Rare Missense Variants of the Human β4 Subunit Alter Nicotinic α3β4 Receptor Plasma Membrane Localisation
Abstract
1. Introduction
2. Results
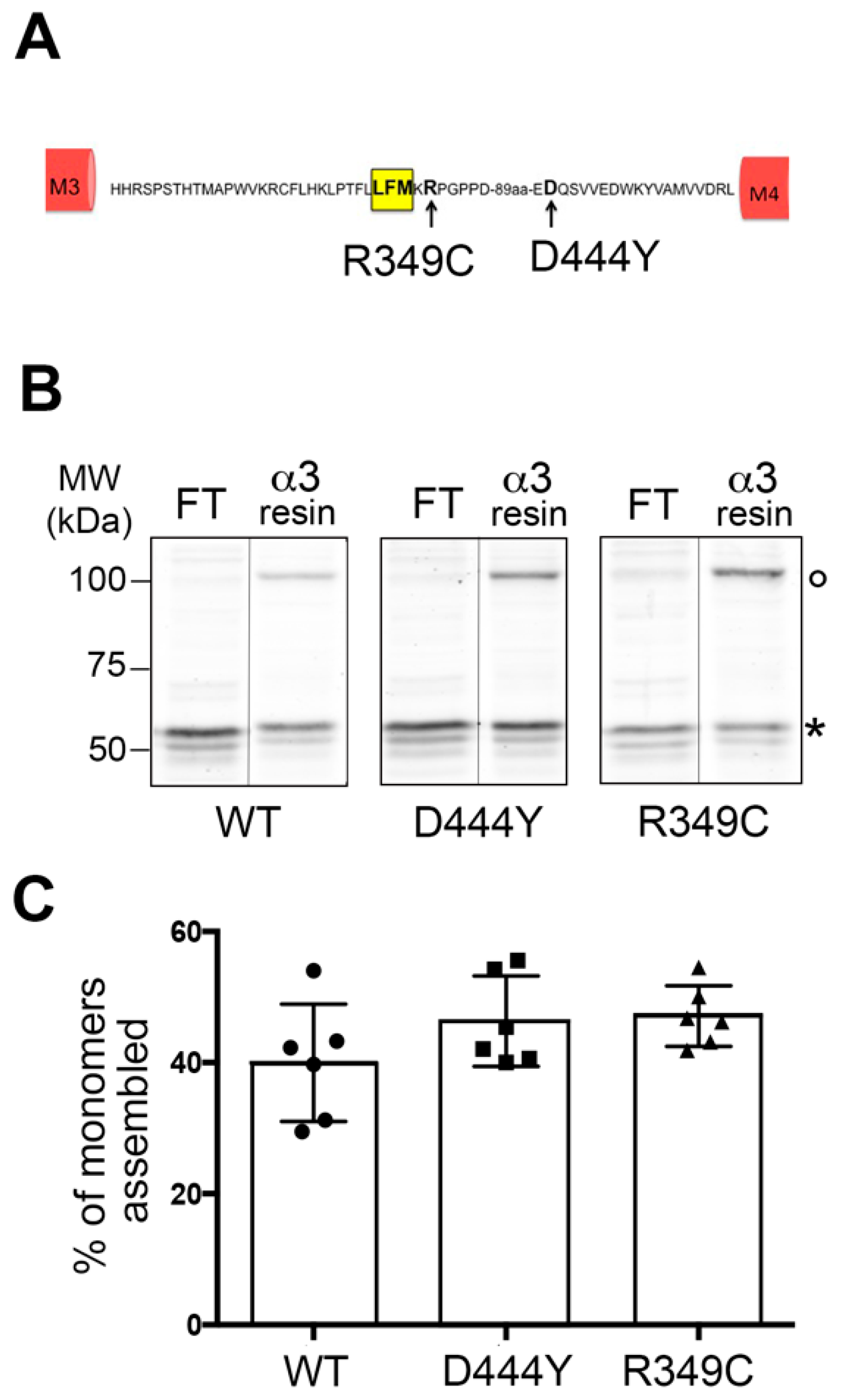
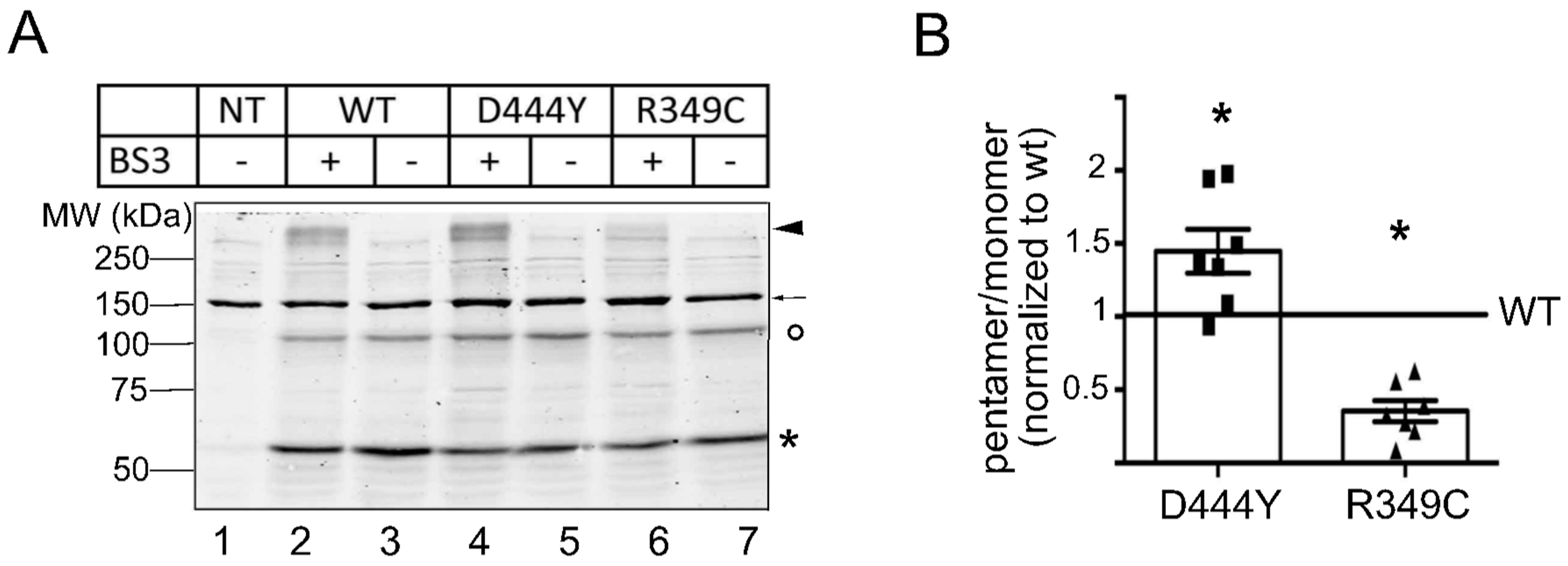
2.1. Expression of α3β4 nAChRs Containing a Mutant Accessory β4 Subunit
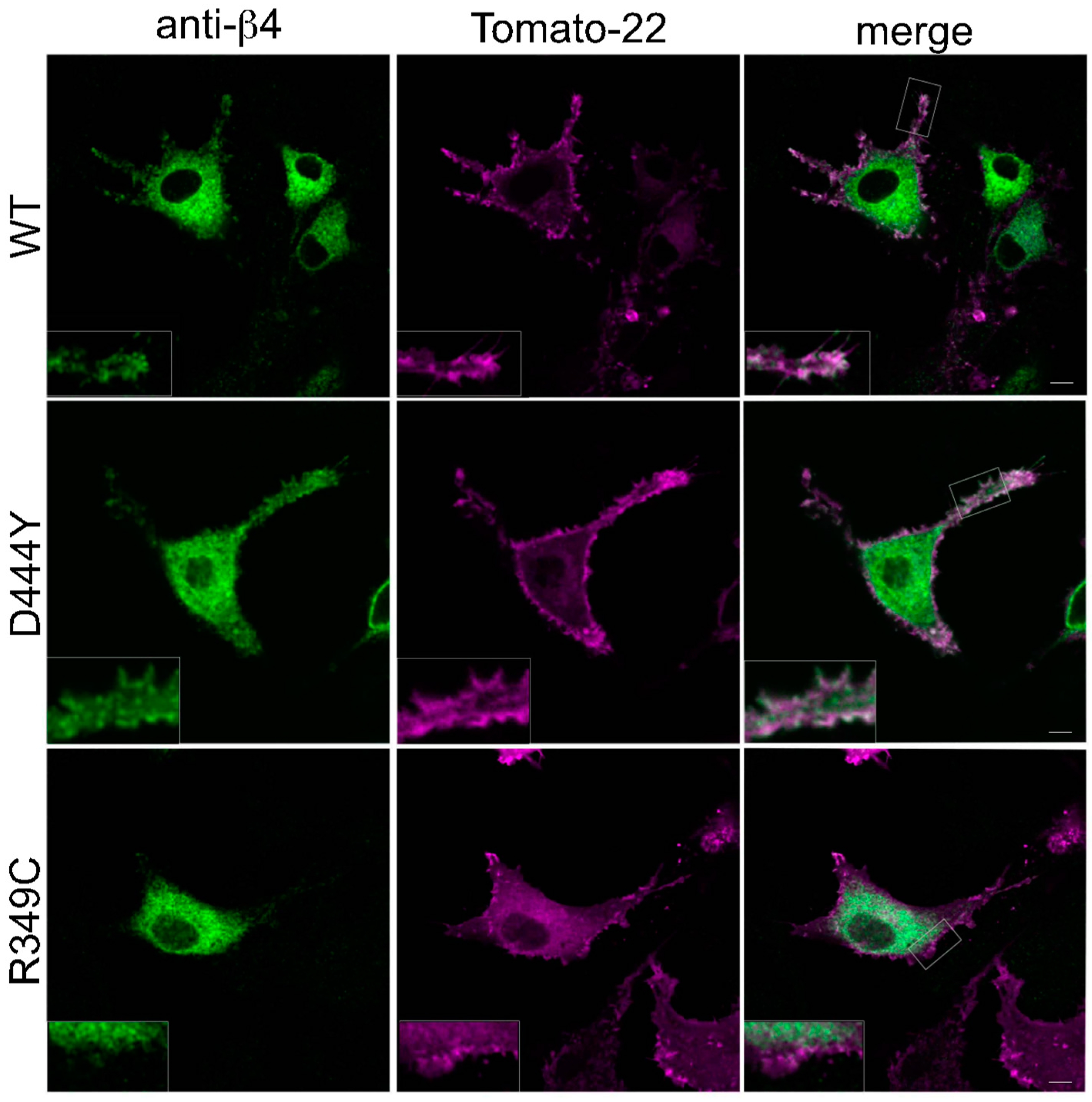
2.2. The Presence of β4 Variants in the Fifth Subunit Alters the Localisation of α3β4 nAChRs
3. Discussion
4. Materials and Methods
4.1. Plasmid Constructions
4.2. Antibodies
4.3. Cell Culture and Transfection
4.4. Biochemical Analysess
4.5. Immunofluorescence and Image Analysis
4.6. Immunoprecipitation Using Cross-Linked Resins
4.7. Crosslinking Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96 Pt B, 302–311. [Google Scholar] [CrossRef]
- Colombo, S.F.; Mazzo, F.; Pistillo, F.; Gotti, C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem. Pharmacol. 2013, 86, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Halevi, S.; McKay, J.; Palfreyman, M.; Yassin, L.; Eshel, M.; Jorgensen, E.; Treinin, M. The, C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002, 21, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.A.; Gu, S.; Davini, W.B.; Bredt, D.S. Nicotinic acetylcholine receptor redux: Discovery of accessories opens therapeutic vistas. Science 2021, 373, 6556. [Google Scholar] [CrossRef]
- Crespi, A.; Colombo, S.F.; Gotti, C. Proteins and chemical chaperones involved in neuronal nicotinic receptor expression and function: An update. Br. J. Pharmacol. 2018, 175, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef]
- Grady, S.R.; Moretti, M.; Zoli, M.; Marks, M.J.; Zanardi, A.; Pucci, L.; Clementi, F.; Gotti, C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 2009, 29, 2272–2282. [Google Scholar] [CrossRef]
- Elayouby, K.S.; Ishikawa, M.; Dukes, A.J.; Smith, A.C.W.; Lu, Q.; Fowler, C.D.; Kenny, P.J. alpha3* Nicotinic Acetylcholine Receptors in the Habenula-Interpeduncular Nucleus Circuit Regulate Nicotine Intake. J. Neurosci. 2021, 41, 1779–1787. [Google Scholar] [CrossRef]
- Husson, M.; Harrington, L.; Tochon, L.; Cho, Y.; Ibanez-Tallon, I.; Maskos, U.; David, V. beta4-Nicotinic Receptors Are Critically Involved in Reward-Related Behaviors and Self-Regulation of Nicotine Reinforcement. J. Neurosci. 2020, 40, 3465–3477. [Google Scholar] [CrossRef]
- Wills, L.; Ables, J.L.; Braunscheidel, K.M.; Caligiuri, S.P.B.; Elayouby, K.S.; Fillinger, C.; Ishikawa, M.; Moen, J.K.; Kenny, P.J. Neurobiological Mechanisms of Nicotine Reward and Aversion. Pharmacol. Rev. 2022, 74, 271–310. [Google Scholar] [CrossRef]
- D’Souza, R.D.; Vijayaraghavan, S. Nicotinic receptor-mediated filtering of mitral cell responses to olfactory nerve inputs involves the alpha3beta4 subtype. J. Neurosci. 2012, 32, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Calarco, C.A.; Picciotto, M.R. Nicotinic Acetylcholine Receptor Signaling in the Hypothalamus: Mechanisms Related to Nicotine’s Effects on Food Intake. Nicotine Tob. Res. 2020, 22, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Perez, L.M.; Kwapiszewski, J.T.; Roberts, M.T. alpha3beta4 (*) Nicotinic Acetylcholine Receptors Strongly Modulate the Excitability of VIP Neurons in the Mouse Inferior Colliculus. Front. Neural. Circuits 2021, 15, 709387. [Google Scholar] [CrossRef] [PubMed]
- Improgo, M.R.; Soll, L.G.; Tapper, A.R.; Gardner, P.D. Nicotinic acetylcholine receptors mediate lung cancer growth. Front. Physiol. 2013, 4, 251. [Google Scholar] [CrossRef] [PubMed]
- Pucci, S.; Zoli, M.; Clementi, F.; Gotti, C. alpha9-Containing Nicotinic Receptors in Cancer. Front. Cell Neurosci. 2021, 15, 805123. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Krasteva-Christ, G. Nicotinic Acetylcholine Receptors in the Respiratory Tract. Molecules 2021, 26, 6097. [Google Scholar] [CrossRef]
- Jall, S.; De Angelis, M.; Lundsgaard, A.M.; Fritzen, A.M.; Nicolaisen, T.S.; Klein, A.B.; Novikoff, A.; Sachs, S.; Richter, E.A.; Kiens, B.; et al. Pharmacological targeting of alpha3beta4 nicotinic receptors improves peripheral insulin sensitivity in mice with diet-induced obesity. Diabetologia 2020, 63, 1236–1247. [Google Scholar] [CrossRef]
- Eom, S.; Kim, C.; Yeom, H.D.; Lee, J.; Lee, S.; Baek, Y.B.; Na, J.; Park, S.I.; Kim, G.Y.; Lee, C.M.; et al. Molecular Regulation of alpha3beta4 Nicotinic Acetylcholine Receptors by Lupeol in Cardiovascular System. Int. J. Mol. Sci. 2020, 21, 4329. [Google Scholar] [CrossRef]
- Krashia, P.; Moroni, M.; Broadbent, S.; Hofmann, G.; Kracun, S.; Beato, M.; Groot-Kormelink, P.J.; Sivilotti, L.G. Human alpha3beta4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells. PloS ONE 2010, 5, e13611. [Google Scholar] [CrossRef]
- Mazzaferro, S.; Benallegue, N.; Carbone, A.; Gasparri, F.; Vijayan, R.; Biggin, P.C.; Moroni, M.; Bermudez, I. Additional acetylcholine (ACh) binding site at alpha4/alpha4 interface of (alpha4beta2)2alpha4 nicotinic receptor influences agonist sensitivity. J. Biol. Chem. 2011, 286, 31043–31054. [Google Scholar] [CrossRef]
- Mazzo, F.; Pistillo, F.; Grazioso, G.; Clementi, F.; Borgese, N.; Gotti, C.; Colombo, S.F. Nicotine-modulated subunit stoichiometry affects stability and trafficking of alpha3beta4 nicotinic receptor. J. Neurosci. 2013, 33, 12316–12328. [Google Scholar] [CrossRef] [PubMed]
- Crespi, A.; Plutino, S.; Sciaccaluga, M.; Righi, M.; Borgese, N.; Fucile, S.; Gotti, C.; Colombo, S.F. The fifth subunit in alpha3beta4 nicotinic receptor is more than an accessory subunit. FASEB J. 2018, 32, 4190–4202. [Google Scholar] [CrossRef]
- Frahm, S.; Slimak, M.A.; Ferrarese, L.; Santos-Torres, J.; Antolin-Fontes, B.; Auer, S.; Filkin, S.; Pons, S.; Fontaine, J.F.; Tsetlin, V.; et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron 2011, 70, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Slimak, M.A.; Ables, J.L.; Frahm, S.; Antolin-Fontes, B.; Santos-Torres, J.; Moretti, M.; Gotti, C.; Ibanez-Tallon, I. Habenular expression of rare missense variants of the beta4 nicotinic receptor subunit alters nicotine consumption. Front. Hum. Neurosci. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.; Treinin, M.; Papke, R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015, 36, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Fossati, M.; Colombo, S.F.; Borgese, N. A positive signal prevents secretory membrane cargo from recycling between the Golgi and the ER. EMBO J. 2014, 33, 2080–2097. [Google Scholar] [CrossRef]
- Sarginson, J.E.; Killen, J.D.; Lazzeroni, L.C.; Fortmann, S.P.; Ryan, H.S.; Schatzberg, A.F.; Murphy, G.M., Jr. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156B, 275–284. [Google Scholar] [CrossRef]
- Sabatelli, M.; Eusebi, F.; Al-Chalabi, A.; Conte, A.; Madia, F.; Luigetti, M.; Mancuso, I.; Limatola, C.; Trettel, F.; Sobrero, F.; et al. Rare missense variants of neuronal nicotinic acetylcholine receptor altering receptor function are associated with sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 2009, 18, 3997–4006. [Google Scholar] [CrossRef]
- Moriconi, C.; Di Angelantonio, S.; Piccioni, A.; Trettel, F.; Sabatelli, M.; Grassi, F. Mutant human beta4 subunit identified in amyotrophic lateral sclerosis patients impairs nicotinic receptor function. Pflug. Arch. 2011, 461, 225–233. [Google Scholar] [CrossRef]
- Richards, C.I.; Srinivasan, R.; Xiao, C.; Mackey, E.D.; Miwa, J.M.; Lester, H.A. Trafficking of alpha4* nicotinic receptors revealed by superecliptic phluorin: Effects of a beta4 amyotrophic lateral sclerosis-associated mutation and chronic exposure to nicotine. J. Biol. Chem. 2011, 286, 31241–31249. [Google Scholar] [CrossRef]
- Curran, J.; Mohler, P.J. Alternative paradigms for ion channelopathies: Disorders of ion channel membrane trafficking and posttranslational modification. Annu. Rev. Physiol. 2015, 77, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.; Lynch, J.W. Phosphorylation mediated structural and functional changes in pentameric ligand-gated ion channels: Implications for drug discovery. Int. J. Biochem. Cell Biol. 2014, 53, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Chrestia, J.F.; Bruzzone, A.; Esandi, M.D.C.; Bouzat, C. Tyrosine phosphorylation differentially fine-tunes ionotropic and metabotropic responses of human alpha7 nicotinic acetylcholine receptor. Cell. Mol. Life Sci. 2021, 78, 5381–5395. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Lane, D. Antibodies: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1998; pp. 522–523. [Google Scholar]
- Boudreau, A.C.; Milovanovic, M.; Conrad, K.L.; Nelson, C.; Ferrario, C.R.; Wolf, M.E. A protein cross-linking assay for measuring cell surface expression of glutamate receptor subunits in the rodent brain after in vivo treatments. Curr. Protoc. Neurosci. 2012, 59, 5.30.1–5.30.19, Chapter 5. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, S.F.; Galli, C.; Crespi, A.; Renzi, M.; Gotti, C. Rare Missense Variants of the Human β4 Subunit Alter Nicotinic α3β4 Receptor Plasma Membrane Localisation. Molecules 2023, 28, 1247. https://doi.org/10.3390/molecules28031247
Colombo SF, Galli C, Crespi A, Renzi M, Gotti C. Rare Missense Variants of the Human β4 Subunit Alter Nicotinic α3β4 Receptor Plasma Membrane Localisation. Molecules. 2023; 28(3):1247. https://doi.org/10.3390/molecules28031247
Chicago/Turabian StyleColombo, Sara Francesca, Cecilia Galli, Arianna Crespi, Massimiliano Renzi, and Cecilia Gotti. 2023. "Rare Missense Variants of the Human β4 Subunit Alter Nicotinic α3β4 Receptor Plasma Membrane Localisation" Molecules 28, no. 3: 1247. https://doi.org/10.3390/molecules28031247
APA StyleColombo, S. F., Galli, C., Crespi, A., Renzi, M., & Gotti, C. (2023). Rare Missense Variants of the Human β4 Subunit Alter Nicotinic α3β4 Receptor Plasma Membrane Localisation. Molecules, 28(3), 1247. https://doi.org/10.3390/molecules28031247





