y.; Xia, R.; He, Q.-Y.; Levermore, P.A.; Huang, W.; Bradley, D.D.C. Enhanced Solid-State Luminescence and Low-Threshold Lasing from Starburst Macromolecular Materials. Adv. Mater. 2009, 21, 355–360. [Google Scholar] [CrossRef]
- Zou, Y.; Zou, J.; Ye, T.; Li, H.; Yang, C.; Wu, H.; Ma, D.; Qin, J.; Cao, Y. Unexpected Propeller-Like Hexakis(fluoren-2-yl)benzene Cores for Six-Arm Star-Shaped Oligofluorenes: Highly Efficient Deep-Blue Fluorescent Emitters and Good Hole-Transporting Materials. Adv. Funct. Mater. 2013, 23, 1781–1788. [Google Scholar] [CrossRef]
- Huang, W.; Tang, F.; Li, B.; Su, J.; Tian, H. Large cyano- and triazine-substituted D–π–A–π–D structures as efficient AIEE solid emitters with large two-photon absorption cross sections. J. Mater. Chem. C 2014, 2, 1141–1148. [Google Scholar] [CrossRef]
- Sharma, G.D.; Angaridis, P.A.; Pipou, S.; Zervaki, G.E.; Nikolaou, V.; Misra, R.; Coutsolelos, A.G. Efficient co-sensitization of dye-sensitized solar cells by novel porphyrin/triazine dye and tertiary aryl-amine organic dye. Org. Electron. 2015, 25, 295–307. [Google Scholar] [CrossRef]
- Sharma, G.D.; Zervaki, G.E.; Angaridis, P.A.; Kitsopoulos, T.N.; Coutsolelos, A.G. Triazine-Bridged Porphyrin Triad as Electron Donor for Solution-Processed Bulk Hetero-Junction Organic Solar Cells. J. Phys. Chem. C 2014, 118, 5968–5977. [Google Scholar] [CrossRef]
- Zassowski, P.; Ledwon, P.; Kurowska, A.; Herman, A.P.; Jarosz, T.; Lapkowski, M.; Cherpak, V.; Stakhira, P.; Peciulyte, L.; Volyniuk, D.; et al. Efficient synthesis and structural effects of ambipolar carbazole derivatives. Synth. Met. 2017, 223, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Zhu, X.; Li, Y.; Chang, J.; Zhu, H.; Ma, L.; Lv, W.; Guo, J. Synthesis and luminescent properties of star-burst D-π-A compounds based on 1,3,5-triazine core and carbazole end-capped phenylene ethynylene arms. J. Lumin. 2014, 156, 130–136. [Google Scholar] [CrossRef]
- Chen, D.; Su, S.-J.; Cao, Y. Nitrogen heterocycle-containing materials for highly efficient phosphorescent OLEDs with low operating voltage. J. Mater. Chem. C 2014, 2, 9565–9578. [Google Scholar] [CrossRef]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. Carbazole Dendrimers as Solution-Processable Thermally Activated Delayed-Fluorescence Materials. Angew. Chem. Int. Ed. 2015, 54, 5677–5682. [Google Scholar] [CrossRef]
- Chang, C.-H.; Kuo, M.-C.; Lin, W.-C.; Chen, Y.-T.; Wong, K.-T.; Chou, S.-H.; Mondal, E.; Kwong, R.C.; Xia, S.; Nakagawa, T.; et al. A dicarbazole–triazine hybrid bipolar host material for highly efficient green phosphorescent OLEDs. J. Mater. Chem. 2012, 22, 3832–3838. [Google Scholar] [CrossRef]
- Ledwon, P. Recent advances of donor-acceptor type carbazole-based molecules for light emitting applications. Org. Electron. 2019, 75, 105422. [Google Scholar] [CrossRef]
- Zassowski, P.; Ledwon, P.; Kurowska, A.; Herman, A.P.; Lapkowski, M.; Cherpak, V.; Hotra, Z.; Turyk, P.; Ivaniuk, K.; Stakhira, P.; et al. 1,3,5-Triazine and carbazole derivatives for OLED applications. Dye. Pigment. 2018, 149, 804–811. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Polycarbazoles: 25 Years of Progress. Macromol. Rapid Commun. 2005, 26, 761–778. [Google Scholar] [CrossRef]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Kim, G.W.; Yang, D.R.; Kim, Y.C.; Yang, H.I.; Fan, J.G.; Lee, C.-H.; Chai, K.Y.; Kwon, J.H. Di(biphenyl)silane and carbazole based bipolar host materials for highly efficient blue phosphorescent OLEDs. Dye. Pigment. 2017, 136, 8–16. [Google Scholar] [CrossRef]
- Lee, C.W.; Im, Y.; Seo, J.-A.; Lee, J.Y. Carboline derivatives with an ortho-linked terphenyl core for high quantum efficiency in blue phosphorescent organic light-emitting diodes. Chem. Commun. 2013, 49, 9860–9862. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, S.-M.; Kang, J.-H.; Lee, J.-H.; Suh, M.C. Bipolar host materials with carbazole and dipyridylamine groups showing high triplet energy for blue phosphorescent organic light emitting diodes. Dye. Pigment. 2017, 141, 217–224. [Google Scholar] [CrossRef]
- Bian, C.; Wang, Q.; Ran, Q.; Liu, X.-Y.; Fan, J.; Liao, L.-S. New carbazole-based bipolar hosts for efficient blue phosphorescent organic light-emitting diodes. Org. Electron. 2018, 52, 138–145. [Google Scholar] [CrossRef]
- Yang, T.; Xu, H.; Wang, K.; Tao, P.; Wang, F.; Zhao, B.; Wang, H.; Xu, B. Bipolar host materials based on diphenylphosphine oxide and carbazole derivatives with high triplet energy: Synthesis, characterization and photoelectronic performance in PhOLEDs. Dye. Pigment. 2018, 153, 67–73. [Google Scholar] [CrossRef]
- Lade, J.; Lee, N.-Y.; Patil, B.; Deshpande, Y.Y.; Pownthurai, B.; Hsieh, C.-A.; Pingale, S.S.; Chen, L.-Y.; Chaskar, A. Novel benzothiadiazine 1,1-dioxide based bipolar host materials for efficient red phosphorescent organic light emitting diodes. Org. Electron. 2021, 92, 106104. [Google Scholar] [CrossRef]
- Trang, N.V.; Tam, N.M.; Dung, T.N.; Nguyen, M.T. A theoretical design of bipolar host materials for blue phosphorescent OLED. J. Mol. Graph. Model. 2021, 105, 107845. [Google Scholar] [CrossRef]
- Luo, J.; Gong, S.; Gu, Y.; Chen, T.; Li, Y.; Zhong, C.; Xie, G.; Yang, C. Multi-carbazole encapsulation as a simple strategy for the construction of solution-processed, non-doped thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2016, 4, 2442–2446. [Google Scholar] [CrossRef]
- Väth, S.; Tvingstedt, K.; Auth, M.; Sperlich, A.; Dabuliene, A.; Grazulevicius, J.V.; Stakhira, P.; Cherpak, V.; Dyakonov, V. Direct Observation of Spin States Involved in Organic Electroluminescence Based on Thermally Activated Delayed Fluorescence. Adv. Opt. Mater. 2017, 5, 1600926. [Google Scholar] [CrossRef]
- Gudeika, D.; Bezvikonnyi, O.; Volyniuk, D.; Grazulevicius, J.V. Differently substituted benzonitriles for non-doped OLEDs. Dye. Pigment. 2020, 172, 107789. [Google Scholar] [CrossRef]
- Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Rutkis, M.; Grazulevicius, J.V. Diphenylsulfone-based hosts for electroluminescent devices: Effect of donor substituents. Dye. Pigment. 2020, 175, 108104. [Google Scholar] [CrossRef]
- Serevičius, T.; Skaisgiris, R.; Fiodorova, I.; Steckis, V.; Dodonova, J.; Banevičius, D.; Kazlauskas, K.; Juršėnas, S.; Tumkevičius, S. Achieving efficient deep-blue TADF in carbazole-pyrimidine compounds. Org. Electron. 2020, 82, 105723. [Google Scholar] [CrossRef]
- Hiraga, Y.; Kuwahara, R.; Hatta, T. Novel indolo[3,2,1-jk]carbazole-based bipolar host material for highly efficient thermally activated delayed-fluorescence organic light-emitting diodes. Tetrahedron 2021, 94, 132317. [Google Scholar] [CrossRef]
- Danyliv, I.; Danyliv, Y.; Lytvyn, R.; Bezvikonnyi, O.; Volyniuk, D.; Simokaitiene, J.; Ivaniuk, K.; Tsiko, U.; Tomkeviciene, A.; Dabulienė, A.; et al. Multifunctional derivatives of donor-substituted perfluorobiphenyl for OLEDs and optical oxygen sensors. Dye. Pigment. 2021, 193, 109493. [Google Scholar] [CrossRef]
- Niu, R.; Li, J.; Liu, D.; Dong, R.; Wei, W.; Tian, H.; Shi, C. A versatile carbazole donor design strategy for blue emission switching from normal fluorescence to thermally activated delayed fluorescence. Dye. Pigment. 2021, 194, 109581. [Google Scholar] [CrossRef]
- Gudeika, D.; Bezvikonnyi, O.; Masimukku, N.; Volyniuk, D.; Chen, C.-H.; Ding, W.-C.; Lee, J.-H.; Chiu, T.-L.; Grazulevicius, J.V. Tetraphenyl ornamented carbazolyl disubstituted diphenyl sulfone as bipolar TADF host for highly efficient OLEDs with low efficiency roll-offs. Dye. Pigment. 2021, 194, 109573. [Google Scholar] [CrossRef]
- Skaisgiris, R.; Serevičius, T.; Dodonova, J.; Banevičius, D.; Kazlauskas, K.; Tumkevičius, S.; Juršėnas, S. Tuning of HOMO-LUMO localization for achieving thermally activated delayed fluorescence. J. Lumin. 2022, 241, 118473. [Google Scholar] [CrossRef]
- Lin, W.-J.; Chen, M.-Y.; Li, B.; Wei, B.; Ding, X.-W. Monomeric carbazolylcyanobenzenes as thermally activated delayed fluorescence emitters: Effect of substitution position on photoluminescent and electroluminescent properties. Mol. Cryst. Liq. Cryst. 2022, 733, 32–38. [Google Scholar] [CrossRef]
- Ranasinghe, C.S.K.; Thamarappalli, A.; Jang, J.; Gao, M.; Koodalingam, M.; Burn, P.L.; Puttock, E.V.; Shaw, P.E. Investigating the donor:acceptor ratio in thermally activated delayed fluorescence light-emitting macromolecules. Org. Electron. 2022, 105, 106500. [Google Scholar] [CrossRef]
- Sun, D.; Saxena, R.; Fan, X.; Athanasopoulos, S.; Duda, E.; Zhang, M.; Bagnich, S.; Zhang, X.; Zysman-Colman, E.; Köhler, A. Regiochemistry of Donor Dendrons Controls the Performance of Thermally Activated Delayed Fluorescence Dendrimer Emitters for High Efficiency Solution-Processed Organic Light-Emitting Diodes. Adv. Sci. 2022, 9, 2201470. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, S.-C.; Lin, B.-Y.; Li, C.-Y.; Kong, Y.-C.; Chen, Y.-S.; Fang, S.-C.; Chiu, C.-H.; Lee, J.-H.; Wong, K.-T.; et al. New bipolar host materials for high power efficiency green thermally activated delayed fluorescence OLEDs. Chem. Eng. J. 2022, 442, 136292. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Gudeika, D.; Kutsiy, S.; Simokaitiene, J.; Butkute, R.; Skhirtladze, L.; Woon, K.L.; Volyniuk, D.; Grazulevicius, J.V. Ornamenting of Blue Thermally Activated Delayed Fluorescence Emitters by Anchor Groups for the Minimization of Solid-State Solvation and Conformation Disorder Corollaries in Non-Doped and Doped Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 40158–40172. [Google Scholar] [CrossRef]
- Li, W.; Pan, Y.; Yao, L.; Liu, H.; Zhang, S.; Wang, C.; Shen, F.; Lu, P.; Yang, B.; Ma, Y. A Hybridized Local and Charge-Transfer Excited State for Highly Efficient Fluorescent OLEDs: Molecular Design, Spectral Character, and Full Exciton Utilization. Adv. Opt. Mater. 2014, 2, 892–901. [Google Scholar] [CrossRef]
- Bai, Y.; Hong, L.; Lei, T.; Zhang, L.; Ouyang, X.; Liu, Z.; Chen, Y.; Li, W.; Ge, Z. Solution-processable, single-layer, blue organic light-emitting diodes employing dual emitting cores of hybridized local and charge-transfer units. Dye. Pigment. 2016, 132, 94–102. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Anudeebhana, J.; Thanikachalam, V.; Sivaraj, S. Multifunctional assistant acceptor modulated pyrenyl phenanthrimidazole derivatives for highly efficient blue and host-sensitized OLEDs. J. Mater. Chem. C 2021, 9, 15683–15697. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Panimozhi, S.; Thanikachalam, V. Hot exciton transition for organic light-emitting diodes: Tailoring excited-state properties and electroluminescence performances of donor-spacer-acceptor molecules. RSC Adv. 2018, 8, 37324–37338. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Han, P.; Xiao, S.; Qu, F.; Yao, J.; Qiao, X.; Yang, D.; Dai, Y.; Sun, Q.; Hu, D.; et al. Efficiency Breakthrough of Fluorescence OLEDs by the Strategic Management of “Hot Excitons” at Highly Lying Excitation Triplet Energy Levels. Adv. Funct. Mater. 2021, 31, 2106912. [Google Scholar] [CrossRef]
- Loythaworn, T.; Petdee, S.; Chasing, P.; Chantanop, N.; Therdkatanyuphong, P.; Waengdongbung, W.; Sudyoadsuk, T.; Promarak, V. An efficient solution-processable non-doped hybridized local and charge-transfer (HLCT) emitter for a simplified organic light-emitting diode. Mater. Chem. Front. 2022, 6, 3225–3236. [Google Scholar] [CrossRef]
- Thanikachalam, V.; Sarojpurani, E.; Jayabharathi, J.; Jeeva, P. Efficient phenanthroimidazole-styryl-triphenylamine derivatives for blue OLEDs: A combined experimental and theoretical study. New J. Chem. 2017, 41, 2443–2457. [Google Scholar] [CrossRef]
- Paterson, L.; May, F.; Andrienko, D. Computer aided design of stable and efficient OLEDs. J. Appl. Phys. 2020, 128, 160901. [Google Scholar] [CrossRef]
- Afonso, C.A.M.; Lourenco, N.M.T.; Rosatella, A.D.A. Synthesis of 2,4,6-Tri-substituted-1,3,5-Triazines. Molecules 2006, 11, 81–102. [Google Scholar] [CrossRef]
- Elguero, J.; Katritzky, A.R.; Denisko, O.V. Prototropic tautomerism of heterocycles: Heteroaromatic tautomerism—General overview and methodology. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 76, pp. 1–84. [Google Scholar]
- Díaz-Ortiz, A.; Elguero, J.; Foces-Foces, C.; de la Hoz, A.; Moreno, A.; Moreno, S.; Sánchez-Migallón, A.; Valiente, G. Synthesis, structural determination and dynamic behavior of 2-chloro-4,6-bis(pyrazolylamino)-1,3,5-triazines. Org. Biomol. Chem. 2003, 1, 4451–4457. [Google Scholar] [CrossRef]
- Antonov, L. Absorption UV–Vis Spectroscopy and Chemometrics: From Qualitative Conclusions to Quantitative Analysis. In Tautomerism; Wiley: Hoboken, NJ, USA, 2013; pp. 25–47. [Google Scholar] [CrossRef]
- Georgiev, A.; Deneva, V.; Yordanov, D.; Völzer, T.; Wolter, S.; Fennel, F.; Lochbrunner, S.; Antonov, L. Benzothiazol picolin/isonicotinamides molecular switches: Expectations and reality. J. Mol. Liq. 2022, 356, 118968. [Google Scholar] [CrossRef]
- Bede, L.A.; Koffi, A.K.; Beke, F.-L.E.D.; Semmeq, A.; Badawi, M. Investigation of tautomerism of 1,3,5-triazine derivative, stability, and acidity of its tautomers from density functional theory. J. Mol. Model. 2021, 27, 147. [Google Scholar] [CrossRef]
- Manolova, Y.; Marciniak, H.; Tschierlei, S.; Fennel, F.; Kamounah, F.S.; Lochbrunner, S.; Antonov, L. Solvent control of intramolecular proton transfer: Is 4-hydroxy-3-(piperidin-1-ylmethyl)-1-naphthaldehyde a proton crane? Phys. Chem. Chem. Phys. 2017, 19, 7316–7325. [Google Scholar] [CrossRef]
- Joshi, H.C.; Antonov, L. Excited-State Intramolecular Proton Transfer: A Short Introductory Review. Molecules 2021, 26, 1475. [Google Scholar] [CrossRef]
- Sandström, J. Dynamic NMR Spectroscopy; Academic Press: London, UK, 1982. [Google Scholar]
- Oki, M. Applications of Dynamic NMR Spectroscopy to Organic Chemistry; VCH Publishers: Weinheim, Germany, 1985. [Google Scholar]
- Abel, E.W.; Coston, T.P.J.; Orrell, K.G.; Šik, V.; Stephenson, D. Two-dimensional NMR exchange spectroscopy. Quantitative treatment of multisite exchanging systems. J. Magn. Reson. 1986, 70, 34–53. [Google Scholar] [CrossRef]
- Orrell, K.G.; Osborne, A.G.; Sik, V.; da Silva, M.W.; Hursthouse, M.B.; Hibbs, D.E.; Malik, K.M.A.; Vassilev, N.G. Stereochemically non-rigid transition metal complexes of 2,6-bis 1-(phenylimino)ethyl pyridine (BIP). Part 3. Dynamic NMR studies of fac- PtXMe3(BIP) (X=Cl, Br, or I). Crystal structure of fac- PtIMe3(BIP). J. Organomet. Chem. 1998, 555, 35–47. [Google Scholar] [CrossRef]
- Orrell, K.G.; Osborne, A.G.; Sik, V.; da Silva, M.W.; Hursthouse, M.B.; Hibbs, D.E.; Malik, K.M.A.; Vassilev, N.G. Stereochemically non-rigid transition metal complexes of 2,6-bis (1-phenylimino)ethyl pyridine (BIP).2. Dynamic NMR studies of fac- ReX(CO)(3)(BIP) (X=Cl, Br, or I). Crystal structure of fac- ReBr(CO)(3)(BIP). J. Organomet. Chem. 1997, 538, 171–183. [Google Scholar] [CrossRef]
- Dangalov, M.; Stoyanova, M.; Petrov, P.; Putala, M.; Vassilev, N.G. Fluxional Pd(II) NHC complexes—Synthesis, structure elucidation and catalytic studies. J. Organomet. Chem. 2016, 817, 1–14. [Google Scholar] [CrossRef]
- Lyapchev, R.; Petrov, P.; Dangalov, M.; Vassilev, N.G. Synthesis and structure elucidation of allyl Pd(II) complexes of NHC ligands derived from substituted imidazo[1,5-a]quinolin-1(2H)-ylidene. J. Organomet. Chem. 2017, 851, 194–209. [Google Scholar] [CrossRef]
- Philipova, I.; Stavrakov, G.; Vassilev, N.; Nikolova, R.; Shivachev, B.; Dimitrov, V. Cytisine as a scaffold for ortho-diphenylphosphinobenzenecarboxamide ligands for Pd-catalyzed asymmetric allylic alkylation. J. Organomet. Chem. 2015, 778, 10–20. [Google Scholar] [CrossRef]
- Philipova, I.; Stavrakov, G.; Dimitrov, V.; Vassilev, N. Galantamine derivatives: Synthesis, NMR study, DFT calculations and application in asymmetric catalysis. J. Mol. Struct. 2020, 1219, 128568. [Google Scholar] [CrossRef]
- Dangalov, M.; Petrov, P.; Vassilev, N.G. Fluxional allyl Pd(II) and Pt(II) complexes of NHC ligands derived from substituted 1,8-naphthalimides—Synthesis and structure elucidation. J. Organomet. Chem. 2016, 824, 104–117. [Google Scholar] [CrossRef]
- Perrin, C.L.; Engler, R.E. Quantitative assessment by 1D-EXSY NMR of stereoelectronic control in acid-catalyzed exchange between stereoisomeric 2-methoxy-1,3-dioxanes and methanol. J. Am. Chem. Soc. 1997, 119, 585–591. [Google Scholar] [CrossRef]
- Vassilev, N.G.; Dimitrov, V.S. Dynamic NMR: Combined use of 1D selective EXSY and complete lineshape analysis of spectra subjected to reference deconvolution and linear prediction or the maximum entropy method. Magn. Reson. Chem. 2001, 39, 607–614. [Google Scholar] [CrossRef]
- Bothner-By, A.A.; Stephens, R.L.; Lee, J.; Warren, C.D.; Jeanloz, R.W. Structure determination of a tetrasaccharide: Transient nuclear Overhauser effects in the rotating frame. J. Am. Chem. Soc. 1984, 106, 811–813. [Google Scholar] [CrossRef]
- Anderson, D.R.; Hickstein, D.D.; O’Leary, D.J.; Grubbs, R.H. Model Compounds of Ruthenium−Alkene Intermediates in Olefin Metathesis Reactions. J. Am. Chem. Soc. 2006, 128, 8386–8387. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shaka, A.J. Cross relaxation without TOCSY: Transverse rotating-frame overhauser effect spectroscopy. J. Am. Chem. Soc. 1992, 114, 3157–3159. [Google Scholar] [CrossRef]
- Boros, S.; Batta, G. Offset-compensated and zero-quantum suppressed ROESY provides accurate 1H–1H distances in small to medium-sized molecules. Magn. Reson. Chem. 2016, 54, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.M.; Petzold, K.; Schleucher, J. EASY ROESY: Reliable Cross-Peak Integration in Adiabatic Symmetrized ROESY. Chem.-Eur. J. 2009, 15, 585–588. [Google Scholar] [CrossRef]
- Baishya, B.; Verma, A. Elimination of Zero-Quantum artifacts and sensitivity enhancement in perfect echo based 2D NOESY. J. Magn. Reson. 2015, 252, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.A.; Colbourne, A.A.; Cassani, J.; Nilsson, M.; Morris, G.A. Decoupling two-dimensional NMR spectroscopy in both dimensions: Pure shift NOESY and COSY. Angew. Chem. Int. Ed. 2012, 51, 6460–6463. [Google Scholar] [CrossRef]
- Ilgen, J.; Nowag, J.; Kaltschnee, L.; Schmidts, V.; Thiele, C.M. Gradient selected pure shift EASY-ROESY techniques facilitate the quantitative measurement of 1H,1H-distance restraints in congested spectral regions. J. Magn. Reson. 2021, 324, 106900. [Google Scholar] [CrossRef] [PubMed]
- Ehn, M.; Vassilev, N.G.; Pašteka, L.F.; Dangalov, M.; Putala, M. Atropisomerism of 2,2′-Diaryl-1,1′-binaphthalenes Containing Three Stereogenic Axes: Experimental and Computational Study. Eur. J. Org. Chem. 2015, 2015, 7935–7942. [Google Scholar] [CrossRef]
- Belyakov, P.A.; Shatin, A.V.; Strelenko, Y.A. Synthesis, structure, and dynamic behavior in solution of arylamino-1,3,5-triazines. 1. Unsymmetrically substituted arylamino-1,3,5- triazines. Russ. Chem. Bull. 2005, 54, 2441–2451. [Google Scholar] [CrossRef]
- Ghiviriga, I.; Oniciu, D.C. Steric hindrance to the solvation of melamines and consequences for non-covalent synthesis. Chem. Commun. 2002, 2002, 2718–2719. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Elguero, J.; de la Hoz, A.; Jiménez, A.; Moreno, A.; Moreno, S.; Sánchez-Migallón, A. Microwave-Assisted Synthesis and Dynamic Behaviour of N2,N4,N6-Tris(1H-pyrazolyl)-1,3,5-triazine-2,4,6-triamines. QSAR Comb. Sci. 2005, 24, 649–659. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Elguero, J.; Foces-Foces, C.; De La Hoz, A.; Moreno, A.; Del Carmen Mateo, M.; Sánchez-Migallón, A.; Valiente, G. Green synthesis and self-association of 2,4-diamino-1,3,5-triazine derivatives. New J. Chem. 2004, 28, 952–958. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Wiggins, P.; Williams, J.A.G.; Tozer, D.J. Excited state surfaces in density functional theory: A new twist on an old problem. J. Chem. Phys. 2009, 131, 091101. [Google Scholar] [CrossRef]
- Improta, R. UV–Visible Absorption and Emission Energies in Condensed Phase by PCM/TD-DFT Methods. In Computational Strategies for Spectroscopy; Wiley: Hoboken, NJ, USA, 2011; pp. 37–75. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Kawauchi, S.; Antonov, L.; Okuno, Y. Prediction of the color of dyes by using time-dependent density functional theory (TD-DFT). Bulg. Chem. Commun 2014, 46, 228–237. [Google Scholar]
- Binsch, G. Band-Shape Analysis. In Dynamic NMR Spectroscopy; Jackman, L.M., Cotton, F.A., Eds.; Academic Press: New York, NY, USA, 1975; pp. 45–82. [Google Scholar]
- Heinzer, J.; Oth, J.F.M. Iterative Least-Squares Lineshape Fitting of H-1-Decoupled C-13-Dnmr Spectra. Helv. Chim. Acta 1981, 64, 258–278. [Google Scholar] [CrossRef]


| Compound | λabsmax [nm] | λemmax [nm] | Δ a [eV] | φeff b [%] | λmax [nm] (f) c | Eg [eV] d |
|---|---|---|---|---|---|---|
| 4 | 300 338 353 358 | 386 | 0.19 | 0.33 | 344 (0.03) | 4.09 |
| 5 | 280 300 338 353 | 386 | 0.25 | 0.90 | 330 (0.04) | 4.32 |
| 6 | 280 300 338 353 | 391 | 0.28 | 5.75 | 334 (0.04) | 4.37 |
| 11 | 290 | - | - | - | 280 (0.54) | 4.97 |
| 12 | 295 | - | - | - | 285 (0.49) | 4.91 |
| 13 | 282 | - | - | - | 267 (0.55) | 5.32 |
| 14 | 282 | - | - | - | 254 (0.33) | 5.08 |
| Comp. | R1 | R2 | Propeller, %, (a, b) | Asymmetric, %, (a, b) | (T, K) |
|---|---|---|---|---|---|
| 6 | CH3 | 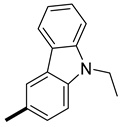 | 14 (0, 50) | 86 (100, 50) | 223 |
| 5 | 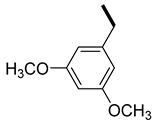 | 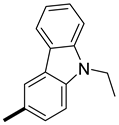 | 17 (100, 94) | 83 (0, 6) | 223 |
| 13 | CH3 | 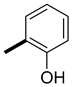 | 37 (26, 88) | 63 (74, 12) | 233 |
| 11 | H |  | 42 (21, 96) | 58 (79, 4) | 223 |
| 12 | H |  | 46 (18, 81) | 54 (82, 19) | 233 |
| 14 | H | 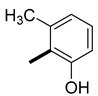 | 48 (11, 99) | 52 (89, 1) | 233 |
| 4 | H |  | 50 (1, 96) | 50 (99, 4) | 223 |
| Comp. | R1 | R2 | Experimental Rotational Barrier, ΔG≠(298K) | Theoretical Rotational Barrier, ΔG≠(298K) a (b) |
|---|---|---|---|---|
| 4 | H | 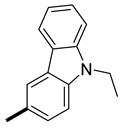 | 13.6 ± 0.1 (AS) c 13.9 ± 0.1 (SA) | 14.5 (11.7) 12.5 (13.2) |
| 5 |  | 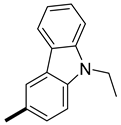 | 14.7 ± 0.1 (AS) 14.3 ± 0.1 (SA) | 17.8 (12.9) 11.4 (14.1) |
| 6 | CH3 | 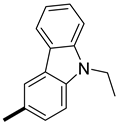 | 12.5 ± 0.1 (AS) 11.7 ± 0.1 (SA) | 9.3 (11.1) 12.6 (11.1) |
| 11 | H |  | 12.7 ± 0.1 (AS) 12.4 ± 0.1 (SA) | 14.8 (14.4) 14.4 (15.9) |
| 12 | H |  | 13.0 ± 0.1 (AS) 13.0 ± 0.1 (SA) | 14.6 (15.0) 14.2 (14.7) |
| 13 | CH3 |  | 13.8 ± 0.1 (AS) 14.1 ± 0.1 (SA) | 14.7 (13.9) 14.2 (13.5) |
| 14 | H | 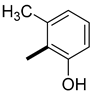 | 14.2 ± 0.1 (AS) 13.9 ± 0.1 (SA) | 14.4 (15.7) 13.5 (16.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrikov, G.M.; Nikolova, Y.; Slavchev, I.; Dangalov, M.; Deneva, V.; Antonov, L.; Vassilev, N.G. Structure and Conformational Mobility of OLED-Relevant 1,3,5-Triazine Derivatives. Molecules 2023, 28, 1248. https://doi.org/10.3390/molecules28031248
Dobrikov GM, Nikolova Y, Slavchev I, Dangalov M, Deneva V, Antonov L, Vassilev NG. Structure and Conformational Mobility of OLED-Relevant 1,3,5-Triazine Derivatives. Molecules. 2023; 28(3):1248. https://doi.org/10.3390/molecules28031248
Chicago/Turabian StyleDobrikov, Georgi M., Yana Nikolova, Ivaylo Slavchev, Miroslav Dangalov, Vera Deneva, Liudmil Antonov, and Nikolay G. Vassilev. 2023. "Structure and Conformational Mobility of OLED-Relevant 1,3,5-Triazine Derivatives" Molecules 28, no. 3: 1248. https://doi.org/10.3390/molecules28031248
APA StyleDobrikov, G. M., Nikolova, Y., Slavchev, I., Dangalov, M., Deneva, V., Antonov, L., & Vassilev, N. G. (2023). Structure and Conformational Mobility of OLED-Relevant 1,3,5-Triazine Derivatives. Molecules, 28(3), 1248. https://doi.org/10.3390/molecules28031248







