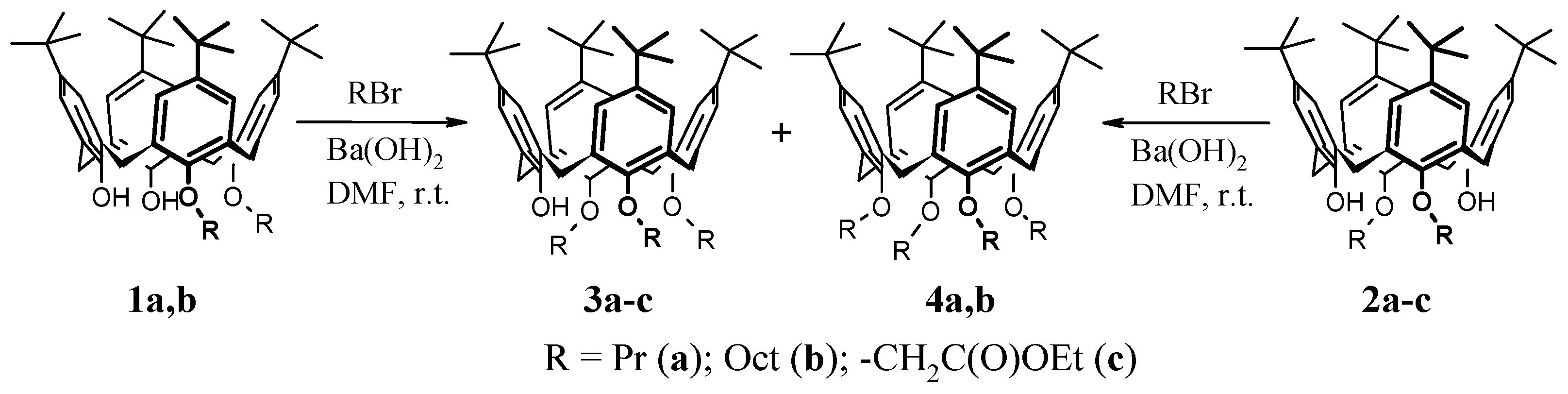The NMR spectra were registered on a Varian VXR-400 spectrometer operating a 400 MHz (1H) using TMS as a reference. The melting points were measured on a Boёtius heating block and are uncorrected. The reactions were carried out in anhydrous solvents. DMF was purified by the azeotropic drying method with benzene. The column chromatography was performed on silica gel that was purchased from Acros Organics (0.040–0.063 mm, pore diameter 6 nm).
3.3. General Procedure for the Selective Alkylation
A mixture of dialkyloxy-calix[4]arene 1, 2, 7, or 10 (0.50 mmol) and anhydrous Ba(OH)2 (0.15 g, 0.65 mmol) in dry DMF (5–10 mL) was stirred at 40–50 °C for 30–40 min. After cooling to room temperature, the suitable alkylating reagent was added [1.50–2.50 mmol of propyl bromine, octyl bromine, or esters of bromoacetic acid; 10.83 mmol (0.2 g) of N-(1-phenylethyl)bromoacetamide]. The mixture was stirred at 20–25 °C for 24 h. After the end of the reaction, water (10 mL) and 30% HCl (1 mL) were added to the reaction mixture and the products were extracted with CHCl3 (3 × 5 mL). The organic phase was washed with water (5 mL), washed with brine (5 mL), and dried over Na2SO4. After the removal of the solvent, the corresponding compounds, or mixtures of compounds, were isolated.
25,26,27-Tripropyloxy-tert-butyl-calix[4]arene 3a had a yield of 95% from
1a and 96% from
2a. The spectral characteristics completely coincide with the literature [
20].
25,26,27-Trioctyloxy-tert-butyl-calix[4]arene 3b had a yield of 96% from
1b and 94% from
2b. The spectral characteristics completely coincide with the literature [
22].
25,26,27-Tri(ethyloxycarbonylmethyloxy)-tert-butyl-calix[4]arene 3c had a yield of 95% from
2c. The spectral characteristics completely coincide with the literature [
15].
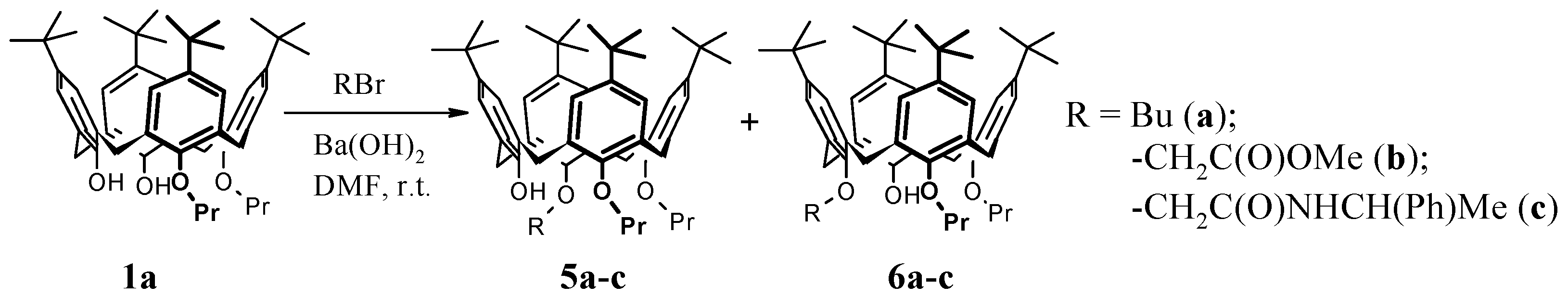
Dipropyloxy-buthyloxy-tert-butyl-calix[4]arenes5a + 6a had a total yield of 94%, m.p. 99–100 °C (MeCN). 1H NMR (CDCl3), δ: 0.81 (s, 18H, t-Bu), 0.94 (t, 3H, MePr, 3JHH = 7.3 Hz), 0.99 (t, 3H, MePr, 3JHH = 7.3 Hz), 1.09 (t, 3H, MeBu, 3JHH = 7.3 Hz), 1.32 (s, 9H, t-Bu), 1.33 (s, 9H, t-Bu), 1.46–1.68 (m, 2H, -CH2-), 1.75–2.00 (m, 4H, -CH2-), 2.25-2.39 (m, 2H, -CH2-), 3.16 (d, 2H, Ar-CH2-eq, 2JHH = 12.7 Hz), 3.22 (d, 2H, Ar-CH2-eq, 2JHH = 13.0 Hz), 3.74 (t, 2H, OCH2, 3JHH = 6.7 Hz), 3.77 (t, 2H, OCH2, 3JHH = 6.7 Hz), 3.83 (t, 2H, OCH2, 3JHH = 8.4 Hz), 4.31 (d, 1H, Ar-CH2-ax, 2JHH = 13.0 Hz), 4.32 (d, 1H, Ar-CH2-ax, 2JHH = 13.0 Hz), 4.35 (d, 1H, Ar-CH2-ax, 2JHH = 12.7 Hz), 4.36 (d, 1H, Ar-CH2-ax, 2JHH = 12.7 Hz), 5.57 (s, 1H, OH), 6.50 (s, 4H, ArH), 7.04 (s, 2H, ArH), 7.13 (s, 2H, ArH). The calc. % was C 82.18; H 9.71. C54H76O4. The found % was C 82.29; H 9.79.
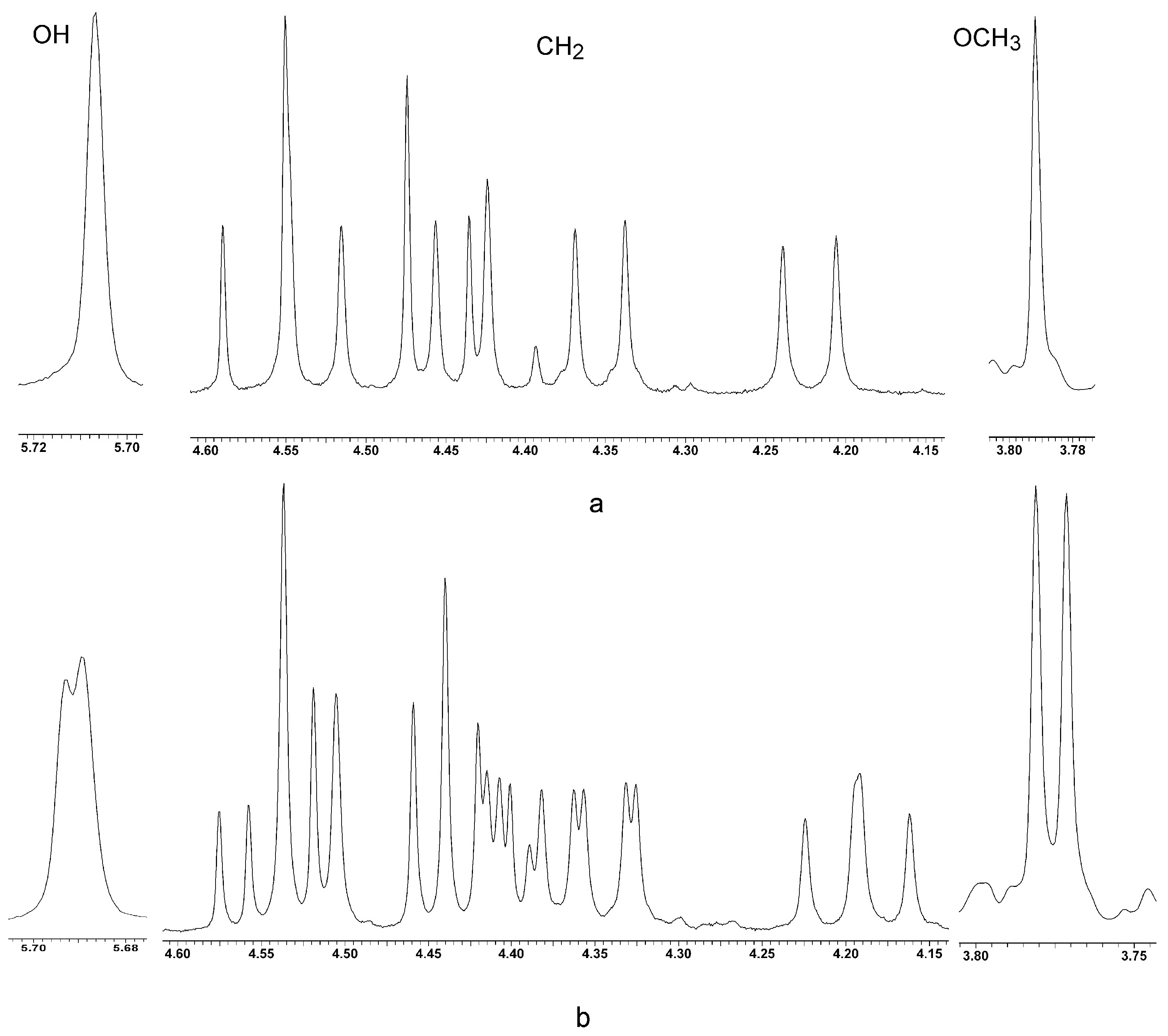
Dipropyloxy-(methyloxycarbonylmethyloxy)-tert-butyl-calix[4]arenes5b + 6b had a total yield of 95%, m.p. 87–89 °C (MeCN). 1H NMR (CDCl3), δ: 0.77 (s, 9H, t-Bu), 0.89 (s, 9H, t-Bu), 0.95 (t, 3H, MePr, 3JHH = 7.3 Hz), 1.09 (t, 3H, MePr, 3JHH = 7.3 Hz), 1.31 (s, 9H, t-Bu), 1.32 (s, 9H, t-Bu), 1.82–2.06 (m, 2H, -CH2-), 2.14–2.37 (m, 2H, -CH2-), 3.17 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.19 (d, 1H, Ar-CH2-eq, 2JHH = 12.9 Hz), 3.22 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.27 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.71-3.90 (m, 4H, OCH2), 3.79 (s, 3H, MeO), 4.22 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.35 (d, 1H, Ar-CH2-ax, 2JHH = 12.9 Hz), 4.44 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.45 (d, 1H, OCH2, 2JHH = 15.6 Hz), 4.53 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.57 (d, 1H, OCH2, 2JHH = 15.6 Hz), 5.71 (s, 1H, OH), 6.45 (s, 2H, ArH), 6.61 (s, 1H, ArH), 6.64 (s, 1H, ArH), 7.01 (s, 1H, ArH), 7.06 (s, 1H, ArH), 7.10 (s, 1H, ArH), 7.12 (s, 1H, ArH). The calc. % was C 79.06; H 9.01. C53H72O6. The found % was C 80.02; H 9.13.
Dipropyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes5c +
6c had a total yield of 94% (
de of 33%) [
17]. After column chromatography (hexane-ethyl acetate, 6:1), the following compounds were isolated:
The first fraction: Calix[4]arene 6c had a yield of 41%, m.p. 106–107 °C (MeCN). 1H NMR (CDCl3), δ: 0.58 (t, 3H, MePr, 3JHH = 7.8 Hz), 0.86 (s, 9H, t-Bu), 0.91 (s, 9H, t-Bu), 0.98 (t, 3H, MePr, 3JHH = 7.5 Hz), 1.27 (s, 9H, t-Bu), 1.32 (s, 9H, t-Bu), 1.55 (d, 3H, MeAmid, 3JHH = 7.2 Hz), 1.73–1.91 (m, 4H, -CH2-), 3.17 (d, 2H, Ar-CH2-eq, 2JHH = 12.4 Hz), 3.22 (d, 1H, Ar-CH2-eq, 2JHH = 12.9 Hz), 3.37 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.71–3.91 (m, 4H, OCH2), 4.08 (d, 1H, CH2CO, 2JHH = 16.0 Hz), 4.16 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.31 (d, 1H, Ar-CH2-ax, 2JHH = 12.9 Hz), 4.33 (d, 2H, Ar-CH2-ax, 2JHH = 12.4 Hz), 4.73 (d, 1H, CH2CO, 2JHH = 16.0 Hz), 5.23–5.34 (m, 1H, CH), 5.75 (s, 1H, OH), 6.59 (s, 1H, ArH), 6.61 (s, 1H, ArH), 6.67 (s, 1H, ArH), 6.71 (s, 1H, ArH), 7.08 (s, 2H, ArH), 7.09 (s, 1H, ArH), 7.11 (s, 1H, ArH), 7.26–7.31 (m, 1H, PhH), 7.33–7.40 (m, 2H, PhH), 7.49 (d, 2H, PhH), 8.68 (d, 1H, NH, 3JHH = 7.5 Hz). The calc. % was C 79.44; H 8.60; N 3.29. C60H79NO5 + CH3CN. The found % was C 79.16; H 8.81; N 3.66.
The second fraction: Calix[4]arene 5c had a yield of 69%, m.p. 104 °C (MeCN). 1H NMR (CDCl3), δ: 0.84 (s, 9H, t-Bu), 0.87 (s, 9H, t-Bu), 0.94 (t, 3H, MePr, 3JHH = 7.6 Hz), 1.09 (t, 3H, MePr, 3JHH = 7.6 Hz), 1.30 (s, 9H, t-Bu), 1.32 (s, 9H, t-Bu), 1.64 (d, 3H, MeAmid, 3JHH = 7.1 Hz), 1.88–2.02 (m, 2H, -CH2-), 2.05–2.17 (m, 2H, -CH2-), 3.04 (d, 1H, Ar-CH2-eq, 2JHH = 12.9 Hz), 3.19 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.21 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.35 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.72–3.80 (m, 1H, OCH2), 3.84–3.94 (m, 2H, OCH2), 3.98–4.06 (m, 1H, OCH2), 4.01 (d, 1H, CH2CO, 2JHH = 15.4 Hz), 4.09 (d, 1H, Ar-CH2-ax, 2JHH = 12.9 Hz), 4.17 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.34 (d, 1H, Ar-CH2-ax, 2JHH = 12.4 Hz), 4.39 (d, 1H, Ar-CH2-ax, 2JHH = 12.4 Hz), 4.69 (d, 1H, CH2CO, 2JHH = 15.4 Hz), 5.24–5.33 (m, 1H, CH), 5.66 (s, 1H, OH), 6.55 (s, 1H, ArH), 6.58 (s, 1H, ArH), 6.62 (s, 1H, ArH), 6.69 (s, 1H, ArH), 7.00–7.08 (m, 5H, PhH), 7.11 (s, 1H, ArH), 7.12 (s, 1H, ArH), 7.30–7.33 (m, 2H, ArH), 8.79 (d, 1H, NH, 3JHH = 8.3 Hz). The calc. % was C 80.58; H 8.90; N 1.57. C60H79NO5. The found % was C 80.92; H 8.83; N 1.36.
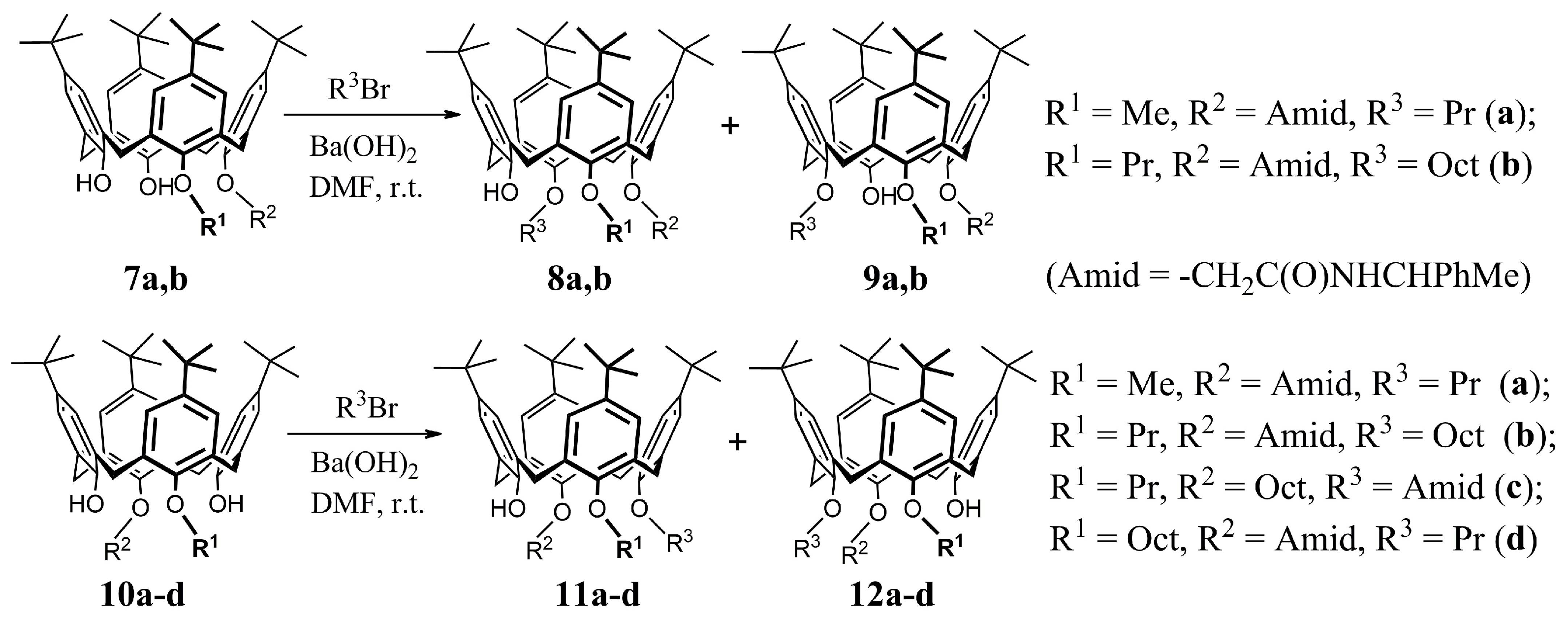
Methyloxy-propyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes8a +
9a had a total yield of 94% [
18]. Product
8a was formed regioselectively with a trace amount of the isomer
9a. Calixarene
8a was isolated after crystallization from MeCN with a yield of 63%. M.p. 206 °C (MeCN).
1H NMR (CDCl
3), δ: 0.80 (s, 9H,
t-Bu), 0.85 (s, 9H,
t-Bu), 1.12 (t, 3H, Me
Pr,
3JHH = 7.5 Hz), 1.34–1.41 (s, 18H,
t-Bu), 1.67 (d, 3H, Me
Amid,
3JHH = 7.2 Hz), 1.96 (m, 2H, -C
H2-), 3.14 (d, 1H, Ar-C
H2-eq,
2JHH = 13.1 Hz), 3.24 (d, 2H, Ar-C
H2-eq), 3.38 (d, 1H, Ar-C
H2-eq,
2JHH = 13.4 Hz), 3.80–3.92 (m, 2H, OC
H2), 3.98 (s, 3H, OMe), 4.11–4.35 (m, 4H, Ar-C
H2-ax + 1H, OC
H2), 4.63 (d, 1H, OC
H2,
2JHH = Hz), 5.30 (m, 1H, C
H), 5.47 (s, 1H, O
H), 6.51–6.62 (m, 4H, Ar
H), 7.09–7.39 (m, 9H, Ar
H + Ph
H), 8.88 (d, 1H, N
H,
3JHH = 8.7 Hz). The calc. % was C 80.42; H 8.73; N 1.62. C
58H
75NO
5. The found % was C 79.98, H 8.36, N 1.68.
Propyloxy-octyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes8b +
9b had a total yield of 96% (the ratio of iteromers is 9:1) [
19]. After column chromatography (hexane-ethyl acetate, 5:1), the following compounds were isolated:
The first fraction: Calix[4]arene 8b had a yield of 58%, m.p. 77–78 °C (MeCN). 1H NMR (CDCl3), δ: 0.75 (s, 9H, t-Bu), 0.85 (s, 9H, t-Bu), 0.86 (t, 3H, MeOct, 3JHH = 6.9 Hz), 0.94 (t, 3H, MePr, 3JHH = 7.4 Hz), 1.06–1.32 (m, 12H, -CH2-Oct), 1.34 (s, 18H, t-Bu), 1.79–1.87 (m, 2H, -CH2-Pr), 1.83 (d, 3H, MeAmid, 3JHH = 7.1 Hz), 3.17 (d, 1H, Ar-CH2-eq, 2JHH = 12.3 Hz), 3.19 (d, 1H, Ar-CH2-eq, 2JHH = 13.2 Hz), 3.24 (d, 1H, Ar-CH2-eq, 2JHH = 12.6 Hz), 3.28–3.38 (m, 2H, OCH2), 3.32 (d, 1H, Ar-CH2-eq, 2JHH = 13.2 Hz), 3.72–3.89 (m, 2H, OCH2), 4.22 (d, 1H, Ar-CH2-ax, 2JHH = 12.6 Hz), 4.23 (d, 1H, Ar-CH2-ax, 2JHH = 13.2 Hz), 4.24 (d, 1H, Ar-CH2-ax, 2JHH = 12.3 Hz), 4.27 (d, 1H, Ar-CH2-ax, 2JHH = 13.2 Hz), 4.37 (d, 1H, CH2CO, 2JHH = 14.3 Hz), 4.45 (d, 1H, CH2CO, 2JHH = 14.3 Hz), 5.34–5.40 (m, 1H, CH), 6.39 (s, 1H, OH), 6.42 (s, 1H, ArH), 6.48 (s, 1H, ArH), 6.57 (s, 1H, ArH), 6.66 (s, 1H, ArH), 7.07 (s, 1H, ArH), 7.10 (s, 1H, ArH), 7.15 (s, 1H, ArH), 7.17 (s, 1H, ArH), 7.23 (t, 1H, PhH, 3JHH = 7.4 Hz), 7.32 (t, 2H, PhH, 3JHH = 7.4 Hz), 7.60 (d, 2H, PhH, 3JHH = 7.4 Hz), 8.63 (s, 1H, NH). The calc. % was C 80.95, H 9.30, N 1.45. C65H89NO5. The found % was C 81.17, H 9.23, N 1.26.
The second fraction: Calix[4]arene 9b had a yield of 13%, m.p. 175–178 °C (MeCN). 1H NMR (CDCl3), δ: 0.82 (s, 9H, t-Bu), 0.85 (s, 9H, t-Bu), 0.86 (t, 3H, MeOct, 3JHH = 6.6 Hz), 0.94 (t, 3H, MePr, 3JHH = 7.4 Hz), 1.22–1.31 (m, 10H, -CH2-Oct), 1.31 (s, 9H, t-Bu), 1.33 (s, 9H, t-Bu), 1.65 (d, 3H, MeAmid, 3JHH = 6.9 Hz), 1.86–2.00 (m, 2H, -CH2-Pr), 2.08–2.16 (m, 2H, -CH2-Pr), 3.03 (d, 1H, Ar-CH2-eq, 2JHH = 13.2 Hz), 3.19 (d, 1H, Ar-CH2-eq, 2JHH = 12.3 Hz), 3.21 (d, 1H, Ar-CH2-eq, 2JHH = 12.3 Hz), 3.35 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.76–3.82 (m, 1H, OCH2), 3.87–3.93 (m, 2H, OCH2), 4.00–4.06 (m, 1H, OCH2), 4.02 (d, 1H, CH2CO, 2JHH = 15.4 Hz), 4.07 (d, 1H, Ar-CH2-ax, 2JHH = 13.2 Hz), 4.18 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.35 (d, 1H, Ar-CH2-ax, 2JHH = 12.3 Hz), 4.39 (d, 1H, Ar-CH2-ax, 2JHH = 12.3 Hz), 4.69 (d, 1H, CH2CO, 2JHH = 15.4 Hz), 5.25–5.31 (m, 1H, CH), 5.45 (s, 1H, OH), 6.52 (s, 1H, ArH), 6.55 (s, 1H, ArH), 6.59 (s, 1H, ArH), 6.65 (s, 1H, ArH), 7.01 (s, 1H, ArH), 7.04 (t, 2H, PhH, 3JHH = 7.0 Hz), 7.05 (t, 1H, PhH, 3JHH = 7.0 Hz), 7.06 (s, 1H, ArH), 7.11 (s, 1H, ArH), 7.13 (s, 1H, ArH), 7.32 (d, 2H, PhH, 3JHH = 7.4 Hz), 8.78 (d, 1H, NH, 3JHH = 8.2 Hz). The calc. % was C 80.95, H 9.30, N 1.45. C65H89NO5. The found % was C 79.37, H 8.93, N 1.52.
Methyloxy-propyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes11a +
12a had a total yield of 92% (the ratio of stereomers was 1:1) [
18]. After column chromatography (hexane-ethyl acetate, 6:1), the following compounds were isolated:
The first fraction: Calix[4]arene 11a had a yield of 38%; m.p. 204–205 °C (MeCN). 1H NMR (CDCl3), δ: 0.62 (t, 3H, MePr, 3JHH = 7.5 Hz), 0.88 (s, 9H, t-Bu), 0.97 (s, 9H, t-Bu), 1.22 (s, 9H, t-Bu), 1.30 (s, 9H, t-Bu), 1.56 (d, 3H, MeAmid, 2JHH = 3.7 Hz), 1.70 (m, 2H, -CH2-), 3.16–3.25 (d, 3H, Ar-CH2-eq), 3.41 (d, 1H, Ar-CH2-eq, 2JHH = 13.7 Hz), 3.60–3.80 (m, 2H, OCH2), 3.68 (s, 3H, OMe), 4.09 (d, 1H, CH2CO, 2JHH = 14.9 Hz), 4.10 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.23 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.31 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.34 (d, 1H, Ar-CH2-ax, 2JHH = 13.1 Hz), 4.77 (d, 1H, CH2CO, 2JHH = 14.9 Hz), 5.33 (m, 1H, CH), 6.65 (s, 1H, OH), 6.75 (s, 1H, ArH), 6.81 (s, 2H, ArH), 7.00 (s, 1H, ArH), 7.05 (s, 2H, PhH), 7.11 (s, 1H, ArH), 7.32 (s, 1H, ArH), 7.40 (m, 3H, PhH), 7.56 (m, 2H, PhH), 8.96 (d, 1H, NH, 3JHH = 8.1 Hz). The calc. % was C 80.42; H 8.73; N 1.62. C58H75NO5. The found, % was C 79.98, H 8.24, N 1.89.
The second fraction: Calix[4]arene 12a had a yield of 22%, m.p. 155–156 °C (MeOH-H2O). 1H NMR (CDCl3), δ: 0.87 (s, 9H, t-Bu), 0.97 (s, 9H, t-Bu), 1.01 (t, 3H, MePr, 3JHH = 7.5 Hz), 1.25 (s, 9H, t-Bu), 1.30 (s, 9H, t-Bu), 1.69 (d, 3H, MeAmid, 3JHH = 6.8 Hz), 2.09 (m, 2H, -CH2-), 3.08 (d, 1H, Ar-CH2-eq, 2JHH = 12.8 Hz), 3.23 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.25 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.38 (d, 1H, Ar-CH2-eq, 2JHH = 13.7 Hz), 3.83-3.96 (m, 2H, OCH2), 3.91 (s, 3H, OMe), 4.03 (d, 1H, CH2CO, 2JHH = 15.3 Hz), 4.05 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz, Ar-CH2-Ar), 4.14 (1H, d, 2JHH = 12.8 Hz, Ar-CH2-Ar), 4.35 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.37 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.75 (d, 1H, CH2CO, 2JHH = 15.3 Hz), 5.29 (m, 1H, CH), 6.60 (s, 1H, ArH), 6.66 (s, 1H, ArH), 6.75 (s, 1H, ArH + 1H, OH), 6.85 (s, 1H, ArH), 7.01–7.06 (m, 3H, PhH), 7.1 (m, 4H, ArH), 7.36 (d, 2H, PhH), 9.03 (d, 1H, NH, 3JHH = 8.1 Hz). The calc. % was C 80.42; H 8.73; N 1.62. C58H75NO5. The found % was C 80.11, H 8.27, N 1.73.
Propyloxy-octyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes11b +
12b had a total yield of 98% (
de of 30%) [
19]. After column chromatography (hexane-ethyl acetate, 10:1), the following compounds were isolated:
The first fraction: Calix[4]arene 12b had a yield of 26%, m.p. 74–76 °C (MeCN). 1H NMR (CDCl3), δ: 0.85 (s, 9H, t-Bu), 0.88 (t, 3H, MeOct, 0.89 (s, 9H, t-Bu), 0.98 (t, 3H, MePr, 3JHH = 7.4 Hz), 1.06–1.24 (m, 10H, -CH2-Oct), 1.27 (s, 9H, t-Bu), 1.31 (s, 9H, t-Bu), 1.54 (d, 3H, MeAmid, 3JHH = 6.9 Hz), 1.71–1.81 (m, 2H, -CH2-Oct), 1.82-1.89 (m, 2H, -CH2-Pr), 3.17 (d, 1H, Ar-CH2-eq, 2JHH = 12.6 Hz), 3.18 (d, 1H, Ar-CH2-eq, 2JHH = 12.6 Hz), 3.22 (d, 1H, Ar-CH2-eq, 2JHH = 12.9 Hz), 3.37 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.74–3.80 (m, 1H, OCH2), 3.81–3.89 (m, 2H, OCH2), 3.90–3.98 (m, 1H, OCH2), 4.10 (d, 1H, CH2CO, 2JHH = 15.4 Hz), 4.19 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.31 (d, 1H, Ar-CH2-ax, 2JHH = 12.6 Hz), 4.34 (d, 1H, Ar-CH2-ax, 2JHH = 12.9 Hz), 4.34 (d, 1H, Ar-CH2-ax, 2JHH = 12.6 Hz), 4.74 (d, 1H, CH2CO, 2JHH = 15.4 Hz), 5.25–5.32 (m, 1H, CH), 5.87 (s, 1H, OH), 6.57 (s, 1H, ArH), 6.59 (s, 1H, ArH), 6.65 (s, 1H, ArH), 6.69 (s, 1H, ArH), 7.05 (s, 1H, ArH), 7.06 (s, 1H, ArH), 7.07 (s, 1H, ArH), 7,10 (s, 1H, ArH), 7.28 (t, 1H, PhH, 3JHH = 7.1 Hz), 7.36 (t, 2H, PhH, 3JHH = 7.4 Hz), 7.49 (d, 2H, PhH, 3JHH = 7.1 Hz), 8.69 (d, 1H, NH, 3JHH = 8.3 Hz). The calc. % was C 80.95, H 9.30, N 1.45. C65H89NO5. The found % was C 81.17, H 8.63, N 1.32.
The second fraction: Calix[4]arene 11b had a yield of 48%, m.p. 171–174 °C (MeCN). 1H NMR (CDCl3), δ: 0.84 (s, 9H, t-Bu), 0.87 (s, 9H, t-Bu), 0.89 (t, 3H, MeOct, 3JHH = 6.7 Hz), 1.09 (t, 3H, MePr, 3JHH = 7.4 Hz), 1.21–1.37 (m, 10H, -CH2-Oct), 1.30 (s, 9H, t-Bu), 1.33 (s, 9H, t-Bu), 1.65 (d, 3H, MeAmid, 3JHH = 7.0 Hz), 1.88-2.00 (m, 2H, -CH2-Oct), 2.00–2.10 (m, 2H, -CH2-Pr), 3.03 (d, 1H, Ar-CH2-eq, 2JHH = 13.2 Hz), 3.19 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.21 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.35 (d, 1H, Ar-CH2-eq, 2JHH = 13.5 Hz), 3.72–3.80 (m, 1H, OCH2), 3.88–3.95 (m, 1H, OCH2), 3.97-4.05 (m, 1H, OCH2), 4.02 (d, 1H, CH2CO, 2JHH = 15.5 Hz), 4.06-4.11 (m, 1H, OCH2), 4.09 (d, 1H, Ar-CH2-ax, 2JHH = 13.2 Hz), 4.18 (d, 1H, Ar-CH2-ax, 2JHH = 13.5 Hz), 4.35 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.40 (d, 1H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.69 (d, 1H, CH2CO, 2JHH = 15.5 Hz), 5.21–5.29 (m, 1H, CH), 5.61 (s, 1H, OH), 6.54 (s, 1H, ArH), 6.57 (s, 1H, ArH), 6.60 (s, 1H, ArH), 6.67 (s, 1H, ArH), 7.02 (s, 1H, ArH), 7.05–7.08 (m, 3H, PhH + 1H, ArH), 7.11 (s, 1H, ArH), 7.12 (s, 1H, ArH), 7.31 (d, 2H, PhH, 3JHH = 7.4 Hz), 8.82 (d, 1H, NH, 3JHH = 8.1 Hz). The calc. % was C 80.95, H 9.30, N 1.45. C65H89NO5. The found % was C 80.37, H 9.19, N 1.12.
Propyloxy-octyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes11c +
12c had a total yield of 97% (the ratio of stereomers was 1:1) [
19]. After column chromatography (hexane-ethyl acetate, 10:1), the following compounds were isolated:
The first fraction: Calix[4]arene 12c had a yield of 31%, m.p. 85–87 °C (MeCN). 1H NMR (CDCl3), δ: 0.55 (t, 3H, MePr, 3JHH = 6.9 Hz), 0.76 (s, 9H, t-Bu), 0.84 (s, 9H, t-Bu), 0.87 (t, 3H, MeOct, 3JHH = 7.0 Hz), 1.22-1.32 (m, 12H, -CH2-Oct), 1.33 (s, 9H, t-Bu), 1.34 (s, 9H, t-Bu), 1.74–1.82 (m, 2H, -CH2-Pr), 1.85 (d, 3H, MeAmid, 3JHH = 6.9 Hz), 3.17 (d, 1H, Ar-CH2-eq, 2JHH = 13.2 Hz), 3.19 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.20–3.29 (m, 2H, OCH2), 3.24 (d, 1H, Ar-CH2-eq, 2JHH = 12.5 Hz), 3.32 (d, 1H, Ar-CH2-eq, 2JHH = 13.6 Hz), 3.74–3.93 (m, 2H, OCH2), 4.18 (d, 1H, Ar-CH2-ax, 2JHH = 13.6 Hz), 4.21 (d, 2H, Ar-CH2-ax, 2JHH = 12.5 Hz), 4.24 (d, 1H, Ar-CH2-ax, 2JHH = 13.2 Hz), 4.44 (d, 1H, CH2CO, 2JHH = 15.0 Hz), 4.54 (d, 1H, CH2CO, 2JHH = 15.0 Hz), 5.36–5.43 (m, 1H, CH), 6.34 (s, 1H, OH), 6.44 (s, 1H, ArH), 6.50 (s, 1H, ArH), 6.54 (s, 1H, ArH), 6.63 (s, 1H, ArH), 7.08 (s, 1H, ArH), 7.09 (s, 1H, ArH), 7.15 (s, 1H, ArH), 7,17 (s, 1H, ArH), 7.25 (t, 1H, PhH, 3JHH = 7.4 Hz), 7.33 (t, 2H, PhH, 3JHH = 7.4 Hz), 7.60 (d, 2H, PhH, 3JHH = 7.4 Hz), 8.77 (s, 1H, NH). The calc. % was C 80.95, H 9.30, N 1.45. C65H89NO5. The found % was C 80.37, H 9.53, N 1.06.
The second fraction: Calix[4]arene 11c had a yield of 33%. The structure and spectral characteristics coincide with compound 8b.
Propyloxy-octyloxy-N-(α-phenylethyl)aminocarbonylmethyloxy-tert-butyl-calix[4]arenes11d +
12d had a total yield of 98% (
de of 20%) [
19]. After column chromatography (hexane-ethyl acetate, 10:1), the following compounds were isolated:
The first fraction: Calix[4]arene 12d had a yield of 28%, m.p. 71–74 °C (MeCN). 1H NMR (CDCl3), δ: 0.55 (t, 3H, MePr, 3JHH = 7.4 Hz), 0.82 (s, 9H, t-Bu), 0.87 (s, 9H, t-Bu + 3H, MeOct, 1.22–1.44 (m, 10H, -CH2-Oct), 1.29 (s, 9H, t-Bu), 1.33 (s, 9H, t-Bu), 1.53 (d, 3H, MeAmid, 3JHH = 6.9 Hz), 1.75–1.93 (m, 2H, -CH2-Oct + 2H, -CH2-Pr), 3.17 (d, 2H, Ar-CH2-eq, 2JHH = 12.6 Hz), 3.22 (d, 1H, Ar-CH2-eq, 2JHH = 13.4 Hz), 3.37 (d, 1H, Ar-CH2-eq, 2JHH = 13.3 Hz), 3.71–3.93 (m, 4H, OCH2), 4.08 (d, 1H, CH2CO, 2JHH = 15.8 Hz), 4.19 (d, 1H, Ar-CH2-ax, 2JHH = 13.4 Hz), 4.30 (d, 1H, Ar-CH2-ax, 2JHH = 12.6 Hz), 4.32 (d, 1H, Ar-CH2-ax, 2JHH = 13.3 Hz), 4.33 (d, 1H, Ar-CH2-ax, 2JHH = 12.6 Hz), 4.69 (d, 1H, CH2CO, 2JHH = 15.8 Hz), 5.22–5.30 (m, 1H, CH), 5.63 (s, 1H, OH), 6.53 (s, 1H, ArH), 6.56 (s, 1H, ArH), 6.62 (s, 1H, ArH), 6.65 (s, 1H, ArH), 7.08 (s, 1H, ArH), 7.09 (s, 2H, ArH), 7,10 (s, 1H, ArH), 7.27 (t, 1H, PhH, 3JHH = 7.0 Hz), 7.37 (t, 2H, PhH, 3JHH = 7.0 Hz), 7.48 (d, 2H, PhH, 3JHH = 7.0 Hz), 8.61 (d, 1H, NH, 3JHH = 7.5 Hz). The calc. % was C 80.95, H 9.30, N 1.45. C65H89NO5. The found % was C 79.37, H 8.93, N 1.56.
The second fraction: Calix[4]arene 11d had a yield of 47%. The structure and spectral characteristics coincide with compound 9b.