Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells
Abstract
1. Introduction
2. Results
2.1. NTs Protect against Neurodegeneration of H2O2-Treated PC-12 Cells
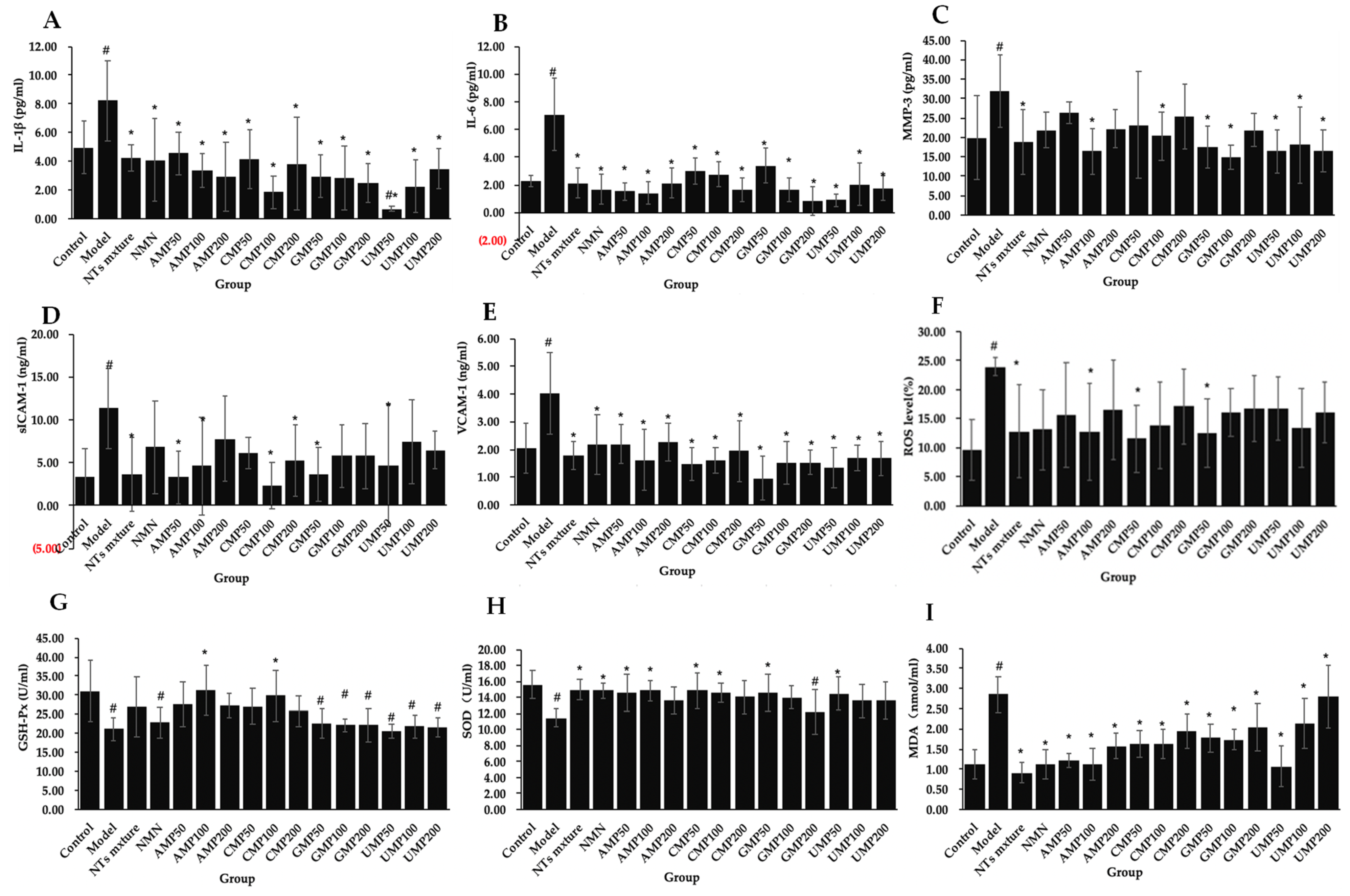
2.2. NTs Suppress Neuroinflammation in H2O2-Treated PC-12 Cells
2.3. NTs Inhibit ROS Production and Enhance Antioxidant Activities in H2O2-Treated PC-12 Cells
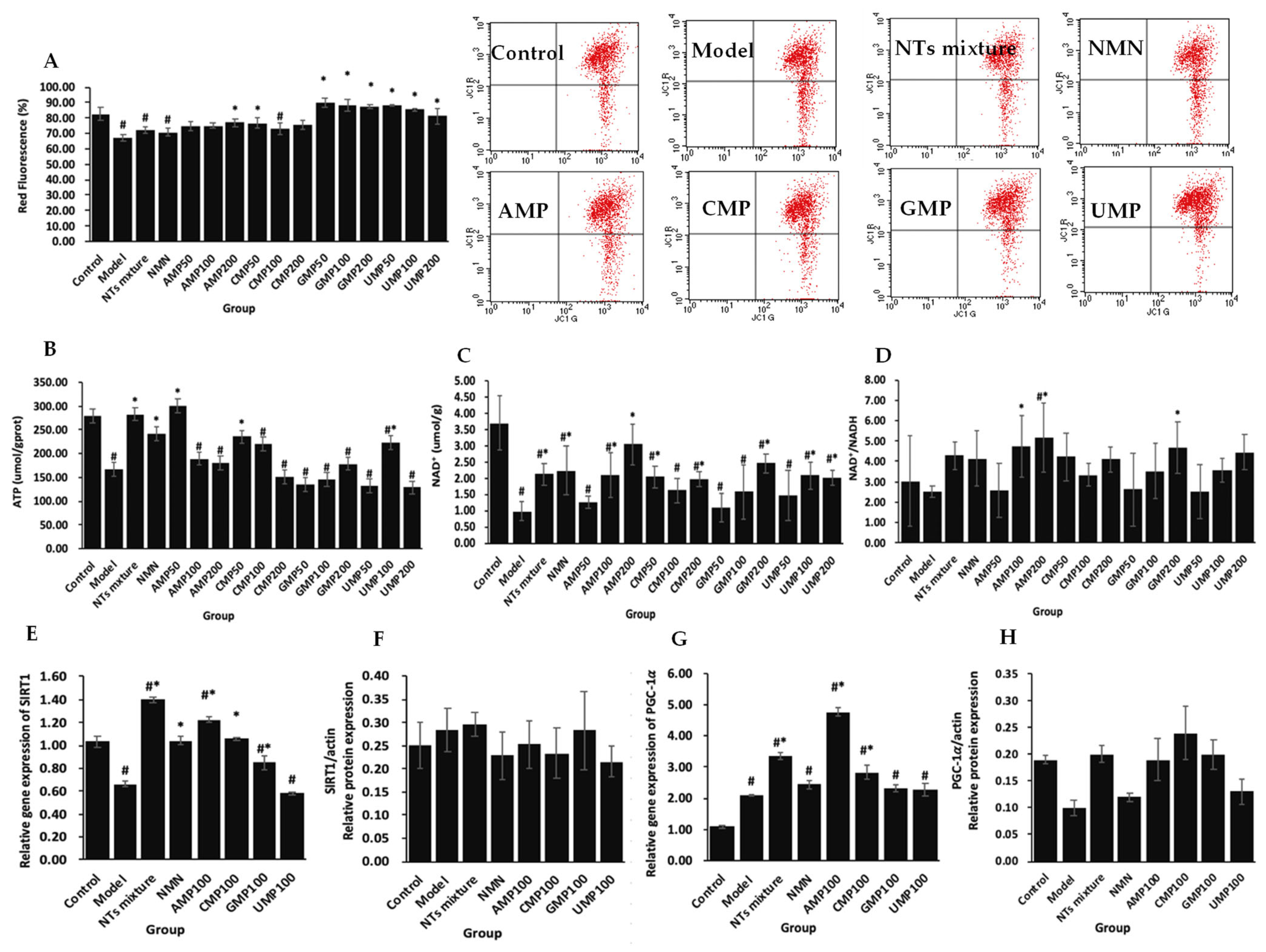
2.4. NTs Improve Mitochondrial Function and Tend to Up-Regulate the Mitochondrial Biogenesis Related Pathway NAD+/SIRT1/PGC-1α in H2O2-Treated PC-12 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cuture and Teatments
4.3. Morphology Observation
4.4. Cell Viability Assay
4.5. Flow Cytometry
4.6. Biochemical Analysis
4.7. Quantitative Real-Time PCR Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurd, M.D.; Martorell, P.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 369, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef]
- Chow, H.M.; Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 2015, 16, 672–684. [Google Scholar] [CrossRef]
- Mather, M.; Harley, C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef]
- Strehler, E.E.; Thayer, S.A. Evidence for a role of plasma membrane calcium pumps in neurodegenerative disease: Recent developments. Neurosci. Lett. 2018, 663, 39–47. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Mattson, M.P.; Chan, S.L.; Duan, W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol. Rev. 2002, 82, 637–672. [Google Scholar] [CrossRef]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000, 71, 630S–636S. [Google Scholar] [CrossRef]
- Bender, A.; Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: End of story? Amino Acids 2016, 48, 1929–1940. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Xu, H.; Luo, X.; Yu, J.; Liu, J.; Chang, R.C. Neuroprotection of Coenzyme Q10 in Neurodegenerative Diseases. Curr. Top. Med. Chem. 2016, 16, 858–866. [Google Scholar] [CrossRef]
- Chandran, A.M.K.; Christina, H.; Das, S.; Mumbrekar, K.D.; Satish Rao, B.S. Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environ. Toxicol. Pharmacol. 2019, 71, 103224. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin against Cerebral Ischemia-Reperfusion via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Liu, T.; Wang, J. Neuroprotective Effects of Ginsenosides against Cerebral Ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef]
- Vohra, M.; Lemieux, G.A.; Lin, L.; Ashrafi, K. The beneficial effects of dietary restriction on learning are distinct from its effects on longevity and mediated by depletion of a neuroinhibitory metabolite. PLoS Biol. 2017, 15, e2002032. [Google Scholar] [CrossRef]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.J.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Pérez, M.J.; Sánchez-Medina, F.; Torres, M.; Gil, A.; Suárez, A. Dietary nucleotides enhance the liver redox state and protein synthesis in cirrhotic rats. J. Nutr. 2004, 134, 2504–2508. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, M.F.; Liang, Y.F.; Komatsu, T.; Chan, Y.C.; Chung, S.Y.; Yamamoto, S. A nucleoside-nucleotide mixture may reduce memory deterioration in old senescence-accelerated mice. J. Nutr. 2000, 130, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Chen, N.; Chiu, P.Y.; Leung, H.Y.; Ko, K.M. Neuroprotection against oxidative injury by a nucleic acid-based health product (Squina DNA) through enhancing mitochondrial antioxidant status and functional capacity. J. Med. Food 2012, 15, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Murakami, Y.; Nakano, T.; Sugawara, M.; Kawakami, H.; Idota, T.; Nakajima, I. Effects of dietary nucleotides on lipid metabolism and learning ability of rats. Biosci. Biotechnol. Biochem. 1995, 59, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Korb, V.; Tep, K.; Escriou, V.; Richard, C.; Scherman, D.; Cynober, L.; Chaumeil, J.; Dumortier, G. Current data on ATP-containing liposomes and potential prospects to enhance cellular energy status for hepatic applications. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 305–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Gong, X.; Le, G.W.; Shi, Y.H. Dietary nucleotides protect thymocyte DNA from damage induced by cyclophosphamide in mice. J. Anim. Physiol. Anim. Nutr. 2008, 92, 211–218. [Google Scholar] [CrossRef]
- Puchałowicz, K.; Tarnowski, M.; Tkacz, M.; Chlubek, D.; Kłos, P.; Dziedziejko, V. Extracellular Adenine Nucleotides and Adenosine Modulate the Growth and Survival of THP-1 Leukemia Cells. Int. J. Mol. Sci. 2020, 21, 4425. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Li, Y.; Tong, L.; Gu, X.C.; Meng, J.; Zhu, Y.; Wu, L.; Feng, M.; Tian, N.; et al. Transketolase Deficiency Protects the Liver from DNA Damage by Increasing Levels of Ribose 5-Phosphate and Nucleotides. Cancer Res. 2019, 79, 3689–3701. [Google Scholar] [CrossRef]
- Jang, K.B.; Kim, S.W. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J. Anim. Sci. 2019, 97, 4875–4882. [Google Scholar] [CrossRef]
- Holen, E.; Bjørge, O.A.; Jonsson, R. Dietary nucleotides and human immune cells. II. Modulation of PBMC growth and cytokine secretion. Nutrition 2005, 21, 1003–1009. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Li, Y.; Wang, J. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr. Res. 2017, 61, 1334485. [Google Scholar] [CrossRef]
- Xu, M.; Liang, R.; Guo, Q.; Wang, S.; Zhao, M.; Zhang, Z.; Wang, J.; Li, Y. Dietary nucleotides extend the life span in Sprague-Dawley rats. J. Nutr. Health Aging 2013, 17, 223–229. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Lv, H.; Zhang, X.; Shen, L. Effects of Nucleotides Supplementation of Infant Formulas on Plasma and Erythrocyte Fatty Acid Composition: A Meta-Analysis. PLoS ONE 2015, 10, e0127758. [Google Scholar] [CrossRef]
- Gil, A.; Lozano, E.; De-Lucchi, C.; Maldonado, J.; Molina, J.A.; Pita, M. Changes in the fatty acid profiles of plasma lipid fractions induced by dietary nucleotides in infants born at term. Eur. J. Clin. Nutr. 1988, 42, 473–481. [Google Scholar]
- Leite, L.H.; Moreira-Vaz, E.; Rosa, G.; Pereira, A.C.; Monteiro, C.R.; Medeiros, F.J.; Chagas, V.L. The influence of dietary nucleotides and long-chain polyunsaturated fatty acids on the incorporation of [3H] arachidonic acid on experimental liver cirrhosis. Arch. Latinoam. De Nutr. 2000, 50, 257–264. [Google Scholar]
- Al-Sabahi, B.N.; Fatope, M.O.; Essa, M.M.; Subash, S.; Al-Busafi, S.N.; Al-Kusaibi, F.S.; Manivasagam, T. Pomegranate seed oil: Effect on 3-nitropropionic acid-induced neurotoxicity in PC12 cells and elucidation of unsaturated fatty acids composition. Nutr. Neurosci. 2017, 20, 40–48. [Google Scholar] [CrossRef]
- Wang, L.Y.; Huang, C.S.; Chen, Y.H.; Chen, C.C.; Chen, C.C.; Chuang, C.H. Anti-Inflammatory Effect of Erinacine C on NO Production Through Down-Regulation of NF-κB and Activation of Nrf2-Mediated HO-1 in BV2 Microglial Cells Treated with LPS. Molecules 2019, 24, 3317. [Google Scholar] [CrossRef]
- Yau, K.I.; Huang, C.B.; Chen, W.; Chen, S.J.; Chou, Y.H.; Huang, F.Y.; Kua, K.E.; Chen, N.; McCue, M.; Alarcon, P.A.; et al. Effect of nucleotides on diarrhea and immune responses in healthy term infants in Taiwan. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 37–43. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, M.; Yang, R.; Zhang, Z.; Li, Y.; Wang, J. Effect of dietary nucleotides on immune function in Balb/C mice. Int. Immunopharmacol. 2013, 17, 50–56. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Datta, D.; Paspalas, C.D.; Arnsten, A.F.T. Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol. Aging 2017, 51, 9–18. [Google Scholar] [CrossRef]
- Pollard, A.K.; Craig, E.L.; Chakrabarti, L. Mitochondrial Complex 1 Activity Measured by Spectrophotometry Is Reduced across All Brain Regions in Ageing and More Specifically in Neurodegeneration. PLoS ONE 2016, 11, e0157405. [Google Scholar] [CrossRef]
- Jafari, A.; Hosseinpourfaizi, M.A.; Houshmand, M.; Ravasi, A.A. Effect of aerobic exercise training on mtDNA deletion in soleus muscle of trained and untrained Wistar rats. Br. J. Sport. Med. 2005, 39, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, A.; López-Pedrosa, J.M.; Torres, M.I.; Gil, A. Dietary nucleotides modulate mitochondrial function of intestinal mucosa in weanling rats with chronic diarrhea. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Herskovits, A.Z.; Guarente, L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013, 23, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Kristian, T. Multi-targeted Effect of Nicotinamide Mononucleotide on Brain Bioenergetic Metabolism. Neurochem. Res. 2019, 44, 2280–2287. [Google Scholar] [CrossRef]
- Chen, X.; Amorim, J.A.; Moustafa, G.A.; Lee, J.J.; Yu, Z.; Ishihara, K.; Iesato, Y.; Barbisan, P.; Ueta, T.; Togka, K.A.; et al. Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment. Aging 2020, 12, 24504–24521. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, X.; Xu, M.; Li, Y. Dietary Nucleotides Retard Oxidative Stress-Induced Senescence of Human Umbilical Vein Endothelial Cells. Nutrients 2021, 13, 3279. [Google Scholar] [CrossRef]
- Pluvinage, J.V.; Wyss-Coray, T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 2020, 21, 93–102. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]

| Primer Sequences | Probes | Sizes of PCR Products |
|---|---|---|
| AMPKα Forward primer | 5′-GGGTGAAGATCGGCCACTAC-3′ | 164bp |
| AMPKα Reverse primer | 5′-CTCTCTGCGGATTTTCCCGA-3′ | |
| PGC-1α Forward primer | 5′-GACTGGCAGGGGCACATCT-3′ | 156bp |
| PGC-1α Reverse Primer | 5′-TGGGATGACCGAAGTGCTT-3′ | |
| SIRT1 Forward primer | 5′-TATGCTCGCCTTGCTGTAGA-3′ | 132bp |
| SIRT1 Reverse Primer | 5′-TGGCTGGAATTGTCCAGGAT-3′ | |
| ULK2 Forward primer | 5′-TTAGTCAGTGCTGCTGTGGA-3′ | 99bp |
| ULK2 Reverse Primer | 5′-AGAGTGACTGTGGTGACTGG-3′ | |
| GAPDH Forward primer | 5′-CAACTCCCTCAAGATTGTCAGCAA-3′ | 128bp |
| GAPDH Reverse primer | 5′-GGCATGGACTGTGGTCATGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Liu, R.; Xu, M.-H.; Li, Y. Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells. Molecules 2023, 28, 1226. https://doi.org/10.3390/molecules28031226
Zhu N, Liu R, Xu M-H, Li Y. Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells. Molecules. 2023; 28(3):1226. https://doi.org/10.3390/molecules28031226
Chicago/Turabian StyleZhu, Na, Riu Liu, Mei-Hong Xu, and Yong Li. 2023. "Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells" Molecules 28, no. 3: 1226. https://doi.org/10.3390/molecules28031226
APA StyleZhu, N., Liu, R., Xu, M.-H., & Li, Y. (2023). Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells. Molecules, 28(3), 1226. https://doi.org/10.3390/molecules28031226







