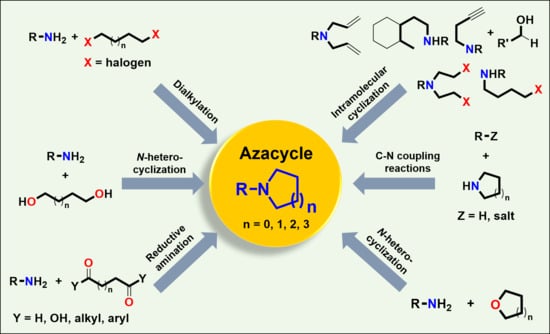
Recent Advances in Synthetic Routes to Azacycles
Abstract
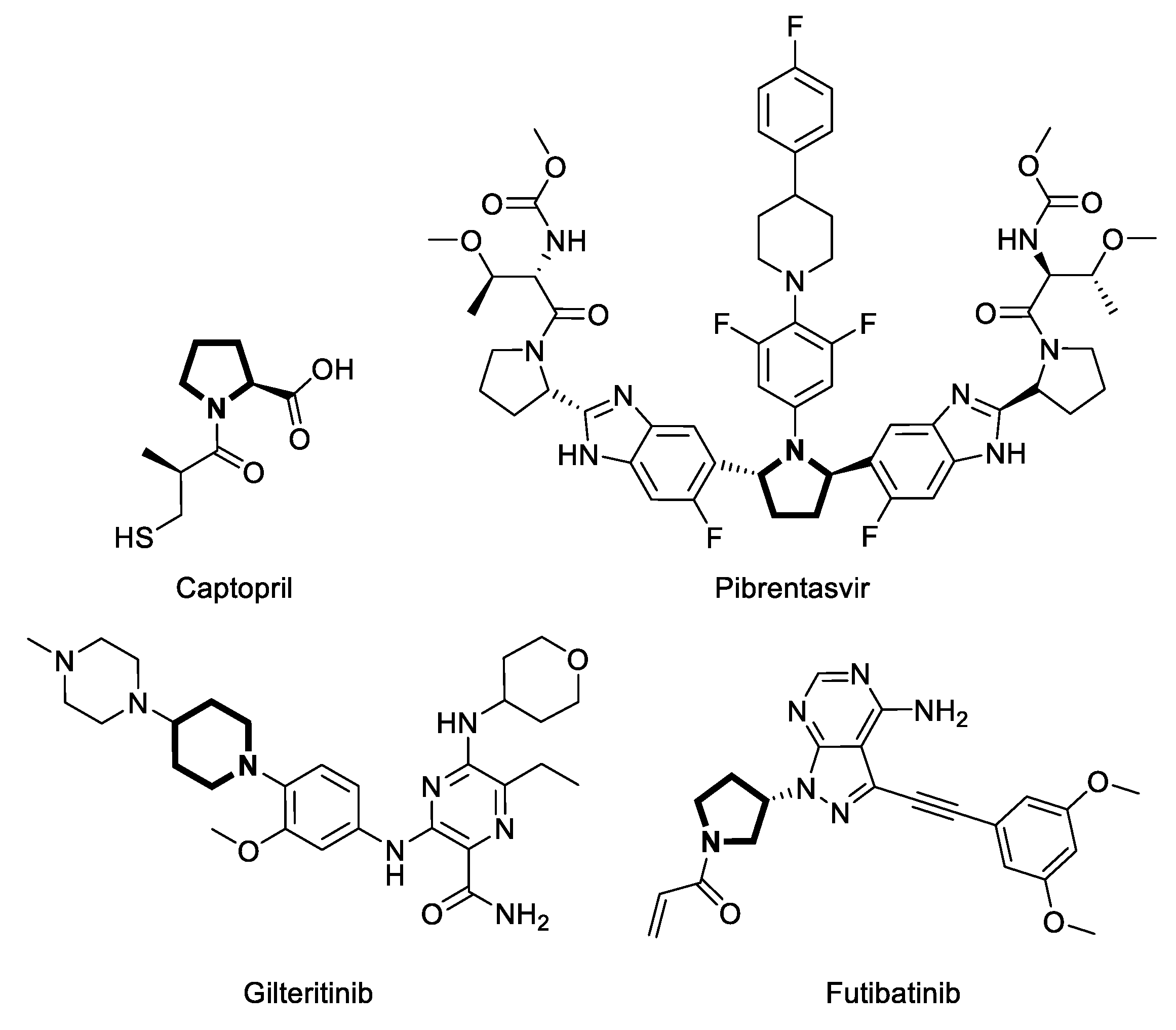
1. Introduction
2. Reactions
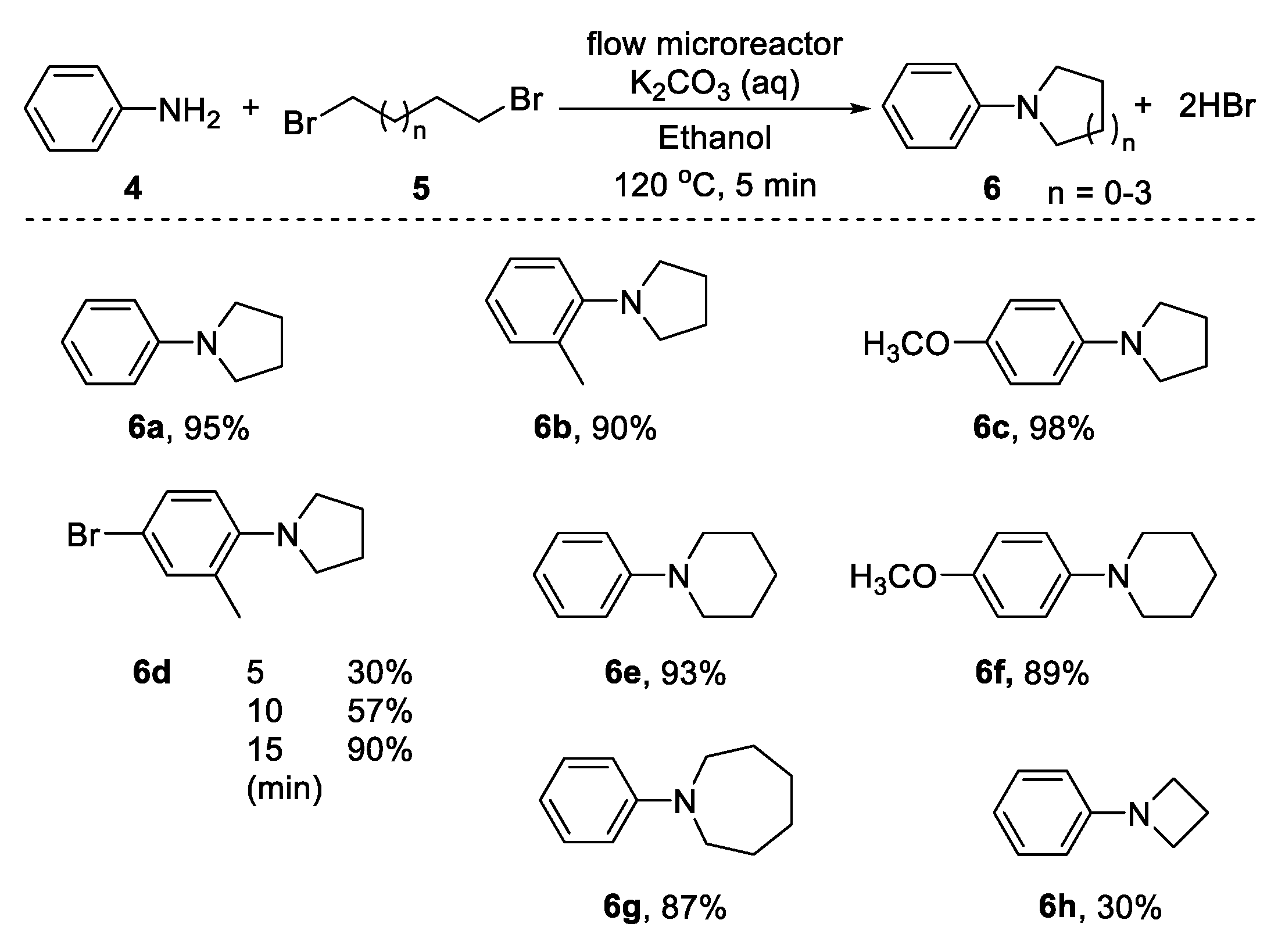
2.1. Dialkylation of Primary Amines with Dihalides
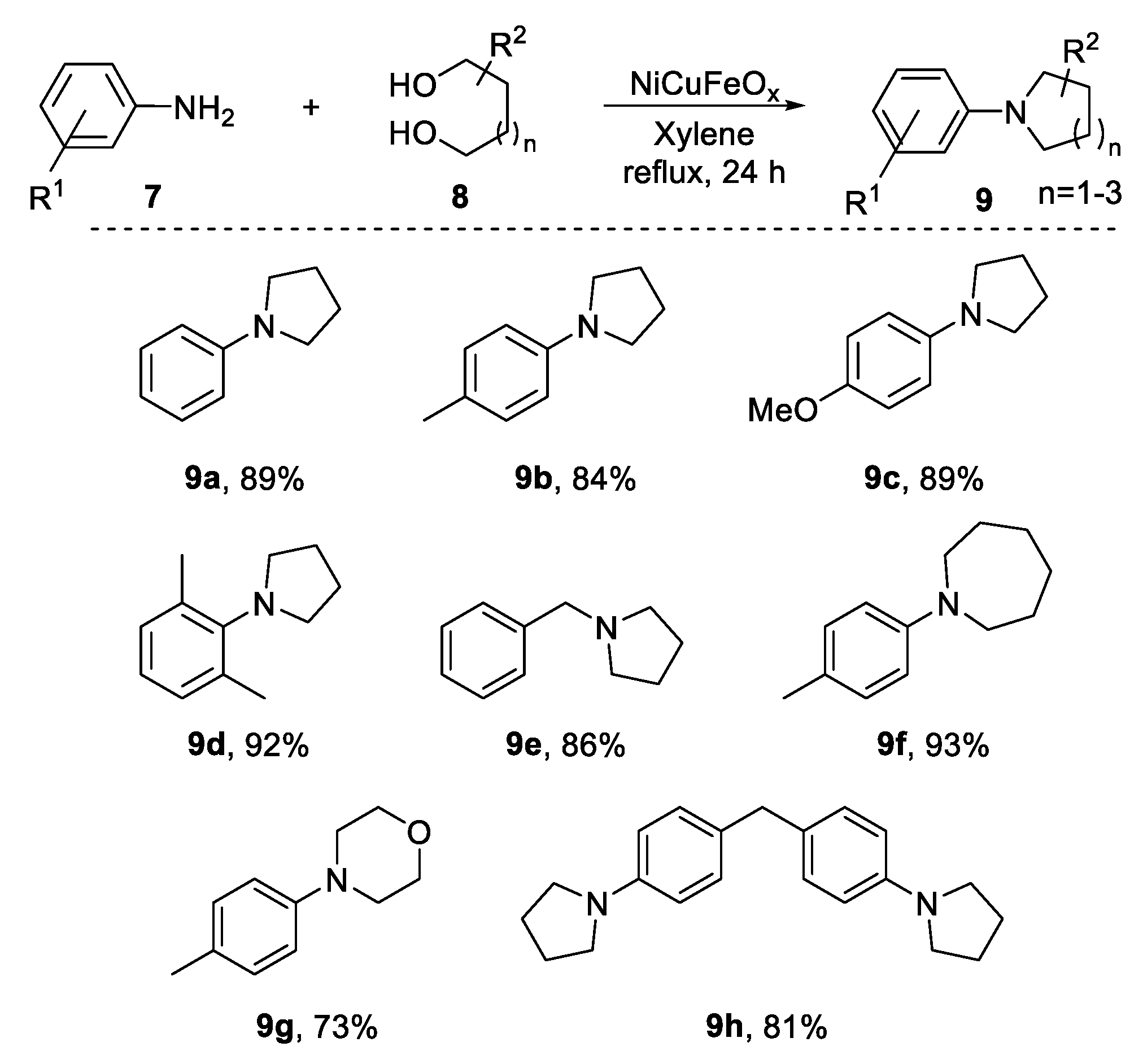
2.2. N-Heterocylization of Primary Amines with Diols
2.3. N-Heterocylization of Primary Amines with Dicarbonyl Compounds
2.3.1. N-Heterocylization of Primary Amines with Dialdehydrides
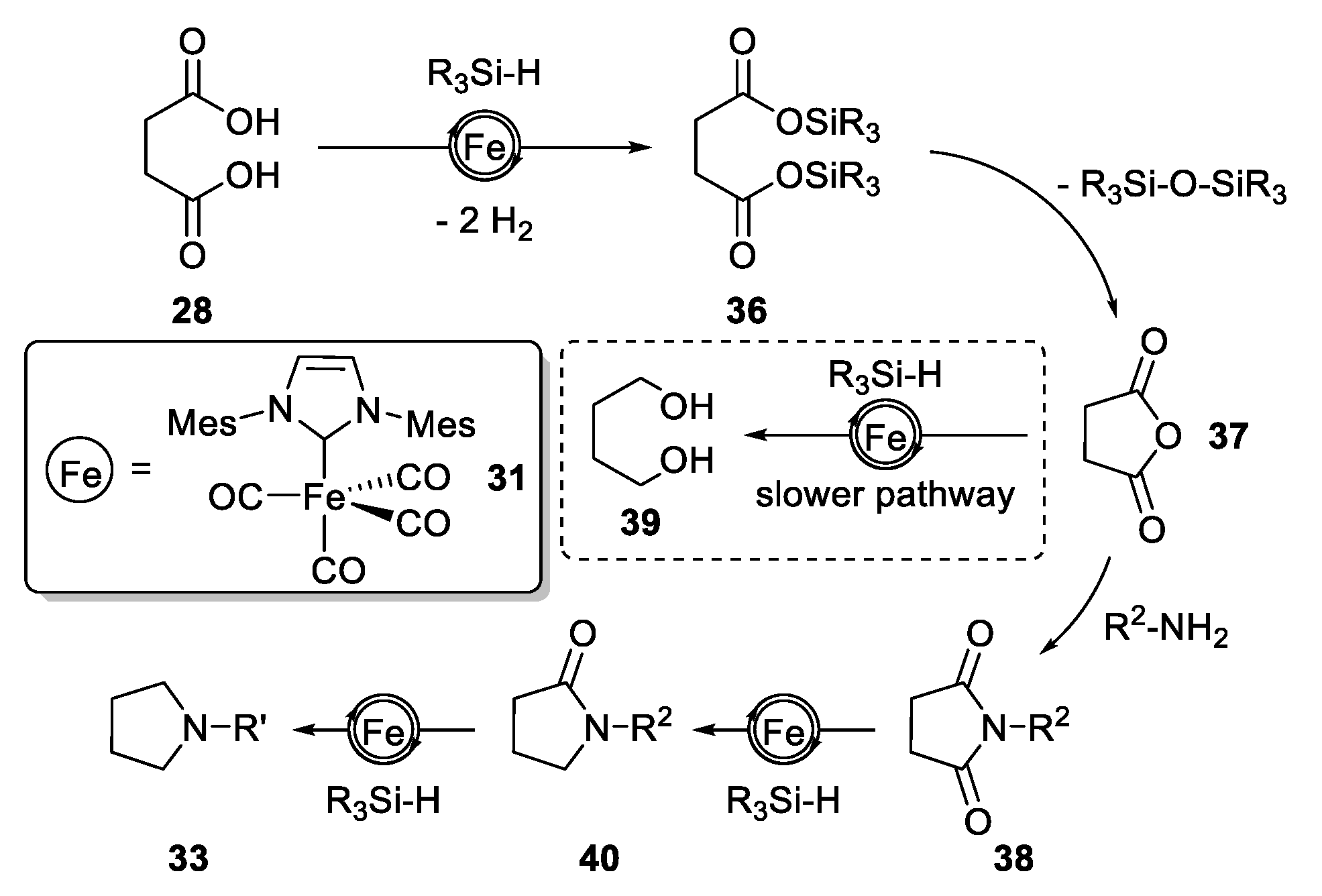
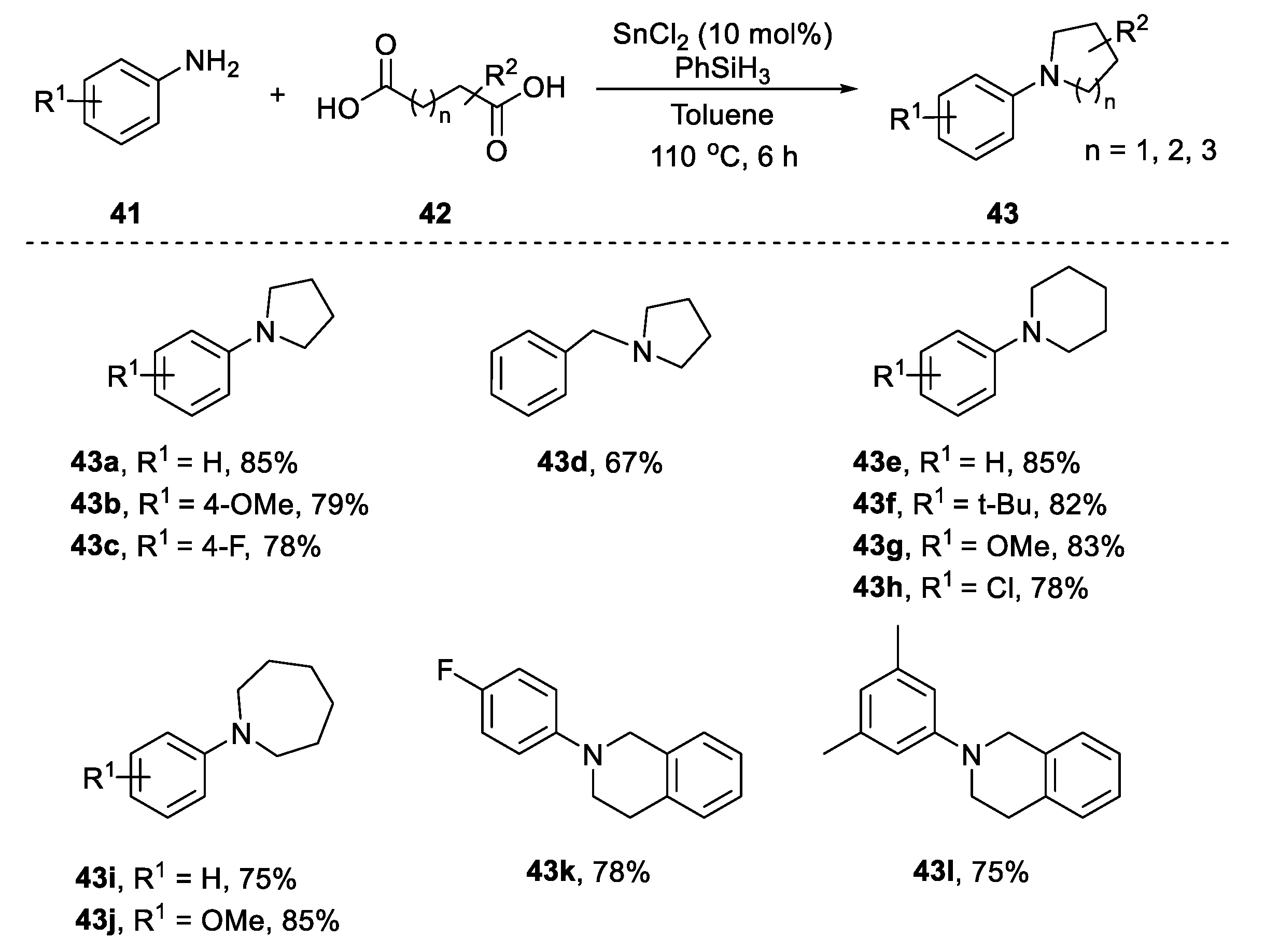
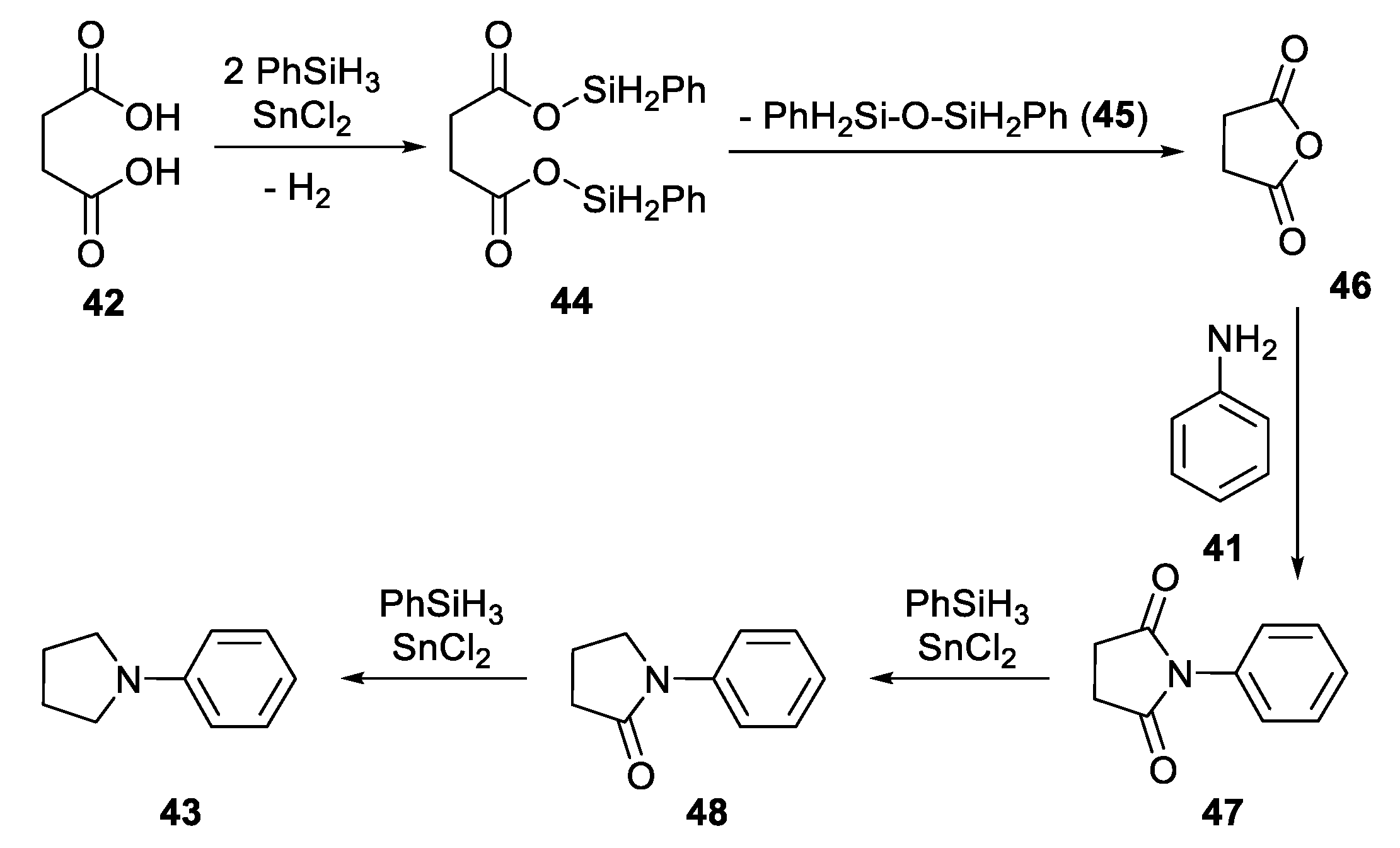
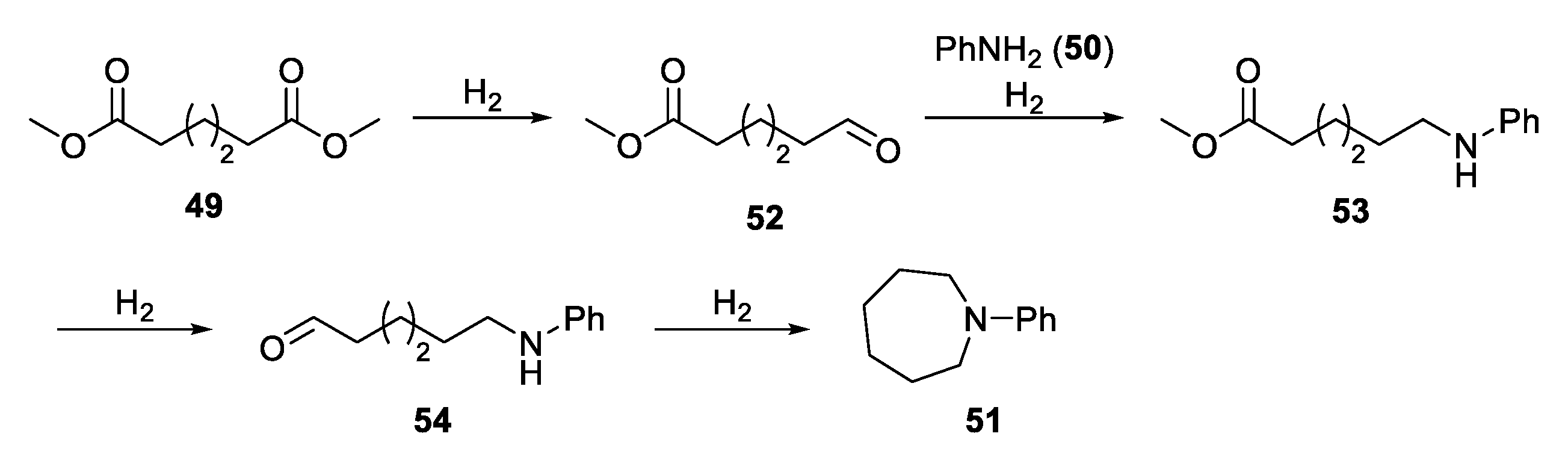
2.3.2. N-Heterocylization of Primary Amines with Dicarboxylic Acids
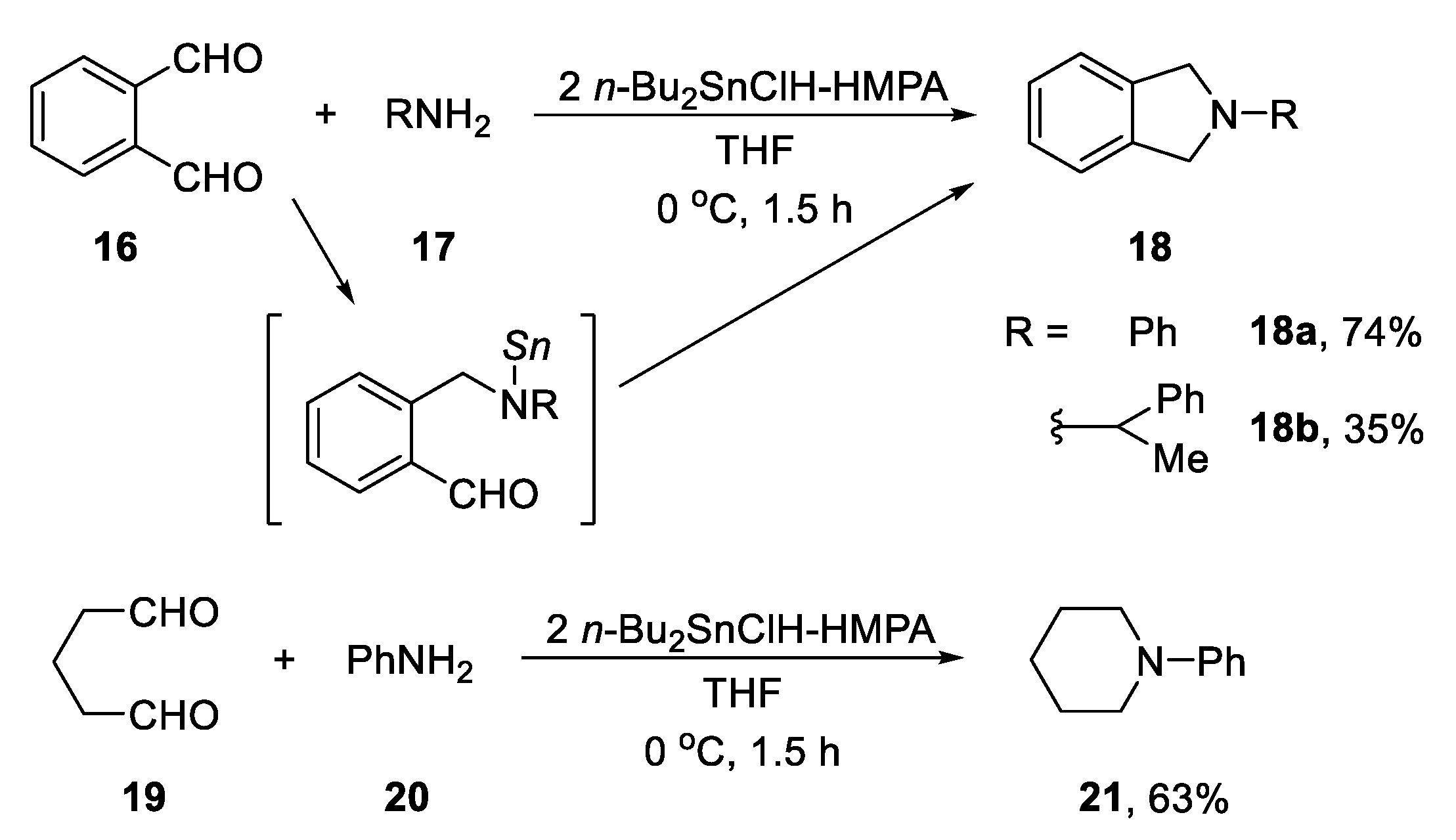
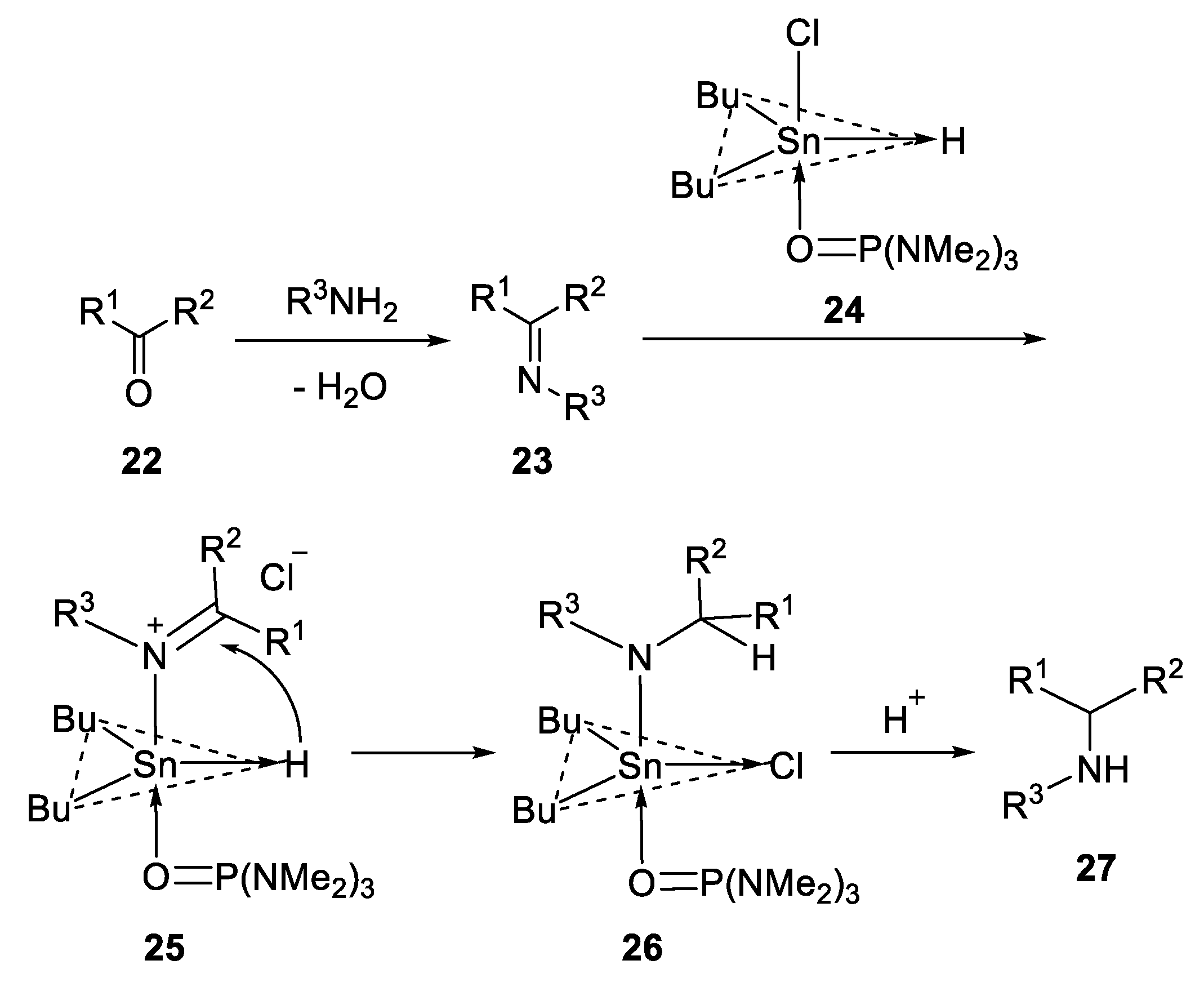
2.3.3. N-Heterocyclization of Primary Amines with Diesters
2.4. N-Heterocyclization of Primary Amines with Cyclic Ethers
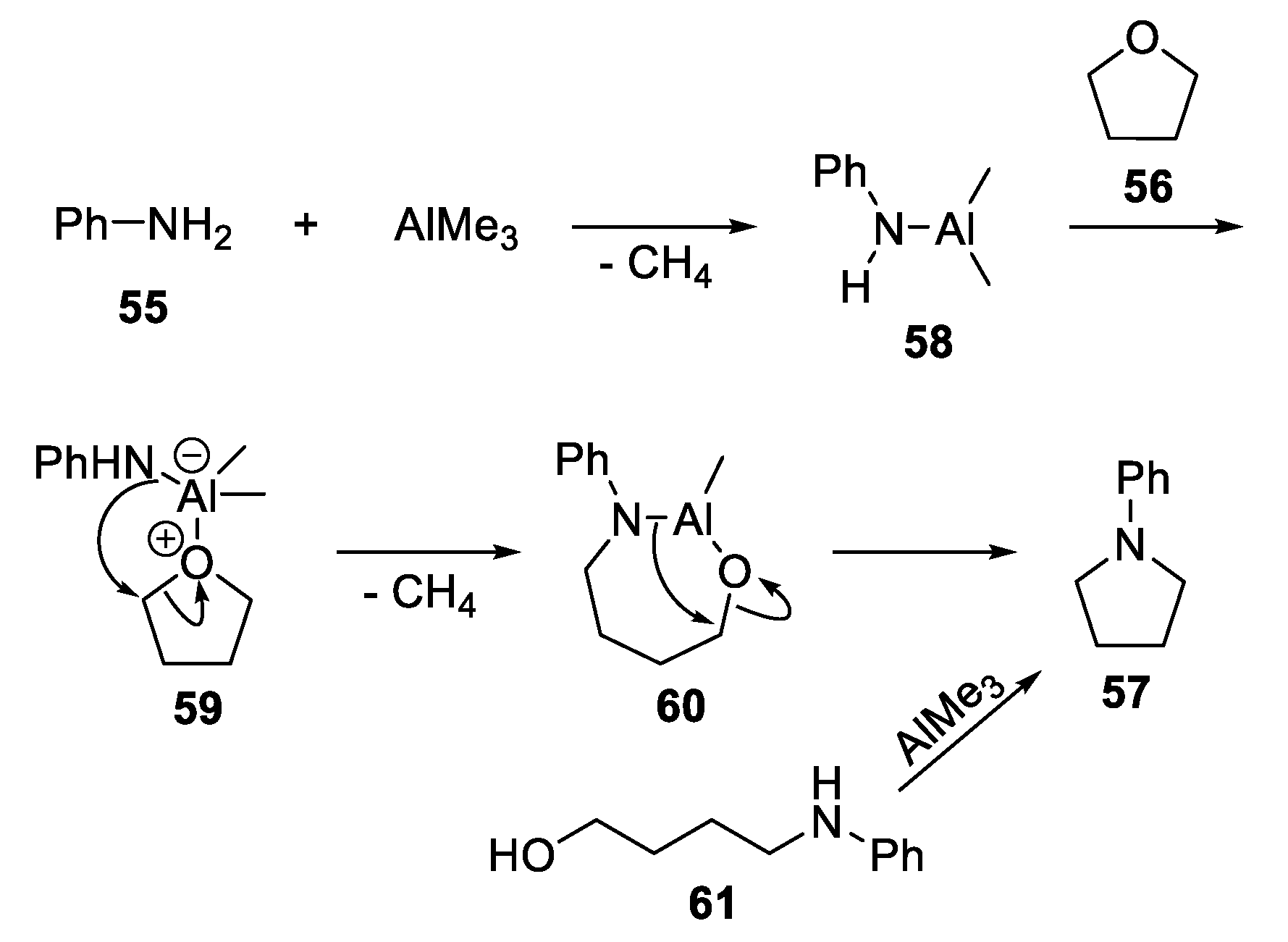
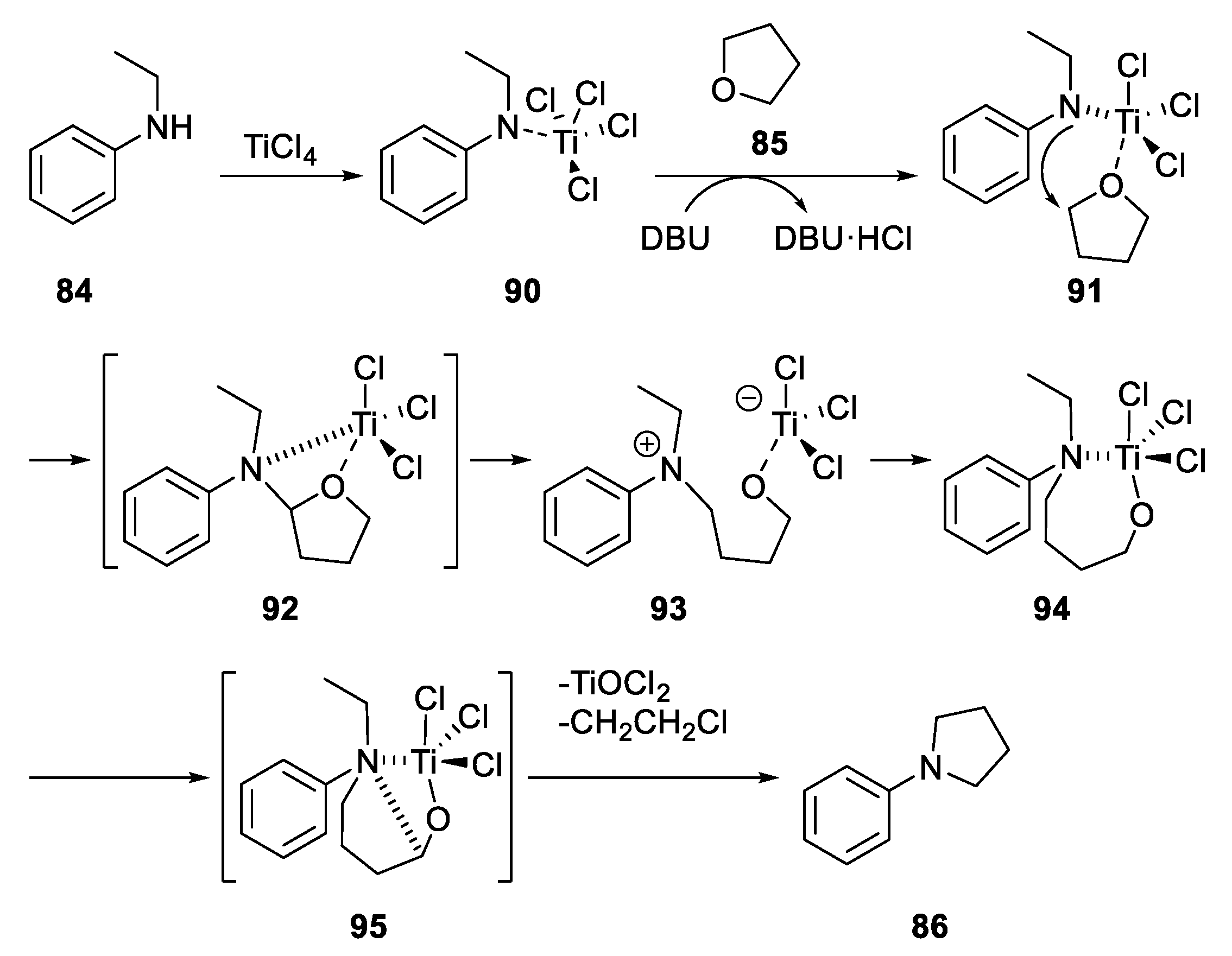
2.4.1. Metal-Based N-Heterocyclization of Primary Amines with Cyclic Ethers
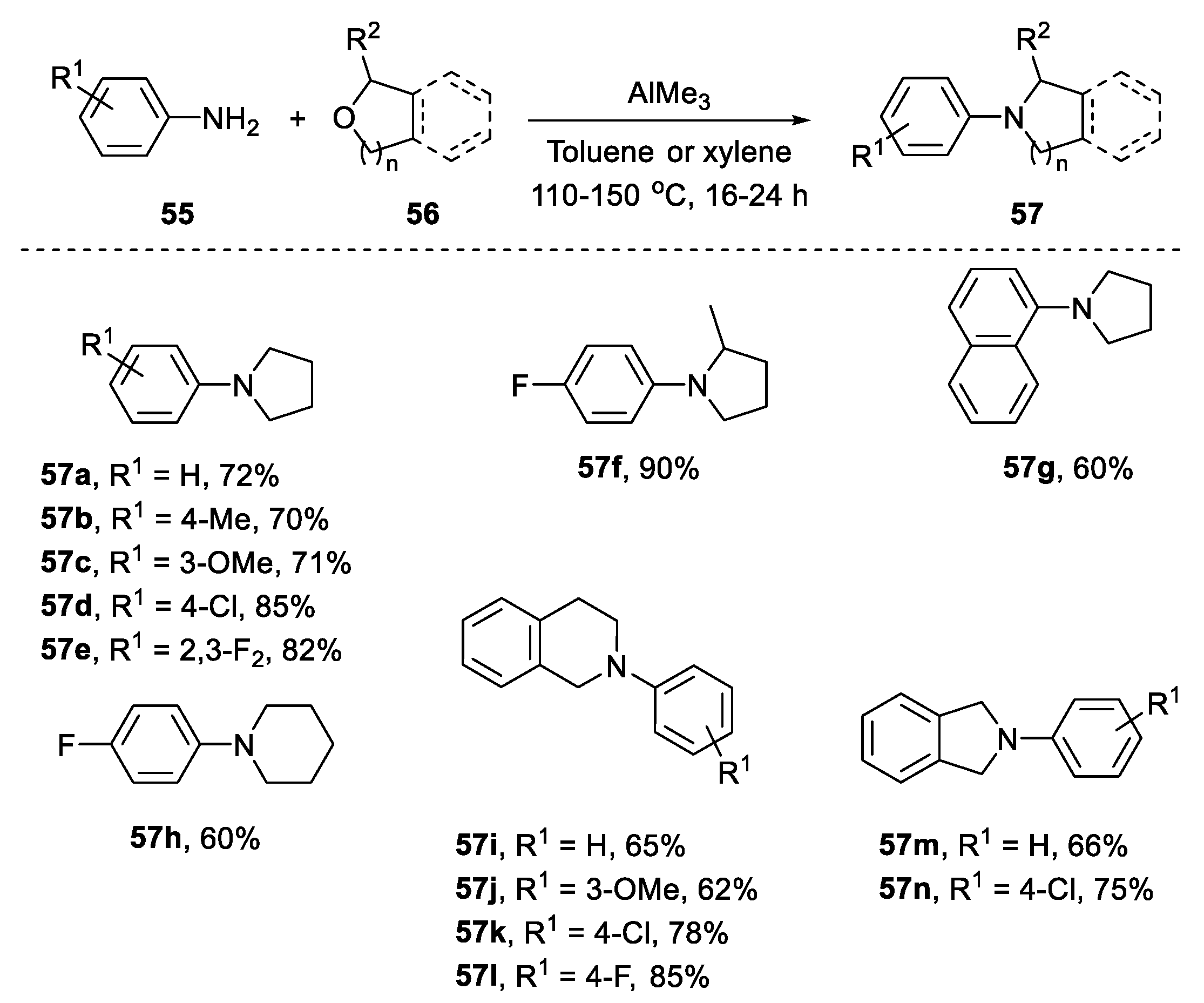
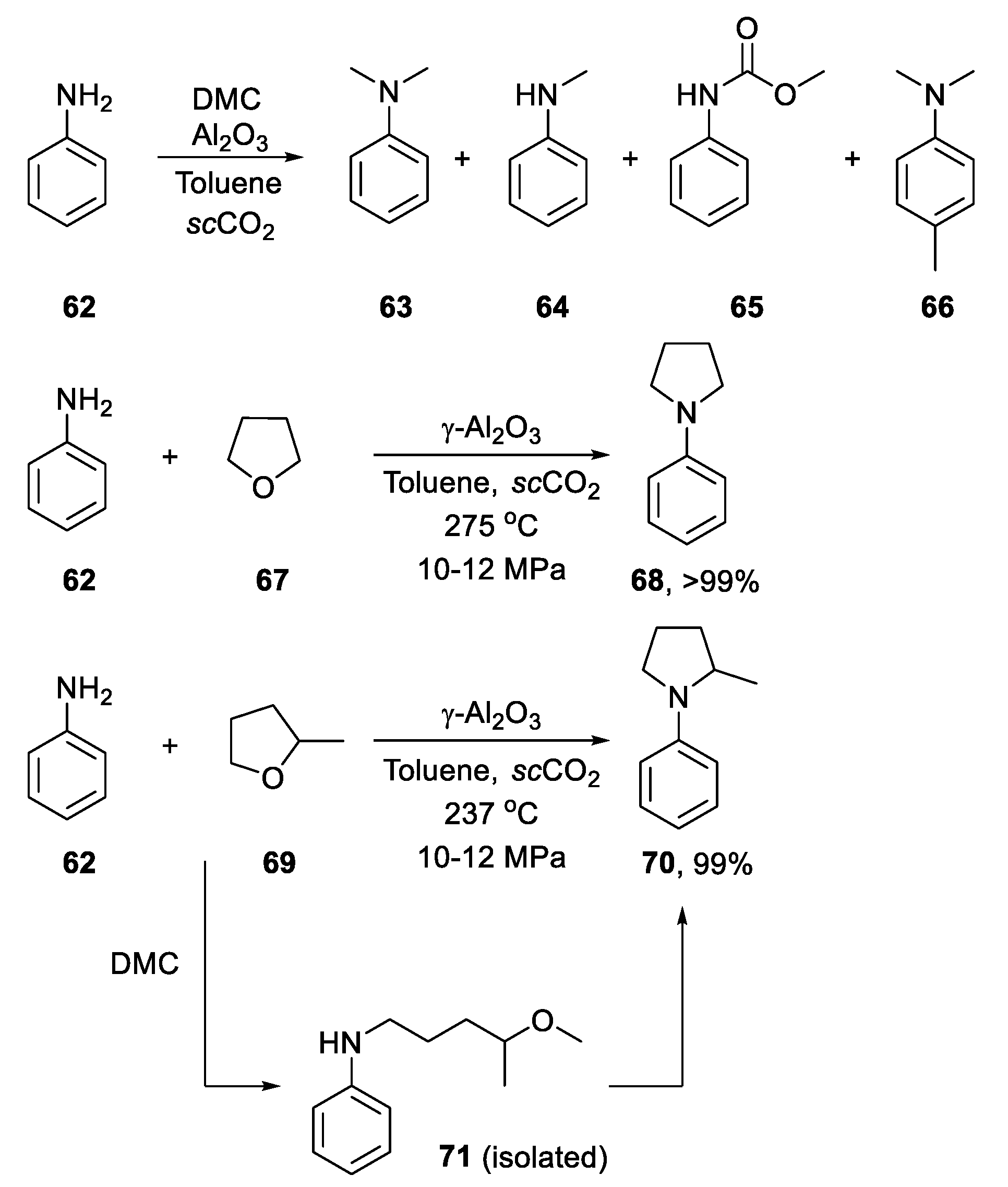
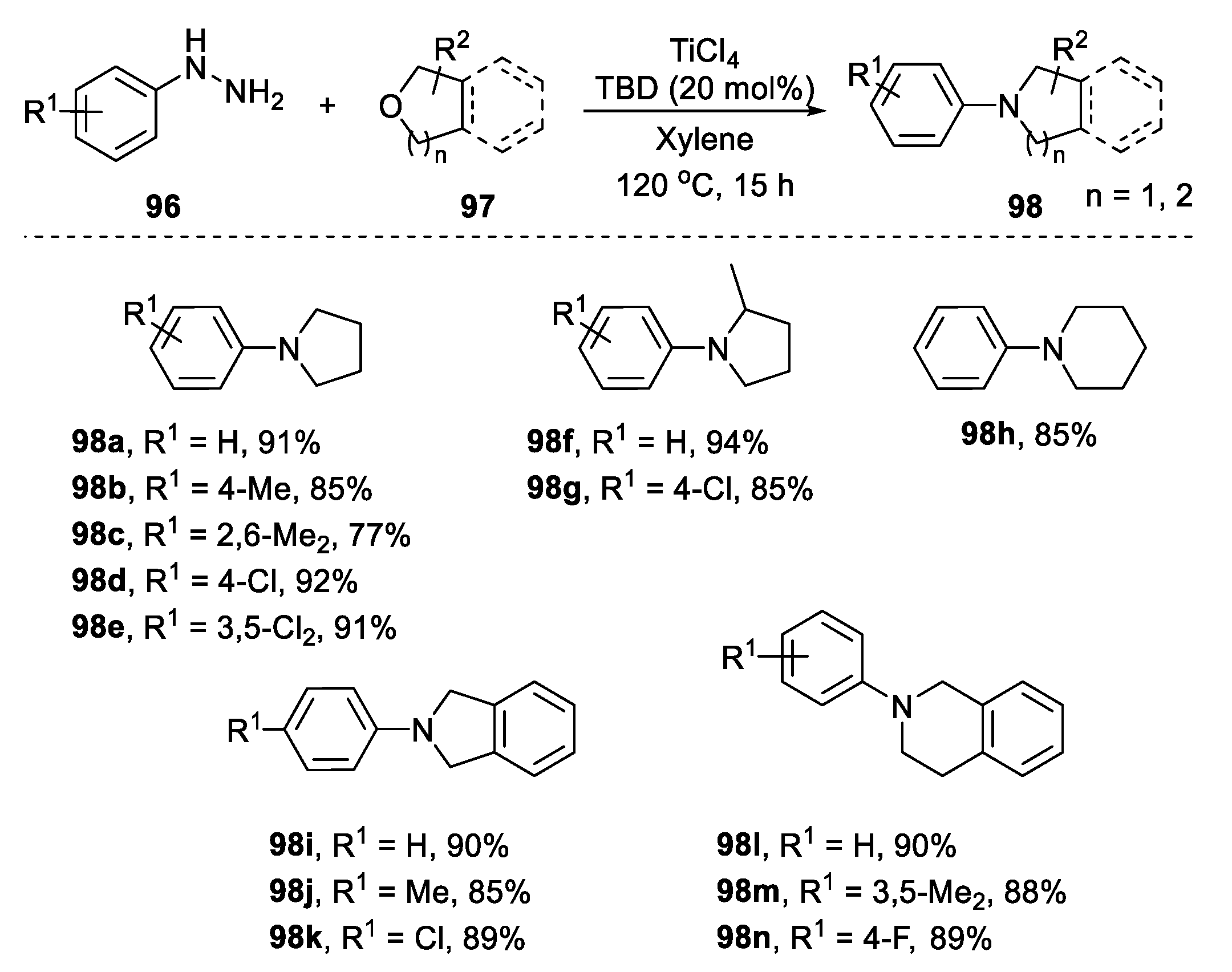
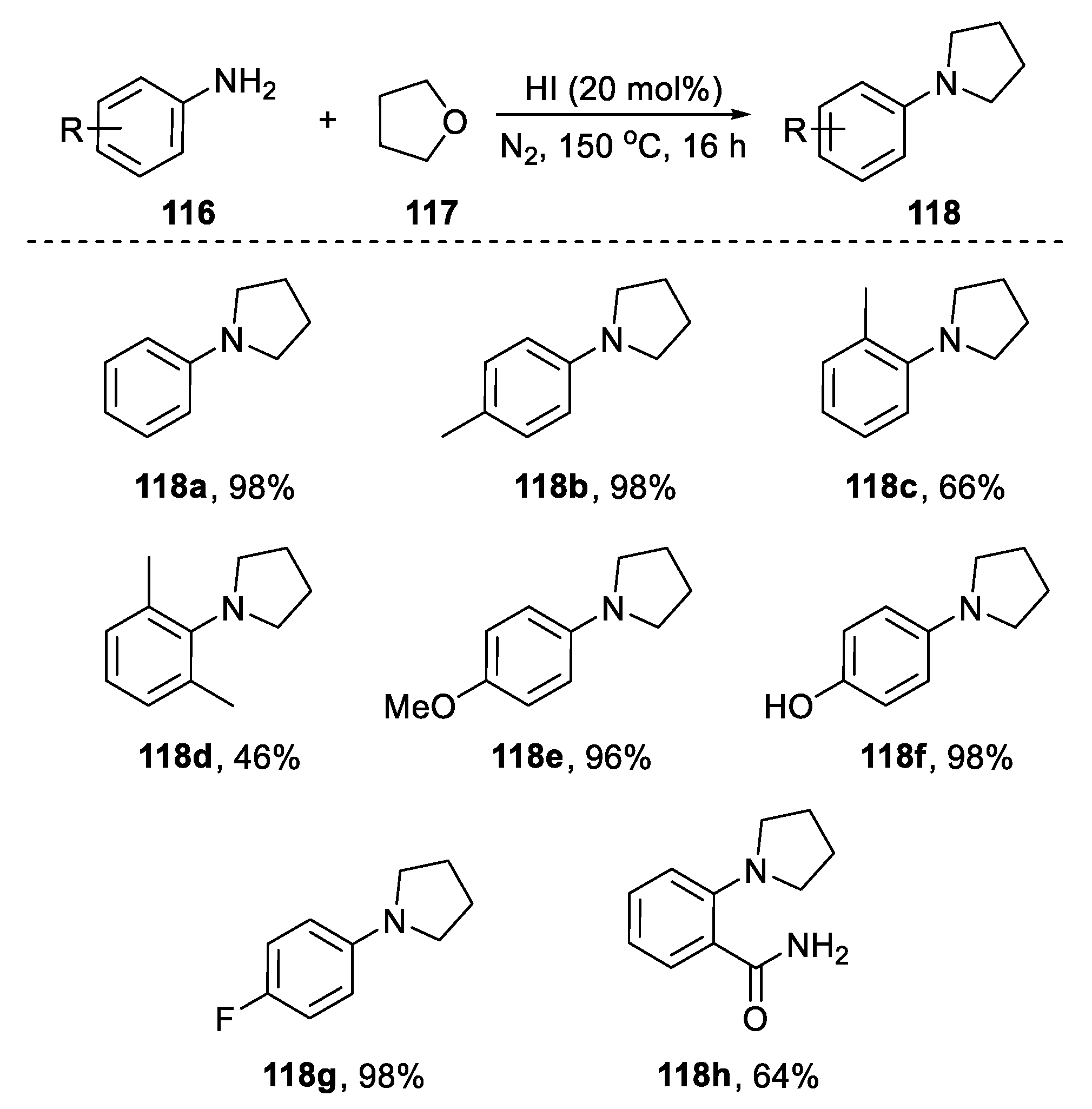
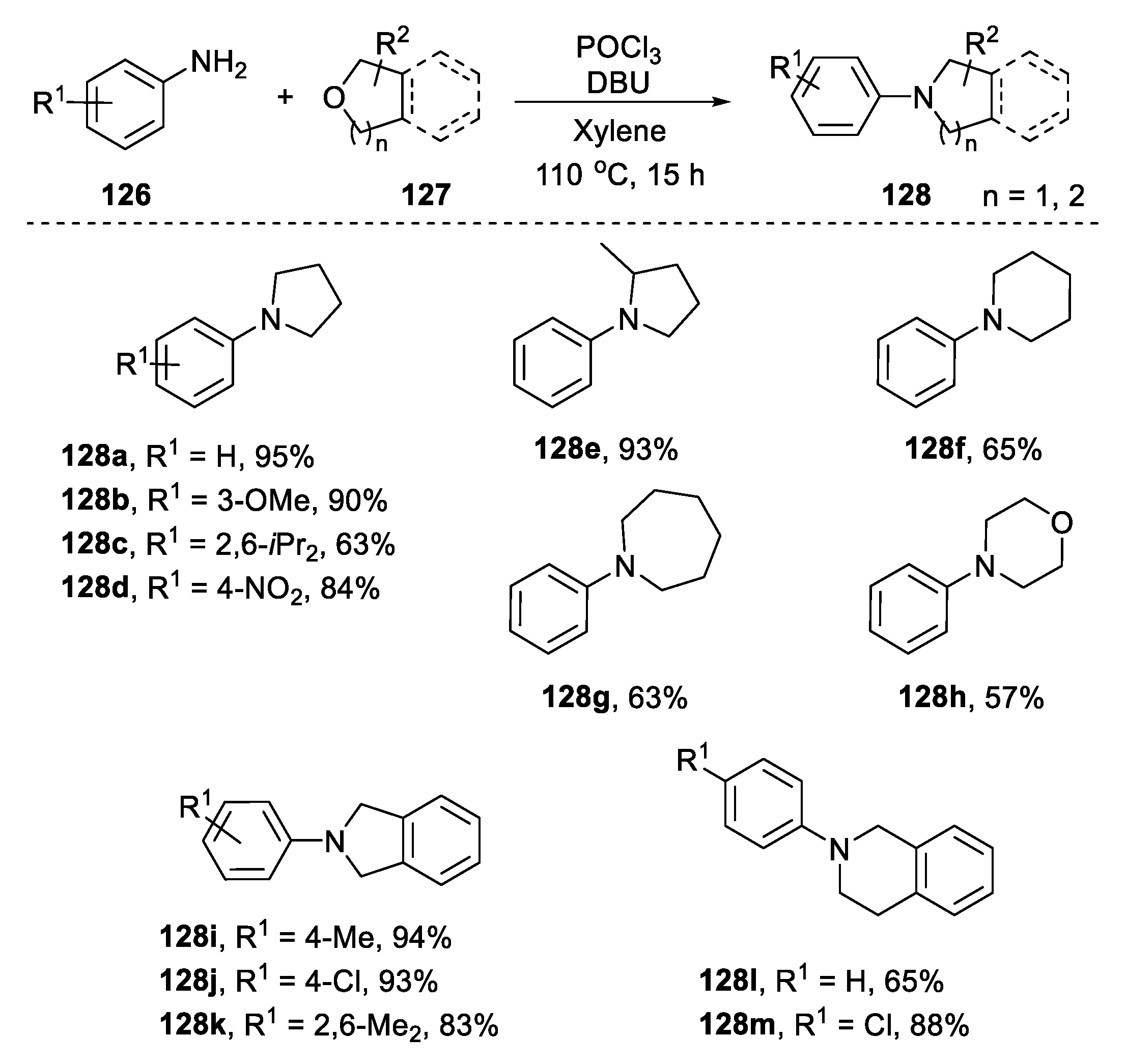
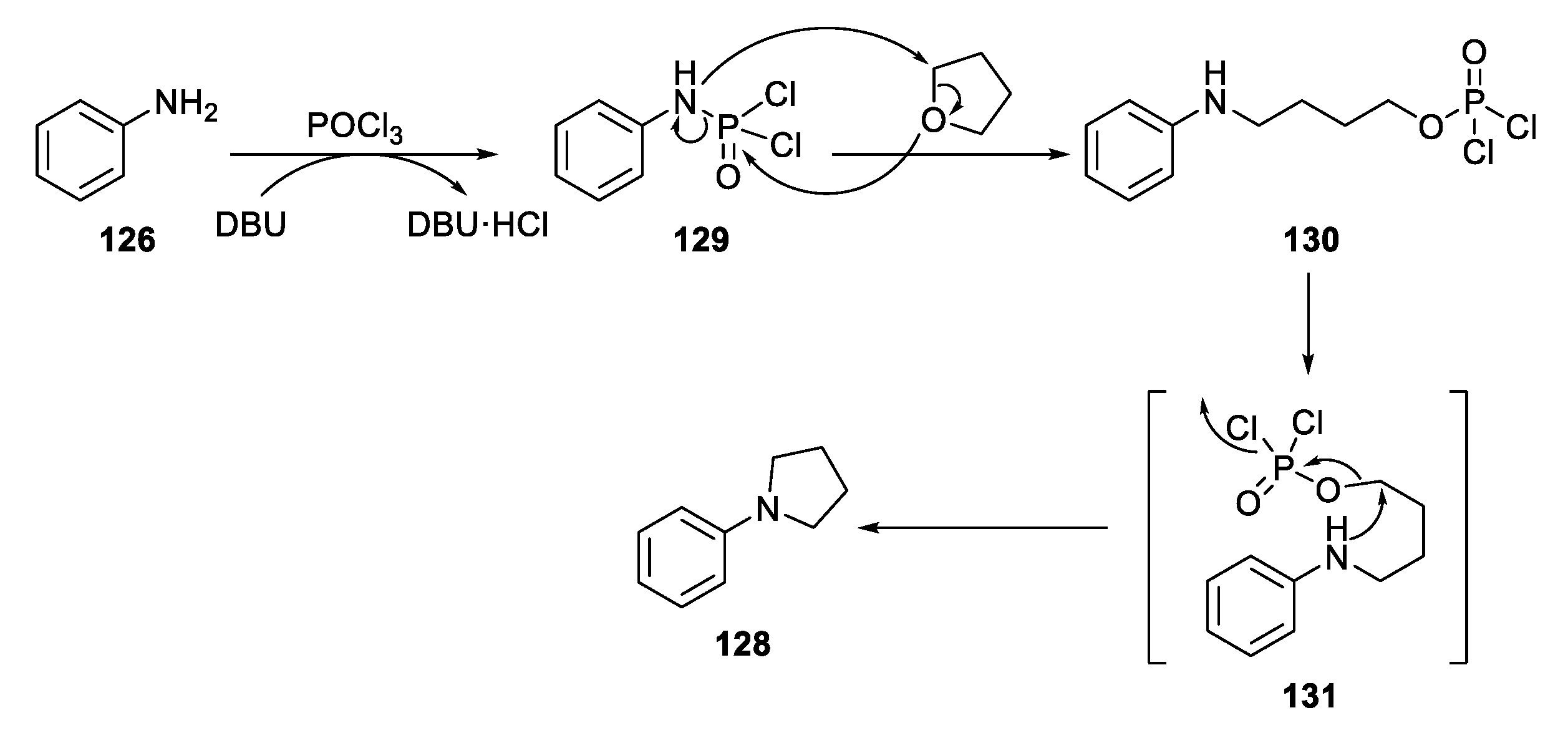
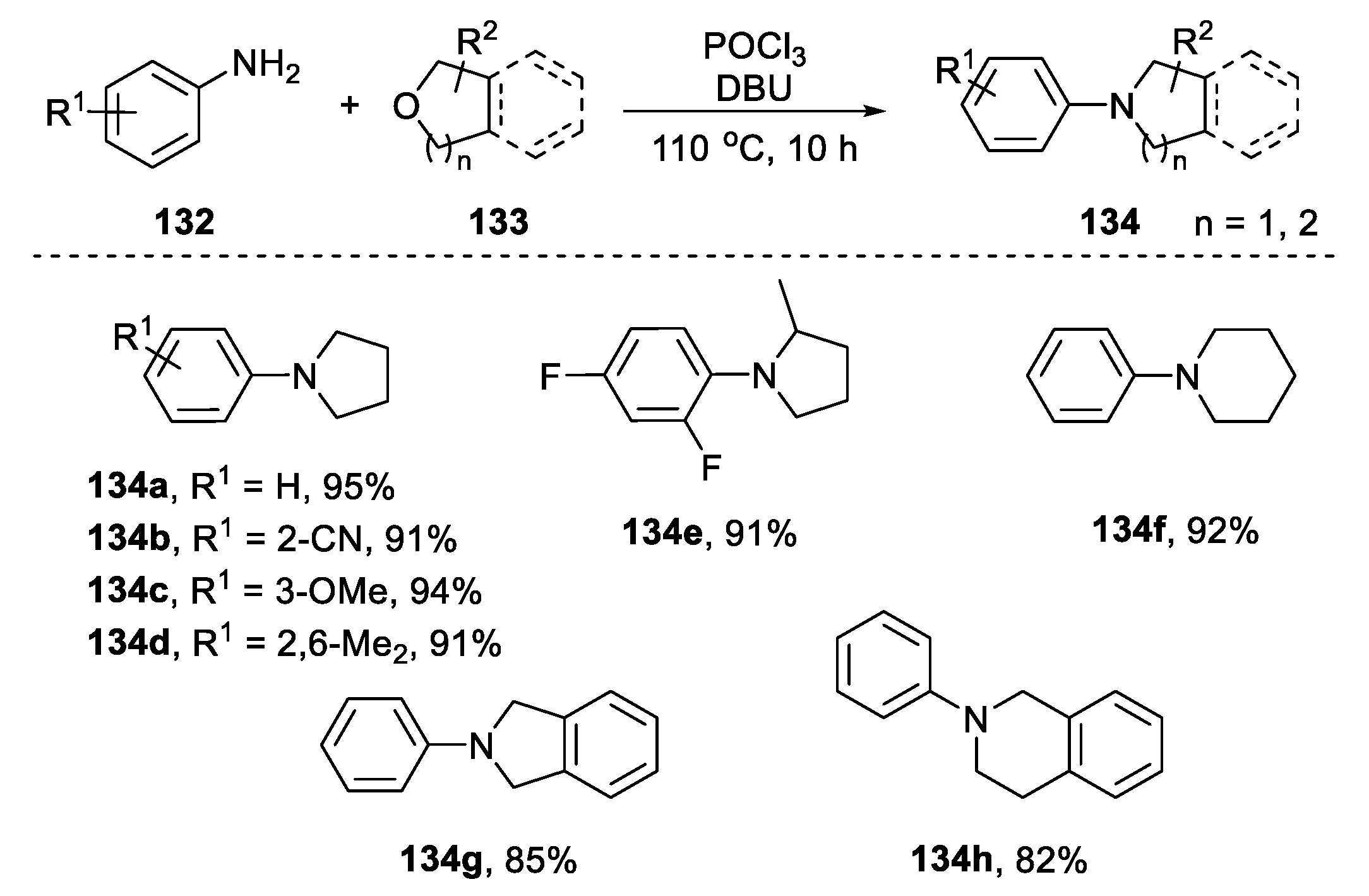
2.4.2. Non-Metal-Based N-Heterocyclization of Primary Amines with Cyclic Ethers
2.5. C–N Coupling Reaction
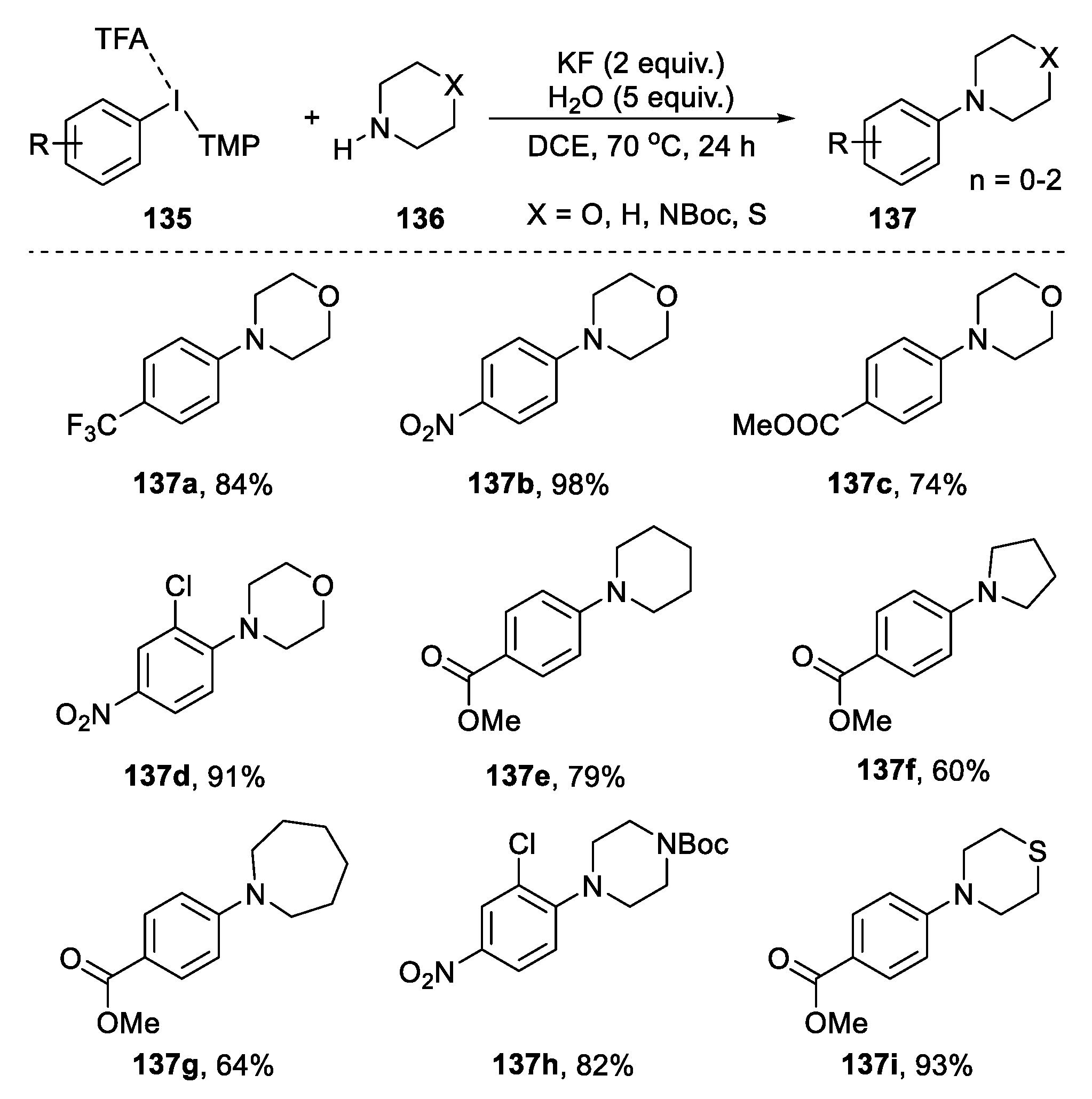
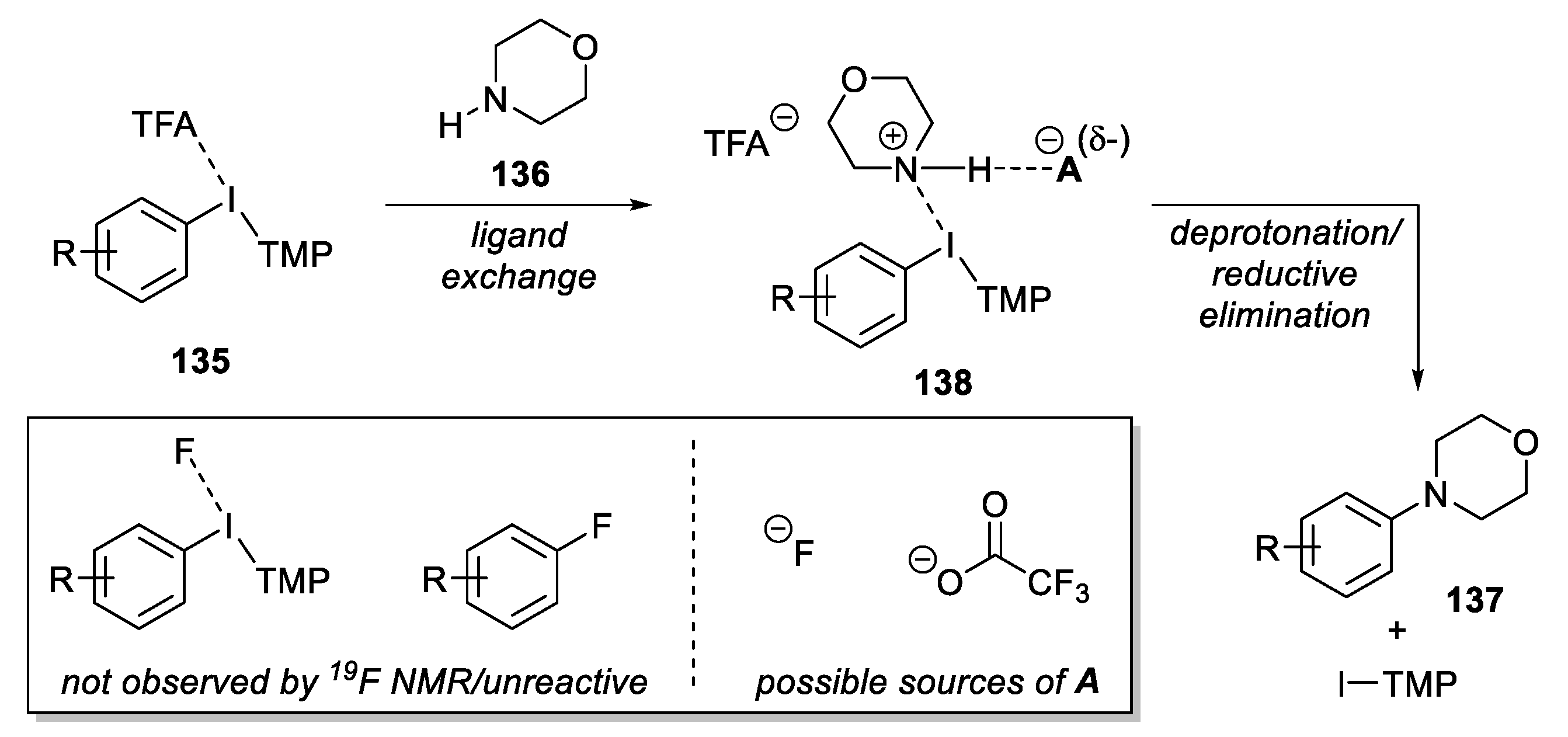
2.5.1. Coupling Reaction from Cyclic Amines and Hypervalent Iodine Compounds
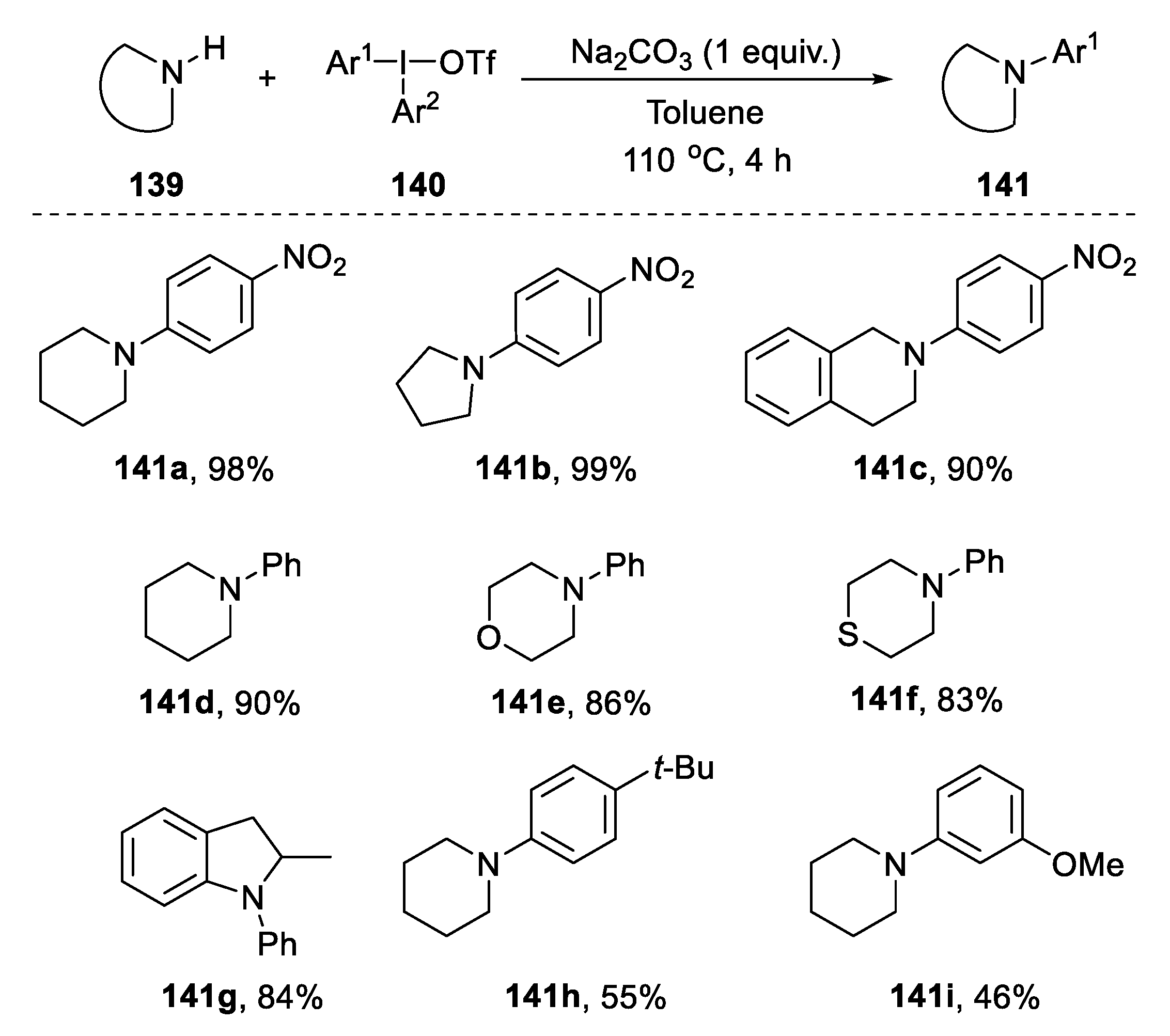
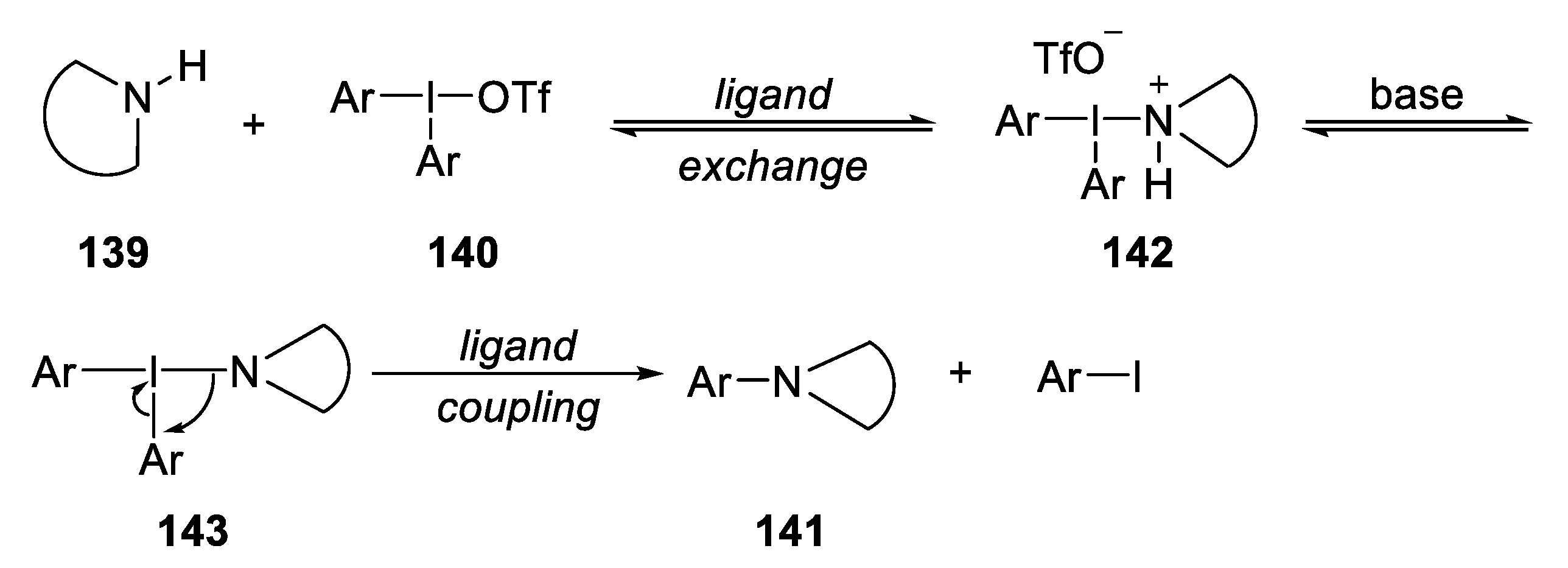
2.5.2. Coupling from Cyclic Amines and Triphenylsulfonium Triflates
2.5.3. Cross-Coupling Reaction of Secondary Amines and Aryl Compounds
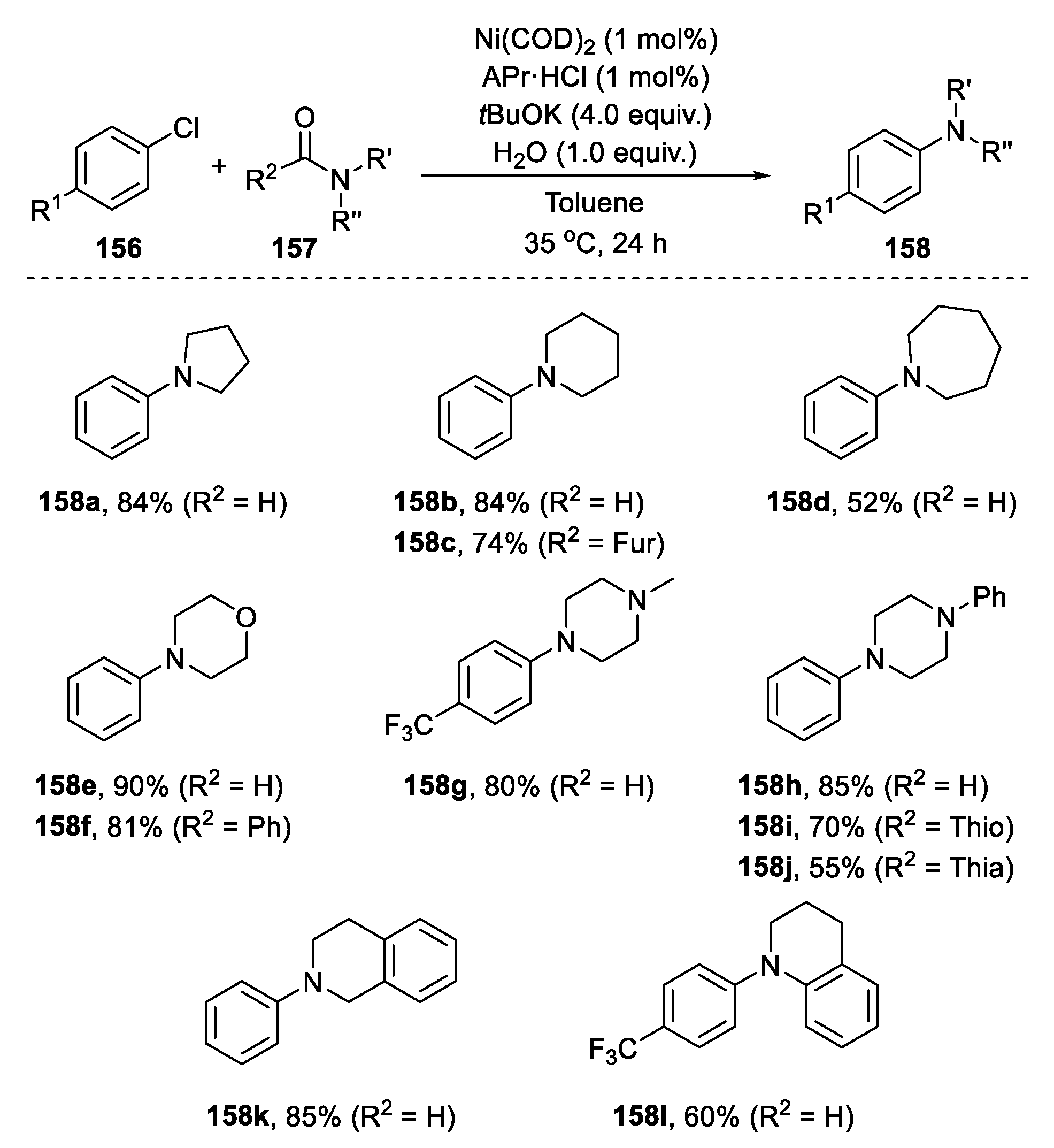
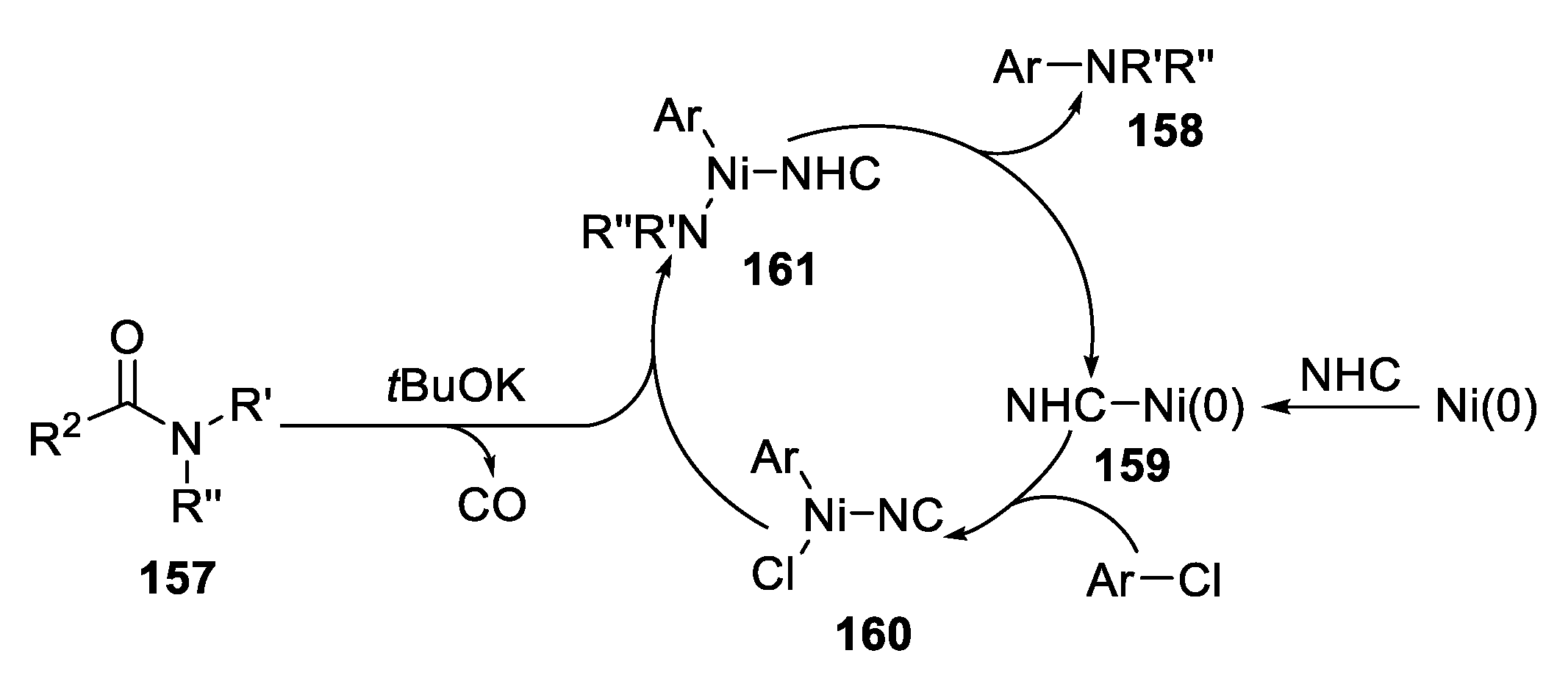
2.5.4. Cross-Coupling Reaction from Aryl Halides and Amides
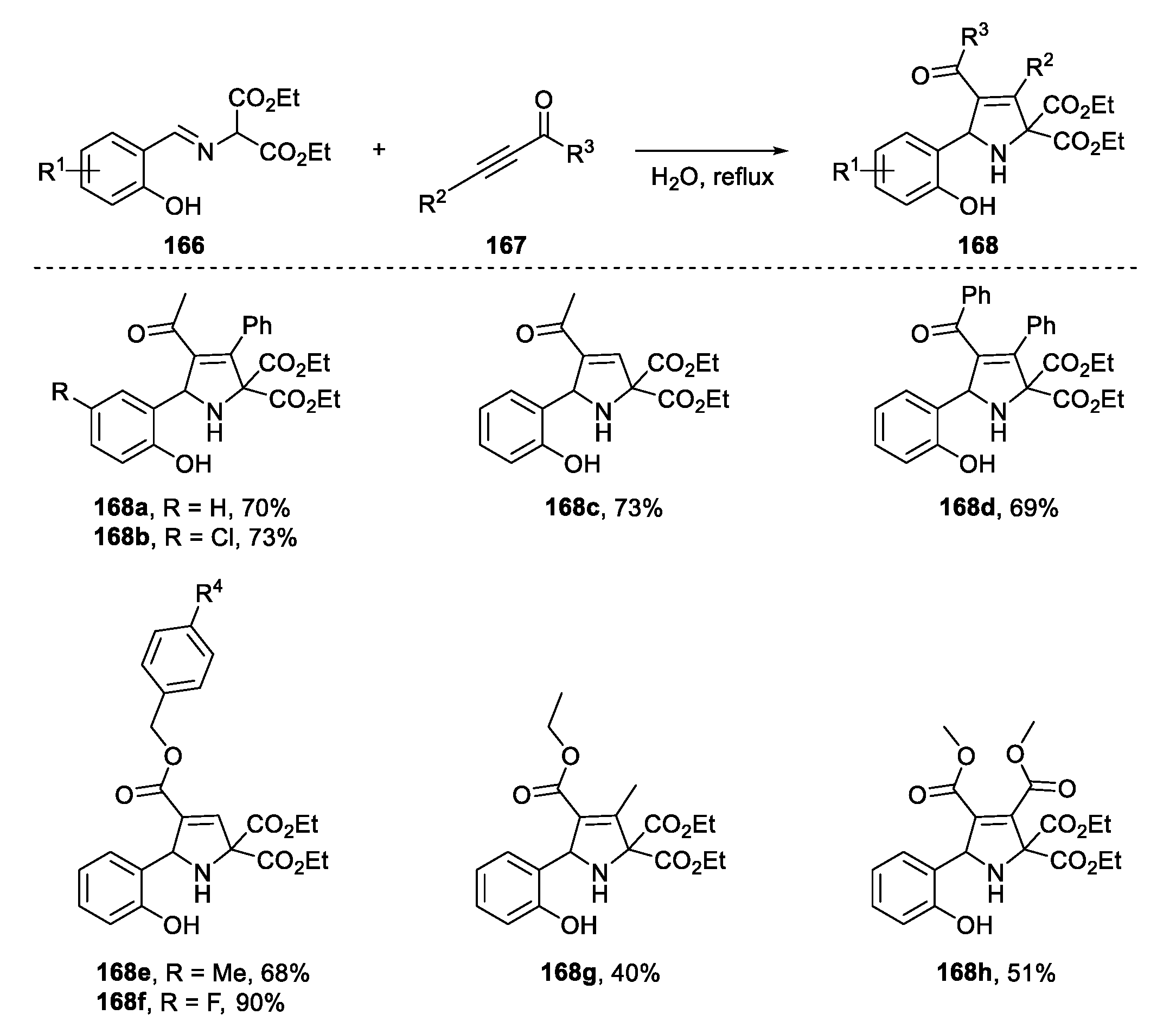
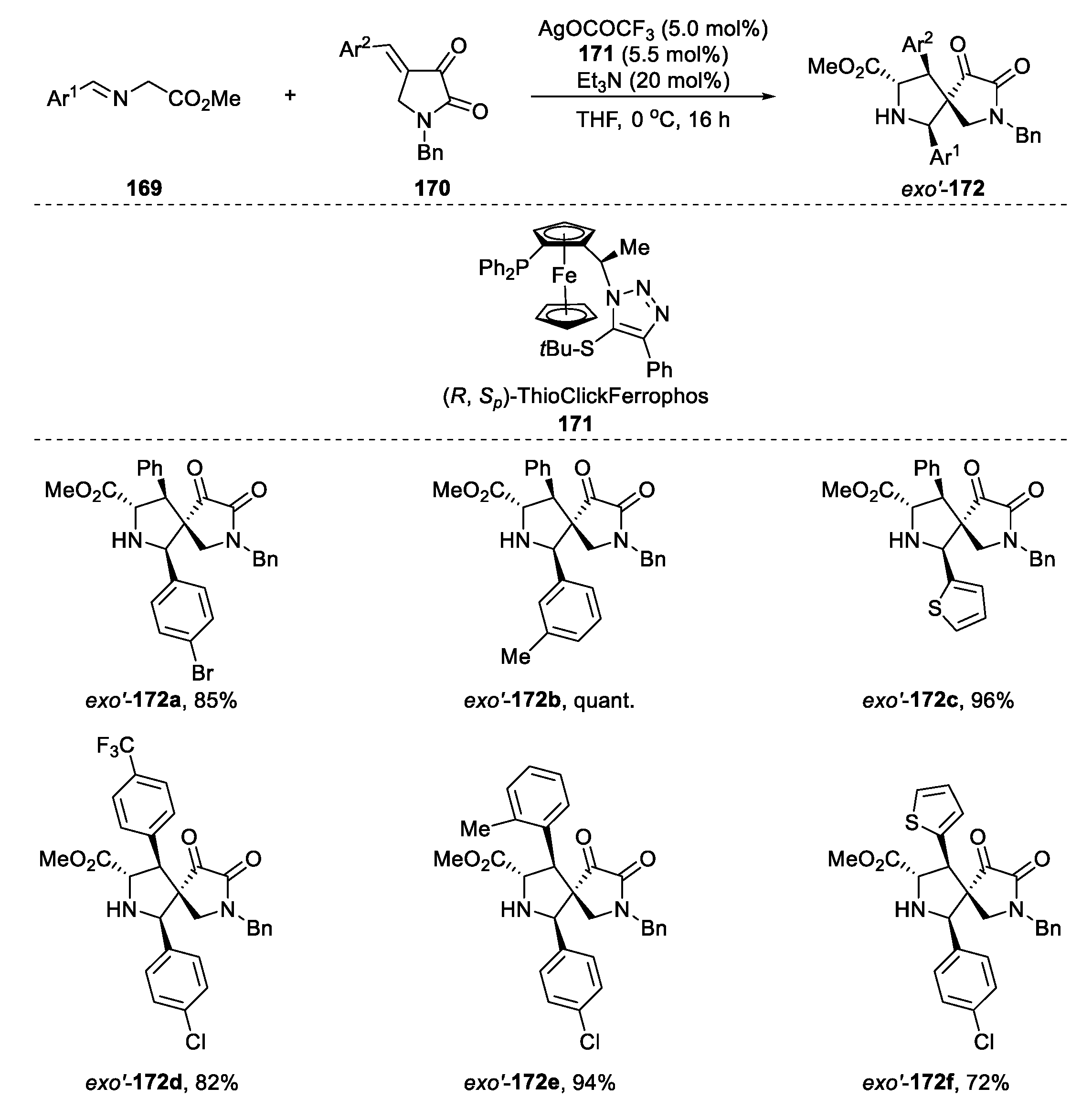
2.6. [3+2] Cycloaddition
2.7. Intramolecular Cyclization
2.7.1. Intramolecular C–N Coupling Reaction
2.7.2. Intramolecular C–N Amination and Cyclization
2.7.3. Intramolecular Cyclization of Dihaloalkanes
2.7.4. Intramolecular Cyclization of Diallyl Compounds
2.7.5. Mitsunobu Cyclodehydration Reaction
2.7.6. Prins Cyclization
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Murray, C.W.; Rees, D.C. The Rise of Fragment-Based Drug Discovery. Nat. Chem. 2009, 1, 187–192. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Sykes, M.; Wang, S. Small-Molecule Inhibitors of Tankyrases as Prospective Therapeutics for Cancer. J. Med. Chem. 2022, 65, 5244–5273. [Google Scholar] [CrossRef]
- Kim, J.; Bae, I.; Song, J.; Kim, Y.; Ahn, Y.; Park, H.J.; Kim, H.H.; Kim, D.K. Design, Synthesis, and Biological Evaluation of Imidazopyrazinone Derivatives as Antagonists of Inhibitor of Apoptosis Proteins (IAPs). Bull. Korean Chem. Soc. 2021, 42, 847–851. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Zhang, H.; Liu, X.; Gao, Y.; Chen, Y.; Liu, L.; Wang, D.; Liang, Z.; Liu, S.; et al. Discovery of Orally Bioavailable N-Benzylpiperidinol Derivatives as Potent and Selective USP7 Inhibitors with in Vivo Antitumor Immunity Activity against Colon Cancer. J. Med. Chem. 2022, 65, 16622–16639. [Google Scholar] [CrossRef] [PubMed]
- La, M.T.; Jeong, B.H.; Kim, H.K. Design and Synthesis of Novel N-(2-Aminophenyl)Benzamide Derivatives as Histone Deacetylase Inhibitors and Their Antitumor Activity Study. Bull. Korean Chem. Soc. 2021, 42, 740–743. [Google Scholar] [CrossRef]
- Zhu, Y.; Shuai, W.; Zhao, M.; Pan, X.; Pei, J.; Wu, Y.; Bu, F.; Wang, A.; Ouyang, L.; Wang, G. Unraveling the Design and Discovery of C-Jun N-Terminal Kinase Inhibitors and Their Therapeutic Potential in Human Diseases. J. Med. Chem. 2022, 65, 3758–3775. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, Z.; Li, X.W.; Zhuang, C. Natural Product-Inspired Targeted Protein Degraders: Advances and Perspectives. J. Med. Chem. 2022, 65, 13533–13560. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Shrestha, R.; Lee, S.; Park, P.H.; Lee, E.S. 6-Hydroxy-Benzofuran-3-(2H)-Ones as Potential Anti-Inflammatory Agents: Synthesis and Inhibitory Activity of LPS-Stimulated ROS Production in RAW 264.7 Macrophage. Bull. Korean Chem. Soc. 2021, 42, 372–375. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Peschiulli, A.; Lefevre, N.; Meerpoel, L.; Maes, B.U.W. Direct α-Functionalization of Saturated Cyclic Amines. Chem. Eur. J. 2012, 18, 10092–10142. [Google Scholar] [CrossRef]
- Ali, I.; Nadeem Lone, M.; Al-Othman, Z.A.; Al-Warthan, A.; Marsin Sanagi, M. Heterocyclic Scaffolds: Centrality in Anticancer Drug Development. Curr. Drug Targets 2015, 16, 711–734. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Becerril, M.; Mo, J.Y.; Sammis, G.M. Free-Radical Synthesis and Functionalization of Heterocycles. In Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 1941; Volume 54, pp. 321–343. [Google Scholar]
- Ghosh, S.; Cho, S.J. Comparative Binding Affinity Analysis of Dual CDK2/FLT3 Inhibitors. Bull. Korean Chem. Soc. 2022, 43, 1320–1327. [Google Scholar] [CrossRef]
- Kang, S.; Kang, B.H. Structure, Function, and Inhibitors of the Mitochondrial Chaperone TRAP1. J. Med. Chem. 2022, 65, 16155–16172. [Google Scholar] [CrossRef]
- Xie, Z.; Hou, S.; Yang, X.; Duan, Y.; Han, J.; Wang, Q.; Liao, C. Lessons Learned from Past Cyclin-Dependent Kinase Drug Discovery Efforts. J. Med. Chem. 2021, 65, 6356–6389. [Google Scholar] [CrossRef]
- Kim, S.L.; Yang, Y.S.; Lee, S.; Kim, N.J. Synthesis and Biological Evaluation of Anilide Derivatives as Epidermal Growth Factor Receptor L858R/T790M and L858R/T790M/C797S Inhibitors. Bull. Korean Chem. Soc. 2022, 43, 1032–1036. [Google Scholar] [CrossRef]
- Xie, W.; Yang, S.; Liang, L.; Wang, M.; Zuo, W.; Lei, Y.; Zhang, Y.; Tang, W.; Lu, T.; Chen, Y.; et al. Discovery of 2-Amino-7-Sulfonyl-7H-Pyrrolo[2,3-d]Pyrimidine Derivatives as Potent Reversible FGFR Inhibitors with Gatekeeper Mutation Tolerance: Design, Synthesis, and Biological Evaluation. J. Med. Chem. 2022, 65, 16570–16588. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.P.; Bojarski, J.; Marcinkowski, K.; Furmanowa, M.; Gutkowska, B.; Kaliszan, R.; Pachecka, J.; Pawlaczyk, J.; Pluta, J.; Wieniawski, W.; et al. Modern Industrial and Pharmacological Applications of Indigo Dye and Its Derivatives—A Review. Acta Pol. Pharm. 2014, 71, 215–221. [Google Scholar]
- Lamberth, C. Heterocyclic Chemistry in Crop Protection. Pest Manag. Sci. 2013, 69, 1106–1114. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, G.; Jiang, X.D. Non-Aryl Substituted Aza-BODIPYs at 1,7- or 3,5-Sites: Synthesis, Structures, Optical Properties, and Applications. J. Mater. Chem. C 2023, 11, 1668–1677. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, J.; An, J.; Li, C.; Bai, S.; Sharma, A.; Deng, G.; Kim, J.S.; Sun, Y. NIR-II Emissive Multifunctional AIEgen with Single Laser-Activated Synergistic Photodynamic/Photothermal Therapy of Cancers and Pathogens. Biomaterials 2020, 259, 120315. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Q.; Shreeve, J.M. Fused Heterocycle-Based Energetic Materials (2012–2019). J. Mater. Chem. A 2020, 8, 4193–4216. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Gopala, L.; Kang, C.; Lee, M.H. Hydrogen Sulfide-Activatable Fluorescence Turn-on Azide-Containing Naphthalimide Derivative. Bull. Korean Chem. Soc. 2022, 43, 1231–1235. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, H.S.; Zou, X.; Huang, L.; Liang, X.; Li, Z.; Kim, J.S.; Lin, W. Harnessing Dual-Fluorescence Lifetime Probes to Validate Regulatory Mechanisms of Organelle Interactions. J. Am. Chem. Soc. 2022, 144, 20854–20865. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, X.; Sun, Z.; Wang, X.; An, Z.; Huang, W. Recent Advances in Metal-Free Phosphorescent Materials for Organic Light-Emitting Diodes. J. Mater. Chem. C 2023, 11, 3143–3161. [Google Scholar] [CrossRef]
- Lee, S.; Shin, E.Y.; Jang, D.; Choi, S.; Park, H.; Kim, J.; Park, S. Production of Mesoporous Carbon Nitrides and Their Photocatalytic Properties for Degradation of Organic Pollutants. Bull. Korean Chem. Soc. 2022, 43, 1124–1129. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.X.; Hou, J.T.; Won, M.; An, J.; Chen, X.; Shu, J.; Kim, J.S. Fluorescence Imaging of Pathophysiological Microenvironments. Chem. Soc. Rev. 2021, 50, 8887–8902. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, J.; Jung, I.H. Synthesis and Characterization of a Copper(II) Phthalocyanine-Based Dye for Organic Photodetectors. Bull. Korean Chem. Soc. 2022, 43, 1130–1135. [Google Scholar] [CrossRef]
- Kumari, S.; Maddeboina, K.; Bachu, R.D.; Boddu, S.H.S.; Trippier, P.C.; Tiwari, A.K. Pivotal Role of Nitrogen Heterocycles in Alzheimer’s Disease Drug Discovery. Drug Discov. Today 2022, 27, 103322. [Google Scholar] [CrossRef]
- Setaki, D.; Tataridis, D.; Stamatiou, G.; Kolocouris, A.; Foscolos, G.B.; Fytas, G.; Kolocouris, N.; Padalko, E.; Neyts, J.; De Clercq, E. Synthesis, Conformational Characteristics and Anti-Influenza Virus A Activity of Some 2-Adamantylsubstituted Azacycles. Bioorg. Chem. 2006, 34, 248–273. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wang, J.; Ouyang, L.; Wang, Y. Polo-like Kinase 1 Inhibitors in Human Cancer Therapy: Development and Therapeutic Potential. J. Med. Chem. 2022, 65, 10133–10160. [Google Scholar] [CrossRef]
- Choi, C.; Park, J.; Jang, S.; Kim, J.; Lee, S.; Min, K.H. Discovery of Novel Thienopyrimidine Derivatives as LRRK2 Inhibitors. Bull. Korean Chem. Soc. 2022, 43, 232–235. [Google Scholar] [CrossRef]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine’s Tool Box. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef]
- Lee, D.H.; Seo, S.H.; Gotina, L.; Pae, A.N.; Lim, S.M. Structural Hybridization for Inhibitors of the Interaction between NRF2 and Keap1. Bull. Korean Chem. Soc. 2022, 43, 1088–1092. [Google Scholar] [CrossRef]
- Padhi, D.; Govindaraju, T. Mechanistic Insights for Drug Repurposing and the Design of Hybrid Drugs for Alzheimer’s Disease. J. Med. Chem. 2022, 65, 7088–7105. [Google Scholar] [CrossRef]
- Serpi, M.; Ferrari, V.; McGuigan, C.; Ghazaly, E.; Pepper, C. Synthesis and Characterization of NUC-7738, an Aryloxy Phosphoramidate of 3′-Deoxyadenosine, as a Potential Anticancer Agent. J. Med. Chem. 2022, 65, 15789–15804. [Google Scholar] [CrossRef]
- Jang, J.; Lee, K.; Koh, B. Investigation of Benzimidazole Anthelmintics as Oral Anticancer Agents. Bull. Korean Chem. Soc. 2022, 43, 750–756. [Google Scholar] [CrossRef]
- Chen, W.; Ji, M.; Cheng, H.; Zheng, M.; Xia, F.; Min, W.; Yang, H.; Wang, X.; Wang, L.; Cao, L.; et al. Discovery, Optimization, and Evaluation of Selective CDK4/6 Inhibitors for the Treatment of Breast Cancer. J. Med. Chem. 2022, 65, 15102–15122. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Shi, L.; Fu, T.; Huang, J.; Pan, Z. Discovery of Novel 7-Azaindole Derivatives as Selective Covalent Fibroblast Growth Factor Receptor 4 Inhibitors for the Treatment of Hepatocellular Carcinoma. J. Med. Chem. 2022, 65, 7278–7295. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, Y.S.; Jeon, S.; Lee, S.I.; Cho, J.E.; Myung, S.; Jang, M.S.; Kim, S.; Song, J.H.; Kim, H.R.; et al. Synthesis and Biological Evaluation of 2-Benzylaminoquinazolin-4(3H)-One Derivatives as a Potential Treatment for SARS-CoV-2. Bull. Korean Chem. Soc. 2022, 43, 412–416. [Google Scholar] [CrossRef]
- Ward, A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Captopril: A Preliminary Review of Its Pharmacological Properties and Therapeutic Efficacy. Drugs 1983, 26, 468–502. [Google Scholar] [CrossRef]
- Hubbard, H.; Lawitz, E. Glecaprevir + Pibrentasvir (ABT493 + ABT-530) for the Treatment of Hepatitis C. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 9–17. [Google Scholar] [CrossRef]
- Perl, A.E.; Altman, J.K.; Cortes, J.; Smith, C.; Litzow, M.; Baer, M.R.; Claxton, D.; Erba, H.P.; Gill, S.; Goldberg, S.; et al. Selective Inhibition of FLT3 by Gilteritinib in Relapsed or Refractory Acute Myeloid Leukaemia: A Multicentre, First-in-Human, Open-Label, Phase 1–2 Study. Lancet Oncol. 2017, 18, 1061–1075. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Futibatinib, an Investigational Agent for the Treatment of Intrahepatic Cholangiocarcinoma: Evidence to Date and Future Perspectives. Expert Opin. Investig. Drugs 2021, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Estévez, V.; Villacampa, M.; Carlos Menéndez, J. Recent Advances in the Synthesis of Pyrroles by Multicomponent Reactions. Chem. Soc. Rev. 2014, 43, 4633–4657. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, A.; Aguiar, A.; Sobral, P.J.M.; Wani, M.Y.; Almeida e Silva, J.; Sobral, A.J.F.N. Paal–Knorr Synthesis of Pyrroles: From Conventional to Green Synthesis. Catal. Rev. 2018, 61, 84–110. [Google Scholar] [CrossRef]
- Xiong, D.; Yang, H.; Zhang, L.; Shao, X.; Xu, X.; Li, Z. One-Pot Hantzsch Synthesis of Unsymmetrical Substituted Pyridines via Condensation of 1, 3-dicarbonyl Compounds with DMF and 1, 1-Dichloro-2-Nitroethene. Tetrahedron Lett. 2023, 116, 154071. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, F.; Gao, X.; Wang, W.; Wu, Y.; Guo, H.; Zheng, B. Base-Mediated Decarboxylative [3+2] Annulation of Ethynyl Benzoxazinanones and Benzimidamides: Synthesis of Imidazole Derivatives. Adv. Synth. Catal. 2021, 363, 2066–2070. [Google Scholar] [CrossRef]
- Xin, X.; Wang, D.; Li, X.; Wan, B. One-Pot Synthesis of Pyridines from 3-Aza-1,5-Enynes. Tetrahedron 2013, 69, 10245–10248. [Google Scholar] [CrossRef]
- Li, T.; Chiou, M.F.; Li, Y.; Ye, C.; Su, M.; Xue, M.; Yuan, X.; Wang, C.; Wan, W.M.; Li, D.; et al. Synthesis of Unsymmetrically Tetrasubstituted Pyrroles and Studies of AIEE in Pyrrolo[1,2-a]Pyrimidine Derivatives. Chem. Sci. 2022, 13, 5667–5673. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, R.; Xiao, X.Q.; Wang, L.; Wang, Z.; Ma, Y. Sustainable Four-Component Annulation for the Synthesis of 2,3,4,6-Tetraarylpyridines. J. Org. Chem. 2021, 86, 3897–3906. [Google Scholar] [CrossRef]
- Borah, B.; Dwivedi, K.D.; Chowhan, L.R. Recent Approaches in the Organocatalytic Synthesis of Pyrroles. RSC Adv. 2021, 11, 13585–13601. [Google Scholar] [CrossRef] [PubMed]
- Vchislo, N.V. α,β-Unsaturated Aldehydes as C-Building Blocks in the Synthesis of Pyridines, 1,4-Dihydropyridines and 1,2-Dihydropyridines. Asian J. Org. Chem. 2019, 8, 1207–1226. [Google Scholar] [CrossRef]
- Mishra, S.; Nair, S.R.; Baire, B. Recent Approaches for the Synthesis of Pyridines and (Iso)Quinolines Using Propargylic Alcohols. Org. Biomol. Chem. 2022, 20, 6037–6056. [Google Scholar] [CrossRef]
- Gao, X.; Wang, P.; Wang, Q.; Chen, J.; Lei, A. Electrochemical Oxidative Annulation of Amines and Aldehydes or Ketones to Synthesize Polysubstituted Pyrroles. Green Chem. 2019, 21, 4941–4945. [Google Scholar] [CrossRef]
- Borghs, J.C.; Azofra, L.M.; Biberger, T.; Linnenberg, O.; Cavallo, L.; Rueping, M.; El-Sepelgy, O. Manganese-Catalyzed Multicomponent Synthesis of Pyrroles through Acceptorless Dehydrogenation Hydrogen Autotransfer Catalysis: Experiment and Computation. ChemSusChem 2019, 12, 3083–3088. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Jesikiewicz, L.T.; Liu, P.; Buchwald, S.L. Synthesis of Pyrroles through the CuH-Catalyzed Coupling of Enynes and Nitriles. J. Am. Chem. Soc. 2020, 142, 9908–9914. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Wang, Y.; Su, X.; He, R.; Chen, C. Copper-Catalyzed [2+2+2] Modular Synthesis of Multisubstituted Pyridines: Alkenylation of Nitriles with Vinyliodonium Salts. Angew. Chem. Int. Ed. 2017, 56, 4824–4828. [Google Scholar] [CrossRef]
- Li, Y.; Yang, K.; Cao, L. Copper-Catalyzed [3+3] Annulation of Ketones with Oxime Acetates for the Synthesis of Pyridines. RSC Adv. 2022, 12, 27546–27549. [Google Scholar] [CrossRef]
- Hamid, M.H.S.A.; Allen, C.L.; Lamb, G.W.; Maxwell, A.C.; Maytum, H.C.; Watson, A.J.A.; Williams, J.M.J. Ruthenium-Catalyzed /V-Alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology. J. Am. Chem. Soc. 2009, 131, 1766–1774. [Google Scholar] [CrossRef]
- Guo, D.; Huang, H.; Xu, J.; Jiang, H.; Liu, H. Efficient Iron-Catalyzed N-Arylation of Aryl Halides with Amines. Org. Lett. 2008, 10, 4513–4516. [Google Scholar] [CrossRef]
- Kubo, T.; Katoh, C.; Yamada, K.; Okano, K.; Tokuyama, H.; Fukuyama, T. A Mild Inter- and Intramolecular Amination of Aryl Halides with a Combination of CuI and CsOAc. Tetrahedron 2008, 64, 11230–11236. [Google Scholar] [CrossRef]
- Hill, A.J.; McKeon, M.-G. Nitrogen-Substituted-3,4-Dihydroxypyrrolidines. J. Am. Chem. Soc. 1954, 76, 3548–3550. [Google Scholar] [CrossRef]
- Romera, J.L.; Cid, J.M.; Trabanco, A.A. Potassium Iodide Catalysed Monoalkylation of Anilines under Microwave Irradiation. Tetrahedron Lett. 2004, 45, 8797–8800. [Google Scholar] [CrossRef]
- Ju, Y.; Varma, R.S. An Efficient and Simple Aqueous N-Heterocyclization of Aniline Derivatives: Microwave-Assisted Synthesis of N-Aryl Azacycloalkanes. Org. Lett. 2005, 7, 2409–2411. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Varma, R.S. Aqueous N-Heterocyclization of Primary Amines and Hydrazines with Dihalides: Microwave-Assisted Syntheses of N-Azacycloalkanes, Isoindole, Pyrazole, Pyrazolidine, and Phthalazine Derivatives. J. Org. Chem. 2006, 71, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Barnard, T.M.; Vanier, G.S.; Collins, M.J. Scale-up of the Green Synthesis of Azacycloalkanes and Isoindolines under Microwave Irradiation. Org. Process Res. Dev. 2006, 10, 1233–1237. [Google Scholar] [CrossRef]
- Marzaro, G.; Guiotto, A.; Chilin, A. Microwave-Promoted Mono-N-Alkylation of Aromatic Amines in Water: A New Efficient and Green Method for an Old and Problematic Reaction. Green Chem. 2009, 11, 774–777. [Google Scholar] [CrossRef]
- Singh, C.B.; Kavala, V.; Samal, A.K.; Patel, B.K. Aqueous-Mediated N-Alkylation of Amines. Eur. J. Org. Chem. 2007, 2007, 1369–1377. [Google Scholar] [CrossRef]
- He, H.; Lin, Q.; Liu, X.; Yang, Y.; Zhou, Y.; Jia, Y.; Gao, X. N-Heterocyclization of Primary Amines with Dihalides Using Microreactors. Synth. Commun. 2012, 42, 2512–2525. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Deng, Y.; Shi, F. Development of a General Non-Noble Metal Catalyst for the Benign Amination of Alcohols with Amines and Ammonia. Chem. Eur. J. 2013, 19, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, C.; Gao, W.C.; Ma, Y.; Wang, X.; Zhang, L.; Yue, J.; Tang, B. Nickel-Catalyzed Borrowing Hydrogen Annulations: Access to Diversified N-Heterocycles. Chem. Commun. 2019, 55, 7844–7847. [Google Scholar] [CrossRef]
- Chamberlain, A.E.R.; Paterson, K.J.; Armstrong, R.J.; Twin, H.C.; Donohoe, T.J. A Hydrogen Borrowing Annulation Strategy for the Stereocontrolled Synthesis of Saturated Aza-Heterocycles. Chem. Commun. 2020, 56, 3563–3566. [Google Scholar] [CrossRef]
- Suwa, T.; Sugiyama, E.; Shibata, I.; Baba, A. Chemoselective Reductive Amination of Aldehydes and Ketones by Dibutylchlorotin Hydride-HMPA Complex. Synthesis 2000, 2000, 789–800. [Google Scholar] [CrossRef]
- Wei, D.; Netkaew, C.; Wu, J.; Darcel, C. Iron-Catalyzed Hydrosilylation of Diacids in the Presence of Amines: A New Route to Cyclic Amines. ChemCatChem 2020, 12, 5449–5455. [Google Scholar] [CrossRef]
- Tran, V.H.; Kim, H.K. Facile Tin(Ii)-Catalyzed Synthesis of N-Heterocycles from Dicarboxylic Acids and Arylamines. Org. Biomol. Chem. 2022, 20, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kamer, P.C.J.; Cole-Hamilton, D.J.; Harvie, M.; Baxter, E.F.; Lim, K.J.C.; Pogorzelec, P. A New Route to N-Aromatic Heterocycles from the Hydrogenation of Diesters in the Presence of Anilines. Chem. Sci. 2017, 8, 6911–6917. [Google Scholar] [CrossRef]
- Olsen, C.J.; Furst, A. N-Phenylpyrrolidine. J. Am. Chem. Soc. 1953, 75, 3026. [Google Scholar] [CrossRef]
- Walkup, R.E.; Searles, S. Synthesis of Sterically Hindered 1-Arylpyrrolidines and 1-Arylpiperidines by Condensation of Primary Aromatic Amines with Cyclic Ethers or Diols. Tetrahedron 1985, 41, 101–106. [Google Scholar] [CrossRef]
- Hargis, D.C.; Shubkin, R.L. Gem-Cyclodialkylation A Facile Synthetic Route to N-Substituted Heterocycles. Tetrahedron Lett. 1990, 31, 2991–2994. [Google Scholar] [CrossRef]
- Korbad, B.L.; Lee, S.H. Synthesis of N-Aryl Substituted, Five- and Six-Membered Azacycles Using Aluminum-Amide Complexes. Chem. Commun. 2014, 50, 8985–8988. [Google Scholar] [CrossRef]
- Amara, Z.; Streng, E.S.; Skilton, R.A.; Jin, J.; George, M.W.; Poliakoff, M. Automated Serendipity with Self-Optimizing Continuous-Flow Reactors. Eur. J. Org. Chem. 2015, 2015, 6141–6145. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, S.; Huo, Y.; Wang, Z. Titanium Tetrachloride-Mediated Synthesis of N-Aryl-Substituted Azacycles from Cyclic Ethers. RSC Adv. 2017, 7, 4363–4367. [Google Scholar] [CrossRef]
- Tran, V.H.; La, M.T.; Kang, S.; Kim, H.K. Practical Direct Synthesis of: N-Aryl-Substituted Azacycles from N-Alkyl Protected Arylamines Using TiCl4 and DBU. Org. Biomol. Chem. 2020, 18, 5008–5016. [Google Scholar] [CrossRef]
- Tran, V.H.; Hong, W.P.; Kim, H.K. Facile Titanium(IV) Chloride and TBD-Mediated Synthesis of N-Aryl-Substituted Azacycles from Arylhydrazines. Bull. Korean Chem. Soc. 2022, 43, 777–783. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, C.; Xia, C.; Sun, W. Synergistic Acid-Catalyzed Synthesis of N-Aryl-Substituted Azacycles from Anilines and Cyclic Ethers. Org. Lett. 2016, 18, 1522–1525. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huo, Y.; Wang, Z. Boron Trifluoride-Mediated Synthesis of N-Aryl-Substituted Pyrrolidines from Tetrahydrofuran and Amines. Chem. Heterocycl. Compd. 2017, 53, 1365–1368. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, C.; Wang, Y.; Liu, Z.; Zhang, Z.; Wang, F. Metal-Free Protocol for the Synthesis of N-Arylpyrrolidines Catalyzed by Hydrogen Iodine. Catal. Commun. 2017, 94, 56–59. [Google Scholar] [CrossRef]
- La, M.T.; Kang, S.; Kim, H.K. Metal-Free Synthesis of N-Aryl-Substituted Azacycles from Cyclic Ethers Using POCl3. J. Org. Chem. 2019, 84, 6689–6696. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; La, M.T.; Kim, H.K. Phosphoryl Chloride-Mediated Solvent-Free Synthesis of N-Aryl-Substituted Azacycles from Arylamines and Cyclic Ethers. Tetrahedron Lett. 2019, 60, 1860–1863. [Google Scholar] [CrossRef]
- Rout, L.; Jammi, S.; Punniyamurthy, T. Novel CuO Nanoparticle Catalyzed C–N Cross Coupling of Amines with Lodobenzene. Org. Lett. 2007, 9, 3397–3399. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yang, L.; Org, J.F.J. Nickel-Catalyzed Amination of Aryl Tosylates. J. Org. Chem. 2008, 73, 1624–1627. [Google Scholar] [CrossRef]
- Khatri, P.K.; Jain, S.L. Glycerol Ingrained Copper: An Efficient Recyclable Catalyst for the N-Arylation of Amines with Aryl Halides. Tetrahedron Lett. 2013, 54, 2740–2743. [Google Scholar] [CrossRef]
- Sandtorv, A.H.; Stuart, D.R. Metal-Free Synthesis of Aryl Amines: Beyond Nucleophilic Aromatic Substitution. Angew. Chem. Int. Ed. 2016, 55, 15812–15815. [Google Scholar] [CrossRef] [PubMed]
- Purkait, N.; Kervefors, G.; Linde, E.; Olofsson, B. Regiospecific N-Arylation of Aliphatic Amines under Mild and Metal-Free Reaction Conditions. Angew. Chem. Int. Ed. 2018, 57, 11427–11431. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Y.; Ming, X.X.; Teng, H.B.; Hu, Y.T.; Zhang, C.P. Transition-Metal-Free N-Arylation of Amines by Triarylsulfonium Triflates. Chem. Eur. J. 2018, 24, 13744–13748. [Google Scholar] [CrossRef]
- Ruffoni, A.; Juliá, F.; Svejstrup, T.D.; McMillan, A.J.; Douglas, J.J.; Leonori, D. Practical and Regioselective Amination of Arenes Using Alkyl Amines. Nat. Chem. 2019, 11, 426–433. [Google Scholar] [CrossRef]
- Li, J.; Huang, C.; Wen, D.; Zheng, Q.; Tu, B.; Tu, T. Nickel-Catalyzed Amination of Aryl Chlorides with Amides. Org. Lett. 2021, 23, 687–691. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Narayan, R.; Potowski, M.; Jia, Z.J.; Antonchick, A.P.; Waldmann, H. Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis. Acc. Chem. Res. 2014, 47, 1296–1310. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent Advances of Catalytic Asymmetric 1,3-Dipolar Cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef] [PubMed]
- Żmigrodzka, M.; Sadowski, M.; Kras, J.; Dresler, E.; Demchuk, O.M.; Kula, K. Polar [3+2] Cycloaddition between N-Methyl Azomethine Ylide and Trans-3,3,3-Trichloro-1-Nitroprop-1-Ene. Sci. Radices 2022, 1, 26–35. [Google Scholar] [CrossRef]
- Lauridsen, V.H.; Ibsen, L.; Blom, J.; Jørgensen, K.A. Asymmetric Brønsted Base Catalyzed and Directed [3+2] Cycloaddition of 2-Acyl Cycloheptatrienes with Azomethine Ylides. Chem. Eur. J. 2016, 22, 3259–3263. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Dresler, E.; Hordyjewicz-Baran, Z.; Kulesza, R.; Jasiński, R. A Unique Example of Noncatalyzed [3+2] Cycloaddition Involving (2E)-3-Aryl-2-Nitroprop-2-Enenitriles. Chem. Heterocycl. Compd. 2017, 53, 1161–1162. [Google Scholar] [CrossRef]
- Zhu, J.; Su, W.; Xiong, C.; Bai, R.; Zhou, Q.; Chen, M. Catalyst-Free [3+2] Cycloaddition of Electron-Deficient Alkynes and o-Hydroxyaryl Azomethine Ylides in Water. ACS Omega 2020, 5, 18244–18253. [Google Scholar] [CrossRef]
- Furuya, S.; Kanemoto, K.; Fukuzawa, S. ichi Exo′-Selective Construction of Spirobipyrrolidines by the Silver-Catalyzed Asymmetric [3+2] Cycloaddition of Imino Esters with 4-Benzylidene-2,3-Dioxopyrrolidines. Chem. Asian J. 2022, 17, e202200239. [Google Scholar] [CrossRef]
- Esteban, F.; Cieślik, W.; Arpa, E.M.; Guerrero-Corella, A.; Díaz-Tendero, S.; Perles, J.; Fernández-Salas, J.A.; Fraile, A.; Alemán, J. Intramolecular Hydrogen Bond Activation: Thiourea-Organocatalyzed Enantioselective 1,3-Dipolar Cycloaddition of Salicylaldehyde-Derived Azomethine Ylides with Nitroalkenes. ACS Catal. 2018, 8, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Biswas, R.G.; Suneja, A.; Sadhu, M.M.; Singh, V.K. (R)-DM-SEGPHOS-Ag(I)-Catalyzed Enantioselective Synthesis of Pyrrolidines and Pyrrolizidines via (1,3)- and Double (1,3)-Dipolar Cycloaddition Reactions. J. Org. Chem. 2018, 83, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; He, F.S.; Deng, H.; Yang, W.L.; Deng, W.P. β-Silyl Acrylates in Asymmetric [3 + 2] Cycloadditions Affording Pyrrolidine Azasugar Derivatives. Org. Lett. 2018, 20, 3838–3842. [Google Scholar] [CrossRef]
- Kalita, S.J.; Cheng, F.; Fan, Q.H.; Shibata, N.; Huang, Y.Y. Diastereodivergent Synthesis of Chiral 4-Fluoropyrrolidines (Exo and Exo′) Based on the Cu(II)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition. J. Org. Chem. 2021, 86, 8695–8705. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.L.; Bartlett, E.S.; Sarpong, R. Intramolecular C(Sp3)-N Coupling by Oxidation of Benzylic C,N-Dianions. Angew. Chem. Int. Ed. 2013, 52, 2194–2197. [Google Scholar] [CrossRef]
- Betz, K.N.; Chiappini, N.D.; Du Bois, J. Intermolecular Sp3-C-H Amination for the Synthesis of Saturated Azacycles. Org. Lett. 2020, 22, 1687–1691. [Google Scholar] [CrossRef]
- Xue, W.; Xu, H.; Liang, Z.; Qian, Q.; Gong, H. Nickel-Catalyzed Reductive Cyclization of Alkyl Dihalides. Org. Lett. 2014, 16, 4984–4987. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, T.; Wang, S.; Xu, H.; Gong, H. Nickel-Catalyzed Reductive Cross-Coupling of Unactivated Alkyl Halides. Org. Lett. 2011, 13, 2138–2141. [Google Scholar] [CrossRef]
- Hoyt, J.M.; Sylvester, K.T.; Semproni, S.P.; Chirik, P.J. Synthesis and Electronic Structure of Bis(Imino)Pyridine Iron Metallacyclic Intermediates in Iron-Catalyzed Cyclization Reactions. J. Am. Chem. Soc. 2013, 135, 4862–4877. [Google Scholar] [CrossRef]
- Schmidt, V.A.; Hoyt, J.M.; Margulieux, G.W.; Chirik, P.J. Cobalt-Catalyzed [2π + 2π] Cycloadditions of Alkenes: Scope, Mechanism, and Elucidation of Electronic Structure of Catalytic Intermediates. J. Am. Chem. Soc. 2015, 137, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M.; Iveson, M.; Collins, I.; Jones, A.M. A Mitsunobu Reaction to Functionalized Cyclic and Bicyclic N-Arylamines. Tetrahedron Lett. 2018, 59, 238–242. [Google Scholar] [CrossRef]
- Miranda, P.O.; Carballo, R.M.; Martin, V.S.; Padrón, J.I. A New Catalytic Prins Cyclization Leading to Oxa- and Azacycles. Org. Lett. 2009, 11, 357–360. [Google Scholar] [CrossRef]
- Liu, G.Q.; Cui, B.; Xu, R.; Li, Y.M. Preparation of Trans-2-Substituted-4-Halopiperidines and Cis-2-Substituted-4-Halotetrahydropyrans via AlCl3-Catalyzed Prins Reaction. J. Org. Chem. 2016, 81, 5144–5161. [Google Scholar] [CrossRef]
- Hasegawa, E.; Hiroi, N.; Osawa, C.; Tayama, E.; Iwamoto, H. Application of Biphasic Reaction Procedure Using Ferric Chloride Dissolved in an Imidazolium Salt and Benzotrifluoride (FeIm-BTF Procedure) to Aza-Prins Cyclization Reaction. Tetrahedron Lett. 2010, 51, 6535–6538. [Google Scholar] [CrossRef]


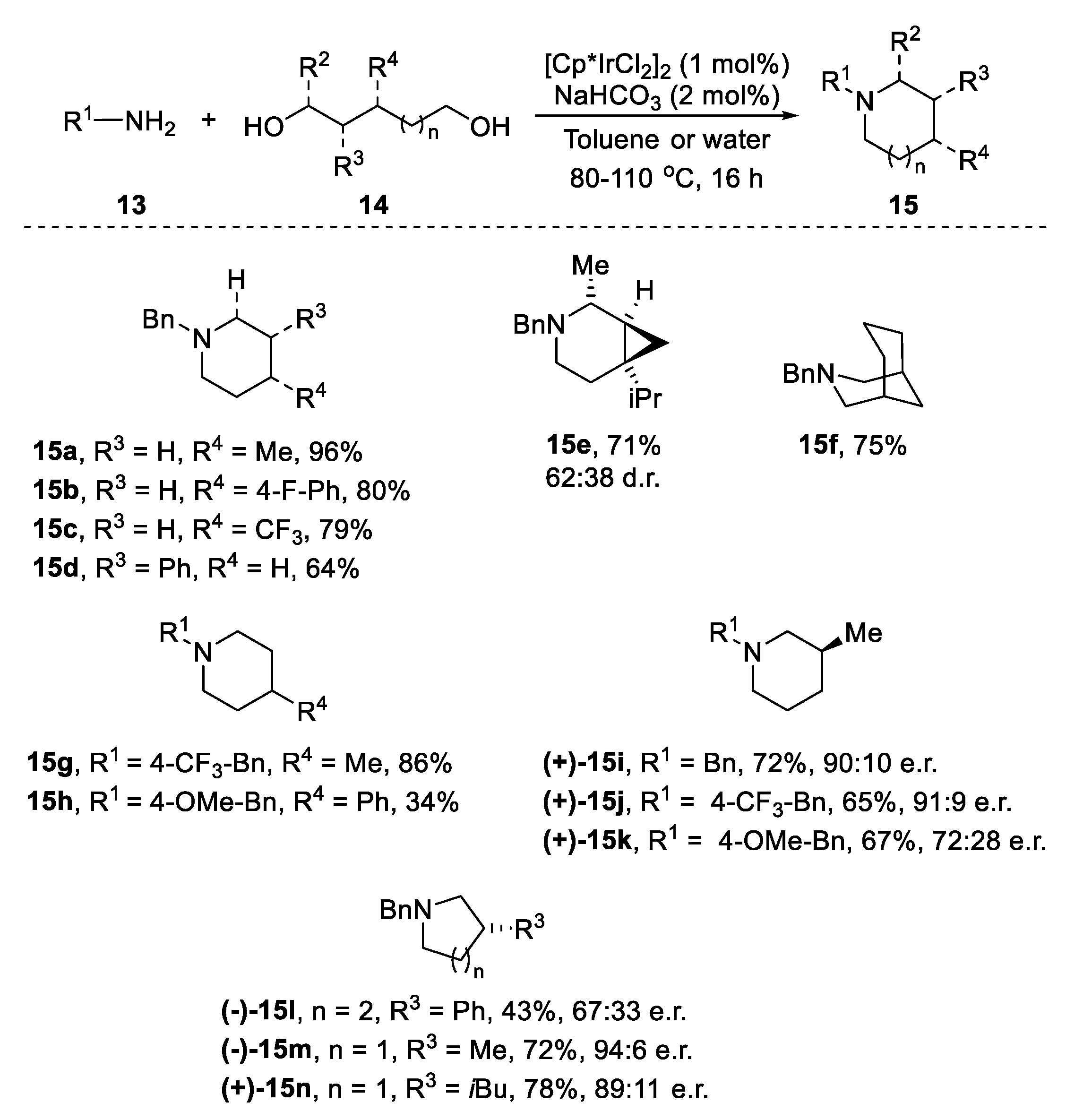





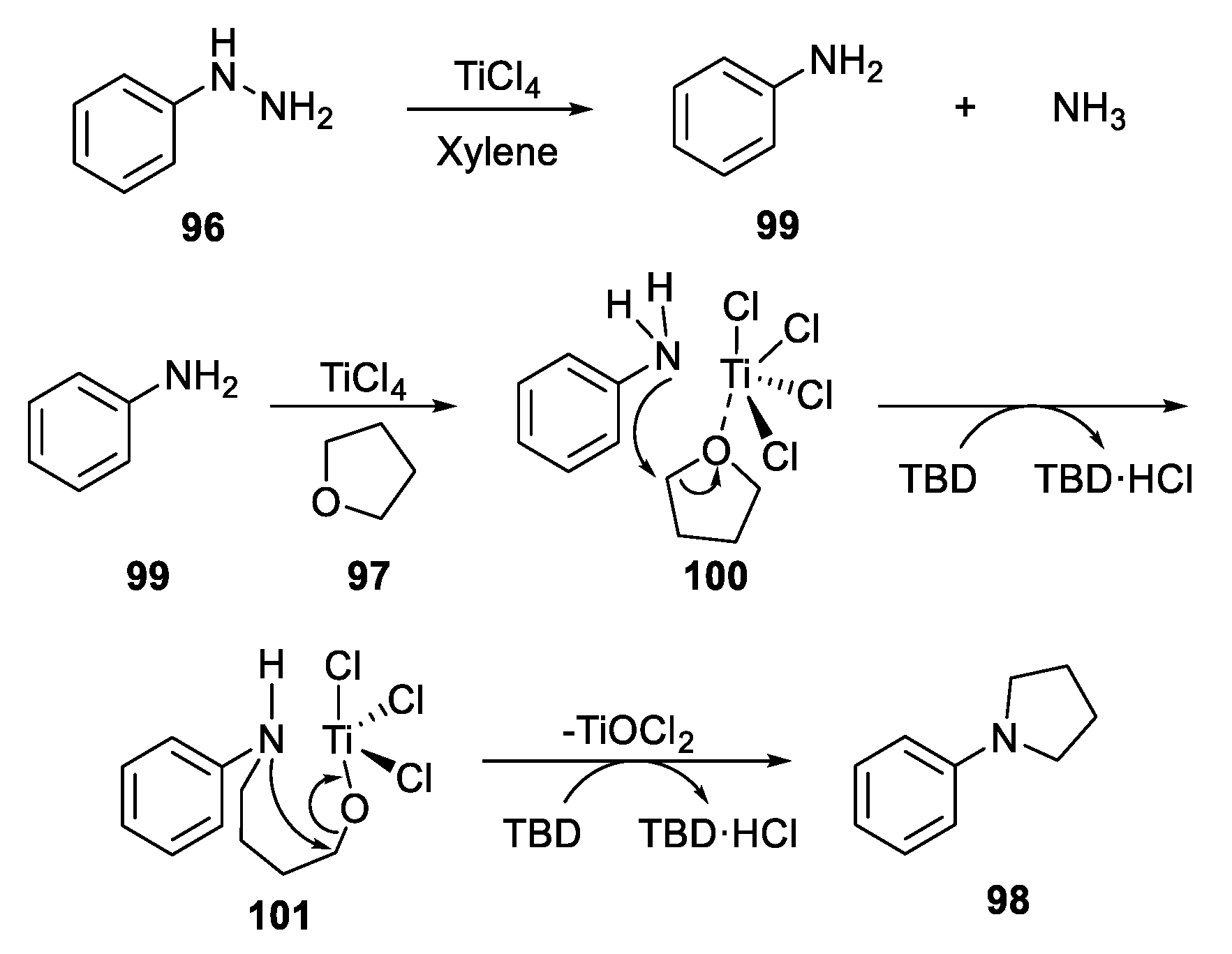

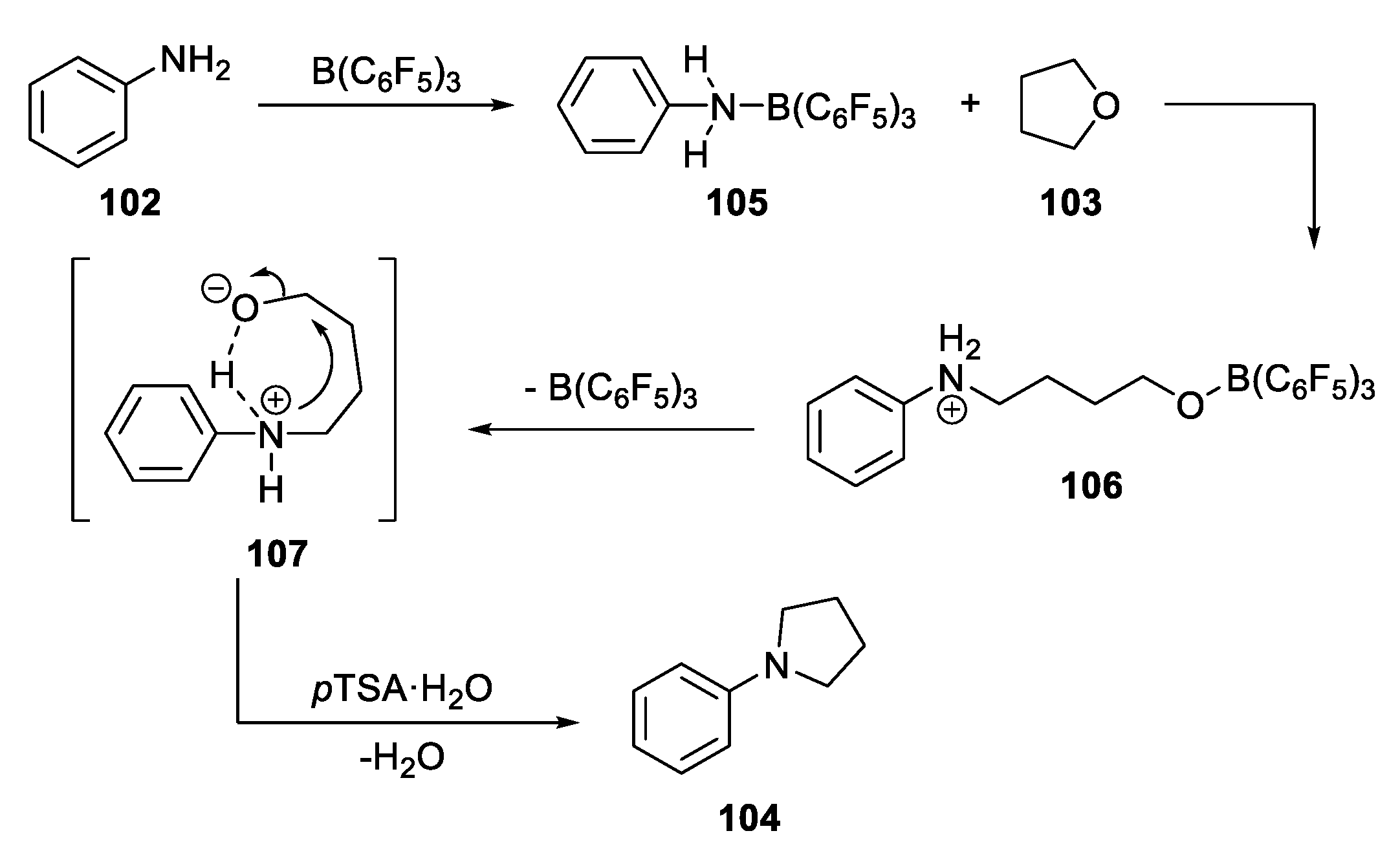
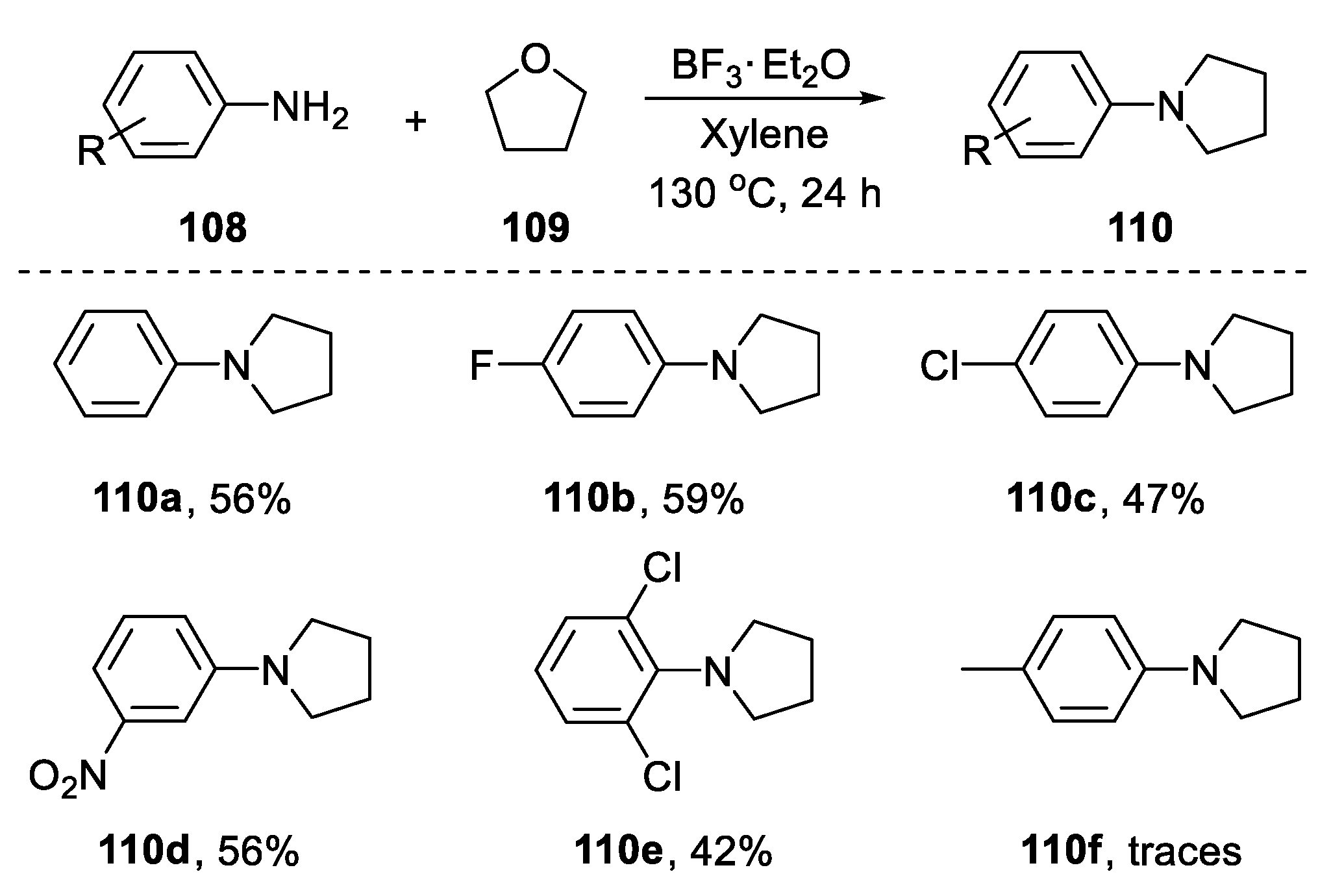
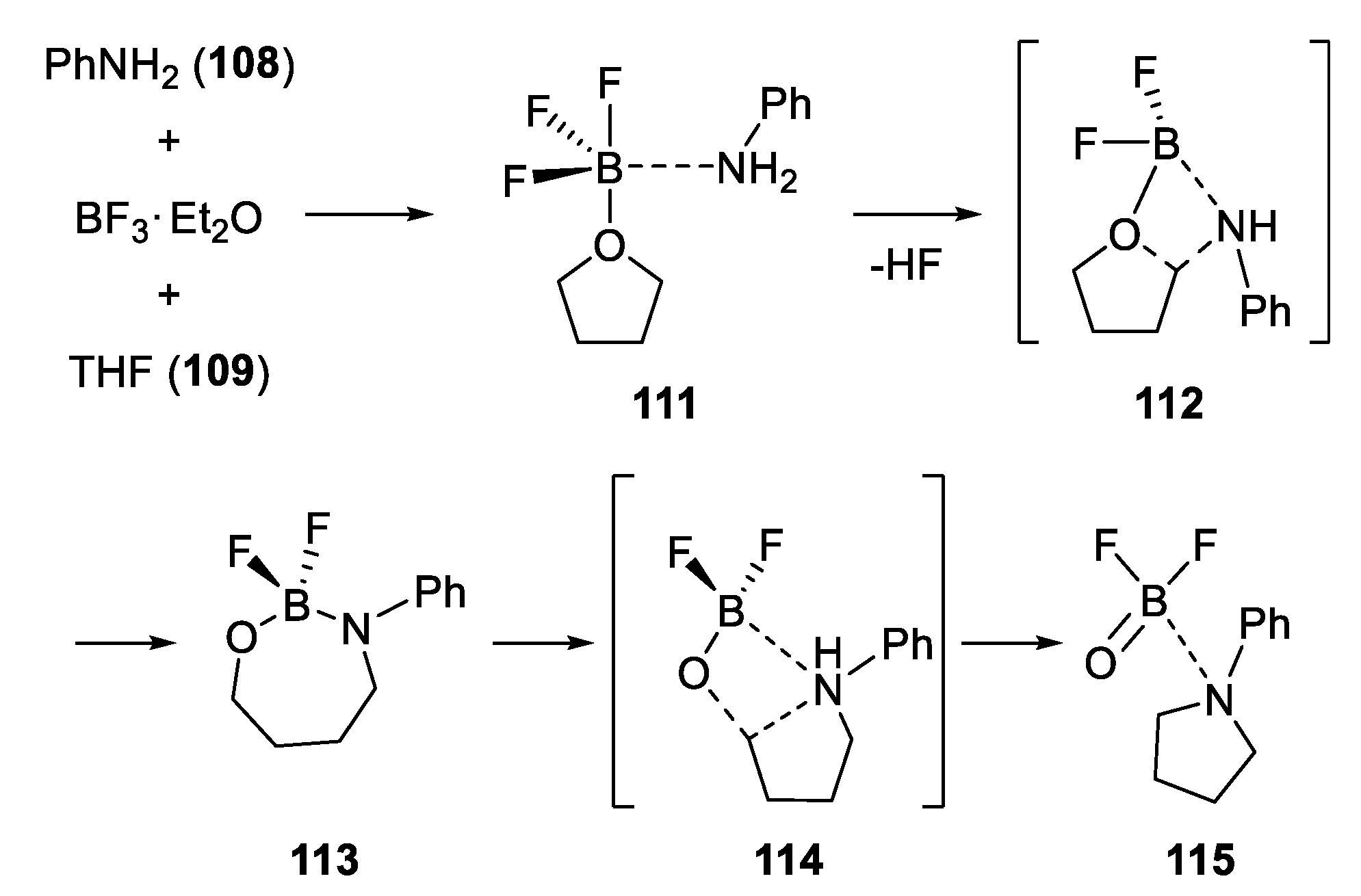





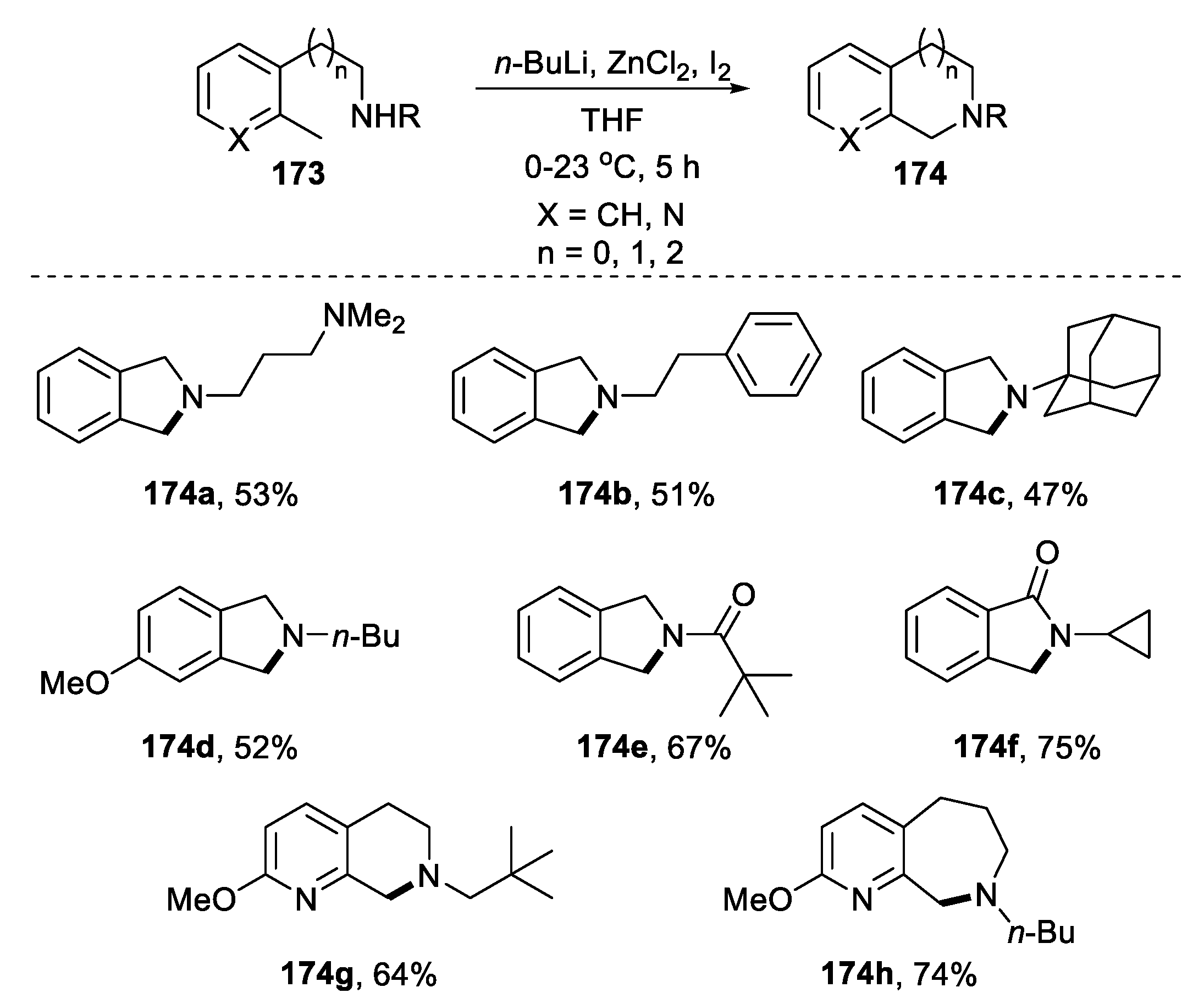
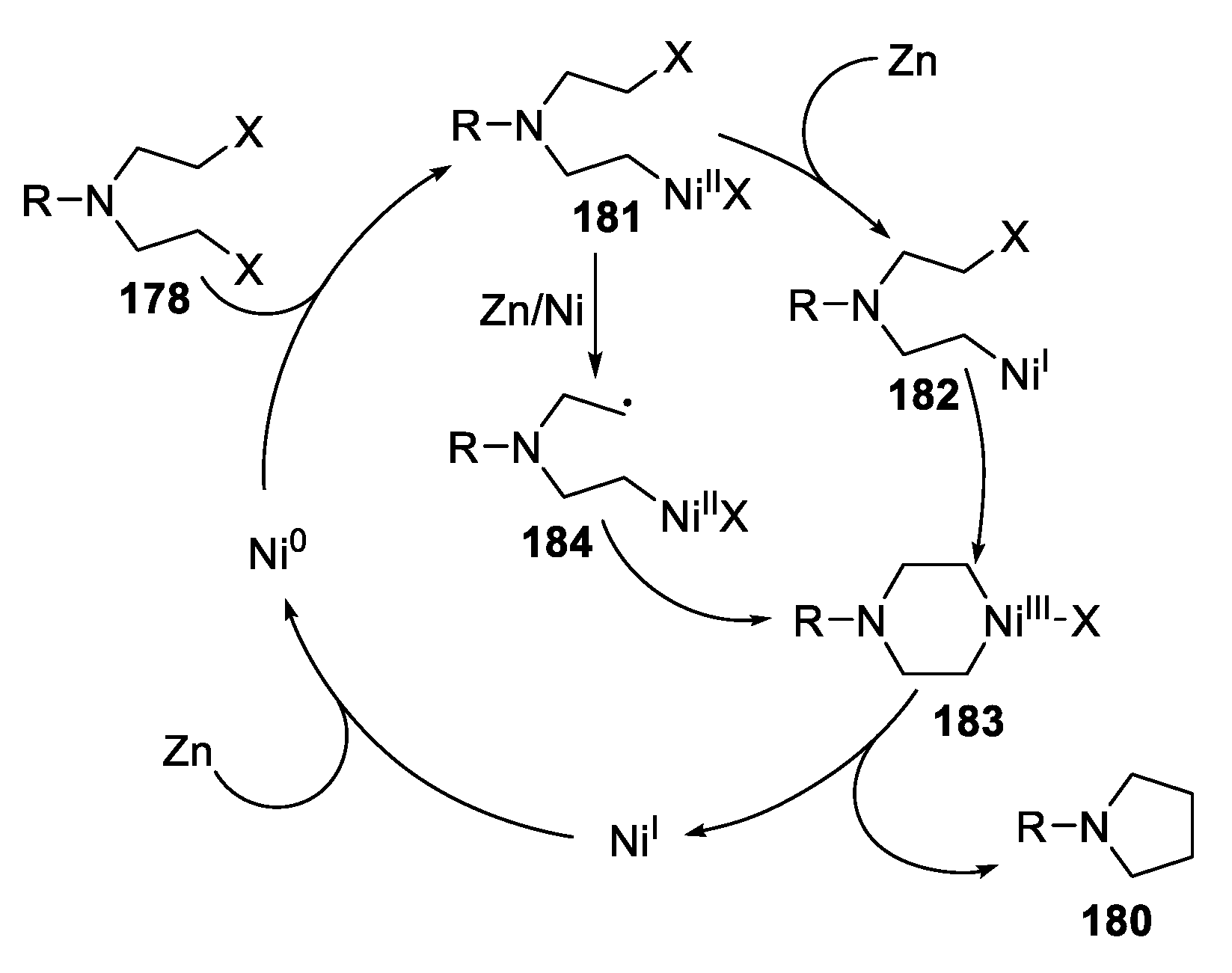
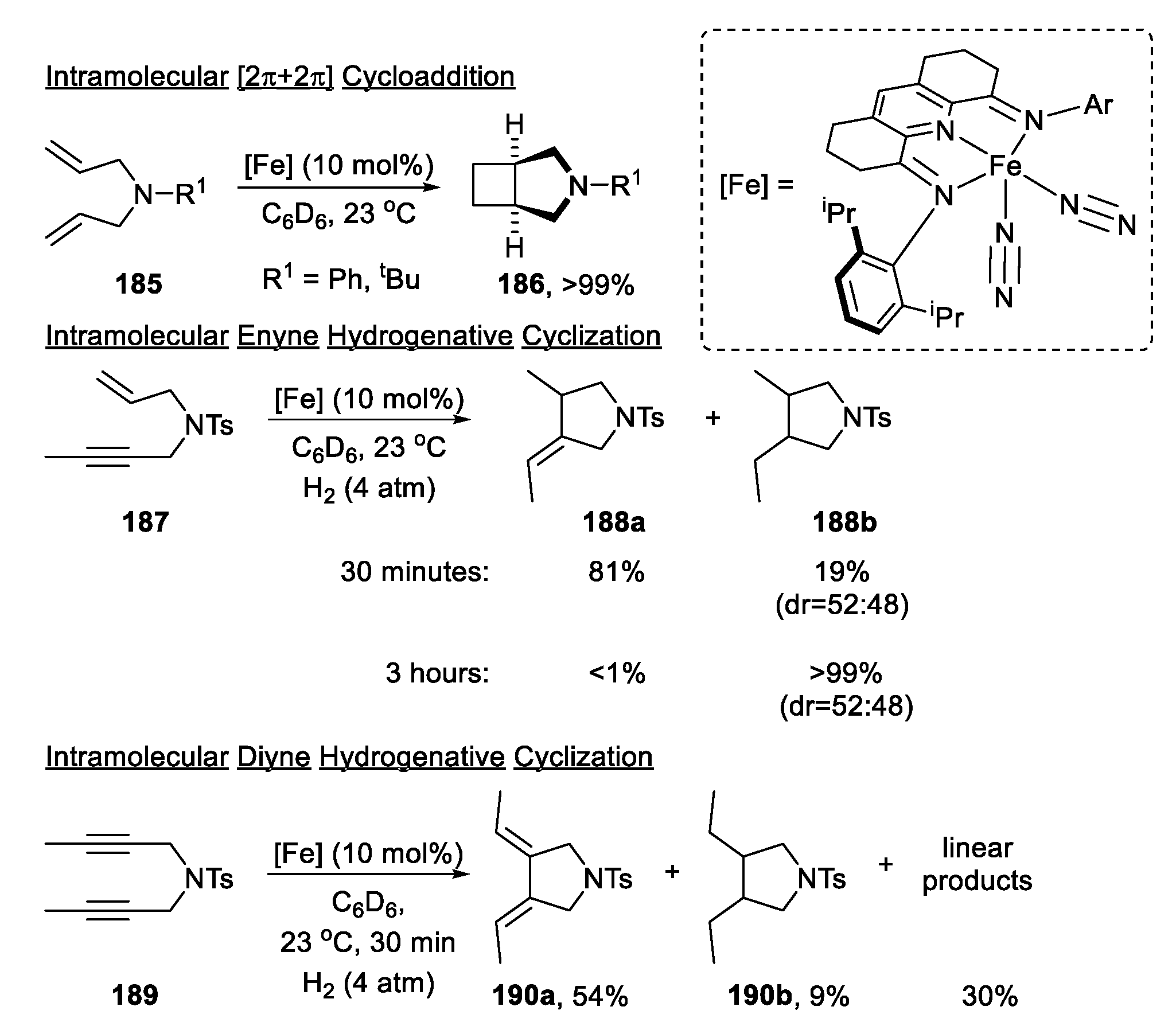



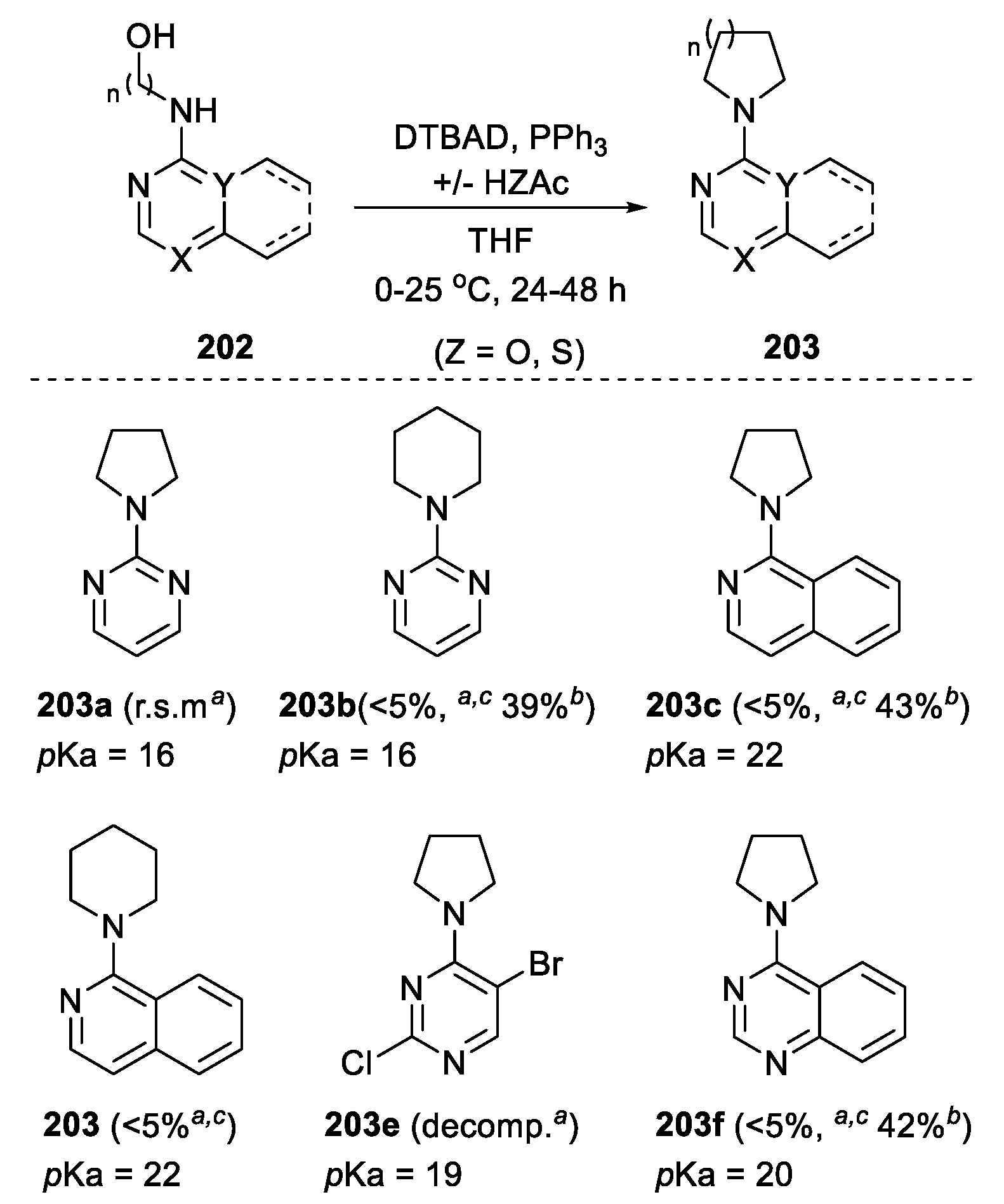

 | |||
| Entry | Substrate | Product | Yield (%) |
| 1 | 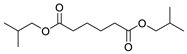 |  | 95 |
| 2 | 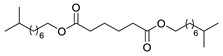 |  | 59 |
| 3 | 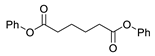 | 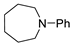 | 92 |
| 4 | 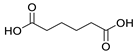 | 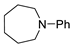 | 13 |
| 5 | 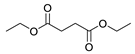 |  | 66 |
| 6 |  |  | 92 |
| 7 | 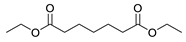 | 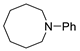 | 66 |
| 8 | 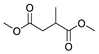 | 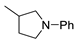 | 75 |
| 9 | 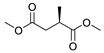 | 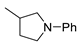 | 78 |
| 10 |  | 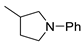 | 79 |
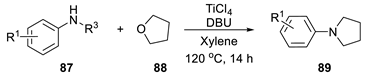 | |||||
| Entry | N-Alkylamine | Product | Yield (%) | ||
| 1 | 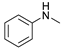 | 87a | 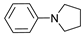 | 89a | 93 |
| 2 | 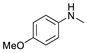 | 87b | 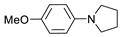 | 89b | 87 |
| 3 | 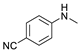 | 87c | 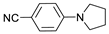 | 89c | 82 |
| 4 | 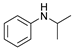 | 87d | 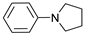 | 89d | 90 |
| 5 |  | 87e | 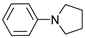 | 89e | 87 |
 | ||||||
| Entry | C – H aminated intermediate | Cyclized product | ||||
| 1 | 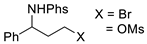 | 176a-1 176a-2 | 64% 38% | 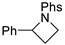 | 177a | 97% 96% |
| 2 | 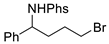 | 176b | 42% | 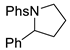 | 177b | 97% |
| 3 |  | 176c | 52% | 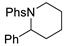 | 177c | 98% |
| 4 | 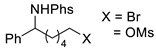 | 176d-1 176d-2 | 65% 42% | 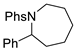 | 177d | 98% 68% |
| 5 | 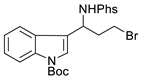 | 176e | 71% | 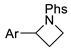 | 177e | 95% |
| 6 | 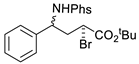 | 176f | 69% | 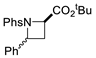 | 177f | 98% |
| 7 | 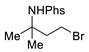 | 176g | 61% | 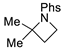 | 177g | 83% |
| 8 |  | 176h | 42% | 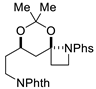 | 177h | 91% |
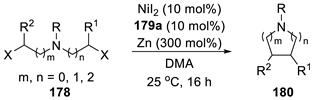 | |||
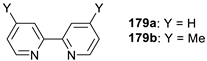 | |||
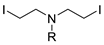
| Entry | Dihaloalkane | Product | Yield (%) |
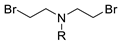 | 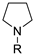 | ||
| 1 2 3 4 5 | 178a, R = PhO(O)C 178b, R = MeO(O)C 178c, R = Ph 178d, R = 4-MePh 178e, R = Ts | 180a 180b 180c 180d 180e | 92 48 54 50 ND |
 |  | ||
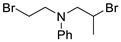
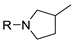
| 6 7 8 | 178f, R = Cbz 178g, R = PhO(O)C 178h, R = 4-MePh | 180f 180g = 180a 180h = 180d | 93 70 58 |
 |  | ||
| 9 10 | 178i, R = Ph 178j, R = Cbz | 180i 180j | 46 61 |
| 11 |  178k | 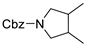 180k | 71 |
| 12 |  178l |  180l | 50 |
| 13 |  178m |  180m | 38 a |
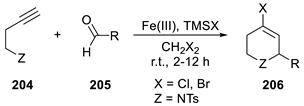 | ||||
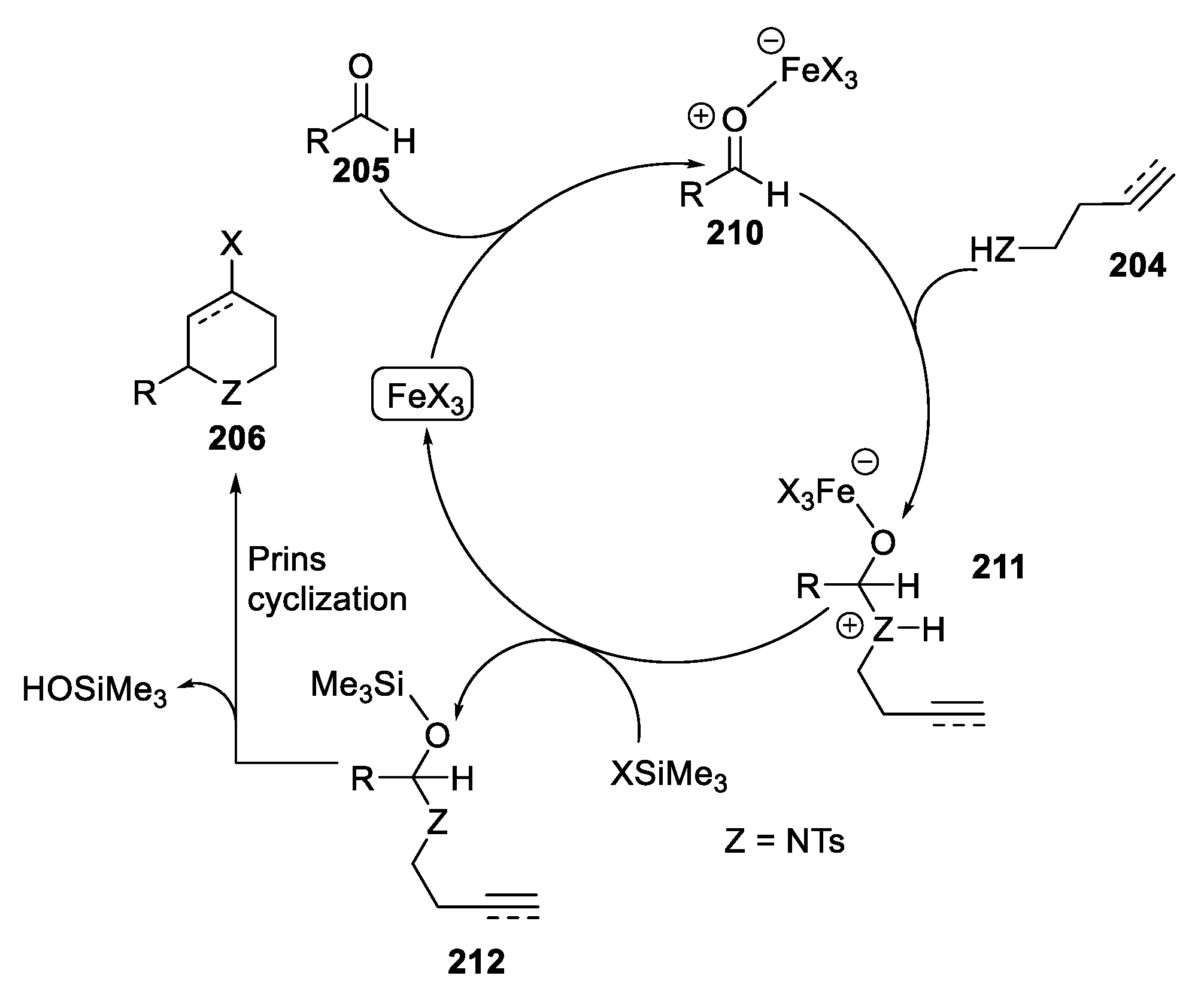
| Entry | X | R | TMSX (equiv.) | Yield (%) |
| 1 | Cl | i-Bu | 1.5 | 80 a |
| 2 | Cl | c-C6H11 | 1.5 | 79 a |
| 3 | Cl | Bn | 1.5 | 65 a |
| 4 | Br | i-Bu | 1.5 | 87 a |
| 5 | Br | c-C6H11 | 1.5 | 81 a |
| 6 | Br | Bn | 1.5 | 88 a |
| 7 | Cl | Bn | 1.5 | 70 b |
| 8 | Br | i-Bu | 1.5 | 85 b |
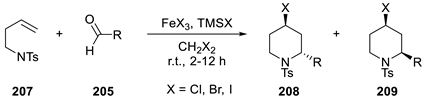 | ||||||
| Entry | X | FeX3 (mol%) | Fe(acac)3 (mol%) | R | 208:209 | Yield (%) |
| 1 | Cl | 10 | 0 | i-Bu | 94:6 | 95 |
| 2 | Cl | 10 | 0 | Bn | 84:16 | 80 |
| 3 | Br | 10 | 0 | i-Bu | 95:5 | 95 |
| 4 | Br | 10 | 0 | Bn | 83:17 | 86 |
| 5 | Cl | 0 | 7.5 | i-Bu | 95:5 | 99 |
| 6 | Cl | 0 | 7.5 | CH2=CH(CH2)2- | 95:5 | 85 |
| 7 | Cl | 0 | 7.5 | BnO(CH2)2- | 95:5 | 85 |
| 8 | I | 0 | 7.5 | i-Bu | 95:5 | 92 |
 | |||
| Entry | R1 | Isolated yield (%) | trans:cis |
| 1 | p-MePh | 83 (215a) | 98:2 |
| 2 | p-MeOPh | 88 (215b) | 91:9 |
| 3 | Ph | 78 (215c) | 95:5 |
| 4 | p-O2NPh | 51 (215d) | 95:5 |
| 5 | CH3 | 66 (215e) | 86:14 |
 | ||||
| Entry | Halide source | R | Isolated yield (%) | trans:cis |
| 1 | TMSCl | 4-MePh | 77 (218a) | 87:13 |
| 2 | TMSCl | 4-MePh | 87 (218b) | 96:4 |
| 3 | TMSCl | 4-FPh | 75 (218c) | 96:4 |
| 4 | TMSCl | H | 90 (218d) | |
| 5 | TMSCl | C2H5 | 86 (218e) | 89:11 |
| 6 | TMSBr | 4-MePh | 82 (218f) | 94:6 |
| 7 | TMSI | 4-MePh | 81 (218g) | 92:8 |
| 8 | BF3·OEt2 | 4-MePh | 80 (218h) | 48:52 |
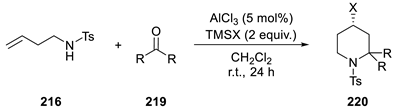 | |||
| Entry | TMSX | R | Isolated yield (%) |
| 1 | TMSCl | Me | No reaction |
| 2 | TMSBr | Me | 46 (220a) |
| 3 | TMSI | Me | <5 |
| 4 | TMSBr | –(CH2)4– | 51 (220b) |
| 5 | TMSBr | –(CH2)5– | 40 (220c) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.T.; Kim, H.-K. Recent Advances in Synthetic Routes to Azacycles. Molecules 2023, 28, 2737. https://doi.org/10.3390/molecules28062737
Nguyen AT, Kim H-K. Recent Advances in Synthetic Routes to Azacycles. Molecules. 2023; 28(6):2737. https://doi.org/10.3390/molecules28062737
Chicago/Turabian StyleNguyen, Anh Thu, and Hee-Kwon Kim. 2023. "Recent Advances in Synthetic Routes to Azacycles" Molecules 28, no. 6: 2737. https://doi.org/10.3390/molecules28062737
APA StyleNguyen, A. T., & Kim, H.-K. (2023). Recent Advances in Synthetic Routes to Azacycles. Molecules, 28(6), 2737. https://doi.org/10.3390/molecules28062737