The Role of the Acetylcholine System in Common Respiratory Diseases and COVID-19
Abstract
1. Introduction
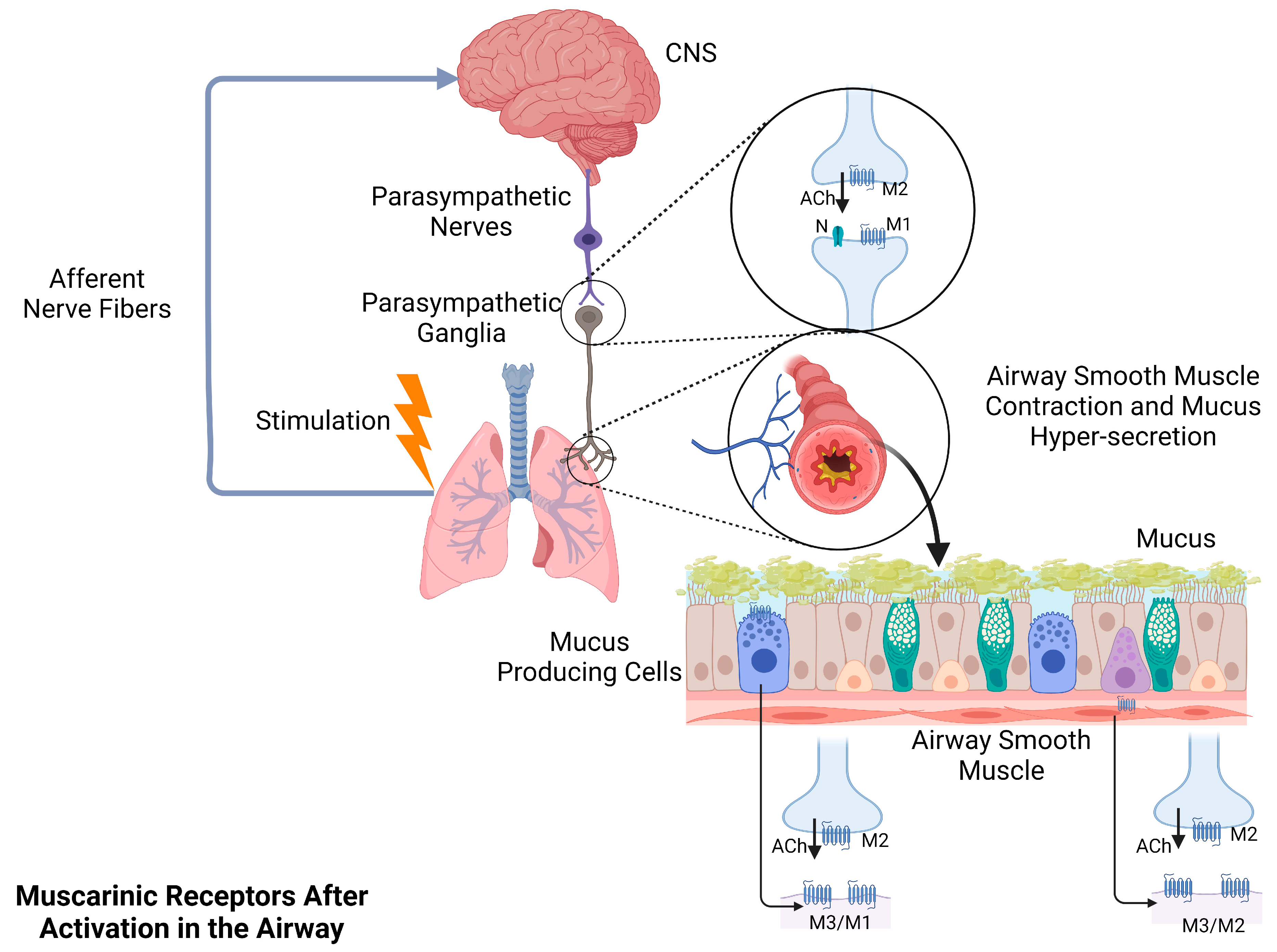
2. The Effects of Muscarinic Acetylcholine Receptors on Structural Cells and Immune Cells in the Human Airway
2.1. Airway Structural Cells
2.2. Airway Immune Cells
3. The Anti-Inflammatory Effects of the Acetylcholine System
3.1. The Composition of the Cholinergic Anti-inflammatory Pathway (CAP)
3.2. The Basic Role of the CAP in Systemic Inflammation and Lung Inflammation
3.3. Molecular Mechanisms and Signal Transduction Pathways of the CAP
4. Acetylcholine System in COPD and Asthma
4.1. Acetylcholine System Participates in Airway Contraction and Mucus Secretion in COPD and Asthma
4.2. Acetylcholine System Participates in Airway Inflammation in COPD and Asthma
5. Acetylcholine System in Lung Cancer
5.1. Muscarinic Acetylcholine Receptors in Lung Cancer
5.2. α7 Nicotinic Acetylcholine Receptors in Lung Cancer
5.3. α5 Nicotinic Acetylcholine Receptors in Lung Cancer
6. Acetylcholine System in Tuberculosis
7. Acetylcholine System in Coronavirus Disease 2019 (COVID-19)
8. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Bader, S.; Klein, J.; Diener, M. Choline acetyltransferase and organic cation transporters are responsible for synthesis and propionate-induced release of acetylcholine in colon epithelium. Eur. J. Pharmacol. 2014, 733, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Szmicseková, K.; Bies Piváčková, L.; Kiliánová, Z.; Slobodová, Ľ.; Křenek, P.; Hrabovská, A. Aortic butyrylcholinesterase is reduced in spontaneously hypertensive rats. Physiol. Res. 2021, 70, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Lewartowski, B.; Mackiewicz, U. The non-neuronal heart’s acetylcholine in health and disease. J. Physiol. Pharmacol. 2015, 66, 773–778. [Google Scholar] [PubMed]
- Ishii, T.; Homma, K.; Mano, A.; Akagi, T.; Shigematsu, Y.; Shimoda, Y.; Inoue, H.; Kakinuma, Y.; Kaneda, M. Novel channel-mediated choline transport in cholinergic neurons of the mouse retina. J. Neurophysiol. 2017, 118, 1952–1961. [Google Scholar] [CrossRef]
- Gaydukov, A.E.; Bogacheva, P.O.; Balezina, O.P. Calcitonin gene-related peptide increases acetylcholine quantal size in neuromuscular junctions of mice. Neurosci. Lett. 2016, 628, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sastry, B.V.; Sadavongvivad, C. Cholinergic systems in non-nervous tissues. Pharmacol. Rev. 1978, 30, 65–132. [Google Scholar]
- Grando, S.A.; Kawashima, K.; Wessler, I. Introduction: The non-neuronal cholinergic system in humans. Life. Sci. 2003, 72, 2009–2012. [Google Scholar] [CrossRef]
- Maeda, S.; Qu, Q.; Robertson, M.J.; Skiniotis, G.; Kobilka, B.K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 2019, 364, 552–557. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Vizi, E.S.; Kobayashi, O.; Töröcsik, A.; Kinjo, M.; Nagashima, H.; Manabe, N.; Goldiner, P.L.; Potter, P.E.; Foldes, F.F. Heterogeneity of presynaptic muscarinic receptors involved in modulation of transmitter release. Neuroscience 1989, 31, 259–267. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Zhang, L.; Shi, H.; Schram, G.; Nattel, S.; Wang, Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol. Pharmacol. 2001, 59, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Lammers, J.W.; Minette, P.; McCusker, M.; Barnes, P.J. The role of pirenzepine-sensitive (M1) muscarinic receptors in vagally mediated bronchoconstriction in humans. Am. Rev. Respir. Dis. 1989, 139, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Walch, L.; Gascard, J.P.; Dulmet, E.; Brink, C.; Norel, X. Evidence for a M(1) muscarinic receptor on the endothelium of human pulmonary veins. Br. J. Pharmacol. 2000, 130, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Hollinger, B. Muscarinic cholinergic receptors in developing rat lung. Pediatr. Res. 1984, 18, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef]
- Hislop, A.A.; Mak, J.C.; Reader, J.A.; Barnes, P.J.; Haworth, S.G. Muscarinic receptor subtypes in the porcine lung during postnatal development. Eur. J. Pharmacol. 1998, 359, 211–221. [Google Scholar] [CrossRef]
- Chelala, J.L.; Kilani, A.; Miller, M.J.; Martin, R.J.; Ernsberger, P. Muscarinic receptor binding sites of the M4 subtype in porcine lung parenchyma. Pharmacol. Toxicol. 1998, 83, 200–207. [Google Scholar] [CrossRef]
- Dixon, W.E. Contributions to the physiology of the lungs: Part I. The bronchial muscles, their innervation, and the action of drugs upon them. J. Physiol. 1903, 29, 97–173. [Google Scholar] [CrossRef]
- Barnes, P.J. Muscarinic receptor subtypes: Implications for lung disease. Thorax 1989, 44, 161–167. [Google Scholar] [CrossRef]
- Gater, P.R.; Alabaster, V.A.; Piper, I. A study of the muscarinic receptor subtype mediating mucus secretion in the cat trachea in vitro. Pulm. Pharmacol. 1989, 2, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Harkness, P.C. Assembly and trafficking of nicotinic acetylcholine receptors (Review). Mol. Membr. Biol. 2008, 25, 279–292. [Google Scholar] [CrossRef]
- Dani, J.A. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gündisch, D.; Eibl, C. Nicotinic acetylcholine receptor ligands, a patent review (2006–2011). Expert Opin. Ther. Pat. 2011, 21, 1867–1896. [Google Scholar] [CrossRef]
- Grando, S.A. Connections of nicotine to cancer. Nat. Rev. Cancer 2014, 14, 419–429. [Google Scholar] [CrossRef]
- Jensen, A.A.; Frølund, B.; Liljefors, T.; Krogsgaard-Larsen, P. Neuronal nicotinic acetylcholine receptors: Structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 2005, 48, 4705–4745. [Google Scholar] [CrossRef]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef]
- Di Lascio, S.; Fornasari, D.; Benfante, R. The Human-Restricted Isoform of the α7 nAChR, CHRFAM7A: A double-edged sword in neurological and inflammatory disorders. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Kummer, W.; Lips, K.S.; Pfeil, U. The epithelial cholinergic system of the airways. Histochem. Cell. Biol. 2008, 130, 219–234. [Google Scholar] [CrossRef]
- Fu, X.W.; Lindstrom, J.; Spindel, E.R. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2009, 41, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gahring, L.C.; Myers, E.J.; Dunn, D.M.; Weiss, R.B.; Rogers, S.W. Lung epithelial response to cigarette smoke and modulation by the nicotinic alpha 7 receptor. PLoS ONE 2017, 12, e0187773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pereira, E.F.; Maus, A.D.; Ostlie, N.S.; Navaneetham, D.; Lei, S.; Albuquerque, E.X.; Conti-Fine, B.M. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol. Pharmacol. 2001, 60, 1201–1209. [Google Scholar] [CrossRef]
- Borkar, N.A.; Roos, B.; Prakash, Y.S.; Sathish, V.; Pabelick, C.M. Nicotinic α7 acetylcholine receptor (α7nAChR) in human airway smooth muscle. Arch. Biochem. Biophys. 2021, 706, 108897. [Google Scholar] [CrossRef]
- Levine, S.M.; Marciniuk, D.D. Global impact of respiratory disease: What can we do, together, to make a difference? Chest 2022, 161, 1153–1154. [Google Scholar] [CrossRef] [PubMed]
- Cortijo, J.; Mata, M.; Milara, J.; Donet, E.; Gavaldà, A.; Miralpeix, M.; Morcillo, E.J. Aclidinium inhibits cholinergic and tobacco smoke-induced MUC5AC in human airways. Eur. Respir. J. 2011, 37, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Profita, M.; Bonanno, A.; Siena, L.; Ferraro, M.; Montalbano, A.M.; Pompeo, F.; Riccobono, L.; Pieper, M.P.; Gjomarkaj, M. Acetylcholine mediates the release of IL-8 in human bronchial epithelial cells by a NFkB/ERK-dependent mechanism. Eur. J. Pharmacol. 2008, 582, 145–153. [Google Scholar] [CrossRef]
- Profita, M.; Bonanno, A.; Montalbano, A.M.; Ferraro, M.; Siena, L.; Bruno, A.; Girbino, S.; Albano, G.D.; Casarosa, P.; Pieper, M.P.; et al. Cigarette smoke extract activates human bronchial epithelial cells affecting non-neuronal cholinergic system signalling in vitro. Life Sci. 2011, 89, 36–43. [Google Scholar] [CrossRef]
- Profita, M.; Albano, G.D.; Montalbano, A.M.; Di Sano, C.; Anzalone, G.; Gagliardo, R.; Riccobono, L.; Bonanno, A.; Siena, L.; Pieper, M.P.; et al. Acetylcholine leads to signal transducer and activator of transcription 1 (STAT-1) mediated oxidative/nitrosative stress in human bronchial epithelial cell line. Biochim. Biophys. Acta 2013, 1832, 1949–1958. [Google Scholar] [CrossRef]
- Gosens, R.; Zaagsma, J.; Grootte Bromhaar, M.; Nelemans, A.; Meurs, H. Acetylcholine: A novel regulator of airway smooth muscle remodelling? Eur. J. Pharmacol. 2004, 500, 193–201. [Google Scholar] [CrossRef]
- Gosens, R.; Rieks, D.; Meurs, H.; Ninaber, D.K.; Rabe, K.F.; Nanninga, J.; Kolahian, S.; Halayko, A.J.; Hiemstra, P.S.; Zuyderduyn, S. Muscarinic M3 receptor stimulation increases cigarette smoke-induced IL-8 secretion by human airway smooth muscle cells. Eur. Respir. J. 2009, 34, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Oenema, T.A.; Smit, M.; Smedinga, L.; Racké, K.; Halayko, A.J.; Meurs, H.; Gosens, R. Muscarinic receptor stimulation augments TGF-β1-induced contractile protein expression by airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L589–L597. [Google Scholar] [CrossRef] [PubMed]
- Matthiesen, S.; Bahulayan, A.; Kempkens, S.; Haag, S.; Fuhrmann, M.; Stichnote, C.; Juergens, U.R.; Racké, K. Muscarinic receptors mediate stimulation of human lung fibroblast proliferation. Am. J. Respir. Cell. Mol. Biol. 2006, 35, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.; Matthiesen, S.; Juergens, U.R.; Racké, K. Muscarinic receptors mediate stimulation of collagen synthesis in human lung fibroblasts. Eur. Respir. J. 2008, 32, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Razani-Boroujerdi, S.; Behl, M.; Hahn, F.F.; Pena-Philippides, J.C.; Hutt, J.; Sopori, M.L. Role of muscarinic receptors in the regulation of immune and inflammatory responses. J. Neuroimmunol. 2008, 194, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Profita, M.; Riccobono, L.; Montalbano, A.M.; Bonanno, A.; Ferraro, M.; Albano, G.D.; Gerbino, S.; Casarosa, P.; Pieper, M.P.; Gjomarkaj, M. In vitro anticholinergic drugs affect CD8+ peripheral blood T-cells apoptosis in COPD. Immunobiology 2012, 217, 345–353. [Google Scholar] [CrossRef]
- Profita, M.; Giorgi, R.D.; Sala, A.; Bonanno, A.; Riccobono, L.; Mirabella, F.; Gjomarkaj, M.; Bonsignore, G.; Bousquet, J.; Vignola, A.M. Muscarinic receptors, leukotriene B4 production and neutrophilic inflammation in COPD patients. Allergy 2005, 60, 1361–1369. [Google Scholar] [CrossRef]
- Koarai, A.; Traves, S.L.; Fenwick, P.S.; Brown, S.M.; Chana, K.K.; Russell, R.E.; Nicholson, A.G.; Barnes, P.J.; Donnelly, L.E. Expression of muscarinic receptors by human macrophages. Eur. Respir. J. 2012, 39, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Koyama, S.; Okubo, Y.; Kubo, K.; Sekiguchi, M. Acetylcholine stimulates alveolar macrophages to release inflammatory cell chemotactic activity. Am. J. Physiol. 1998, 274, L970–L979. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Cervera, A.; de Diego, A.; Sanz, C.; Juan, G.; Gavaldà, A.; Miralpeix, M.; Morcillo, E.; Cortijo, J. Non-neuronal cholinergic system contributes to corticosteroid resistance in chronic obstructive pulmonary disease patients. Respir. Res. 2016, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Radosa, J.; Dyck, W.; Goerdt, S.; Kurzen, H. The cholinergic system in guttate psoriasis with special reference to mast cells. Exp. Dermatol. 2011, 20, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Reinheimer, T.; Möhlig, T.; Zimmermann, S.; Höhle, K.D.; Wessler, I. Muscarinic control of histamine release from airways. Inhibitory M1-receptors in human bronchi but absence in rat trachea. Am. J. Respir. Crit. Care Med. 2000, 162, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. The cholinergic anti-inflammatory pathway: Towards innovative treatment of rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 229–232. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Vida, G.; Peña, G.; Deitch, E.A.; Ulloa, L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 2011, 186, 4340–4346. [Google Scholar] [CrossRef]
- Huston, J.M.; Ochani, M.; Rosas-Ballina, M.; Liao, H.; Ochani, K.; Pavlov, V.A.; Gallowitsch-Puerta, M.; Ashok, M.; Czura, C.J.; Foxwell, B.; et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 2006, 203, 1623–1628. [Google Scholar] [CrossRef]
- Huston, J.M.; Wang, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ashok, M.; Yang, L.; Tracey, K.J.; Yang, H. Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J. Immunol. 2008, 181, 3535–3539. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Ochani, M.; Parrish, W.R.; Ochani, K.; Harris, Y.T.; Huston, J.M.; Chavan, S.; Tracey, K.J. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 2008, 105, 11008–11013. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Vida, G.; Peña, G.; Kanashiro, A.; Thompson-Bonilla Mdel, R.; Palange, D.; Deitch, E.A.; Ulloa, L. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011, 25, 4476–4485. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ichinose, M. The cholinergic anti-inflammatory pathway: An innovative treatment strategy for respiratory diseases and their comorbidities. Curr. Opin. Pharmacol. 2018, 40, 18–25. [Google Scholar] [CrossRef]
- Pinheiro, N.M.; Santana, F.P.; Almeida, R.R.; Guerreiro, M.; Martins, M.A.; Caperuto, L.C.; Câmara, N.O.; Wensing, L.A.; Prado, V.F.; Tibério, I.F.; et al. Acute lung injury is reduced by the α7nAChR agonist PNU-282987 through changes in the macrophage profile. FASEB J. 2017, 31, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Peng, Z.; Zhou, W.; Hu, B.; Rao, X.; Yang, X.; Li, J. GTS-21 reduces inflammation in acute lung injury by regulating M1 polarization and function of alveolar macrophages. Shock 2019, 51, 389–400. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, X.; Su, E.M.; Huang, Y.; Li, L.; Matthay, M.A.; Su, X. Signals of vagal circuits engaging with AKT1 in α7 nAChR(+)CD11b(+) cells lessen E. coli and LPS-induced acute inflammatory injury. Cell. Discov. 2017, 3, 17009. [Google Scholar] [CrossRef]
- Antunes, G.L.; Silveira, J.S.; Kaiber, D.B.; Luft, C.; da Costa, M.S.; Marques, E.P.; Ferreira, F.S.; Breda, R.V.; Wyse, A.T.S.; Stein, R.T.; et al. Cholinergic anti-inflammatory pathway confers airway protection against oxidative damage and attenuates inflammation in an allergic asthma model. J. Cell. Physiol. 2020, 235, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Zi, S.F.; Li, J.H.; Liu, L.; Deng, C.; Ao, X.; Chen, D.D.; Wu, S.Z. Dexmedetomidine-mediated protection against septic liver injury depends on TLR4/MyD88/NF-κB signaling downregulation partly via cholinergic anti-inflammatory mechanisms. Int. Immunopharmacol. 2019, 76, 105898. [Google Scholar] [CrossRef]
- Wang, W.; Xu, H.; Lin, H.; Molnar, M.; Ren, H. The role of the cholinergic anti-inflammatory pathway in septic cardiomyopathy. Int. Immunopharmacol. 2021, 90, 107160. [Google Scholar] [CrossRef]
- de Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef]
- Bencherif, M.; Lippiello, P.M.; Lucas, R.; Marrero, M.B. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell. Mol. Life Sci. 2011, 68, 931–949. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; León, R.; Lopez, M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Parada, E.; Egea, J.; Buendia, I.; Negredo, P.; Cunha, A.C.; Cardoso, S.; Soares, M.P.; López, M.G. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid. Redox. Signal. 2013, 19, 1135–1148. [Google Scholar] [CrossRef]
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.H.; Hudson, L.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 2008, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, R.; Cheng, W.; Hu, Y.; Li, J.; Pan, X.; Peng, J.; Zhang, P. GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-κB signaling pathway through the α7 nicotinic acetylcholine receptor. Int. Immunopharmacol. 2015, 29, 504–512. [Google Scholar] [CrossRef]
- Wang, H.; Liao, H.; Ochani, M.; Justiniani, M.; Lin, X.; Yang, L.; Al-Abed, Y.; Wang, H.; Metz, C.; Miller, E.J.; et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004, 10, 1216–1221. [Google Scholar] [CrossRef]
- Wazea, S.A.; Wadie, W.; Bahgat, A.K.; El-Abhar, H.S. Galantamine anti-colitic effect: Role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-κB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Sci. Rep. 2018, 8, 5110. [Google Scholar] [CrossRef]
- Hylkema, M.N.; Sterk, P.J.; de Boer, W.I.; Postma, D.S. Tobacco use in relation to COPD and asthma. Eur. Respir. J. 2007, 29, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Siroux, V.; Pin, I.; Oryszczyn, M.P.; Le Moual, N.; Kauffmann, F. Relationships of active smoking to asthma and asthma severity in the EGEA study. Epidemiological study on the Genetics and Environment of Asthma. Eur. Respir. J. 2000, 15, 470–477. [Google Scholar] [CrossRef]
- Silverman, R.A.; Boudreaux, E.D.; Woodruff, P.G.; Clark, S.; Camargo, C.A.J. Cigarette smoking among asthmatic adults presenting to 64 emergency departments. Chest 2003, 123, 1472–1479. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: A population-based cohort study. Lancet Respir. Med. 2018, 6, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, M.R.; Israël-Assayag, E.; Cormier, Y. Modulation of airway inflammation and resistance in mice by a nicotinic receptor agonist. Eur. Respir. J. 2005, 26, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Jiang, L.; Li, Q.; Sokulsky, L.; Wanyan, Y.; Wang, L.; Liu, X.; Zhou, L.; Tay, H.L.; Zhang, G.; et al. A Selective α7 Nicotinic Acetylcholine Receptor Agonist, PNU-282987, Attenuates ILC2s Activation and Alternaria-Induced Airway Inflammation. Front. Immunol. 2020, 11, 598165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, S.; Xia, G.; Wu, J.; Shen, Y.; Wang, Y.; Ostrom, R.S.; Du, A.; Shen, C.; Xu, C. Anti-inflammatory effects of α7-nicotinic ACh receptors are exerted through interactions with adenylyl cyclase-6. Br. J. Pharmacol. 2021, 178, 2324–2338. [Google Scholar] [CrossRef]
- Kwok, H.H.; Gao, B.; Chan, K.H.; Ip, M.S.; Minna, J.D.; Lam, D.C. Nicotinic Acetylcholine Receptor Subunit α7 Mediates Cigarette Smoke-Induced PD-L1 Expression in Human Bronchial Epithelial Cells. Cancers 2021, 13, 5345. [Google Scholar] [CrossRef]
- Rennard, S.I. Cigarette smoke in research. Am. J. Respir. Cell. Mol. Biol. 2004, 31, 479–480. [Google Scholar] [CrossRef]
- Borgerding, M.; Klus, H. Analysis of complex mixtures--cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 43–73. [Google Scholar] [CrossRef]
- Roffel, A.F.; Elzinga, C.R.; Van Amsterdam, R.G.; De Zeeuw, R.A.; Zaagsma, J. Muscarinic M2 receptors in bovine tracheal smooth muscle: Discrepancies between binding and function. Eur. J. Pharmacol. 1988, 153, 73–82. [Google Scholar] [CrossRef]
- Pera, T.; Penn, R.B. Crosstalk between beta-2-adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr. Opin. Pharmacol. 2014, 16, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.T.; Vincent, S.G.; Gomeza, J.; Yamada, M.; Wess, J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004, 18, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.F. Mucus hypersecretion in chronic obstructive pulmonary disease. Novartis. Found. Symp. 2001, 234, 65–83. [Google Scholar] [CrossRef]
- Rogers, D.F. Airway mucus hypersecretion in asthma: An undervalued pathology? Curr. Opin. Pharmacol. 2004, 4, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.C.; Barnes, P.J. Autoradiographic visualization of muscarinic receptor subtypes in human and guinea pig lung. Am. Rev. Respir. Dis. 1990, 141, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, A.; Partanen, M.; Hervonen, A.; Laitinen, L.A. Electron microscopic study on the innervation of the human lower respiratory tract: Evidence of adrenergic nerves. Eur. J. Respir. Dis. 1985, 67, 209–215. [Google Scholar]
- Jeffery, P.K. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2004, 1, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Cazzola, M.; Matera, M.G.; Rogliani, P. Adding a LAMA to ICS/LABA Therapy: A Meta-analysis of Triple Combination Therapy in COPD. Chest 2019, 155, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Oda, N.; Yokoe, T.; Tanaka, A.; Yamamoto, Y.; Watanabe, Y.; Minoguchi, K.; Ohnishi, T.; Hirose, T.; Nagase, H.; et al. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin. Exp. Allergy 2010, 40, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Kistemaker, L.E.; Bos, I.S.; Hylkema, M.N.; Nawijn, M.C.; Hiemstra, P.S.; Wess, J.; Meurs, H.; Kerstjens, H.A.; Gosens, R. Muscarinic receptor subtype-specific effects on cigarette smoke-induced inflammation in mice. Eur. Respir. J. 2013, 42, 1677–1688. [Google Scholar] [CrossRef]
- Kistemaker, L.E.; Bos, S.T.; Mudde, W.M.; Hylkema, M.N.; Hiemstra, P.S.; Wess, J.; Meurs, H.; Kerstjens, H.A.; Gosens, R. Muscarinic M₃ receptors contribute to allergen-induced airway remodeling in mice. Am. J. Respir. Cell. Mol. Biol. 2014, 50, 690–698. [Google Scholar] [CrossRef]
- Xu, Z.P.; Yang, K.; Xu, G.N.; Zhu, L.; Hou, L.N.; Zhang, W.H.; Chen, H.Z.; Cui, Y.Y. Role of M3 mAChR in in vivo and in vitro models of LPS-induced inflammatory response. Int. Immunopharmacol. 2012, 14, 320–327. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Pan, J.; Liu, N.; Qin, Y.; Qiu, L.; Liu, M.; Wang, T. The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases. Front. Immunol. 2021, 12, 586078. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, L.; Zhao, C.; Pan, M.; Qian, Z.; Su, X. Deficiency of α7 nicotinic acetylcholine receptor attenuates bleomycin-induced lung fibrosis in mice. Mol. Med. 2017, 23, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Vicary, G.W.; Ritzenthaler, J.D.; Panchabhai, T.S.; Torres-González, E.; Roman, J. Nicotine stimulates collagen type I expression in lung via α7 nicotinic acetylcholine receptors. Respir. Res. 2017, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Peng, G.; Hao, B.; Liao, B.; Zhao, Z.; Zhou, Y.; Peng, F.; Ye, X.; Huang, L.; Zheng, M.; et al. Nicotine-Induced Airway Smooth Muscle Cell Proliferation Involves TRPC6-Dependent Calcium Influx Via α7 nAChR. Cell. Physiol. Biochem. 2017, 43, 986–1002. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef]
- Zhao, M.; He, X.; Yang, Y.H.; Yu, X.J.; Bi, X.Y.; Yang, Y.; Xu, M.; Lu, X.Z.; Sun, Q.; Zang, W.J. Acetylcholine protects mesenteric arteries against hypoxia/reoxygenation injury via inhibiting calcium-sensing receptor. J. Pharmacol. Sci. 2015, 127, 481–488. [Google Scholar] [CrossRef]
- Zhao, Q.; Gu, X.; Zhang, C.; Lu, Q.; Chen, H.; Xu, L. Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC). Cancer. Biol. Ther. 2015, 16, 634–643. [Google Scholar] [CrossRef]
- Zhao, Q.; Yue, J.; Zhang, C.; Gu, X.; Chen, H.; Xu, L. Inactivation of M2 AChR/NF-κB signaling axis reverses epithelial-mesenchymal transition (EMT) and suppresses migration and invasion in non-small cell lung cancer (NSCLC). Oncotarget 2015, 6, 29335–29346. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef]
- Takiguchi, Y.; Sekine, I.; Iwasawa, S.; Kurimoto, R.; Tatsumi, K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J. Clin. Oncol. 2014, 5, 660–666. [Google Scholar] [CrossRef]
- Lin, G.; Sun, L.; Wang, R.; Guo, Y.; Xie, C. Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, J.; Yao, L.; Lang, Y.; Liang, Y.; Chen, L.; Zhang, J.; Wang, F.; Wang, Y.; Chen, H.; et al. High expression of M3 muscarinic acetylcholine receptor is a novel biomarker of poor prognostic in patients with non-small cell lung cancer. Tumour Biol. 2013, 34, 3939–3944. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Chen, N.; Pang, H.; Jiang, T.; Jiang, W.; Tian, P.; Yao, L.; Chen, Y.; DeBerardinis, R.J.; Li, W.; et al. Targeting acetylcholine signaling modulates persistent drug tolerance in EGFR-mutant lung cancer and impedes tumor relapse. J. Clin. Invest. 2022, 132. [Google Scholar] [CrossRef]
- Schaal, C.; Chellappan, S. Nicotine-Mediated Regulation of Nicotinic Acetylcholine Receptors in Non-Small Cell Lung Adenocarcinoma by E2F1 and STAT1 Transcription Factors. PLoS ONE 2016, 11, e0156451. [Google Scholar] [CrossRef]
- Brown, K.C.; Perry, H.E.; Lau, J.K.; Jones, D.V.; Pulliam, J.F.; Thornhill, B.A.; Crabtree, C.M.; Luo, H.; Chen, Y.C.; Dasgupta, P. Nicotine induces the up-regulation of the α7-nicotinic receptor (α7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J. Biol. Chem. 2013, 288, 33049–33059. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.; Girard, L.; Ramirez, R.; Chau, W.S.; Suen, W.S.; Sheridan, S.; Tin, V.P.; Chung, L.P.; Wong, M.P.; Shay, J.W.; et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007, 67, 4638–4647. [Google Scholar] [CrossRef]
- Doll, R.; Hill, A.B. Smoking and carcinoma of the lung; preliminary report. Br. Med. J. 1950, 2, 739–748. [Google Scholar] [CrossRef]
- Heeschen, C.; Jang, J.J.; Weis, M.; Pathak, A.; Kaji, S.; Hu, R.S.; Tsao, P.S.; Johnson, F.L.; Cooke, J.P. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001, 7, 833–839. [Google Scholar] [CrossRef]
- Villablanca, A.C. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J. Appl. Physiol. 1998, 84, 2089–2098. [Google Scholar] [CrossRef]
- Maneckjee, R.; Minna, J.D. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc. Natl. Acad. Sci. USA 1990, 87, 3294–3298. [Google Scholar] [CrossRef]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, X.P.; Zhao, Q.N.; Yang, X.J.; An, S.M.; Wang, H.; Xu, L.; Zhu, L.; Chen, H.Z. Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget 2016, 7, 59199–59208. [Google Scholar] [CrossRef] [PubMed]
- Mucchietto, V.; Fasoli, F.; Pucci, S.; Moretti, M.; Benfante, R.; Maroli, A.; Di Lascio, S.; Bolchi, C.; Pallavicini, M.; Dowell, C.; et al. α9- and α7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br. J. Pharmacol. 2018, 175, 1957–1972. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, E.; Irigoyen, M.; Ansó, E.; Martínez-Irujo, J.J.; Rouzaut, A. Recurrent exposure to nicotine differentiates human bronchial epithelial cells via epidermal growth factor receptor activation. Toxicol. Appl. Pharmacol. 2008, 228, 334–342. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Y.; Zhang, Y.; Zhou, D.; Sun, H.; Ma, X. α5-nAChR contributes to epithelial-mesenchymal transition and metastasis by regulating Jab1/Csn5 signalling in lung cancer. J. Cell. Mol. Med. 2020, 24, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Q.; Liu, Z.; Pan, P.; Jia, Y.; Zhu, P.; Jiao, Y.; Kang, G.; Ma, X. The role of α5-nicotinic acetylcholine receptor/NLRP3 signaling pathway in lung adenocarcinoma cell proliferation and migration. Toxicology 2022, 469, 153120. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Pan, P.; Zhang, X.; Jia, Y.; Zhu, P.; Chen, X.; Jiao, Y.; Kang, G.; Zhang, L.; et al. α5-nAChR associated with Ly6E modulates cell migration via TGF-β1/Smad signaling in non-small cell lung cancer. Carcinogenesis 2022, 43, 393–404. [Google Scholar] [CrossRef]
- Ma, X.; Jia, Y.; Zu, S.; Li, R.; Jia, Y.; Zhao, Y.; Xiao, D.; Dang, N.; Wang, Y. α5 Nicotinic acetylcholine receptor mediates nicotine-induced HIF-1α and VEGF expression in non-small cell lung cancer. Toxicol. Appl. Pharmacol. 2014, 278, 172–179. [Google Scholar] [CrossRef]
- Sun, H.; Ma, X. α5-nAChR modulates nicotine-induced cell migration and invasion in A549 lung cancer cells. Exp. Toxicol. Pathol. 2015, 67, 477–482. [Google Scholar] [CrossRef]
- Sun, H.J.; Jia, Y.F.; Ma, X.L. Alpha5 nicotinic acetylcholine receptor contributes to nicotine-induced lung cancer development and progression. Front. Pharmacol. 2017, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Jin, Z.; Kang, G.; Jia, Y.; Liu, D.; Zhang, Q.; Guo, F.; Jia, Y.; Jiao, Y.; Li, J.; et al. Alpha5 nicotinic acetylcholine receptor mediated immune escape of lung adenocarcinoma via STAT3/Jab1-PD-L1 signalling. Cell Commun. Signal. 2022, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Stitzel, J.A.; Bai, A.; Zambrano, C.A.; Phillips, M.; Marrack, P.; Chan, E.D. Nicotine impairs macrophage control of Mycobacterium tuberculosis. Am. J. Respir. Cell Mol. Biol. 2017, 57, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Miramontes, C.V.; Rodríguez-Carlos, A.; Marin-Luévano, S.P.; Trejo Martínez, L.A.; de Haro Acosta, J.; Enciso-Moreno, J.A.; Rivas-Santiago, B. Nicotine promotes the intracellular growth of Mycobacterium tuberculosis in epithelial cells. Tuberculosis 2021, 127, 102026. [Google Scholar] [CrossRef]
- Islas-Weinstein, L.; Marquina-Castillo, B.; Mata-Espinosa, D.; Paredes-González, I.S.; Chávez, J.; Balboa, L.; Marín Franco, J.L.; Guerrero-Romero, D.; Barrios-Payan, J.A.; Hernandez-Pando, R. The cholinergic system contributes to the immunopathological progression of experimental pulmonary tuberculosis. Front. Immunol. 2020, 11, 581911. [Google Scholar] [CrossRef]
- Valdez-Miramontes, C.E.; Trejo Martínez, L.A.; Torres-Juárez, F.; Rodríguez Carlos, A.; Marin-Luévano, S.P.; de Haro-Acosta, J.P.; Enciso-Moreno, J.A.; Rivas-Santiago, B. Nicotine modulates molecules of the innate immune response in epithelial cells and macrophages during infection with M. tuberculosis. Clin. Exp. Immunol. 2020, 199, 230–243. [Google Scholar] [CrossRef]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018, 23, 130–137. [Google Scholar] [CrossRef]
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Farsalinos, K.; Niaura, R.; Le Houezec, J.; Barbouni, A.; Tsatsakis, A.; Kouretas, D.; Vantarakis, A.; Poulas, K. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 2020, 7, 658–663. [Google Scholar] [CrossRef]
- Farsalinos, K.; Eliopoulos, E.; Tzartos, S.; Poulas, K. Nicotinic Cholinergic System and COVID-19: Identification of a Potentially Crucial Snake Toxin-Like Sequence in the SARS-CoV-2 Spike Glycoprotein. Preprints 2020, 2020050301. [Google Scholar] [CrossRef]
- Courties, A.; Boussier, J.; Hadjadj, J.; Yatim, N.; Barnabei, L.; Péré, H.; Veyer, D.; Kernéis, S.; Carlier, N.; Pène, F.; et al. Regulation of the acetylcholine/α7nAChR anti-inflammatory pathway in COVID-19 patients. Sci. Rep. 2021, 11, 11886. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.M.; Pimentel, V.E.; Fuzo, C.A.; da Silva-Neto, P.V.; Toro, D.M.; Fraga-Silva, T.F.C.; Gardinassi, L.G.; Oliveira, C.N.S.; Souza, C.O.S.; Torre-Neto, N.T.; et al. Acetylcholine, fatty acids, and lipid mediators are linked to COVID-19 severity. J. Immunol. 2022, 209, 250–261. [Google Scholar] [CrossRef]
- Gauthier, A.G.; Lin, M.; Wu, J.; Kennedy, T.P.; Daley, L.A.; Ashby, C.R.J.; Mantell, L.L. From nicotine to the cholinergic anti-inflammatory reflex—Can nicotine alleviate the dysregulated inflammation in COVID-19? J. Immunotoxicol. 2021, 18, 23–29. [Google Scholar] [CrossRef]
- Cattaruzza, M.S.; Zagà, V.; Gallus, S.; D’Argenio, P.; Gorini, G. Tobacco smoking and COVID-19 pandemic: Old and new issues. A summary of the evidence from the scientific literature. Acta Biomed. 2020, 91, 106–112. [Google Scholar] [CrossRef]
- Mehranfard, D.; Speth, R.C. Cholinergic anti-inflammatory pathway and COVID-19. Bioimpacts 2022, 12, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Balint, E.M.; Grüner, B.; Haase, S.; Kaw-Geppert, M.; Thayer, J.F.; Gündel, H.; Jarczok, M.N. A randomized clinical trial to stimulate the cholinergic anti-inflammatory pathway in patients with moderate COVID-19-pneumonia using a slow-paced breathing technique. Fron.t Immunol. 2022, 13, 928979. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Dormoy, V.; Perotin, J.M.; Gosset, P.; Maskos, U.; Polette, M.; Deslée, G. Nicotinic receptors as SARS-CoV-2 spike co-receptors? Med Hypotheses 2022, 158, 110741. [Google Scholar] [CrossRef]
- Changeux, J.P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus. Biol. 2020, 343, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
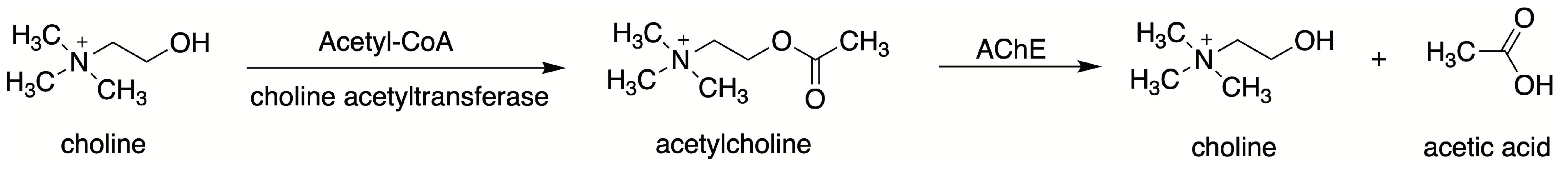

| Cell Type | Muscarinic Receptor Subtypes | Function | References |
|---|---|---|---|
| airway epithelial cells | M1/M2/M3 | facilitation of CXCL8, LTB4 release activation of oxidative/nitrosative stress, mucus secretion | [21,36,37,38,39] |
| airway smooth muscle | M2/M3 | contraction, proliferation, facilitation of IL-6 and CXCL8 release | [40,41,42] |
| fibroblast cells | M1/M2/M3 | proliferation, collagen production | [43,44] |
| lymphocytes | M1/M2/M3/M4/M5 | proliferation, chemotactic, apoptosis regulation | [45,46,47] |
| macrophages | M1/M2/M3/M4/M5 | facilitation of LTB4 release | [48,49,50] |
| neutrophils | M1/M2/M3/M4/M5 | facilitation of CXCL8 release | [48,51] |
| mast cells | M1 | inhibitory regulation of histamine release | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wu, J.; Xiong, X. The Role of the Acetylcholine System in Common Respiratory Diseases and COVID-19. Molecules 2023, 28, 1139. https://doi.org/10.3390/molecules28031139
Li D, Wu J, Xiong X. The Role of the Acetylcholine System in Common Respiratory Diseases and COVID-19. Molecules. 2023; 28(3):1139. https://doi.org/10.3390/molecules28031139
Chicago/Turabian StyleLi, Dehu, Jianghua Wu, and Xianzhi Xiong. 2023. "The Role of the Acetylcholine System in Common Respiratory Diseases and COVID-19" Molecules 28, no. 3: 1139. https://doi.org/10.3390/molecules28031139
APA StyleLi, D., Wu, J., & Xiong, X. (2023). The Role of the Acetylcholine System in Common Respiratory Diseases and COVID-19. Molecules, 28(3), 1139. https://doi.org/10.3390/molecules28031139







