Mannitol Is a Good Anticaking Agent for Spray-Dried Hydroxypropyl-Beta-Cyclodextrin Microcapsules
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Microstructure of Microcapsule Powders with Different Anticaking Agents
2.2. Particle Size Distribution, Moisture Content, and Hygroscopicity of Microcapsules with Different Anticaking Agents
2.2.1. Particle Size Distribution of Microcapsules with Different Anticaking Agents
2.2.2. Moisture Content of Microcapsules with Different Anticaking Agents
2.2.3. Hygroscopicity of Microcapsules with Different Anticaking Agents
2.3. Powder Flowability of Microcapsules with Different Anticaking Agents
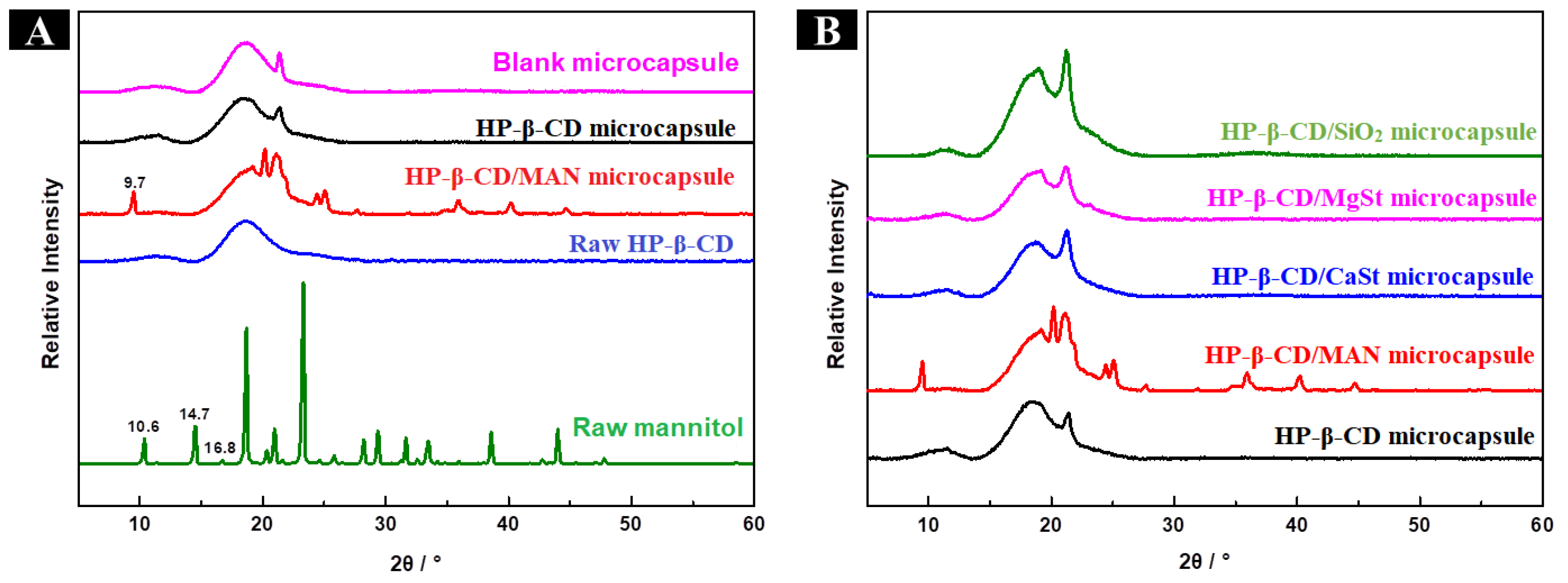
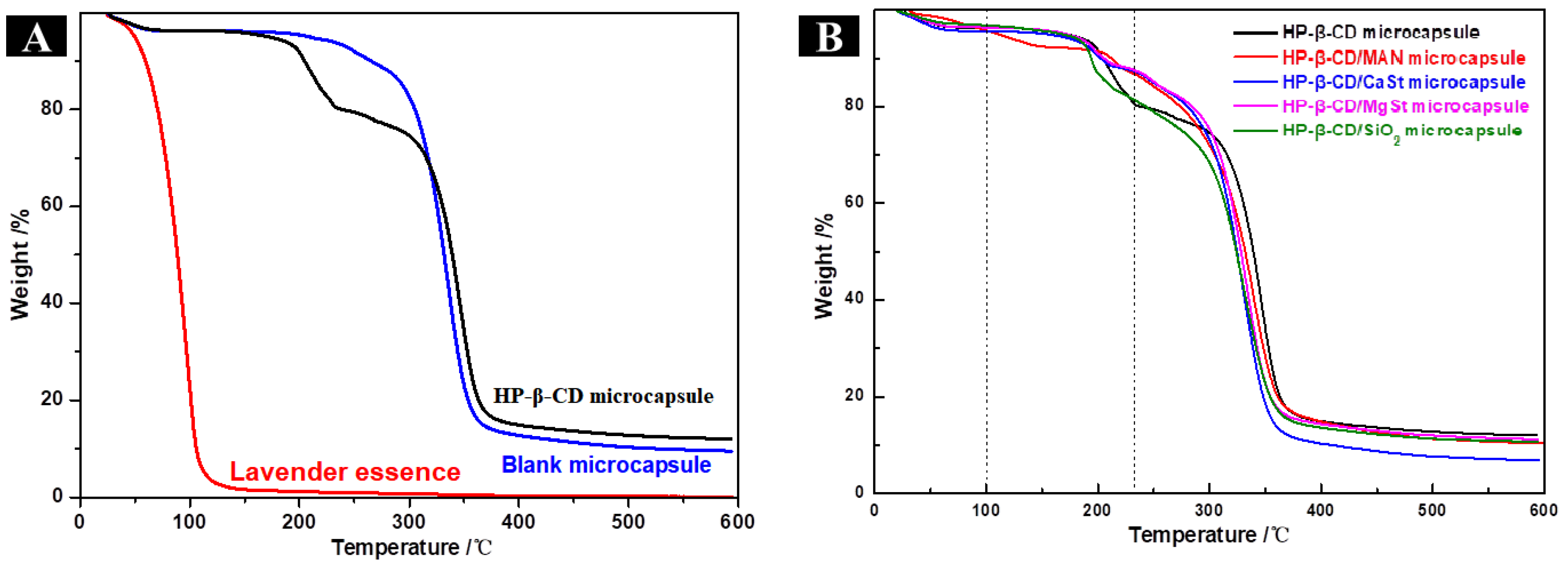
2.4. FTIR, Thermal, and XRD Analysis of Microcapsules with Different Anticaking Agents
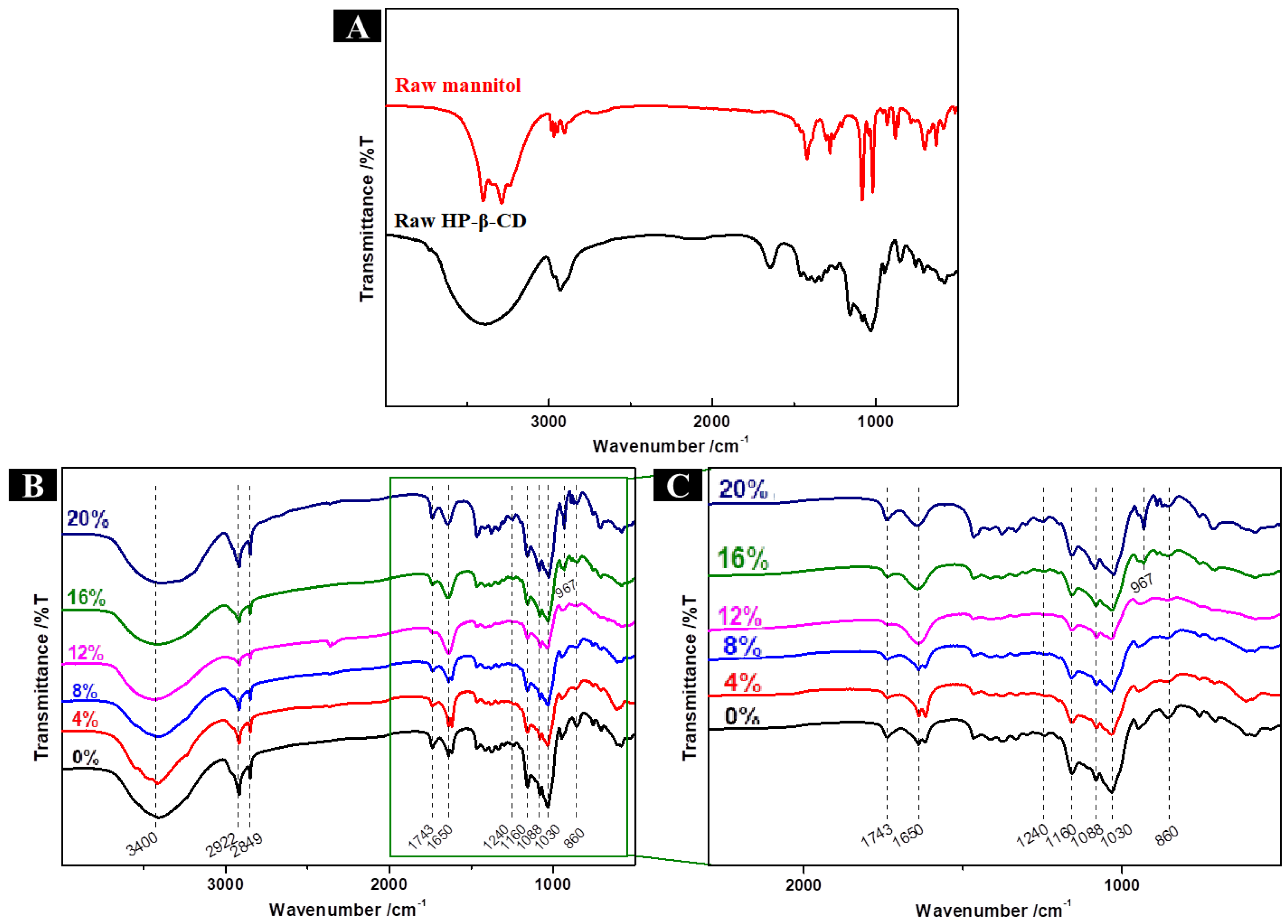
2.4.1. FTIR Analysis of HP-β-CD Microcapsules
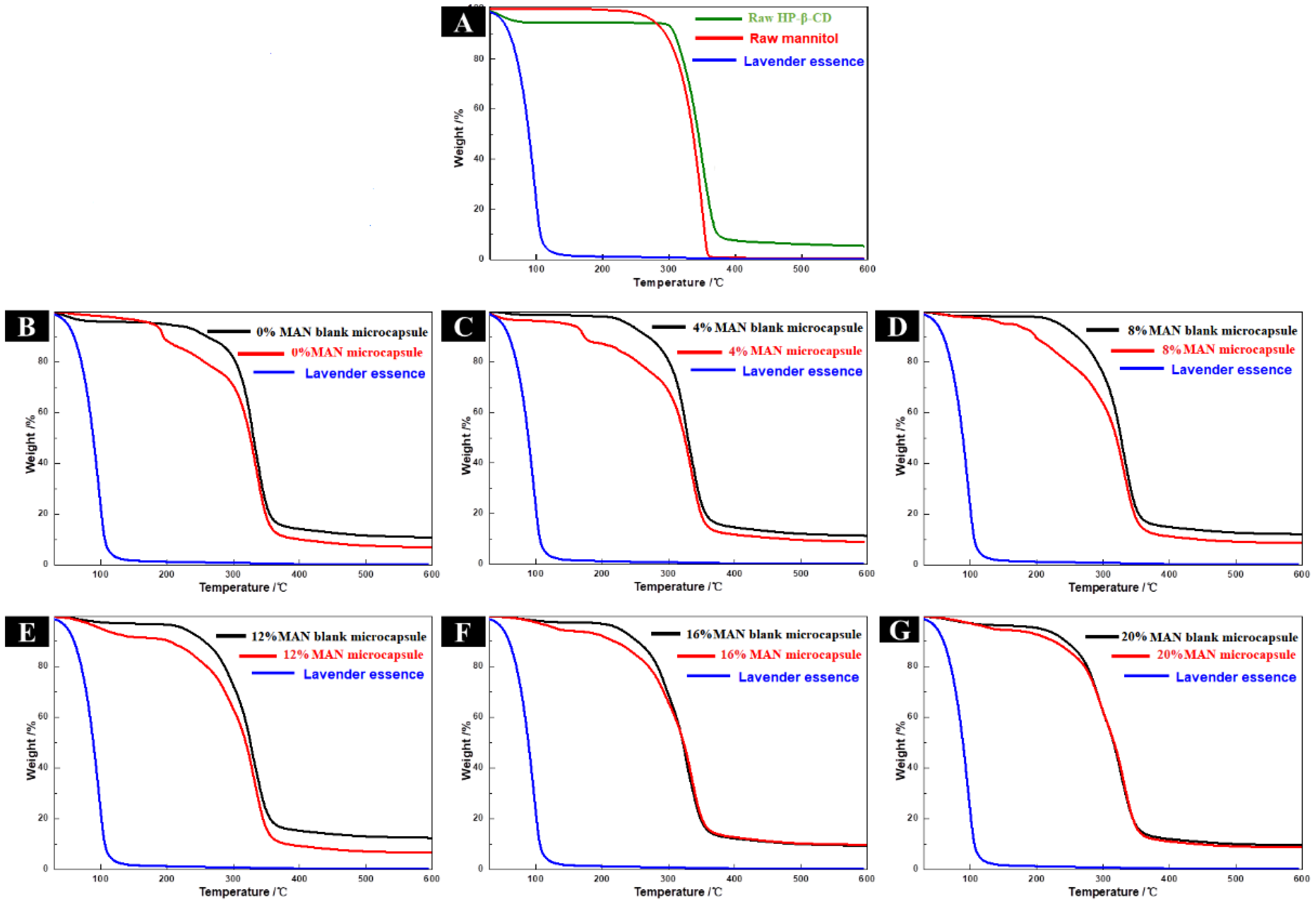
2.4.2. Thermal Analysis of Microcapsules with Different Anticaking Agents
2.4.3. XRD Analysis of Microcapsules with Different Anticaking Agents
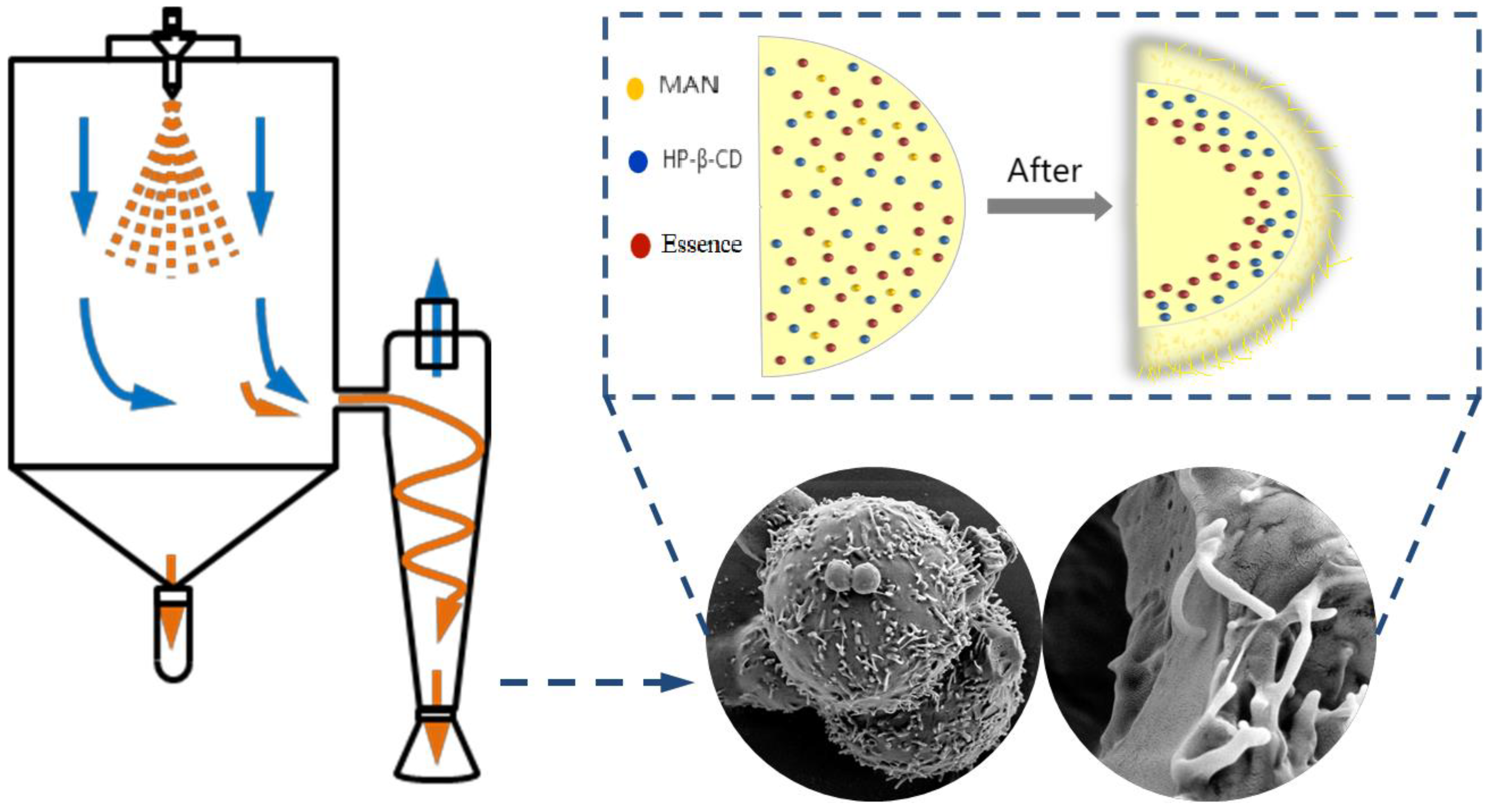
2.5. Morphology and Microstructure of HP-β-CD/MAN Microcapsules
2.6. Particle Size Distribution, Moisture Content, and Fluidity of HP-β-CD/MAN Microcapsules
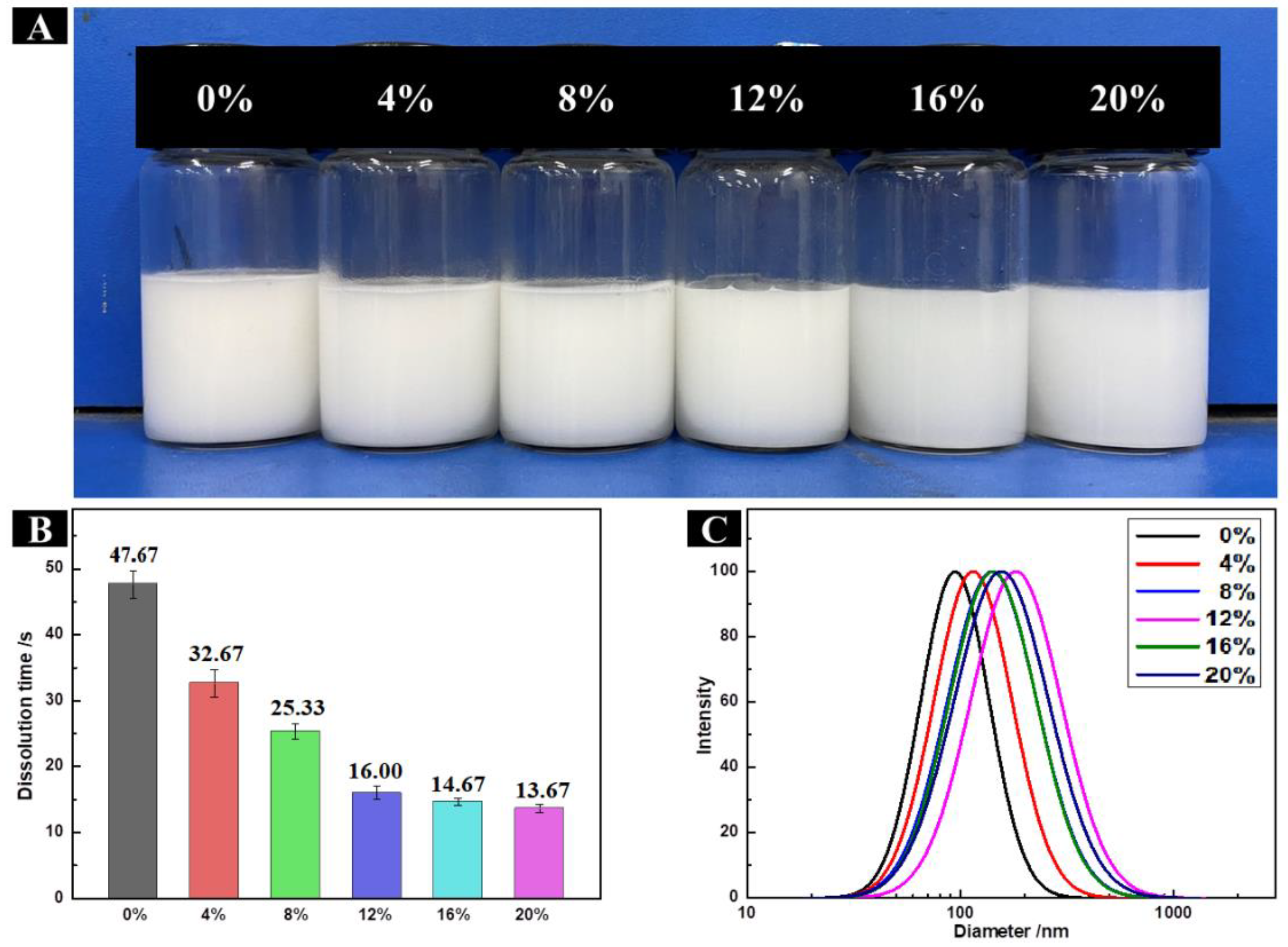
2.6.1. Particle Size Distribution of HP-β-CD/MAN Microcapsules
2.6.2. Moisture Content of HP-β-CD/MAN Microcapsules
2.6.3. Hygroscopicity Content of HP-β-CD/MAN Microcapsules
2.6.4. Powder Flowability of HP-β-CD/MAN Microcapsules
2.7. Solubility of HP-β-CD/MAN Microcapsules
2.8. Thermal, XRD, and FTIR Analyses of HP-β-CD/MAN Microcapsules
2.8.1. Thermal Analysis of HP-β-CD/MAN Microcapsules
2.8.2. XRD Analysis of HP-β-CD/MAN Microcapsules
2.8.3. FTIR Analysis of HP-β-CD/MAN Microcapsules
3. Materials and Methods
3.1. Materials
3.2. Preparation of Microcapsule Emulsion and Spray-Drying Conditions
3.3. Characterisation of Microcapsule Powder
3.3.1. Process Yield
3.3.2. Surface Morphology and Internal Structure of the Microcapsules
3.3.3. Particle Size Distribution of Microcapsule Emulsion and Powder
3.3.4. Moisture Content and Hygroscopicity of Microcapsule Powder
3.3.5. Fourier Transform Infrared Spectroscopy Analysis
3.3.6. Thermal Analysis
3.3.7. X-ray Diffraction (XRD) Measurement
3.3.8. Flowability
3.3.9. Solubility
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellinghausen, R. Spray drying from yesterday to tomorrow: An industrial perspective. Dry. Technol. 2019, 37, 612–622. [Google Scholar] [CrossRef]
- Böger, B.R.; Acre, L.B.; Viegas, M.C.; Kurozawa, L.E.; Benassi, M.T. Roasted coffee oil microencapsulation by spray drying and complex coacervation techniques: Characteristics of the particles and sensory effect. Innov. Food Sci. Emerg. Technol. 2021, 72, 102739. [Google Scholar] [CrossRef]
- Littringer, E.M.; Zellnitz, S.; Hammernik, K.; Adamer, V.; Friedl, H.; Urbanetz, N.A. Spray Drying of Aqueous Salbutamol Sulfate Solutions Using the Nano Spray Dryer B-90—The Impact of Process Parameters on Particle Size. Dry. Technol. 2013, 31, 1346–1353. [Google Scholar] [CrossRef]
- Lucas, J.; Ralaivao, M.; Estevinho, B.N.; Rocha, F. A new approach for the microencapsulation of curcumin by a spray drying method, in order to value food products. Powder Technol. 2020, 362, 428–435. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; dos Santos, T.O.; de Paula, J.R.; Conceição, E.C.; Bara, M.T.F. Microencapsulation of Essential Oil from Fruits of Pterodon emarginatus Using Gum Arabic and Maltodextrin as Wall Materials: Composition and Stability. Dry. Technol. 2014, 32, 96–105. [Google Scholar] [CrossRef]
- Weiler, C.; Budde, C.; Schiewe, J. Solvent evaporation kinetics in spray drying and how to consider heat loss. Powder Technol. 2021, 388, 434–441. [Google Scholar] [CrossRef]
- Sadek, C.; Tabuteau, H.; Schuck, P.; Fallourd, Y.; Pradeau, N.; Le Floch-Fouéré, C.; Jeantet, R. Shape, Shell, and Vacuole Formation during the Drying of a Single Concentrated Whey Protein Droplet. Langmuir 2013, 29, 15606–15613. [Google Scholar] [CrossRef]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Cuq, B.; Rondet, E.; Abecassis, J. Food powders engineering, between knowhow and science: Constraints, stakes and opportunities. Powder Technol. 2011, 208, 244–251. [Google Scholar] [CrossRef]
- Jubaer, H.; Xiao, J.; Chen, X.D.; Selomulya, C.; Woo, M.W. Identification of regions in a spray dryer susceptible to forced agglomeration by CFD simulations. Powder Technol. 2019, 346, 23–37. [Google Scholar] [CrossRef]
- Turchiuli, C.; Gianfrancesco, A.; Palzer, S.; Dumoulin, E. Evolution of particle properties during spray drying in relation with stickiness and agglomeration control. Powder Technol. 2011, 208, 433–440. [Google Scholar] [CrossRef]
- Palzer, S. The effect of glass transition on the desired and undesired agglomeration of amorphous food powders. Chem. Eng. Sci. 2005, 60, 3959–3968. [Google Scholar] [CrossRef]
- Palzer, S. Agglomeration of pharmaceutical, detergent, chemical and food powders—Similarities and differences of materials and processes. Powder Technol. 2011, 206, 2–17. [Google Scholar] [CrossRef]
- Addo, K.A.; Bi, J.; Chen, Q.; Bhandari, B.; Lyu, J.; Wu, X.; Jin, X.; Qiao, Y.; Hou, H.; Li, C. Assessment of Anticaking Agent on Caking Behavior of Jujube Amorphous Powder via Glass Transition and State Diagram. Food Bioprocess Technol. 2020, 13, 1588–1599. [Google Scholar] [CrossRef]
- Wilkowska, A.; Czyżowska, A.; Ambroziak, W.; Adamiec, J. Structural, physicochemical and biological properties of spray-dried wine powders. Food Chem. 2017, 228, 77–84. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.-Q.; Fan, H.-F.; Xia, Q. Comparison of Release Behaviors of Fragrance/Hydroxypropyl-β-cyclodextrin Inclusion Complex and Fragrance Microcapsules. Integr. Ferroelectr. 2014, 152, 81–89. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- Walton, D.E.; Mumford, C.J. Spray Dried Products—Characterization of Particle Morphology. Chem. Eng. Res. Des. 1999, 77, 21–38. [Google Scholar] [CrossRef]
- Chew, N.Y.K.; Chan, H.-K. Use of Solid Corrugated Particles to Enhance Powder Aerosol Performance. Pharm. Res. 2001, 18, 1570–1577. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Cui, Y.; Huang, Y.; Huang, Z.; Wang, G.; Liang, R.; Pan, X.; Tao, L.; Wu, C. Hydroxypropyl-β-cyclodextrin as anti-hygroscopicity agent inamorphous lactose carriers for dry powder inhalers. Powder Technol. 2019, 358, 29–38. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- da Costa, J.M.G.; Silva, E.K.; Toledo Hijo, A.A.C.; Azevedo, V.M.; Malta, M.R.; Ferreira Alves, J.G.L.; Borges, S.V. Microencapsulation of Swiss cheese bioaroma by spray-drying: Process optimization and characterization of particles. Powder Technol. 2015, 274, 296–304. [Google Scholar] [CrossRef]
- Quispe-Condori, S.; Saldaña, M.D.A.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT-Food Sci. Technol. 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Alavi, S.; Caussat, B. Experimental study on fluidization of micronic powders. Powder Technol. 2005, 157, 114–120. [Google Scholar] [CrossRef]
- Ishida, M.; Uchiyama, J.; Isaji, K.; Suzuki, Y.; Ikematsu, Y.; Aoki, S. A novel approach to a fine particle coating using porous spherical silica as core particles. Drug Dev. Ind. Pharm. 2014, 40, 1054–1064. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Griesser, U.; Walzel, P.; Urbanetz, N.A. Spray Drying of Mannitol as a Drug Carrier—The Impact of Process Parameters on Product Properties. Dry. Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Serra, C.; Remuñán-López, C. Chitosan Nanoparticle-Loaded Mannitol Microspheres: Structure and Surface Characterization. Biomacromolecules 2007, 8, 2072–2079. [Google Scholar] [CrossRef]
- Mönckedieck, M.; Kamplade, J.; Fakner, P.; Urbanetz, N.A.; Walzel, P.; Steckel, H.; Scherließ, R. Spray drying of mannitol carrier particles with defined morphology and flow characteristics for dry powder inhalation. Dry. Technol. 2017, 35, 1843–1857. [Google Scholar] [CrossRef]
- Gaspar, D.P.; Gaspar, M.M.; Eleutério, C.V.; Grenha, A.; Blanco, M.; Gonçalves, L.M.D.; Taboada, P.; Almeida, A.J.; Remuñán-López, C. Microencapsulated Solid Lipid Nanoparticles as a Hybrid Platform for Pulmonary Antibiotic Delivery. Mol. Pharm. 2017, 14, 2977–2990. [Google Scholar] [CrossRef]
- Forny, L.; Marabi, A.; Palzer, S. Wetting, disintegration and dissolution of agglomerated water soluble powders. Powder Technol. 2011, 206, 72–78. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Wu, J.X.; Yang, M.; Young, P.M.; van den Berg, F.; Rantanen, J. Particle size dependence of polymorphism in spray-dried mannitol. Eur. J. Pharm. Sci. 2011, 44, 41–48. [Google Scholar] [CrossRef]
- Lu, W.; Wang, S.; Lin, R.; Yang, X.; Cheng, Z.; Liu, W. Unveiling the importance of process parameters on droplet shrinkage and crystallization behaviors of easily crystalline material during spray drying. Dry. Technol. 2020, 40, 326–336. [Google Scholar] [CrossRef]
- Abiad, M.G.; Campanella, O.H.; Carvajal, M.T. Effect of Spray Drying Conditions on the Physicochemical Properties and Enthalpy Relaxation of α-Lactose. Int. J. Food Prop. 2014, 17, 1303–1316. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, W.; Ma, J.; Zhao, Y.; Niu, Y.; Kou, X.; Ke, Q. Double-Encapsulated Microcapsules for the Adsorption to Cotton Fabrics. Coatings 2021, 11, 426. [Google Scholar] [CrossRef]
- Santana, A.A.; Kurozawa, L.E.; de Oliveira, R.A.; Park, K.J. Influence of Process Conditions on the Physicochemical Properties of Pequi Powder Produced by Spray Drying. Dry. Technol. 2013, 31, 825–836. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Ren, J.; Liao, M.; Ma, L.; Chen, F.; Liao, X.; Hu, X.; Miao, S.; Fitzpatrick, J.; Ji, J. Effect of spray freeze drying on the structural modification and rehydration characteristics of micellar casein powders. Innov. Food Sci. Emerg. Technol. 2022, 80, 103093. [Google Scholar] [CrossRef]
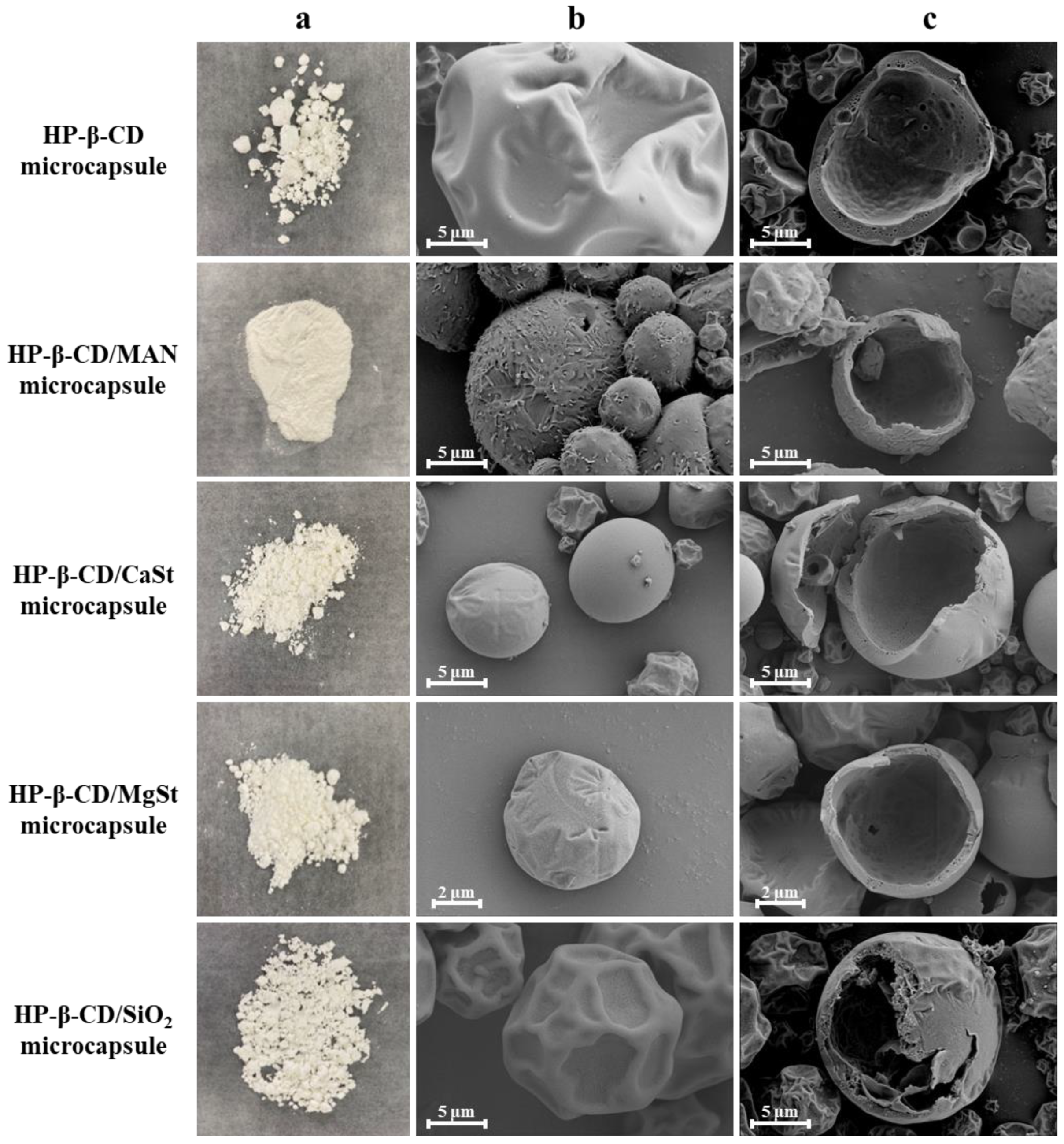

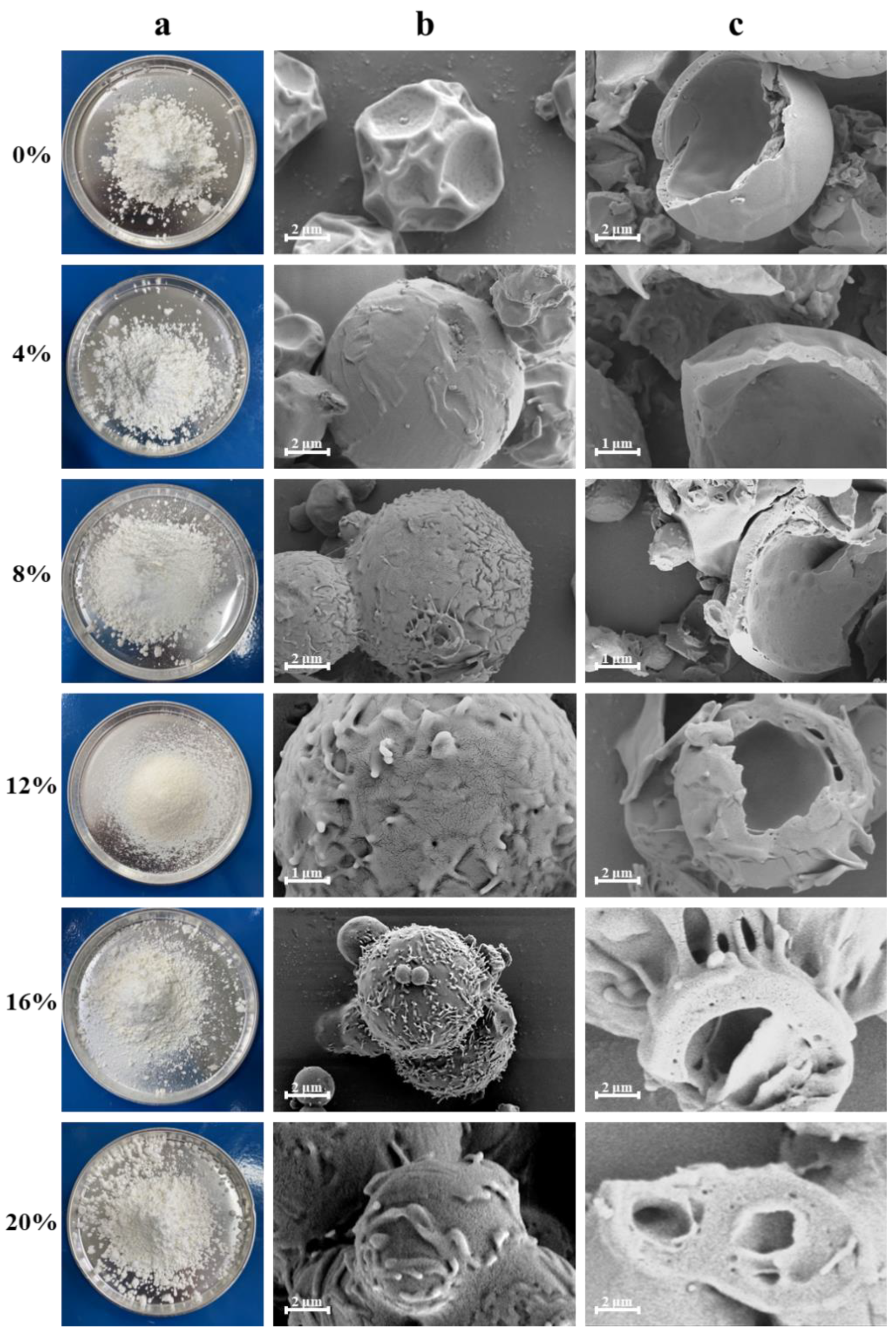

| Microcapsule Formulation | Yield (%) | Particle Size of Emulsion (nm) | Particle Size of Powder (μm) | Moisture Content (%) | Hygroscopicity (%) | Hausner Ratio | Angle of Repose (°) |
|---|---|---|---|---|---|---|---|
| HP-β-CD | 50.65 ± 2.48 a | 212.80 ± 3.76 a | 8.90 ± 0.29 b | 6.99 ± 0.14 a | 4.38 ± 0.04 a | 2.21 ± 0.07 a | 49.68 ± 1.00 a |
| HP-β-CD/MAN | 50.80 ± 1.75 a | 197.62 ± 4.77 b | 11.13 ± 0.38 a | 4.03 ± 0.12 b | 3.97 ± 0.10 c | 1.33 ± 0.03 d | 32.90 ± 0.85 d |
| HP-β-CD/CaSt | 37.30 ± 3.62 c | 180.49 ± 4.22 d | 11.53 ± 2.31 a | 3.43 ± 0.11 c | 2.97 ± 0.05 d | 1.42 ± 0.06 b | 35.27 ± 0.39 c |
| HP-β-CD/MgSt | 40.20 ± 2.97 b | 194.26 ± 3.31 b | 11.83 ± 2.00 a | 3.56 ± 0.05 c | 3.50 ± 0.04 c | 1.43 ± 0.06 b | 36.17 ± 0.99 c |
| HP-β-CD/SiO2 | 49.27 ± 1.09 a | 186.36 ± 2.23 c | 8.21 ± 0.58 b | 4.55 ± 0.14 b | 4.05 ± 0.12 ab | 1.72 ± 0.10 ab | 41.97 ± 0.29 b |
| MAN Ratio (%) | Yield (%) | Particle Size of Emulsion (nm) | Particle Size of Powder (μm) | Moisture Content (%) | Hygroscopicity (%) | Hausner Ratio | Angle of Repose (°) | Relative crystallinity (%) |
|---|---|---|---|---|---|---|---|---|
| 0 | 49.22 ± 4.35 ab | 219.10 ± 3.70 a | 8.78 ± 1.06 c | 6.99 ± 0.20 a | 5.34 ± 0.31 a | 2.21 ± 0.07 a | 49.54 ± 1.00 a | 15.83 e |
| 4 | 50.57 ± 0.90 a | 210.45 ± 3.71 bc | 8.53 ± 1.07 c | 3.21 ± 0.12 b | 5.29 ± 0.13 a | 1.78 ± 0.02 b | 48.58 ± 0.50 a | 33.43 d |
| 8 | 48.55 ± 2.14 b | 212.16 ± 3.55 b | 7.17 ± 0.65 cd | 2.55 ± 0.10 c | 4.37 ± 0.31 b | 1.75 ± 0.05 b | 44.54 ± 0.96 ab | 51.97 c |
| 12 | 51.14 ± 2.17 a | 207.72 ± 5.73 c | 8.00 ± 0.45 cd | 2.47 ± 0.18 c | 3.99 ± 0.30 c | 1.29 ± 0.02 c | 33.65 ± 0.45 b | 64.18 b |
| 16 | 33.92 ± 2.31 b | 212.82 ± 2.25 b | 24.57 ± 3.64 b | 2.31 ± 0.07 c | 3.68 ± 0.16 bc | 1.24 ± 0.08 c | 22.62 ± 1.29 c | 66.20 a |
| 20 | 19.79 ± 2.29 d | 213.71 ± 2.17 b | 57.67 ± 5.39 a | 1.48 ± 0.11 d | 3.23 ± 0.31 d | 1.20 ± 0.03 c | 21.25 ± 0.81 d | 67.43 a |
| MAN Ratio of Microcapsules | Anticaking Agent | HP-β-CD (% w/w) | Mannitol (% w/w) |
|---|---|---|---|
| 0 | Mannitol | 30 | 0 |
| 4 | Mannitol | 28 | 2 |
| 8 | Mannitol | 26 | 4 |
| 12 | Mannitol | 24 | 6 |
| 16 | Mannitol | 22 | 8 |
| 20 | Mannitol | 20 | 10 |
| Microcapsule Formulation | Anticaking Agent | HP-β-CD (% w/w) | Anticaking Agent (% w/w) |
|---|---|---|---|
| HP-β-CD microcapsule | None | 5 | 1 |
| HP-β-CD/MAN microcapsule | Mannitol | 5 | 1 |
| HP-β-CD/CaSt microcapsule | Calcium stearate | 5 | 1 |
| HP-β-CD/MgSt microcapsule | Magnesium stearate | 5 | 1 |
| HP-β-CD/SiO2 microcapsule | Silica | 5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, X.; Zhang, X.; Cheng, Y.; Yu, M.; Meng, Q.; Ke, Q. Mannitol Is a Good Anticaking Agent for Spray-Dried Hydroxypropyl-Beta-Cyclodextrin Microcapsules. Molecules 2023, 28, 1119. https://doi.org/10.3390/molecules28031119
Kou X, Zhang X, Cheng Y, Yu M, Meng Q, Ke Q. Mannitol Is a Good Anticaking Agent for Spray-Dried Hydroxypropyl-Beta-Cyclodextrin Microcapsules. Molecules. 2023; 28(3):1119. https://doi.org/10.3390/molecules28031119
Chicago/Turabian StyleKou, Xingran, Xinping Zhang, Ying Cheng, Miao Yu, Qingran Meng, and Qinfei Ke. 2023. "Mannitol Is a Good Anticaking Agent for Spray-Dried Hydroxypropyl-Beta-Cyclodextrin Microcapsules" Molecules 28, no. 3: 1119. https://doi.org/10.3390/molecules28031119
APA StyleKou, X., Zhang, X., Cheng, Y., Yu, M., Meng, Q., & Ke, Q. (2023). Mannitol Is a Good Anticaking Agent for Spray-Dried Hydroxypropyl-Beta-Cyclodextrin Microcapsules. Molecules, 28(3), 1119. https://doi.org/10.3390/molecules28031119





