A Review of Plant Selenium-Enriched Proteins/Peptides: Extraction, Detection, Bioavailability, and Effects of Processing
Abstract
1. Introduction
2. Preparation and Detection of Se-Enriched Proteins/Peptides
2.1. Extraction and Preparation of Plant Se-Enriched Proteins/Peptides
2.1.1. Liquid-Phase Extraction
2.1.2. Enzymatic Extraction
2.1.3. Auxiliary Extraction
2.2. Separation and Detection of Se-Enriched Proteins/Peptides
2.2.1. Separation Methods
Liquid Chromatography
Gas Chromatography
Capillary Electrophoresis
2.2.2. Testing and Identification Methods
Mass Spectrometry
Hydride Generation Atomic Fluorescence Spectrometry
2.2.3. Other Methods
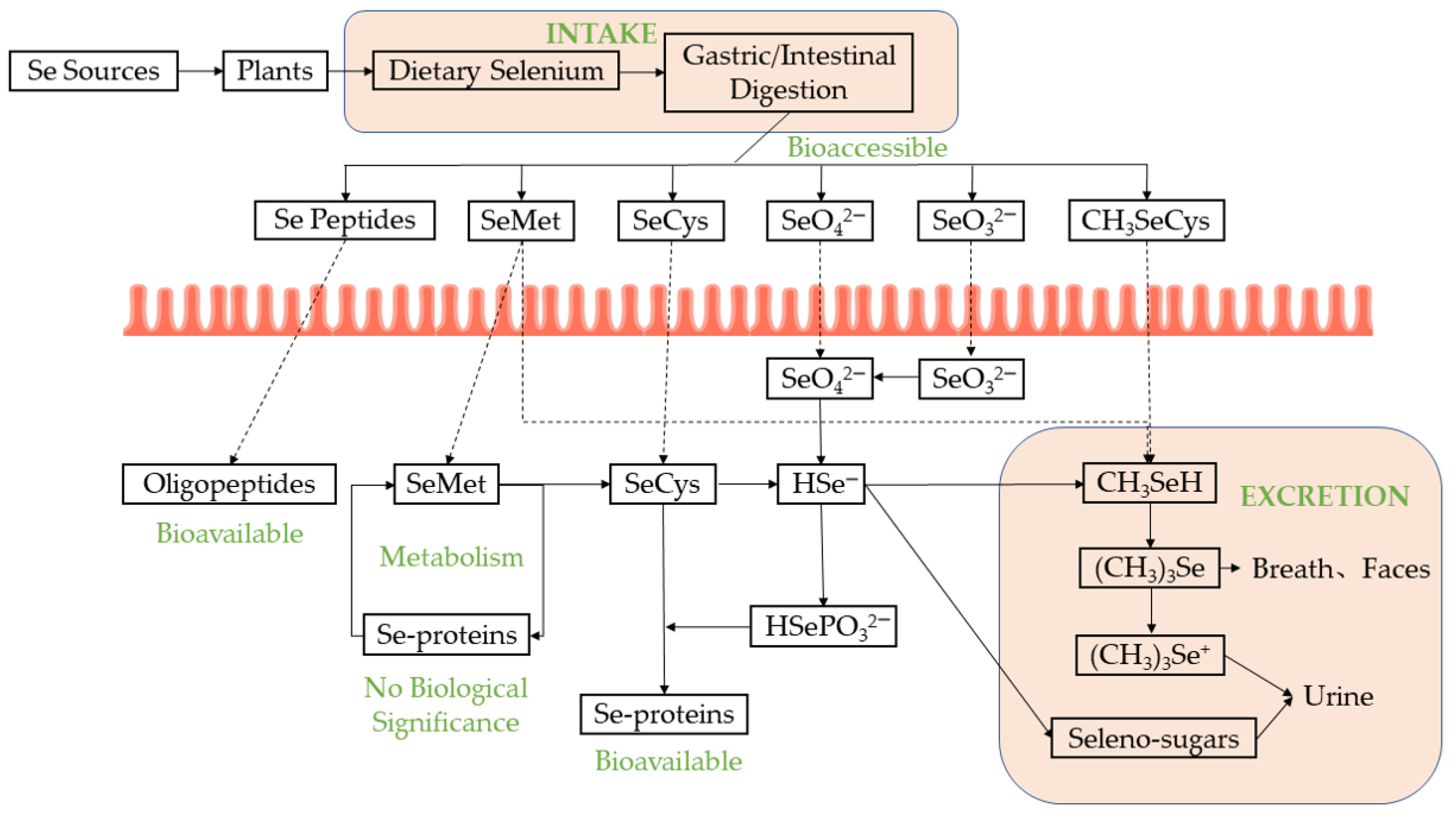
3. Bioaccessibility and Bioavailability of Se-Enriched Proteins/Peptides
3.1. In Vitro Evaluation
3.2. In Vivo Evaluation
4. Effect of Processing on Se-Enriched Proteins/Peptides
4.1. Effect of Processing on the Structure of Se-Enriched Proteins/Peptides
4.2. Effect of Processing on the Bioactivity of Se-Enriched Proteins/Peptides
4.3. Effect of Processing on the Digestion of Se-Enriched Proteins/Peptides
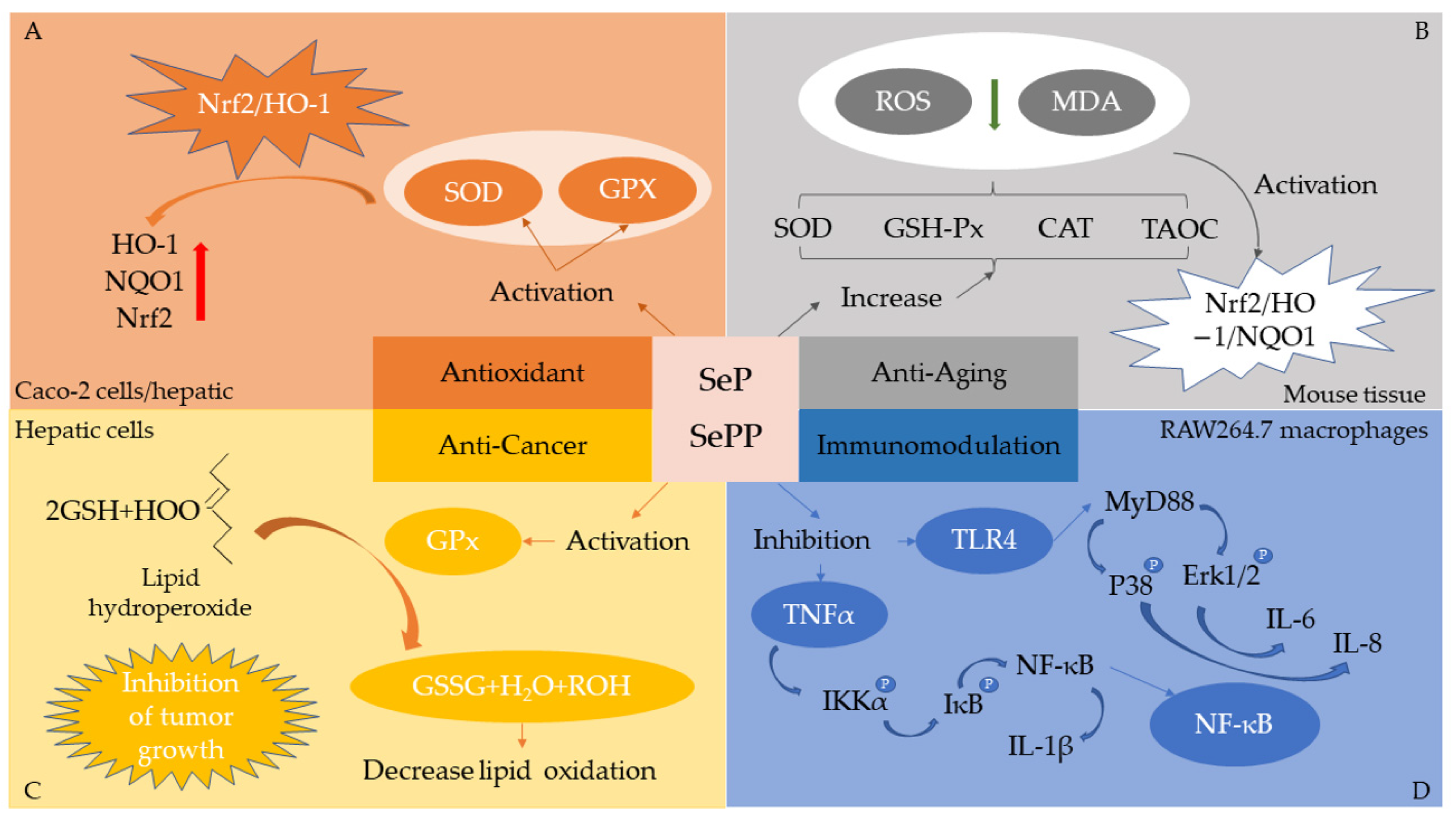
5. Bioactivity of Se-Enriched Proteins/Peptides
5.1. Antioxidant
5.2. Anticancer
5.3. Neuroprotective
5.4. Immunomodulation
5.5. Other Bioactivities
6. Exploitation and Utilization of Se-Enriched Proteins/Peptides
7. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-Containing Proteins/Peptides from Plants: A Review on the Structures and Functions. J. Agric. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Lavu, R.V.; Van De Wiele, T.; Pratti, V.L.; Tack, F.; Du Laing, G. Selenium bioaccessibility in stomach, small intestine and colon: Comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem. 2016, 197, 382–387. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Q.B. A Preliminary Study on the Antioxidant Activity of Selenoprotein in Cordyceps militaris Rich in Selenium. Adv. Mater. Res. 2011, 396–398, 157–161. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, L.; Li, S.P.; Zhang, Z.; Wu, Y.L. The Analysis of Selenoprotein and Se-Polysaccharide in Selenium-Enriched Lentinan edodes. Adv. Mater. Res. 2012, 524–527, 2325–2329. [Google Scholar] [CrossRef]
- Zeng, R.; Farooq, M.U.; Zhang, G.; Tang, Z.; Zheng, T.; Su, Y.; Hussain, S.; Liang, Y.; Ye, X.; Jia, X.; et al. Dissecting the Potential of Selenoproteins Extracted from Selenium-Enriched Rice on Physiological, Biochemical and Anti-Ageing Effects In Vivo. Biol. Trace Elem. Res. 2020, 196, 119–130. [Google Scholar] [CrossRef]
- Chunhieng, T.; Pétritis, K.; Elfakir, C.; Brochier, J.; Goli, T.; Montet, D. Study of Selenium Distribution in the Protein Fractions of the Brazil Nut, Bertholletia excelsa. J. Agric. Food Chem. 2004, 52, 4318–4322. [Google Scholar] [CrossRef]
- Mounicou, S.; Dernovics, M.; Bierla, K.; Szpunar, J. A sequential extraction procedure for an insight into selenium speciation in garlic. Talanta 2009, 77, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Gergely, V.; Kubachka, K.M.; Mounicou, S.; Fodor, P.; Caruso, J.A. Selenium speciation in Agaricus bisporus and Lentinula edodes mushroom proteins using multi-dimensional chromatography coupled to inductively coupled plasma mass spectrometry. J. Chromatogr. A 2006, 1101, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, Y.; Zhang, Y.; Guo, T.; Zhang, Z.; Zhang, W.J.; Zhang, X.G.; Ashraf, M.A. The extraction of different proteins in selenium enriched peanuts and their antioxidant properties. Saudi J. Biol. Sci. 2016, 23, 353–357. [Google Scholar] [CrossRef]
- Zhu, J.; Du, M.; Wu, M.; Yue, P.; Yang, X.; Wei, X.; Wang, Y. Preparation, physicochemical characterization and identification of two novel mixed ACE-inhibiting peptides from two distinct tea alkali-soluble protein. Eur. Food Res. Technol. 2020, 246, 1483–1494. [Google Scholar] [CrossRef]
- Kitaguchi, T.; Ogra, Y.; Iwashita, Y.; Suzuki, K.T. Speciation of selenium in selenium-enriched seeds, buckwheat (Fagopyrum esculentum Moench) and quinoa (Chenopodium quinoa Willdenow). Eur. Food Res. Technol. 2008, 227, 1455–1460. [Google Scholar] [CrossRef]
- Du, M.; Wang, C.; Hu, X.S.; Zhao, G.H. Biological properties of different protein extracts from selenium-enriched Ganoderma lucidum. Int. J. Food Sci. Nutr. 2008, 59, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Tie, M.; Li, B.; Sun, T.; Guan, W.; Liang, Y.; Li, H. HPLC-ICP-MS speciation of selenium in Se-cultivated Flammulina velutipes. Arab. J. Chem. 2020, 13, 416–422. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, X.; Zhao, E.; Shi, Y.; Shen, X.; Wu, J.; Pei, F.; Hu, Q.; Qiu, W. Isolation and identification of immunomodulatory selenium-containing peptides from selenium-enriched rice protein hydrolysates. Food Chem. 2019, 275, 696–702. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, W.; Lin, Y.; Du, C.; Huang, D.; Chen, S.; Yu, T.; Cong, X. Antioxidant and anti-fatigue activities of selenium-enriched peptides isolated from Cardamine violifolia protein hydrolysate. J. Funct. Foods 2021, 79, 104412. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, S.; Li, H.; Cao, M.; Li, W.; Liu, X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021, 9, 6322–6334. [Google Scholar] [CrossRef]
- Montes-Bayon, M.; Molet, M.J.; Gonzalez, E.B.; Sanz-Medel, A. Evaluation of different sample extraction strategies for selenium determination in selenium-enriched plants (Allium sativum and Brassica juncea) and Se speciation by HPLC-ICP-MS. Talanta 2006, 68, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhu, Z.; Li, S.; Cai, J.; Cong, X.; Yu, T.; Yang, W.; He, J.; Cheng, S. Green recovery of Se-rich protein and antioxidant peptides from Cardamine violifolia: Composition and bioactivity. Food Biosci. 2020, 38, 100743. [Google Scholar] [CrossRef]
- Peachey, E.; McCarthy, N.; Goenaga-Infante, H. Acceleration of enzymatic hydrolysis of protein-bound selenium by focused microwave energy. J. Anal. At. Spectrom. 2008, 23, 487–492. [Google Scholar] [CrossRef]
- Guzmán Mar, J.L.; Hinojosa Reyes, L.; Mizanur Rahman, G.M.; Kingston, H.M.S. Simultaneous Extraction of Arsenic and Selenium Species From Rice Products by Microwave-Assisted Enzymatic Extraction and Analysis by Ion Chromatography-Inductively Coupled Plasma-Mass Spectrometry. J. Agric. Food Chem. 2009, 57, 3005–3013. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, W.T.; Tian, X.; Mi, R.F.; Hu, X.S.; Wu, J.H. Preparation of Isolate Protein from Selemiun-enriched Peanut. J. Chin. Inst. Food Sci. Technol. 2017, 17, 9. [Google Scholar] [CrossRef]
- Wang, J.; Dong, W.B.; Yang, F.L.; Gao, R.D.; Gou, J.X. Process investigation on selemiun-protein from selenium-enriched tea by repeated freezing and thawing alkali extraction. Food Mach. 2015, 31, 211–215. [Google Scholar] [CrossRef]
- Guerin, T.; Astruc, A.; Astruc, M. Speciation of arsenic and selenium compounds by HPLC hyphenated to specific detectors a review of the main separation techniques. Talanta 1999, 50, 1–24. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. Analytical Problems in Separation of Selenomethionine and Its Oxidative Product in HILIC HPLC. Molecules 2021, 26, 5073. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Y.; Sun, R.; Wang, J. An analysis of the content changes in free and combinative forms of organic selenium in radish sprouts cultivated with solutions of selenoamino acids. Food Res. Int. 2022, 158, 111558. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Acharya, R.; Reddy, R.A.; Prakash, N.T. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010, 58, 2295–2301. [Google Scholar] [CrossRef]
- Gao, H.H.; Chen, M.X.; Hu, X.Q.; Chai, S.S.; Qin, M.L.; Cao, Z.Y. Separation of selenium species and their sensitive determination in rice samples by ion-pairing reversed-phase liquid chromatography with inductively coupled plasma tandem mass spectrometry. J. Sep. Sci. 2018, 41, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ariza, J.L.; Sanchez-Rodas, D.; Torre, M.A.C.D.L.; Giraldez, I.; Morales, E. Column-switching system for selenium speciation by coupling reversed-phase and ion-exchange high-performance liquid chromatography. J. Chromatogr. 2000, 889, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ayouni, L.; Barbier, F.; Imbert, J.L.; Gauvrit, J.Y.; Lanteri, P.; Grenier-Loustalot, M.F. New separation method for organic and inorganic selenium compounds based on anion exchange chromatography followed by inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2006, 385, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Csapó, J.; Albert, C. Determination of seleno-amino acids by ion-exchange column chromatography and high-performance liquid chromatography (Preliminary study). Acta Univ. Sapientiae-Aliment. 2013, 6, 45–51. [Google Scholar]
- Zhang, Y.; Frankenberger, W.T. Speciation of selenium in plant water extracts by ion exchange chromatography-hydride generation atomic absorption spectrometry. Sci. Total Environ. 2001, 269, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Quijano, M.A.; Gutiérrez, A.M.; Pérez-Conde, M.C.; Cámara, C. Study of selenium species distribution in biological tissues by size exclusion and ion exchange chromatagraphy inductively coupled plasma–mass spectrometry. Anal. Chim. Acta 2004, 524, 315–327. [Google Scholar] [CrossRef]
- Kapolna, E.; Shah, M.; Caruso, J.; Fodor, P. Selenium speciation studies in Se-enriched chives (Allium schoenoprasum) by HPLC-ICP–MS. Food Chem. 2007, 101, 1398–1406. [Google Scholar] [CrossRef]
- Moreno-Martin, G.; Sanz-Landaluze, J.; Leon-Gonzalez, M.E.; Madrid, Y. In-vivo solid phase microextraction for quantitative analysis of volatile organoselenium compounds in plants. Anal. Chim. Acta 2019, 1081, 72–80. [Google Scholar] [CrossRef]
- Lee, J.; Finley, J.W.; Harnly, J.M. Effect of Selenium Fertilizer on Free Amino Acid Composition of Broccoli (Brassica oleracea cv. Majestic) Determined by Gas Chromatography with Flame Ionization and Mass Selective Detection. J. Agric. Food Chem. 2005, 53, 9105–9111. [Google Scholar] [CrossRef]
- Dilli, S.; Sutikno, I. Analysis of selenium at the ultra-trace level by gas chromatography. J. Chromatogr. A 1984, 300, 265–302. [Google Scholar] [CrossRef]
- Nakashima, S.; Tôei, K. Determination of ultramicro amounts of selenium by gas chromatography. Talanta 1968, 15, 1475–1476. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Wang, G.R.; Huang, Q.P.; Liu, C.Y. Speciation of selenium compounds by open tubular capillary electrochromatography-inductively coupled plasma mass spectrometry. Electrophoresis 2006, 27, 4257–4265. [Google Scholar] [CrossRef]
- Deng, B.; Shen, C.; Qin, X.; Liang, S.; Liang, Y. Selenium speciation in ginger using capillary electrophoresis online coupled with electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 2014, 29, 1889–1896. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, J.; Yang, M.; Yang, G.; Wu, Y.; Fu, F. Speciation analysis of selenium in rice samples by using capillary electrophoresis-inductively coupled plasma mass spectrometry. Talanta 2011, 84, 983–988. [Google Scholar] [CrossRef]
- Pyrzyńska, K. Analysis of selenium species by capillary electrophoresis. Talanta 2001, 55, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Kondo, M.; Chihara, Y.; Yuji, M.; Hattori, H.; Yoshida, M. Preparation of selenium-enriched sprouts and identification of their selenium species by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Biosci. Biotechnol. Biochem. 2004, 68, 193–199. [Google Scholar] [CrossRef]
- Thosaikham, W.; Jitmanee, K.; Sittipout, R.; Maneetong, S.; Chantiratikul, A.; Chantiratikul, P. Evaluation of selenium species in selenium-enriched pakchoi (Brassica chinensis Jusl var parachinensis (Bailey) Tsen & Lee) using mixed ion-pair reversed phase HPLC-ICP-MS. Food Chem. 2014, 145, 736–742. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Paśko, P.; Domínguez-Álvarez, E.; Salardón-Jiménez, N.; Sevilla-Hernández, C.; Sanmartín, C.; Bierła, K.; Łobiński, R.; Szpunar, J.; Handzlik, J.; et al. Synthesis of novel organic selenium compounds and speciation of their metabolites in biofortified kale sprouts. Microchem. J. 2022, 172, 106962. [Google Scholar] [CrossRef]
- Tie, M.; Li, B.; Zhuang, X.; Han, J.; Liu, L.; Hu, Y.; Li, H. Selenium speciation in soybean by high performance liquid chromatography coupled to electrospray ionization–tandem mass spectrometry (HPLC–ESI–MS/MS). Microchem. J. 2015, 123, 70–75. [Google Scholar] [CrossRef]
- Zou, X.; Shen, K.; Wang, C.; Wang, J. Molecular recognition and quantitative analysis of free and combinative selenium speciation in selenium-enriched millets using HPLC-ESI-MS/MS. J. Food Compos. Anal. 2022, 106, 104333. [Google Scholar] [CrossRef]
- Kápolna, E.; Hillestrøm, P.R.; Laursen, K.H.; Husted, S.; Larsen, E.H. Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem. 2009, 115, 1357–1363. [Google Scholar] [CrossRef]
- Sánchez-Rodas, D.; Corns, W.T.; Chen, B.; Stockwell, P.B. Atomic Fluorescence Spectrometry: A suitable detection technique in speciation studies for arsenic, selenium, antimony and mercury. J. Anal. At. Spectrom. 2010, 25, 933–946. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yu, X.P.; Guo, Y.F.; Deng, T.L. Selenium Species Analysis by Chromatography Hyphenated Techniques. Appl. Mech. Mater. 2011, 71–78, 2614–2617. [Google Scholar] [CrossRef]
- Bodó, E.T.; Stefánka, Z.; Ipolyi, I.; Sörös, C.; Dernovics, M.; Fodor, P. Preparation, homogeneity and stability studies of a candidate LRM for Se speciation. Anal. Bioanal. Chem. 2003, 377, 32–38. [Google Scholar] [CrossRef]
- Ipolyi, I.; Fodor, P. Development of analytical systems for the simultaneous determination of the speciation of arsenic [As(III), methylarsonic acid, dimethylarsinic acid, As(V)] and selenium [Se(IV), Se(VI)]. Anal. Chim. Acta 2000, 413, 13–23. [Google Scholar] [CrossRef]
- Grijalba, C.A.; Fiorentini, E.F.; Wuilloud, R.G. Ionic liquid-assisted separation and determination of selenium species in food and beverage samples by liquid chromatography coupled to hydride generation atomic fluorescence spectrometry. J. Chromatogr. A 2017, 1491, 117–125. [Google Scholar] [CrossRef]
- Rievaj, M.; Culkova, E.; Sandorova, D.; Lukacova-Chomistekova, Z.; Bellova, R.; Durdiak, J.; Tomcik, P. Electroanalytical Techniques for the Detection of Selenium as a Biologically and Environmentally Significant Analyte-A Short Review. Molecules 2021, 26, 1768. [Google Scholar] [CrossRef]
- Beni, V.; Collins, G.; Arrigan, D.W. Investigation into the voltammetric behaviour and detection of selenium(IV) at metal electrodes in diverse electrolyte media. Anal. Chim. Acta 2011, 699, 127–133. [Google Scholar] [CrossRef]
- Cavalli, S.; Cardellicchio, N. Direct determination of seleno-amino acids in biological tissues by anion-exchange separation and electrochemical detection. J. Chromatogr. A 1995, 706, 429–436. [Google Scholar] [CrossRef]
- Yang, Z.; Bk, A.; Zhao, W.; Shi, L.; Wu, H.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility and bioavailability changes of phenolic compounds in pumpkins (Cucurbita moschata): A review. Food Biosci. 2022, 47, 101753. [Google Scholar] [CrossRef]
- Pollini, L.; Juan-García, A.; Blasi, F.; Mañes, J.; Cossignani, L.; Juan, C. Assessing bioaccessibility and bioavailability in vitro of phenolic compounds from freeze-dried apple pomace by LC-Q-TOF-MS. Food Biosci. 2022, 48, 101799. [Google Scholar] [CrossRef]
- Poland, C.A.; Lombaert, N.; Mackie, C.; Renard, A.; Sinha, P.; Verougstraete, V.; Lourens, N.J.J. Bioaccessibility as a determining factor in the bioavailability and toxicokinetics of cadmium compounds. Toxicology 2021, 463, 152969. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fan, F.; Sun, X.; Li, P.; Xu, T.; Ding, J.; Fang, Y. Effect of ultrasonic treatment on the stability and release of selenium-containing peptide TSeMMM-encapsulated nanoparticles in vitro and in vivo. Ultrason. Sonochem. 2022, 83, 105923. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Nomura, C.S.; Naozuka, J. Evaluation of selenium enrichment of adzuki bean (Vigna angularis) sprouts: Translocation, bioaccessibility and Se-protein speciation. Microchem. J. 2017, 134, 19–26. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Dong, G.; Wu, H. Effect of boiling and frying on the selenium content, speciation, and in vitro bioaccessibility of selenium-biofortified potato (Solanum tuberosum L.). Food Chem. 2021, 348, 129150. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef]
- Delaqua, D.; Carnier, R.; Cadore, S.; Sanches, V.L.; Berton, R.S.; Corbi, F.C.A.; Coscione, A.R. In vitro bioaccessibility and bioavailability of selenium in agronomic biofortified wheat. J. Food Compos. Anal. 2022, 105, 104253. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, X.; Luo, X.; Ding, J.; Fan, F.; Li, P.; Shen, X.; Fang, Y. Encapsulation of selenium-containing peptides in xanthan gum-lysozyme nanoparticles as a powerful gastrointestinal delivery system. Food Res. Int. 2022, 156, 111351. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.; Hou, T. Screening and bioavailability evaluation of anti-oxidative selenium-containing peptides from soybeans based on specific structures. Food Funct. 2022, 13, 5252–5261. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Reeves, P.G.; Johnson, L.K. Assessment of selenium bioavailability from naturally produced high-selenium soy foods in selenium-deficient rats. J. Trace Elem. Med. Biol. 2010, 24, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ari, B.; Oz, E.; Can, S.Z.; Bakirdere, S. Bioaccessibility and bioavailability of selenium species in Se-enriched leeks (Allium Porrum) cultivated by hydroponically. Food Chem. 2022, 372, 131314. [Google Scholar] [CrossRef]
- Lyons, G.H.; Genc, Y.; Stangoulis, J.C.R.; Palmer, L.T.; Graham, R.D. Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biol. Trace Elem. Res. 2005, 103, 155–168. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wang, C.Y.; Dong, H.F. Effect of Treatment Process Parameters on Soybean and Edamame Enriched with Selenium. Adv. Mater. Res. 2013, 864–867, 563–566. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Nie, B.; Lyu, C.; Liu, X. Selenium loss and changes in product quality during cooking of selenium enriched potato tubers. J. Food Compos. Anal. 2021, 96, 103728. [Google Scholar] [CrossRef]
- Kápolna, E.; Gergely, V.; Dernovics, M.; Illés, A.; Fodor, P. Fate of selenium species in sesame seeds during simulated bakery process. J. Food Eng. 2007, 79, 494–501. [Google Scholar] [CrossRef]
- Pérez, M.B.; Maniero, M.Á.; Londonio, A.; Smichowski, P.; Wuilloud, R.G. Effects of common cooking heat treatments on selenium content and speciation in garlic. J. Food Compos. Anal. 2018, 70, 54–62. [Google Scholar] [CrossRef]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef]
- Min, Z.; Chunli, L.; Ping, C. Effects of processing conditions of the green-leafy vegetable juice enriched with selenium on its quality stability. J. Food Eng. 2004, 62, 393–398. [Google Scholar] [CrossRef]
- Roberge, M.T.; Borgerding, A.J.; Finley, J.W. Speciation of Selenium Compounds from High Selenium Broccoli Is Affected by the Extracting Solution. J. Agric. Food Chem. 2003, 51, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzynska, K. Stability of selenium compounds in aqueous extracts of dietary supplements during storage. J. Pharm. Biomed. Anal. 2022, 214, 114714. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Zamorano, M.; Barrientos, H.; Reyes, A. Optimization of a process to obtain selenium-enriched freeze-dried broccoli with high antioxidant properties. LWT 2012, 47, 267–273. [Google Scholar] [CrossRef]
- Liu, K.; Ning, M. Antioxidant activity stability and digestibility of protein from Se-enriched germinated brown rice. LWT 2021, 142, 111032. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, C.; Wang, T.; Sun, Y.; Li, T.; Fan, G. Improvement of Biological Activity of Morchella esculenta Protein Hydrolysate by Microwave-Assisted Selenization. J. Food Sci. 2019, 84, 73–79. [Google Scholar] [CrossRef]
- Fang, L.C.; Liu, M.J.; Wu, Z.L.; Huang, W.P.; Huang, C.Q.; Huang, H. Effects of Microwave Drying at Different Power and Temperature on Selenium Content and Nutritional Quality of Moringa oleifera Leaves. Southwest China J. Agric. Sci. 2021, 34, 6. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Influence of domestic processing on the bioaccessibility of selenium from selected food grains and composite meals. J. Food Sci. Technol. 2016, 53, 1634–1639. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Q.; Wang, M.; Liu, N.; Dinh, Q.T.; Zhai, H.; Xue, M.; Liang, D. Influence of processing methods and exogenous selenium species on the content and in vitro bioaccessibility of selenium in Pleurotus eryngii. Food Chem. 2021, 338, 127661. [Google Scholar] [CrossRef]
- Funes-Collado, V.; Rubio, R.; Lopez-Sanchez, J.F. Does boiling affect the bioaccessibility of selenium from cabbage? Food Chem. 2015, 181, 304–309. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Q.; Cheng, X.; Selomulya, C.; Bai, C.; Zhu, X.; Li, X.; Xiong, H. Antioxidant activities of Se-SPI produced from soybean as accumulation and biotransformation reactor of natural selenium. Food Chem. 2014, 146, 531–537. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, J.; Hogenkamp, A.; Knippels, L.M.J.; Garssen, J.; Bai, J.; Yang, A.; Wu, Y.; Chen, H. Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway. Foods 2021, 10, 2542. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, J.; Zhu, S.; Zhang, X.; Zhu, Z.Z.; Cheng, S.; Cong, X. Protective effects of selenium-enriched peptides from Cardamine violifolia on d-galactose-induced brain aging by alleviating oxidative stress, neuroinflammation, and neuron apoptosis. J. Funct. Foods 2020, 75, 104277. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Antunes-Ricardo, M.; Rocha-Pizaña, M.R.; Martínez-Torres, A.-C.; Gutiérrez-Uribe, J.A.; Serna Saldivar, S.O. Chickpea (Cicer arietinum L.) sprouts containing supranutritional levels of selenium decrease tumor growth of colon cancer cells xenografted in immune-suppressed mice. J. Funct. Foods 2019, 53, 76–84. [Google Scholar] [CrossRef]
- Wu, J.; Ding, J.; Shi, Y.; Fang, Y.; Li, P.; Fan, F.; Zhao, E.; Sun, X.; Shen, X.; Hu, Q. Inhibition of immunotoxicity of Pb(2+)-induced RAW264.7 macrophages by selenium species in selenium-enriched rice. Food Chem. Toxicol. 2021, 148, 111943. [Google Scholar] [CrossRef]
- Abdulah, R.; Faried, A.; Kobayashi, K.; Yamazaki, C.; Suradji, E.W.; Ito, K.; Suzuki, K.; Murakami, M.; Kuwano, H.; Koyama, H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer 2009, 9, 414. [Google Scholar] [CrossRef]
- Wu, M.; Wu, X.; Zhu, J.; Li, F.; Wei, X.; Wang, Y. Selenium-enriched and ordinary green tea extracts prevent high blood pressure and alter gut microbiota composition of hypertensive rats caused by high-salt diet. Food Sci. Hum. Wellness 2022, 11, 738–751. [Google Scholar] [CrossRef]
- Liu, W.; Hou, T.; Shi, W.; Guo, D.; He, H. Hepatoprotective effects of selenium-biofortified soybean peptides on liver fibrosis induced by tetrachloromethane. J. Funct. Foods 2018, 50, 183–191. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Shi, Y.; Fang, Y.; Zhu, Y.; Fan, F.; Pei, F.; Xia, J.; Xie, M.; Hu, Q. Neuroprotective effects of two selenium-containing peptides, TSeMMM and SeMDPGQQ, derived from selenium-enriched rice protein hydrolysates on Pb(2+)-induced oxidative stress in HT22 cells. Food Chem. Toxicol. 2020, 135, 110932. [Google Scholar] [CrossRef]
- Feng, M.; Wang, X.; Xiong, H.; Qiu, T.; Zhang, H.; Guo, F.; Jiang, L.; Sun, Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264.7 macrophages via NF-κB/MAPKs signaling pathways. J. Funct. Foods 2021, 76, 104320. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, H.; Zhang, R.; Shi, M.; Ren, R.; Cheng, S.; Dun, C. Extraction optimization and antioxidant activity evaluation of se-enriched walnut proteins. J. Food Process. Preserv. 2022, 46, e16719. [Google Scholar] [CrossRef]
- Du, M.; Zhao, L.; Li, C.; Zhao, G.; Hu, X. Purification and characterization of a novel fungi Se-containing protein from Se-enriched Ganoderma Lucidum mushroom and its Se-dependent radical scavenging activity. Eur. Food Res. Technol. 2006, 224, 659–665. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lin, T.-H. Comparison of selenomethionine and hydroxyselenomethionine on tissue selenium retention, and antioxidative capacity of giant grouper, Epinephelus lanceolatus, fed diet with soybean meal. Aquac. Nutr. 2021, 27, 2567–2574. [Google Scholar] [CrossRef]
- Rahmanto, A.S.; Davies, M.J. Selenium-containing amino acids as direct and indirect antioxidants. IUBMB Life 2012, 64, 863–871. [Google Scholar] [CrossRef]
- Xia, Y.J.; Ma, Y.Y.; He, X.J.; Wang, H.J.; Ye, Z.Y.; Tao, H.Q. Suppression of selenium-binding protein 1 in gastric cancer is associated with poor survival. Hum. Pathol. 2011, 42, 1620–1628. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, R.; Bei, L.; Yang, J.; Zhao, X.; Hu, Z.; Chen, L.; Meng, H.; Zhang, Q.; Niu, G.; et al. Selenium binding protein 1 inhibits tumor angiogenesis in colorectal cancers by blocking the Delta-like ligand 4/Notch1 signaling pathway. Transl. Oncol. 2022, 18, 101365. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Younes, M.; Liu, H.; Chen, C.; Yao, Q. Reduced selenium-binding protein 1 in breast cancer correlates with poor survival and resistance to the anti-proliferative effects of selenium. PLoS ONE 2013, 8, e63702. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Gundimeda, U.; Zhou, S.; Bui, H.; Holmgren, A. Redox regulation of protein kinase C by selenometabolites and selenoprotein thioredoxin reductase limits cancer prevention by selenium. Free Radic. Biol. Med. 2018, 127, 55–61. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Amemori, T.; Jendelova, P.; Ruzicka, J.; Urdzikova, L.M.; Sykova, E. Alzheimer’s Disease: Mechanism and Approach to Cell Therapy. Int. J. Mol. Sci. 2015, 16, 26417–26451. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu. Rev. Cell Biol. 1994, 10, 373–403. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Nunomura, A.; Hirai, K.; Zhu, X.; Prez, M.; Avila, J.; Castellani, R.J.; Atwood, C.S.; Aliev, G.; Sayre, L.M.; et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic. Biol. 2002, 33, 1475–1479. [Google Scholar] [CrossRef]
- Yarilina, A.; Xu, K.; Chen, J.; Ivashkiv, L.B. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 1573–1578. [Google Scholar] [CrossRef]
- Reeves, M.A.; Bellinger, F.P.; Berry, M.J. The Neuroprotective Functions of Selenoprotein M and its Role in Cytosolic Calcium Regulation. Antioxid. Redox Signal. 2010, 12, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, N. Selenoprotins and Neurodegenerative Diseases. Curr. Biotechnol. 2017, 5, 511–517. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Franke, S.I.; Bordin, D.L.; Pra, D.; Henriques, J.A. Biological functions of selenium and its potential influence on Parkinson’s disease. Acad. Bras. Cienc. 2016, 88, 1655–1674. [Google Scholar] [CrossRef]
- Hemmati-Dinarvand, M.; Saedi, S.; Valilo, M.; Kalantary-Charvadeh, A.; Alizadeh Sani, M.; Kargar, R.; Safari, H.; Samadi, N. Oxidative stress and Parkinson’s disease: Conflict of oxidant-antioxidant systems. Neurosci. Lett. 2019, 709, 134296. [Google Scholar] [CrossRef]
- Chang, C.H.; Wei, C.C.; Ho, C.T.; Liao, V.H. N-gamma-(L-glutamyl)-L-selenomethionine shows neuroprotective effects against Parkinson’s disease associated with SKN-1/Nrf2 and TRXR-1 in Caenorhabditis elegans. Phytomedicine 2021, 92, 153733. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. 2020, 14, 367–382. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox. Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, X.; Luo, P.; Pei, F.; Kimatu, B.M.; Liu, K.; Du, M.; Qiu, W.; Hu, Q. The Correlation Between In Vitro Antioxidant Activity and Immunomodulatory Activity of Enzymatic Hydrolysates from Selenium-Enriched Rice Protein. J. Food Sci. 2017, 82, 517–522. [Google Scholar] [CrossRef]
- Zhou, X.; Ji, W.J.; Zhu, Y.; He, B.; Li, H.; Huang, T.G.; Li, Y.M. Enhancement of endogenous defenses against ROS by supra-nutritional level of selenium is more safe and effective than antioxidant supplementation in reducing hypertensive target organ damage. Med. Hypotheses 2007, 68, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, N.; Satoh, K.; Satoh, T.; Yaoita, N.; Siddique, M.A.H.; Omura, J.; Kurosawa, R.; Nogi, M.; Sunamura, S.; Miyata, S.; et al. Diagnostic and Prognostic Significance of Serum Levels of SeP (Selenoprotein P) in Patients With Pulmonary Hypertension. Arter. Thromb. Vasc. Biol. 2019, 39, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hou, T.; Zhang, X.; He, H. TGF-β1/Smad7 signaling pathway and cell apoptosis: Two key aspects of Selenium-biofortified soybean peptide attenuating liver fibrosis. J. Funct. Foods 2019, 63, 103583. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohe, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition, S. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Chilimba, A.D.; Young, S.D.; Black, C.R.; Rogerson, K.B.; Ander, E.L.; Watts, M.J.; Lammel, J.; Broadley, M.R. Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi. Sci. Rep. 2011, 1, 72. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Huang, Y.; Lin, Z.-q.; Bañuelos, G.S.; Lam, M.H.-W.; Yin, X. Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem. 2011, 126, 1088–1093. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.-J.; Xu, Q.-L.; Tang, H.-Y.; Jiang, W.-Y.; Su, D.-X.; He, S.; Zeng, Q.-Z.; Yuan, Y. Development and stability of novel selenium colloidal particles complex with peanut meal peptides. LWT 2020, 126, 109280. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, D.; Li, S.; Yin, J.; Xu, Z.; Zhang, X. Effect of dietary selenium on postprandial protein deposition in the muscle of juvenile rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2021, 125, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.I.; Allison, R.; Cottrill, B.; Blake, J.S. Enhancing the selenium content of bovine milk through alteration of the form and concentration of selenium in the diet of the dairy cow. J. Sci. Food Agric. 2004, 84, 811–817. [Google Scholar] [CrossRef]
- Chen, J.; Tian, M.; Guan, W.; Wen, T.; Yang, F.; Chen, F.; Zhang, S.; Song, J.; Ren, C.; Zhang, Y.; et al. Increasing selenium supplementation to a moderately-reduced energy and protein diet improves antioxidant status and meat quality without affecting growth performance in finishing pigs. J. Trace Elem. Med. Biol. 2019, 56, 38–45. [Google Scholar] [CrossRef]
| Extraction Methods | Specific Processes | Application Examples | Advantages/ Disadvantages |
|---|---|---|---|
| Water extraction | The sample is crushed and added to distilled water at a certain ratio, extracted at a certain extraction temperature with stirring for a certain time, and centrifuged; the clear liquid is collected. Ammonium sulfate is added to saturate the precipitation degree and separate the precipitation fraction before dialysis, before drying to obtain water-soluble protein samples. | Cordyceps militaris [7] Lentinus edodes [8] Rice [9] Brazil nut [10] Garlic [11] | Advantages: better protection of biological activity of SePs; no pollution; simple operation Disadvantages: low extraction rate; time-consuming |
| Alkali extraction | The sample is crushed and extracted with a certain concentration of alkali at a certain temperature with stirring, the supernatant is centrifuged, and the pH is adjusted to the isoelectric point, before centrifuging and drying to obtain the protein samples. | Cordyceps militaris [7] Lentinus edodes [8] Mushroom [12] Rice [9] Peanut [13] Tea [14] Buckwheat [15] Quinoa [15] | Advantages: high extraction rate; suitable for the extraction of most plant SePs Disadvantages: high alkali concentration can cause protein to produce lysine, which changes its physiological function; time-consuming |
| Salt extraction | The sample is crushed and extracted with a certain concentration of NaCl solution at a certain temperature with stirring, the supernatant is centrifuged, and a certain amount of sulfuric acid is added to saturate the precipitation, before centrifuging and drying after dialysis to obtain the protein samples. | Cordyceps militaris [7] Lentinus edodes [8] Rice [9] Ganoderma lucidum [16] | Advantages: suitable for salt-soluble protein extraction; often used for further processing of residue after water-soluble protein extraction Disadvantages: low extraction rate; more suitable for animal-derived SeP extraction |
| Alcohol extraction | The sample is crushed and added to 75% ethanol at a certain ratio, extracted at room temperature with stirring, and centrifuged at 4 °C. The supernatant is added to a certain amount of distilled water and left to rest overnight, centrifuged at 4 °C to obtain the precipitate, and dried to obtain the protein samples. | Cordyceps militaris [7] Rice [9] Ganoderma lucidum [16] | Advantages: suitable for alcohol-soluble protein extraction; good effect with alkali method Disadvantages: low extraction rate using the method alone; easy loss of protein; organic solvents lead to pollution |
| Buffer solution Extraction | The sample is crushed and extracted with a certain concentration of liquid buffer (PBS, Tris-HCl, etc.) at a certain temperature with stirring, the supernatant is centrifuged, the pH is adjusted to precipitate the protein, and the precipitate is dried to obtain the protein samples. | Mushroom [12] Buckwheat [15] Quinoa [15] Flammulina velutipe [17] | Advantages: less damage to protein; suitable for soluble protein extraction Disadvantages: need to use organic solvent or other reagents for protein precipitation treatment |
| Name | Structure |
|---|---|
| Selenomethionine, SeMet | 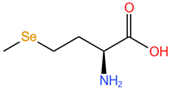 |
| Selenocystine, SeCys2 | 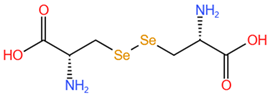 |
| Selenocysteine, SeCys | 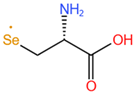 |
| Se-methylselenocysteine, SeMeSeCys | 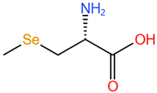 |
| Source | Evaluation Methods | Evaluation Object | Results | Reference |
|---|---|---|---|---|
| Mushroom | Gastrointestinal digestion | Bioaccessibility | 75% of the Se in the mushroom was potentially bioaccessible, and SeMet was the main form | [63] |
| Rice | Gastrointestinal digestion | Bioaccessibility | 80.69% of the rice SePPs were potentially bioaccessible | [64] |
| Adzuki bean sprouts | Gastrointestinal digestion | Bioaccessibility | 100% of the Se in the Adzuki bean sprouts was potentially bioaccessible | [65] |
| Potato | Gastrointestinal digestion | Bioaccessibility | The bioaccessibility of total Se, Se(IV), SeMet, SeCys2, and SeMeCys was 18.3%, 32.3%, 7.0%, 23.6%, and 27.6%, respectively | [66] |
| Cereals; pulses; green leafy vegetables | Gastrointestinal digestion | Bioaccessibility | The bioaccessibility of Se in the cereals, pulses, and green leafy vegetables was 10–24%, 12–29%, 10–30%, respectively | [67] |
| Wheat | Gastrointestinal digestion; Caco-2 cells | Bioaccessibility Bioavailability | 62.6–82.3% of the Se in the wheat was potentially bioaccessible, and only 19.6% was bioavailable | [68] |
| Rice | Gastrointestinal digestion; Caco-2 cells | 91.5% and 38.95% of the two rice SePPs (TSeMMM and SeMDPGQQ) were potentially bioaccessible, and the apparent permeability coefficient of the two was 0.58 × 10−6 cm/s and 1.060.58 × 10−6 cm/s | [69] | |
| Soybean | Caco-2 cells | Bioavailability | The transport rate of Se was 9.467% ± 0.97% | [70] |
| Soybean | Caco-2 cells | Bioavailability | The transport rate of soybean SePPs was 8.63% ± 1.41% | [71] |
| Soy protein isolate; tofu | Se-deficient rats | Bioavailability | The bioavailability of soy protein isolate and tofu was 101% and 94%, relative to SeMet | [72] |
| Source | Bioactivities | Identification Approach | Se Status | Sequence | Reference |
|---|---|---|---|---|---|
| Broccoli sprout | Anticancer | HPLC/ICP-MS | SeMeSeCys | Not studied | [96] |
| Cardamine violifolia | Anti-fatigue Antioxidant | LC–MS/MS | SeCys SeMet MeSeCys | Tyr–Leu–Pro–Gly–SeMet–Val; Phe–SeCys–Leu–Val–Glu–Ser–Thr; Val–His–Thr–SeCys–Pro–Ile–SeCys–Thr–Ser; Leu–Leu–Thr–MeSeCys–Pro–Ala; Ser–Val–Ile–Ala–Thr–Ile–SeMet–Val–Pro; Ser–SeCys–SeCys–Ser–Pro–Thr–Pro; Lys–Lys–SeCys–Ser–Leu; Cys–Pro–Gln–Ser–MeSeCys–Lys; Asn–SeCys–Val–Ala–Ser–Pro–Leu; Asn–Leu–Ile–Val–Asn–SeMet–Lys–Asn | [19] |
| Green tea | Antihypertensive | Not studied | Not studied | Not studied | [97] |
| Soybean | Hepatoprotective | HPLC–ESI-MS/MS | SeCys SeMet | SeMet–Val–Val–SeCys; Ser–SeCys–Arg–Asp–Cys–Val; Phe–Ile/Leu–Phe–SeCys–Phe; SeCys–Ile/Leu–Ser–SeCys | [98] |
| Rice | Antiaging | Not studied | Not Studied | Not Studied | [9] |
| Rice | Immunomodulatory | RP-UPLC triple-TOF MS/MS | SeMet MeSeCys | SeMet–Pro–Ser; Met–MeSeCys–Glu; SeMet–MeSeCys–Glu | [95] |
| Rice | Immunomodulatory | RP-UPLC triple-TOF MS/MS | SeMet | SeMet–Aps–Pro–Gly–Gln–Gln; Thr–SeMet–Met–Met | [18] |
| Rice | Neuroprotective | RP-UPLC triple-TOF MS/MS | SeMet | SeMet–Aps–Pro–Gly–Gln–Gln; Thr–SeMet–Met–Met | [99] |
| Cardamine violifolia | Neuroprotective | Not studied | Not studied | Not studied | [93] |
| Brown rice | Anti-inflammatory | Triple-TOF LC-MS/MS | Not studied | Ala–Leu–Leu–Leu–Gln–Ala–Val–Gln–Ser–Gln–Tyr–Glu–Glu–Lys | [100] |
| Soybean | Antioxidant | Not studied | Not studied | Not studied | [92] |
| Walnut | Antioxidant | LC–HG-AFS | MeSeCys SeMet SeCys2 | Not studied | [101] |
| Ganoderma lucidum mushroom | Antioxidant | Not studied | Not studied | Asp–Ile–Asn–Gly–Gly–Gly–Ala–Thr–Leu–Pro–Gln–Lys–Leu–Tyr–Leu–Thr–Pro–Asp–Val–Leu | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Huang, Y.; Li, L.; Liu, Y.; Liu, L.; Wang, L.; Tong, L.; Wang, F.; Fan, B. A Review of Plant Selenium-Enriched Proteins/Peptides: Extraction, Detection, Bioavailability, and Effects of Processing. Molecules 2023, 28, 1223. https://doi.org/10.3390/molecules28031223
Xiong Y, Huang Y, Li L, Liu Y, Liu L, Wang L, Tong L, Wang F, Fan B. A Review of Plant Selenium-Enriched Proteins/Peptides: Extraction, Detection, Bioavailability, and Effects of Processing. Molecules. 2023; 28(3):1223. https://doi.org/10.3390/molecules28031223
Chicago/Turabian StyleXiong, Yangyang, Yatao Huang, Lin Li, Yanfang Liu, Liya Liu, Lili Wang, Litao Tong, Fengzhong Wang, and Bei Fan. 2023. "A Review of Plant Selenium-Enriched Proteins/Peptides: Extraction, Detection, Bioavailability, and Effects of Processing" Molecules 28, no. 3: 1223. https://doi.org/10.3390/molecules28031223
APA StyleXiong, Y., Huang, Y., Li, L., Liu, Y., Liu, L., Wang, L., Tong, L., Wang, F., & Fan, B. (2023). A Review of Plant Selenium-Enriched Proteins/Peptides: Extraction, Detection, Bioavailability, and Effects of Processing. Molecules, 28(3), 1223. https://doi.org/10.3390/molecules28031223