Recent Advances in Catalytic and Technology-Driven Radical Addition to N,N-Disubstituted Iminium Species
Abstract
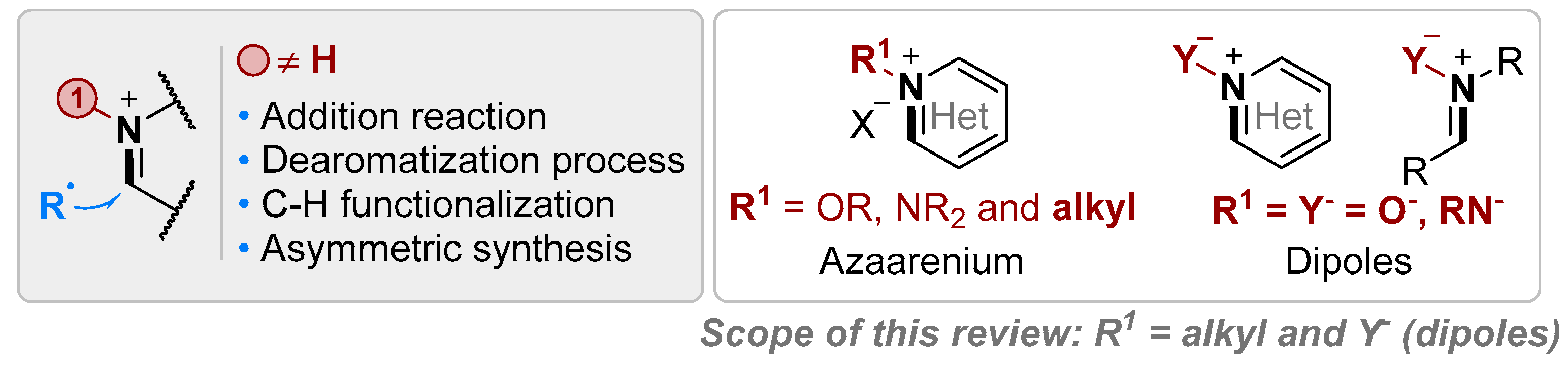
1. Introduction
2. Dipole Type Iminium Species
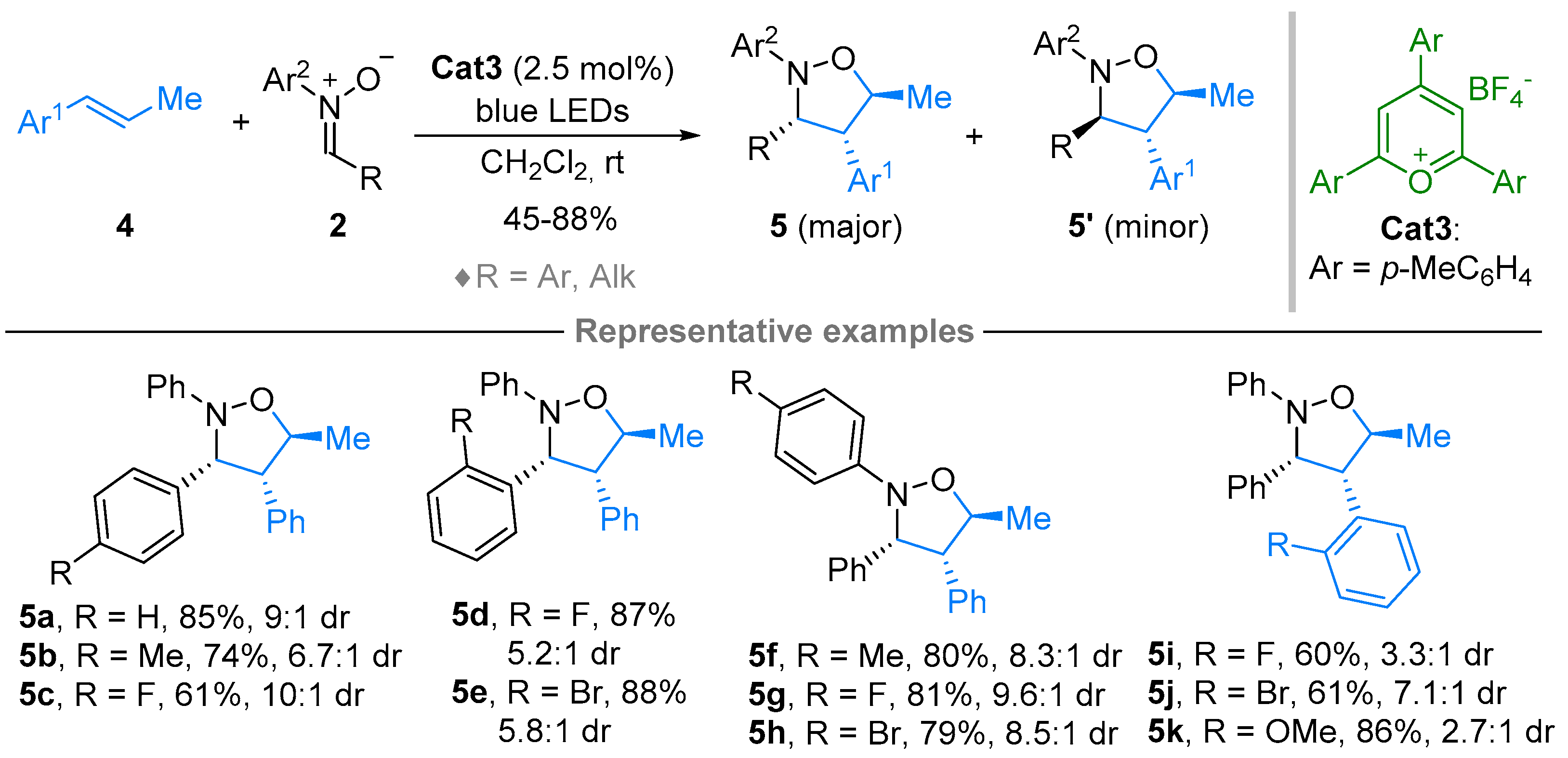
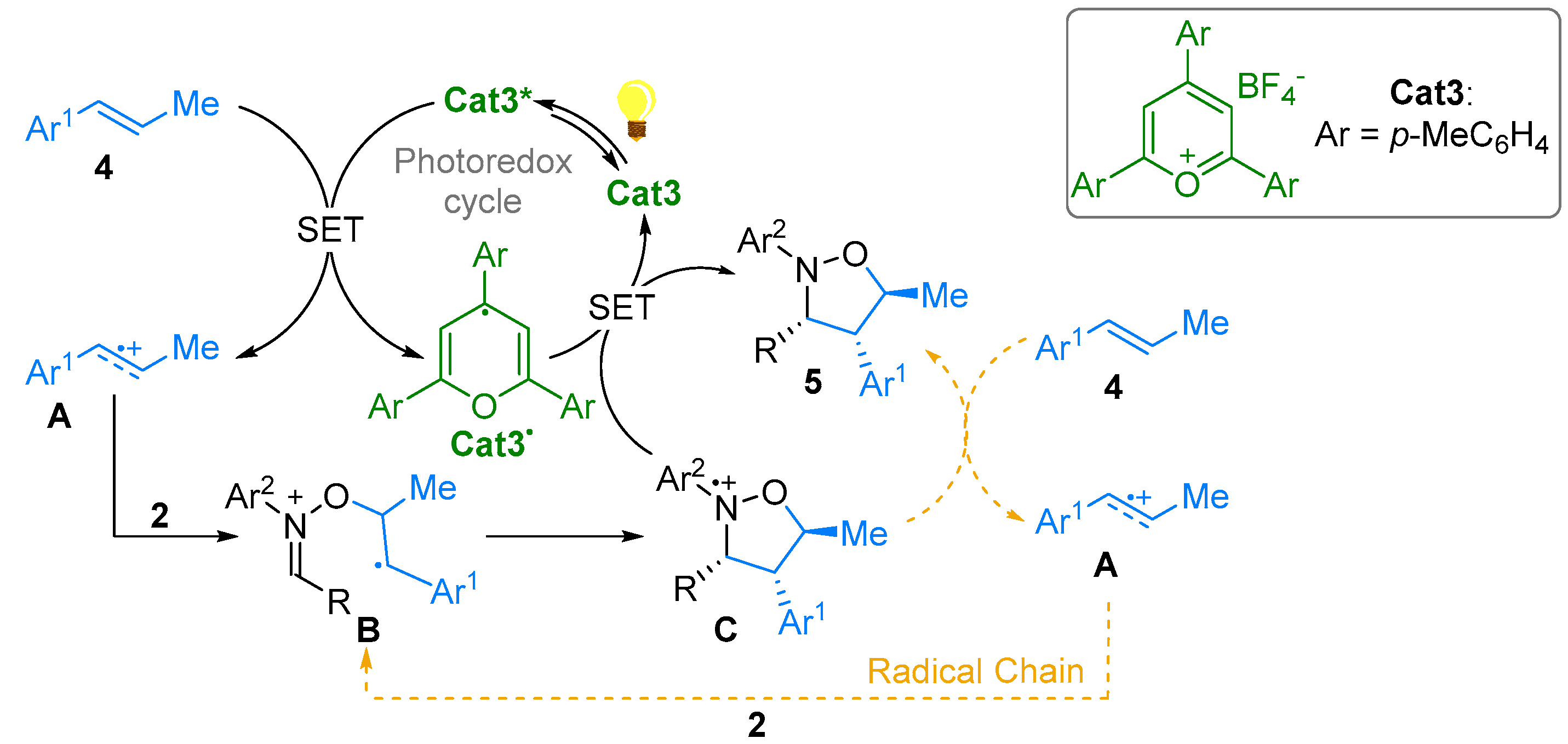
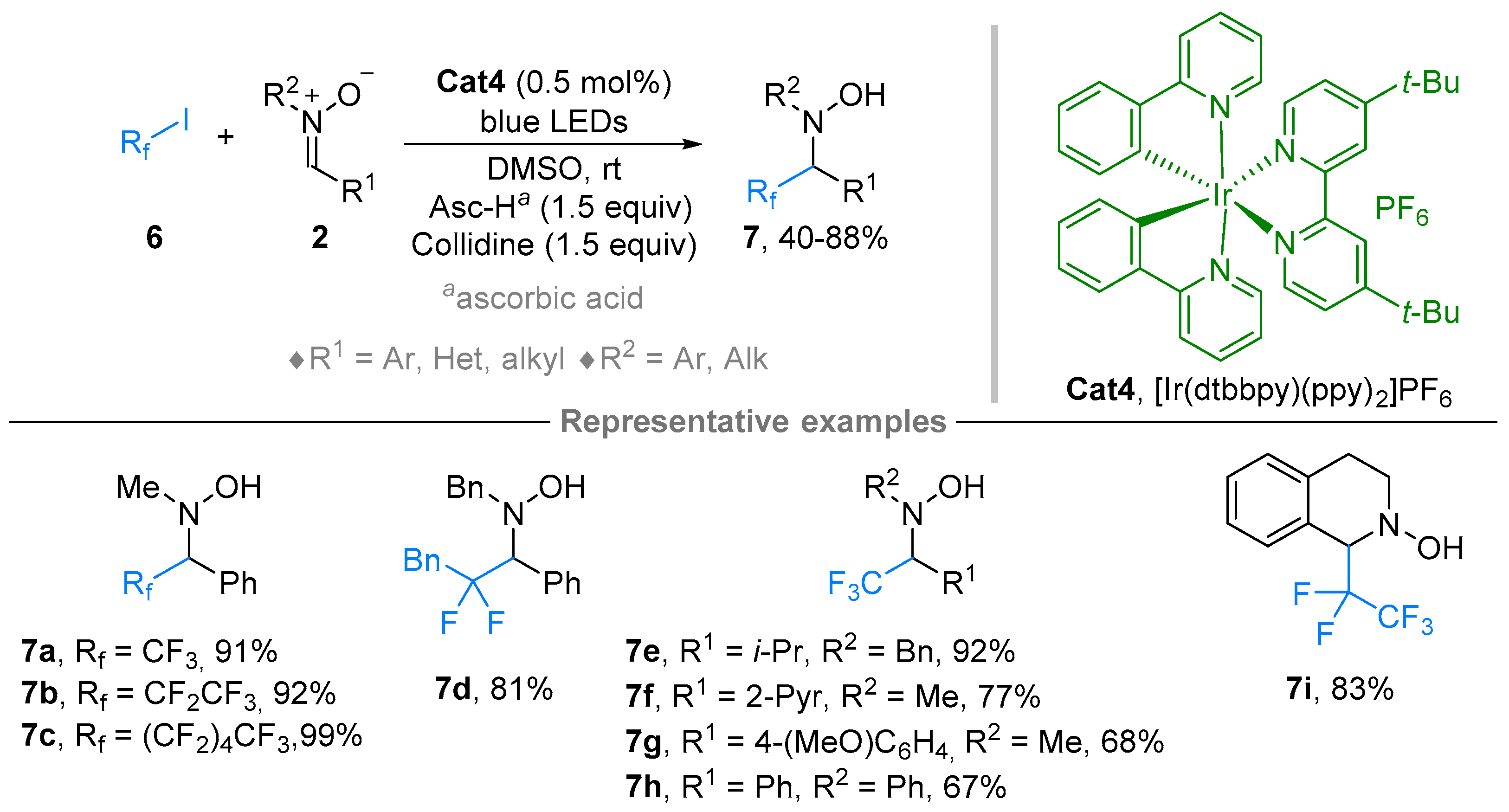
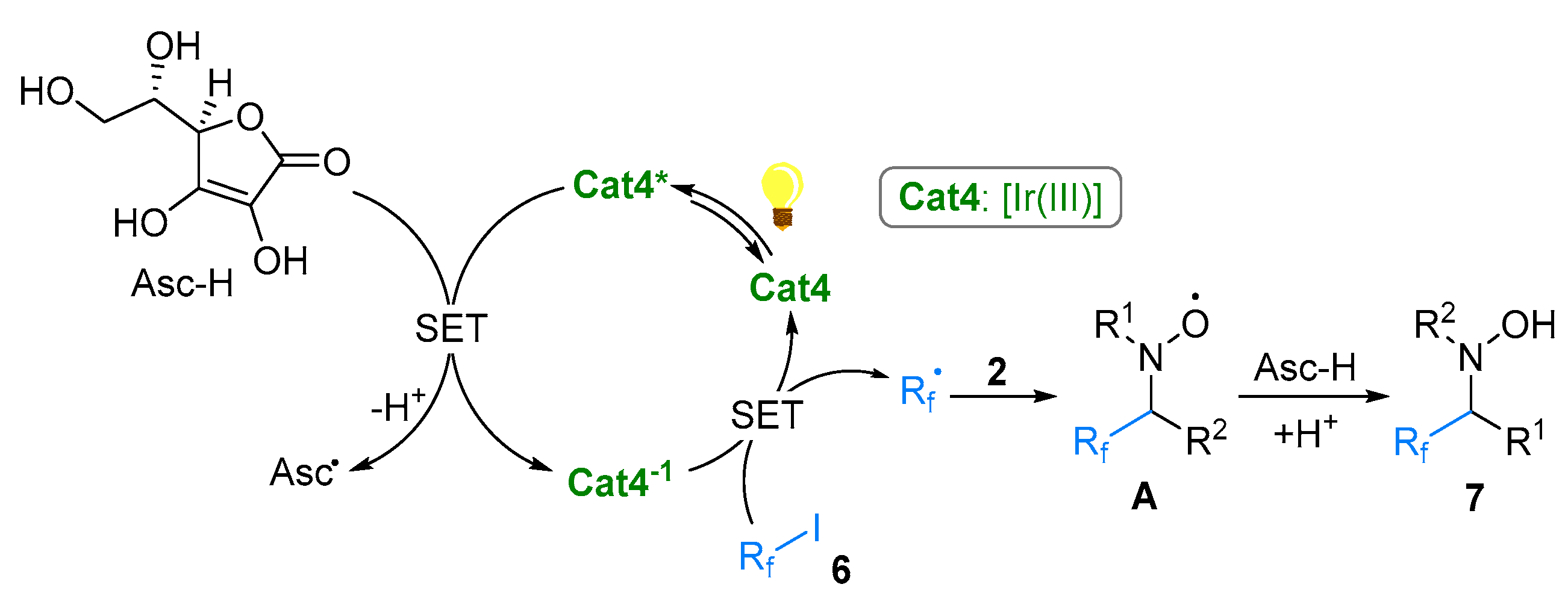
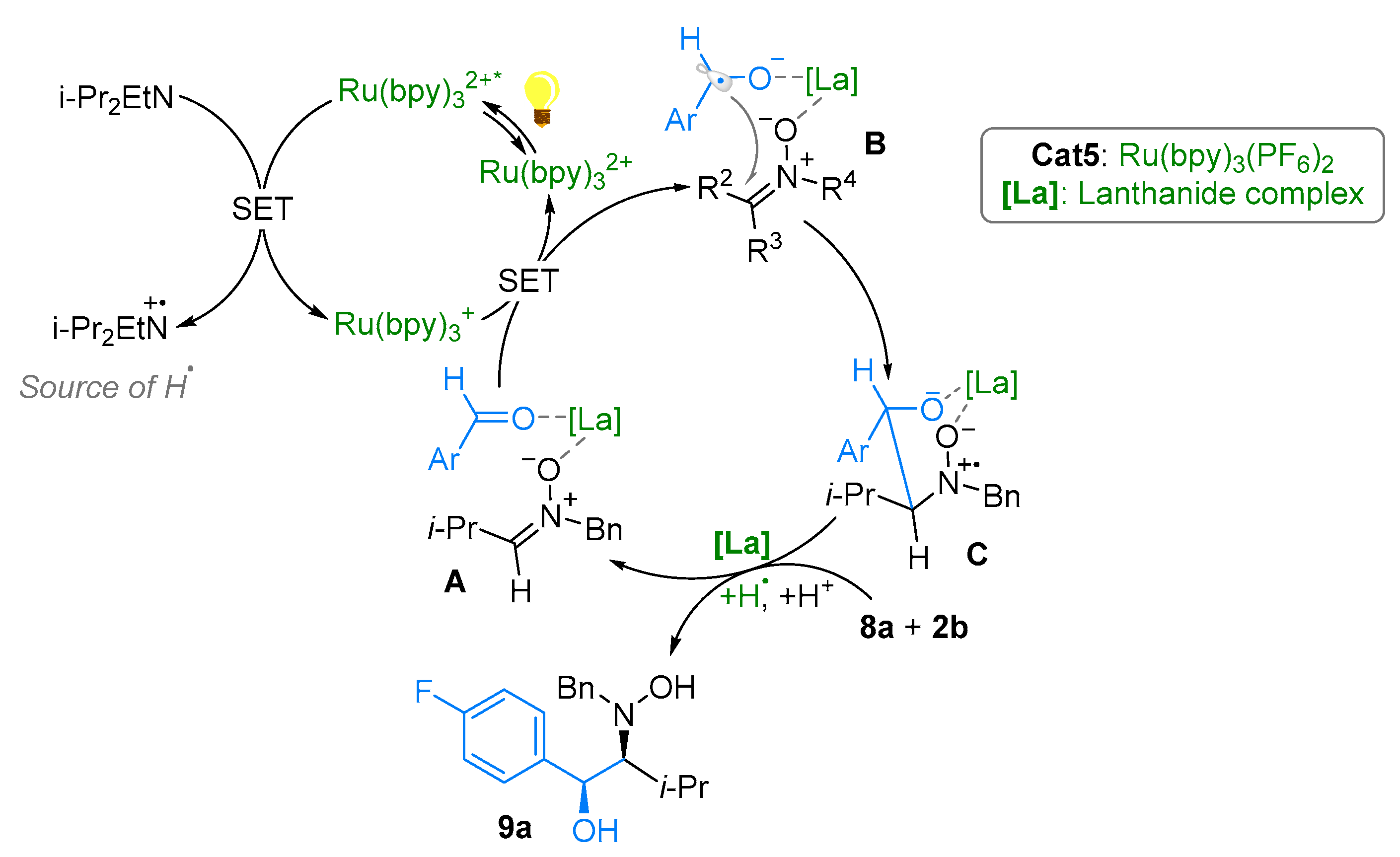
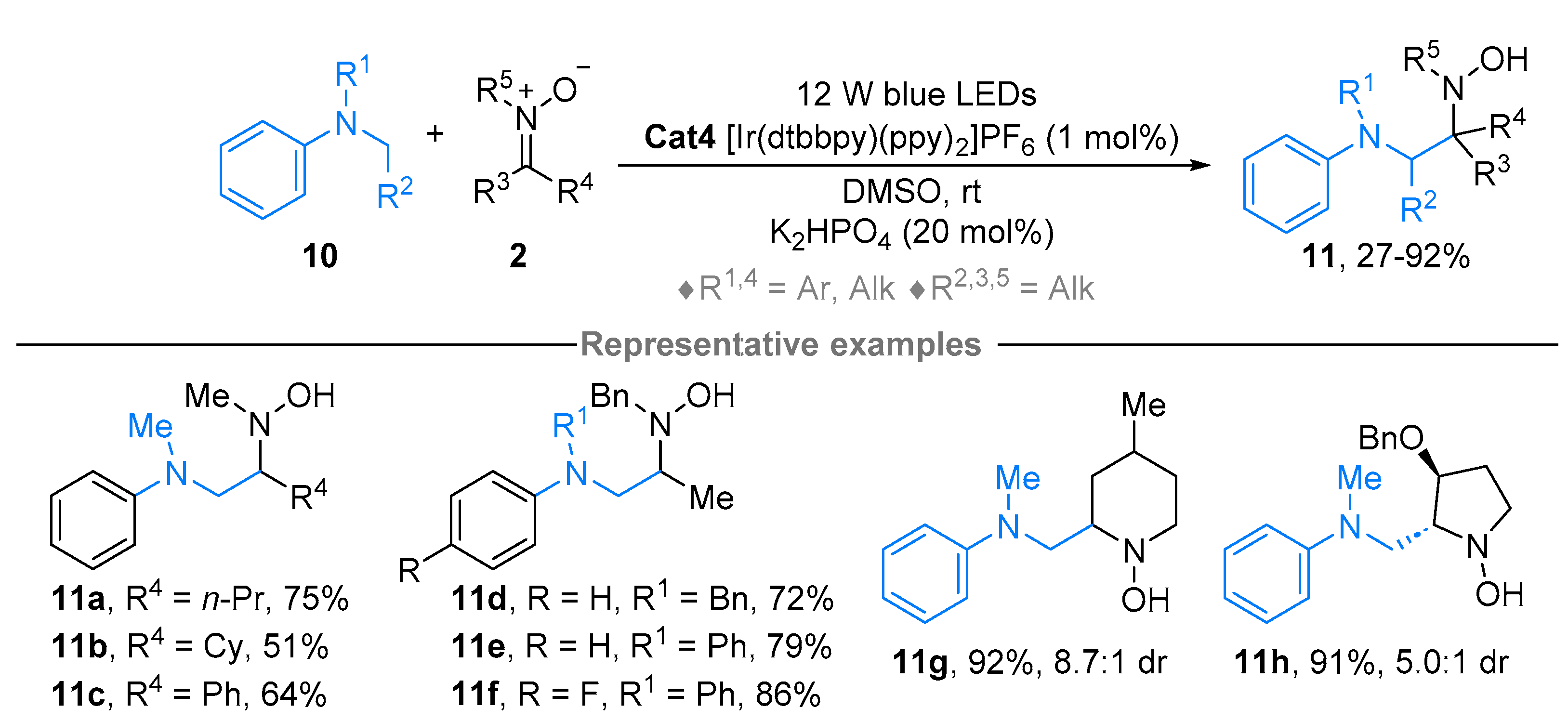
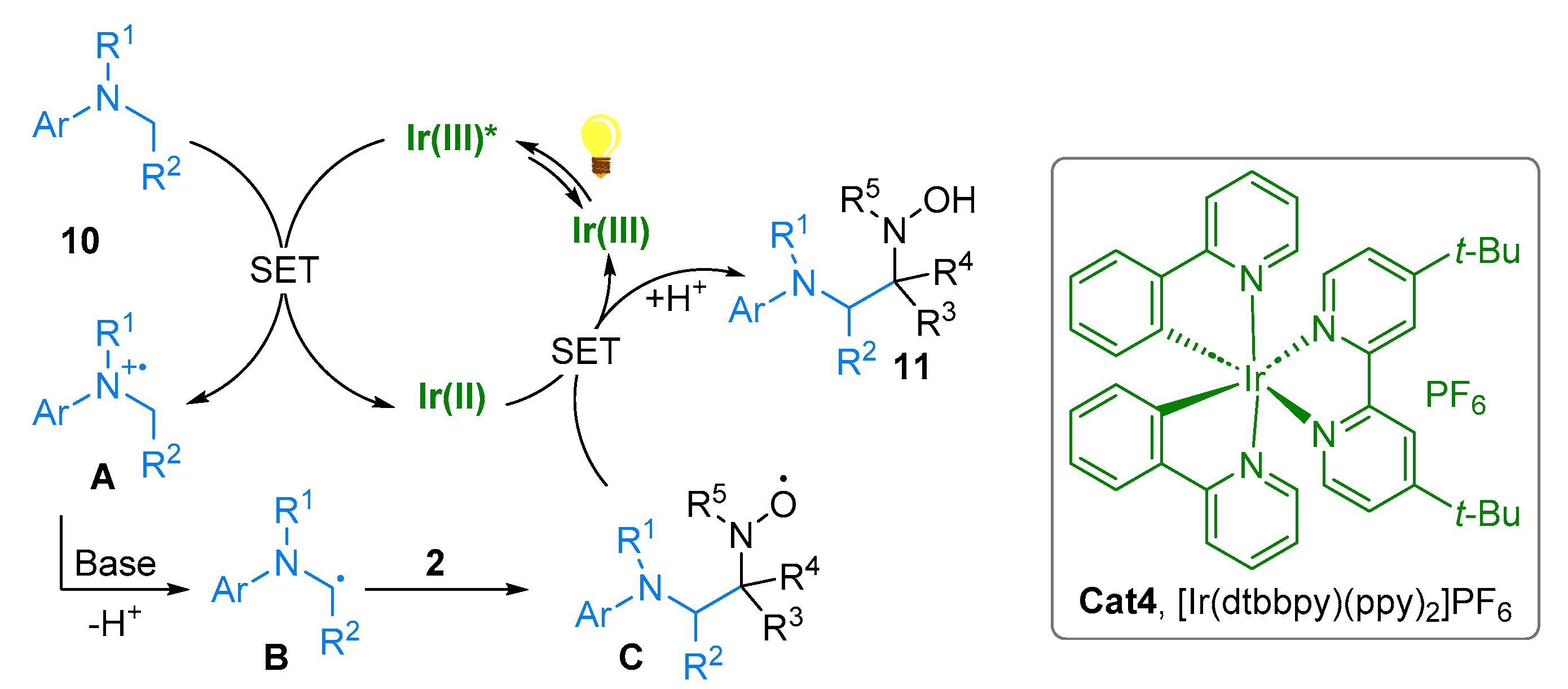
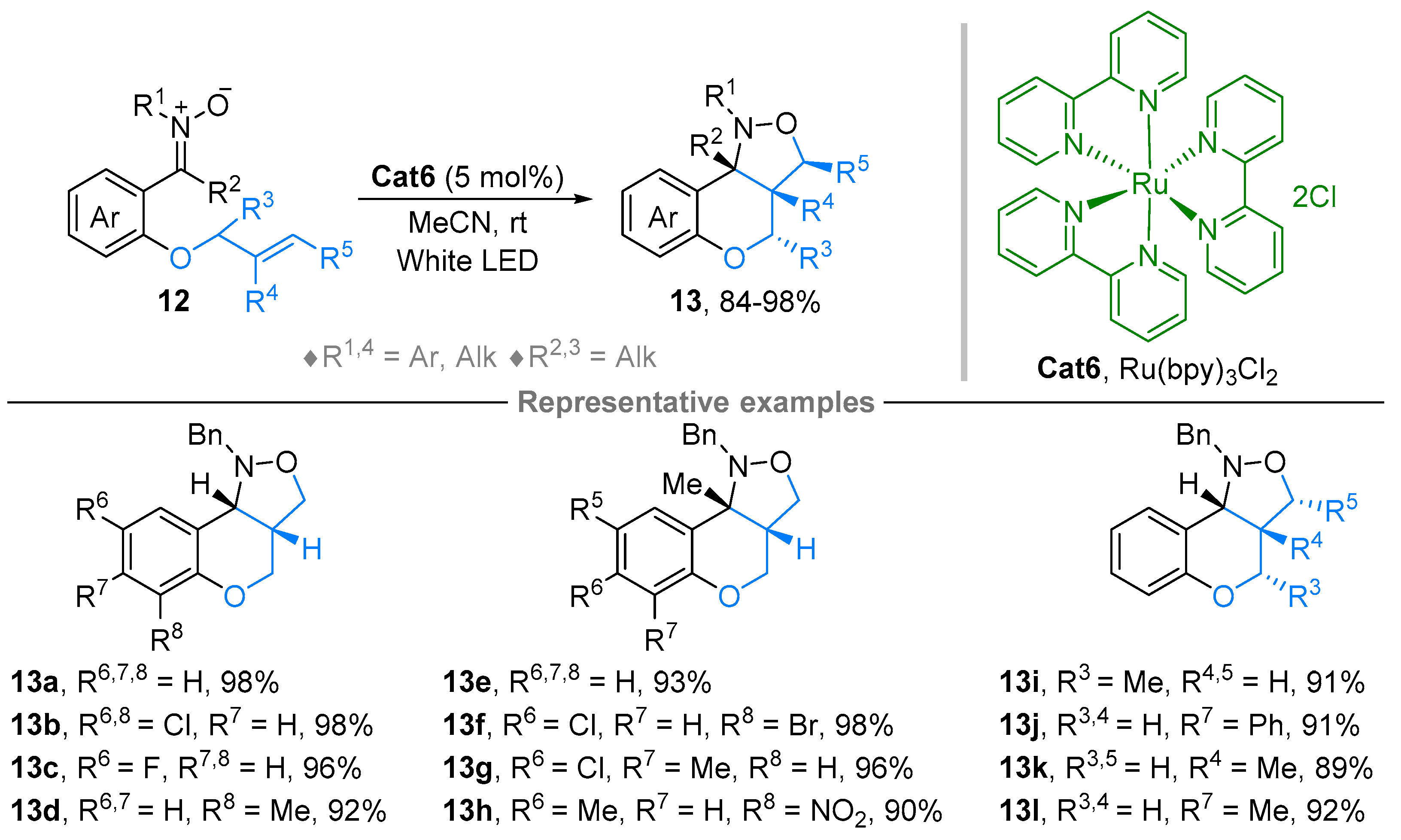
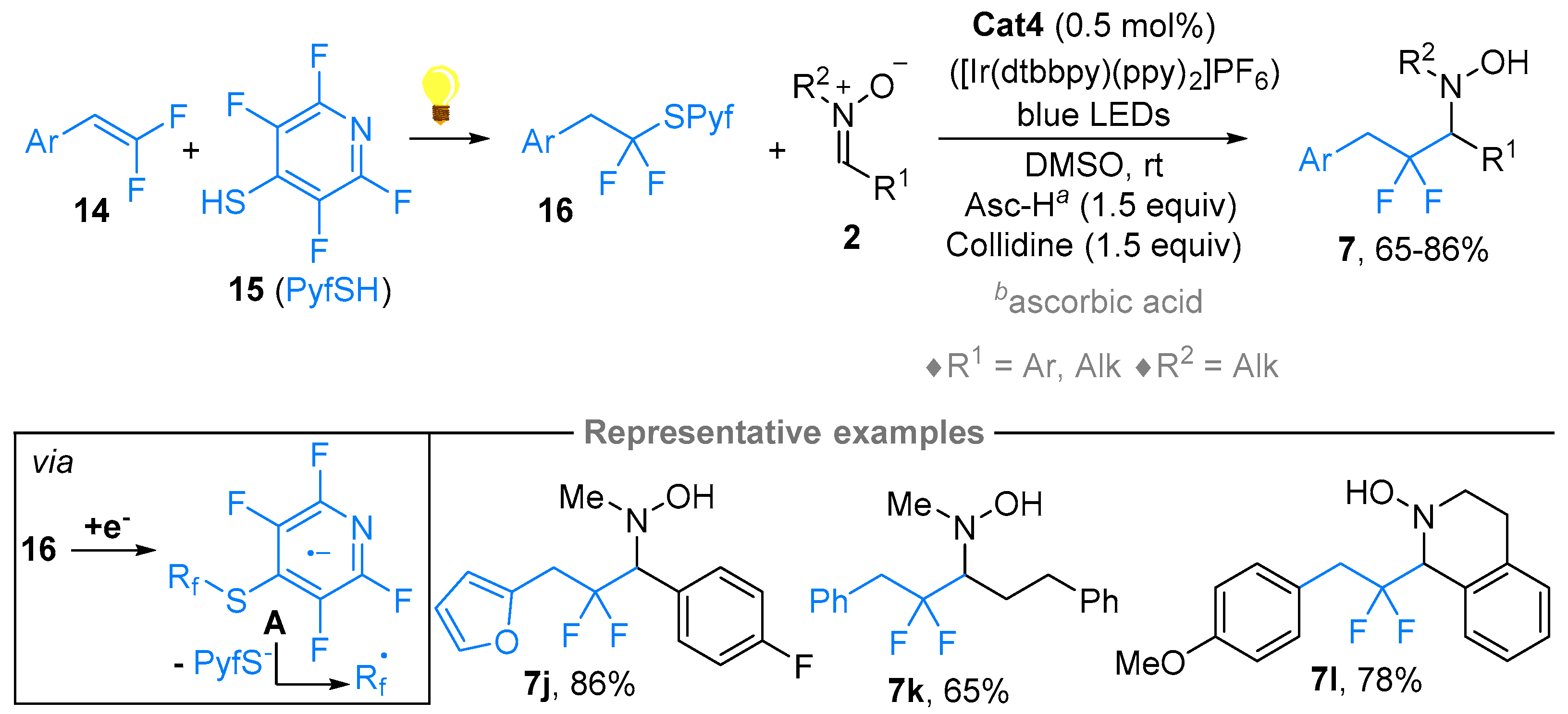
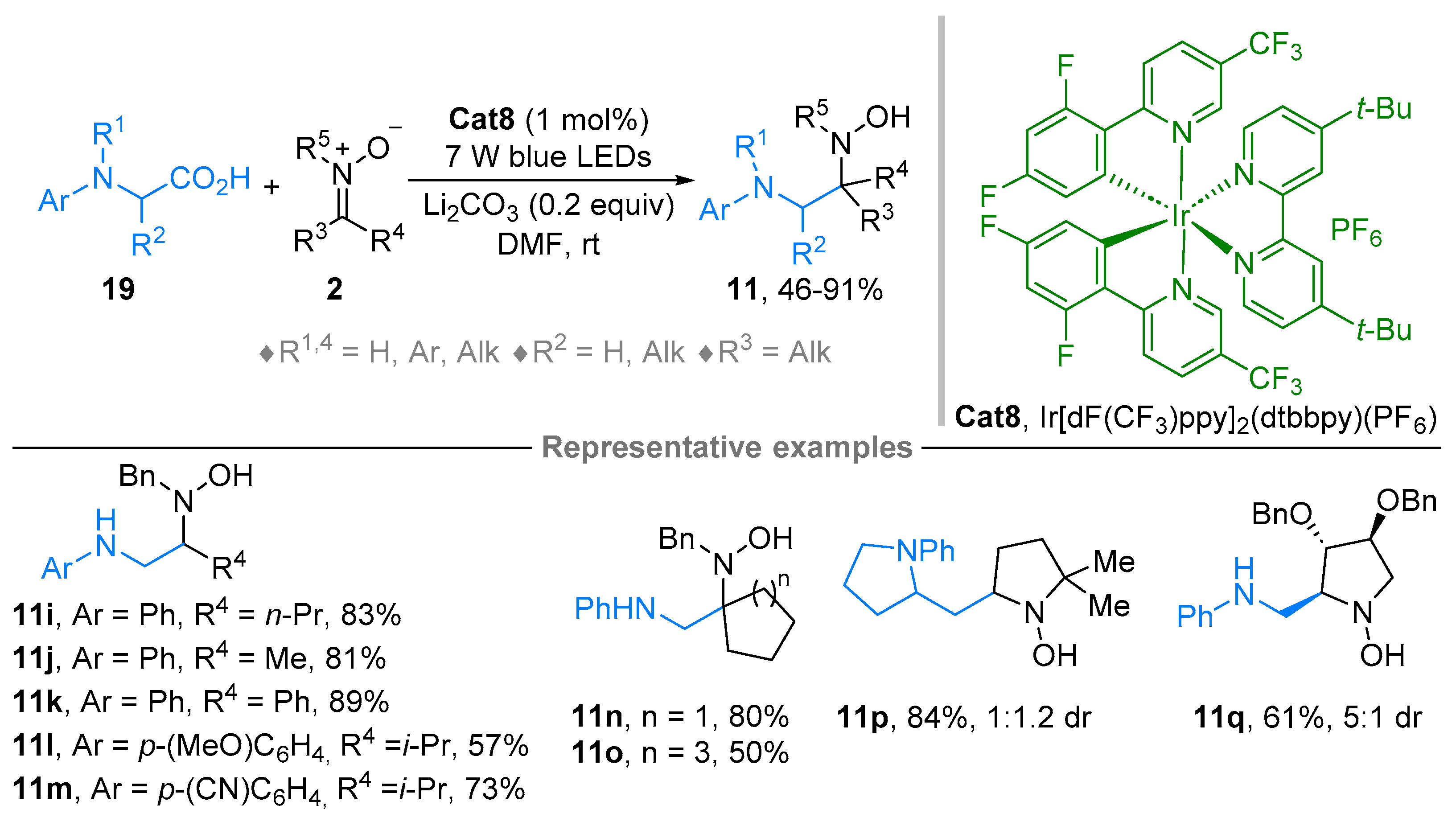
2.1. Nitrones
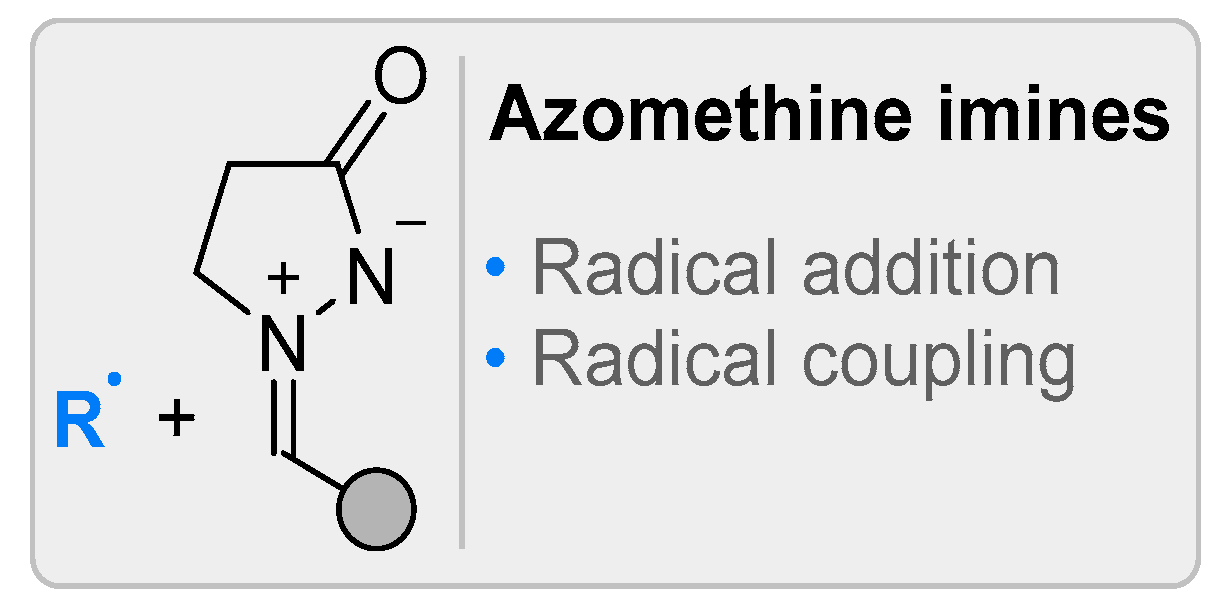
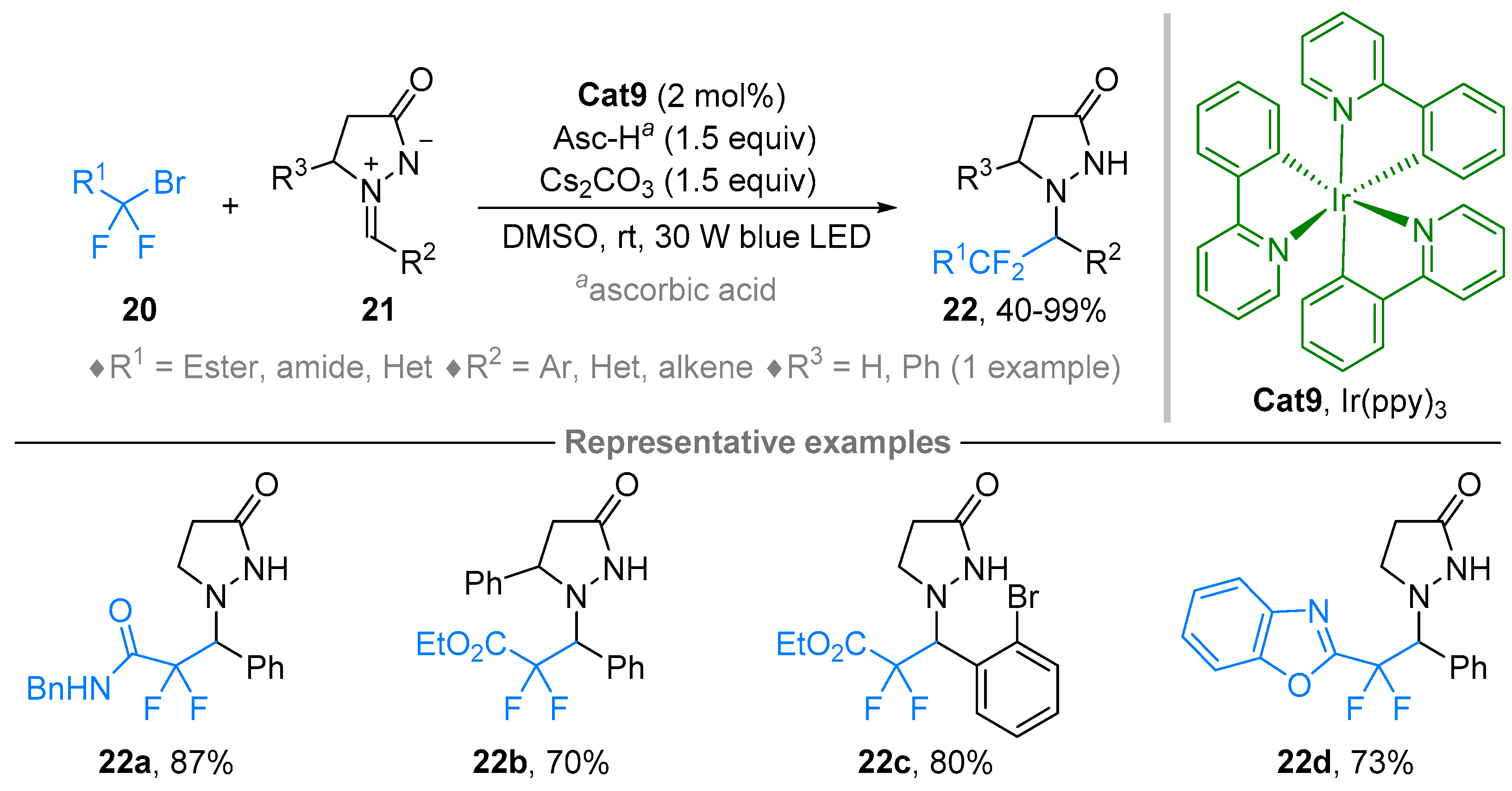
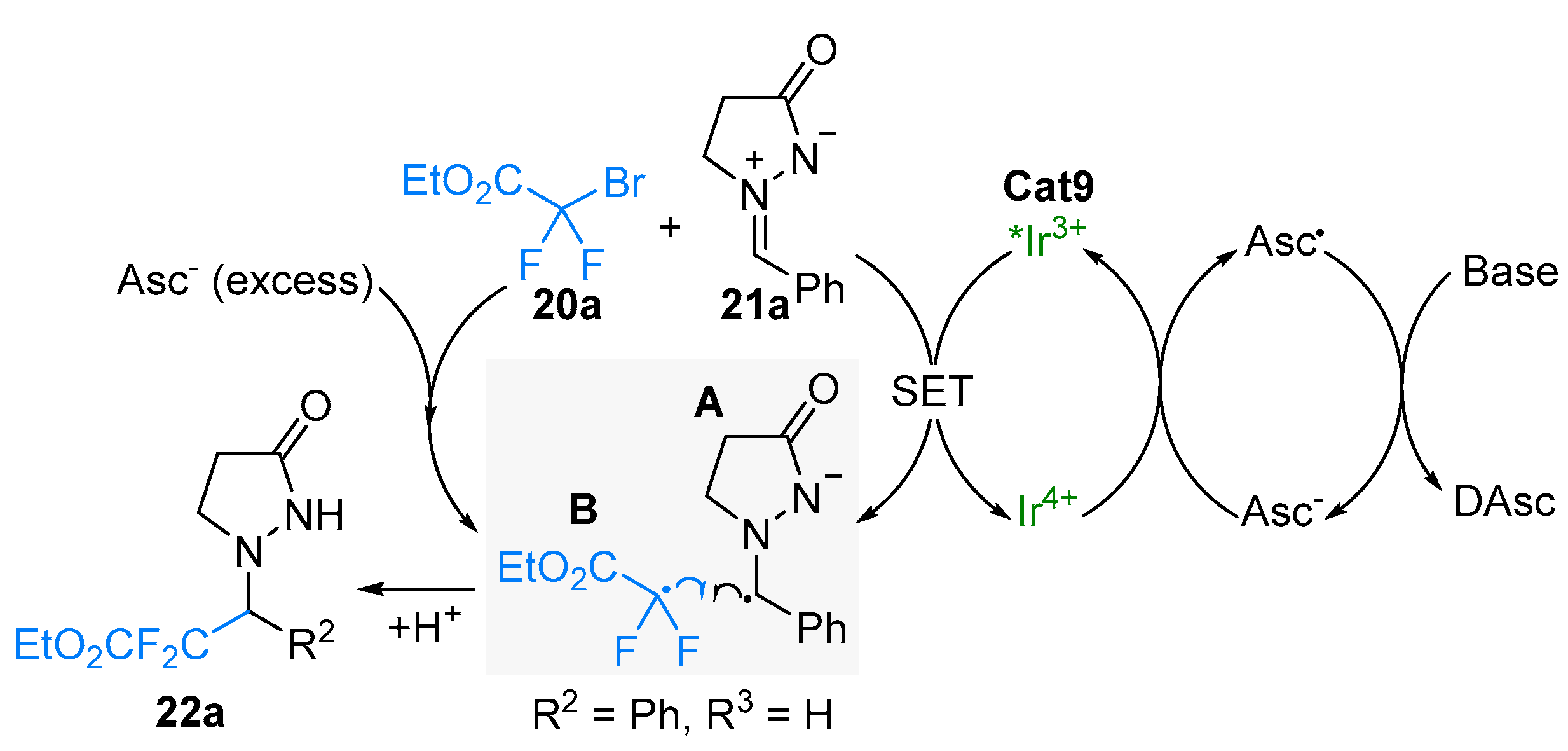
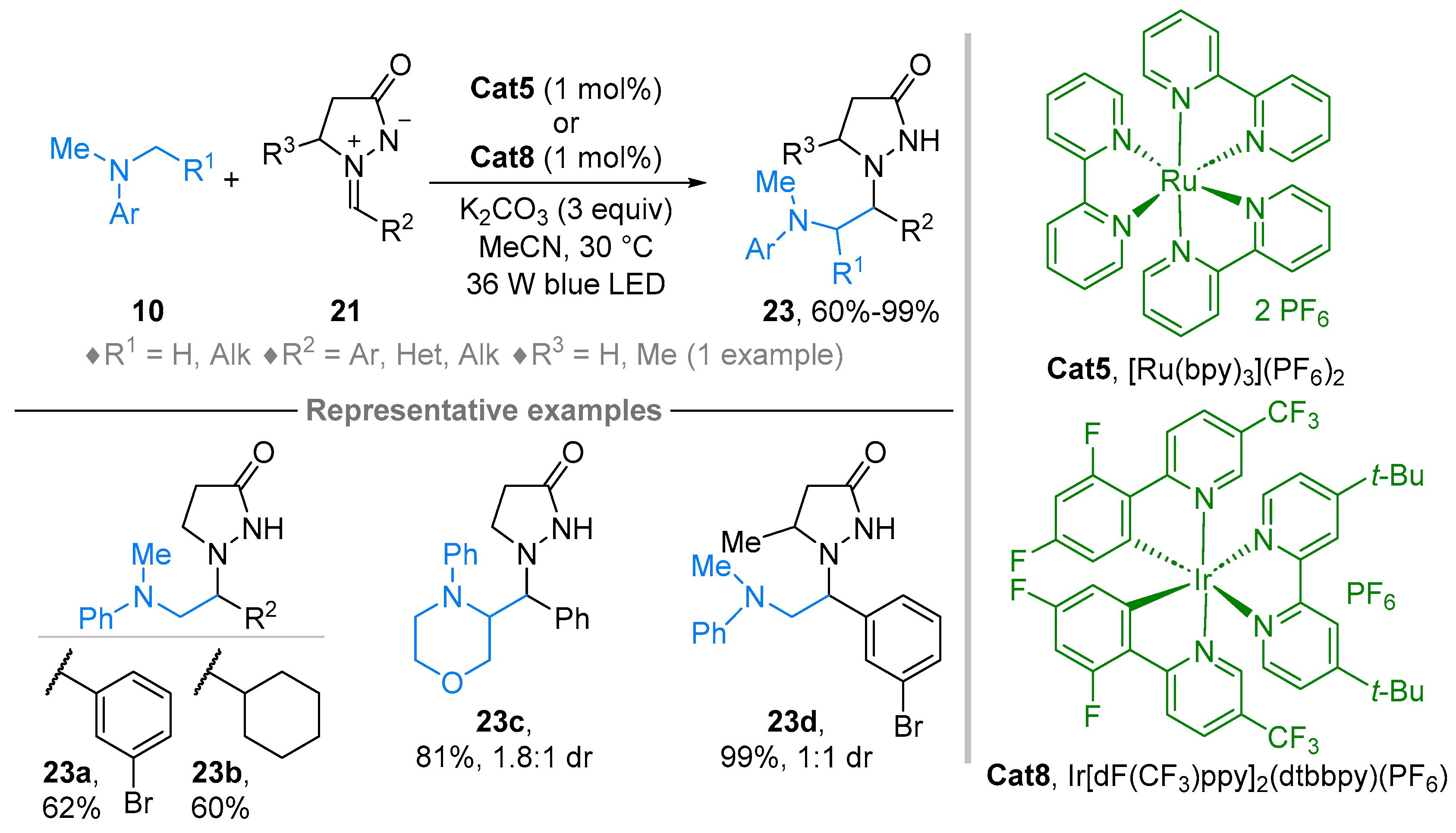
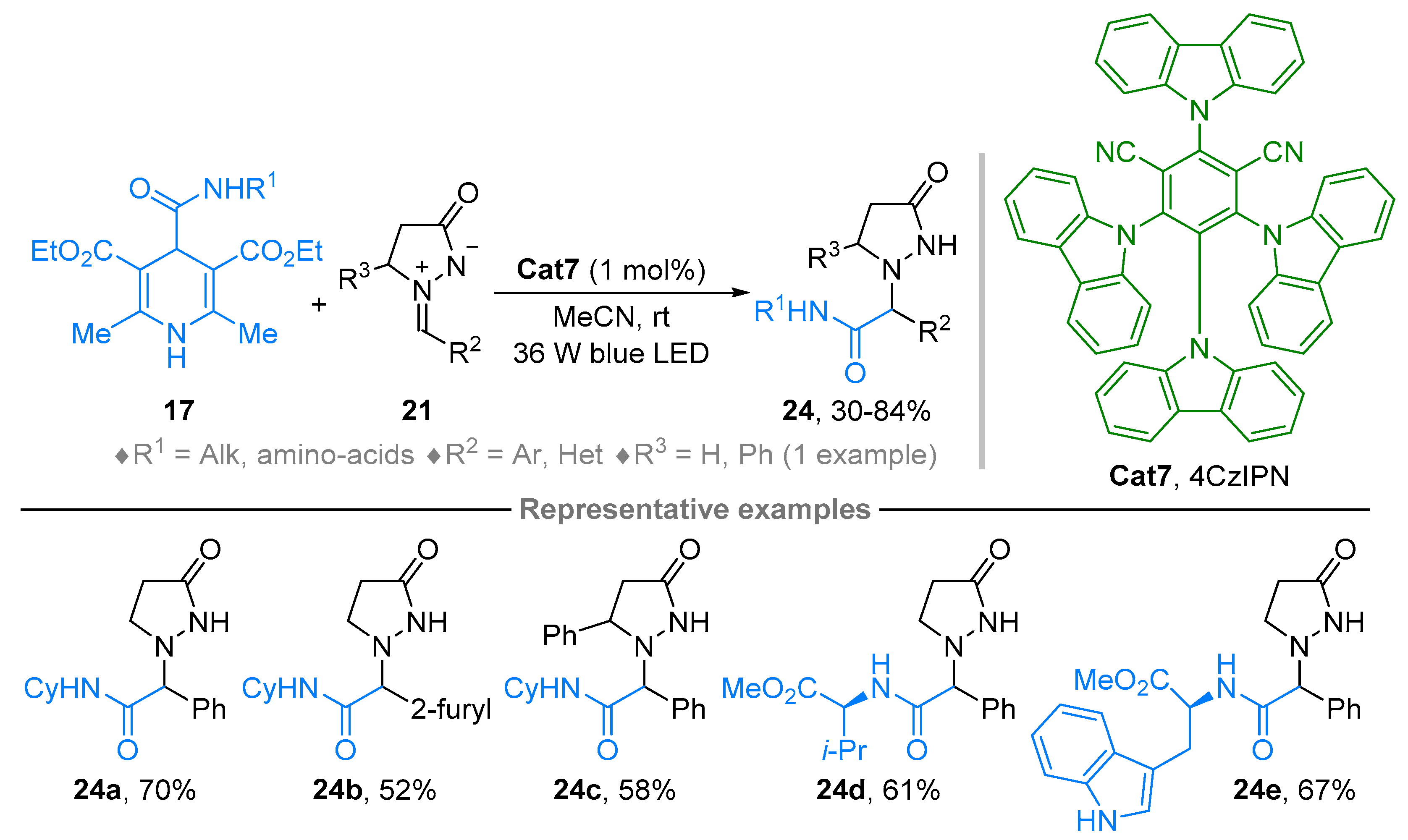
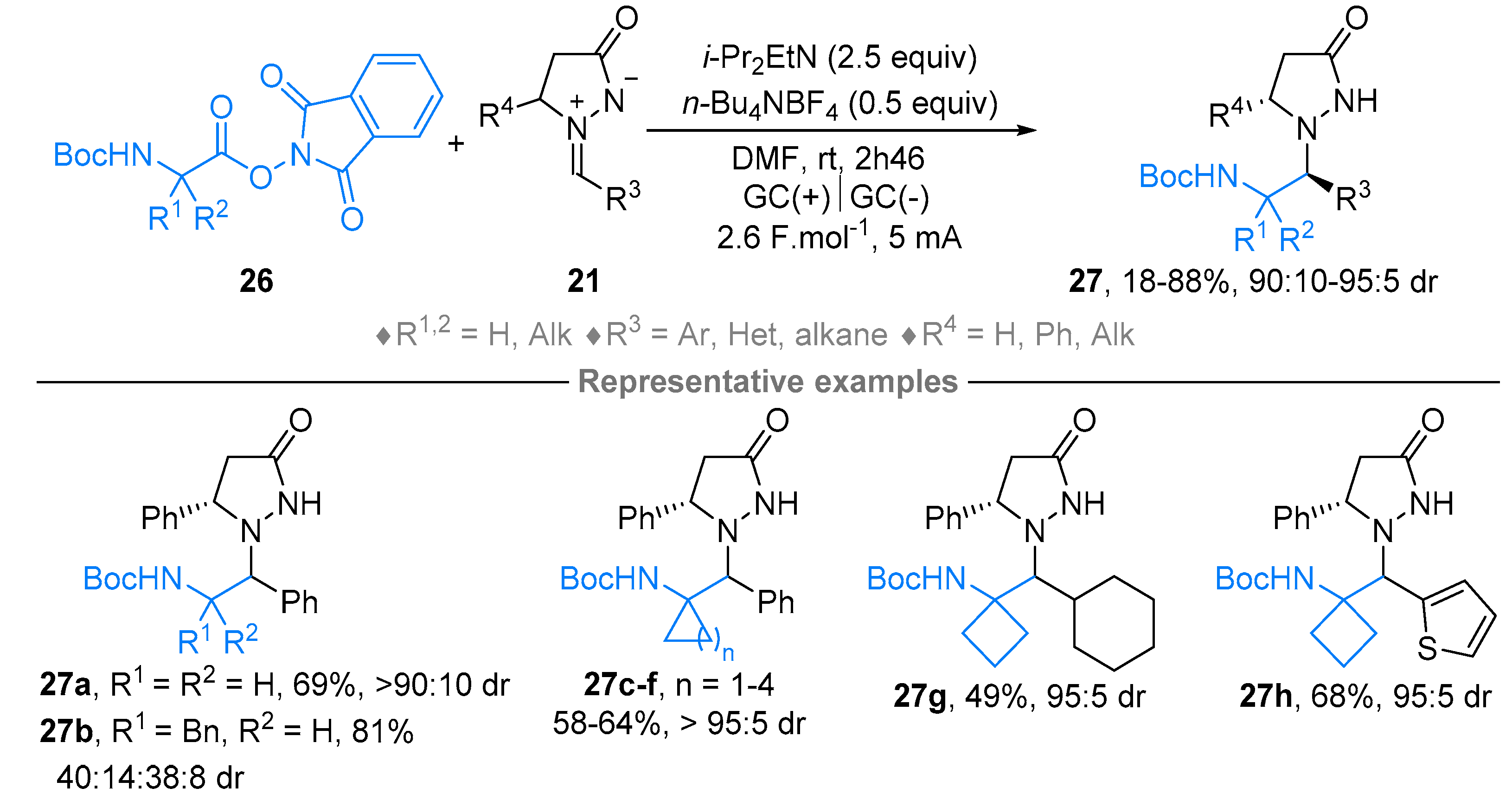
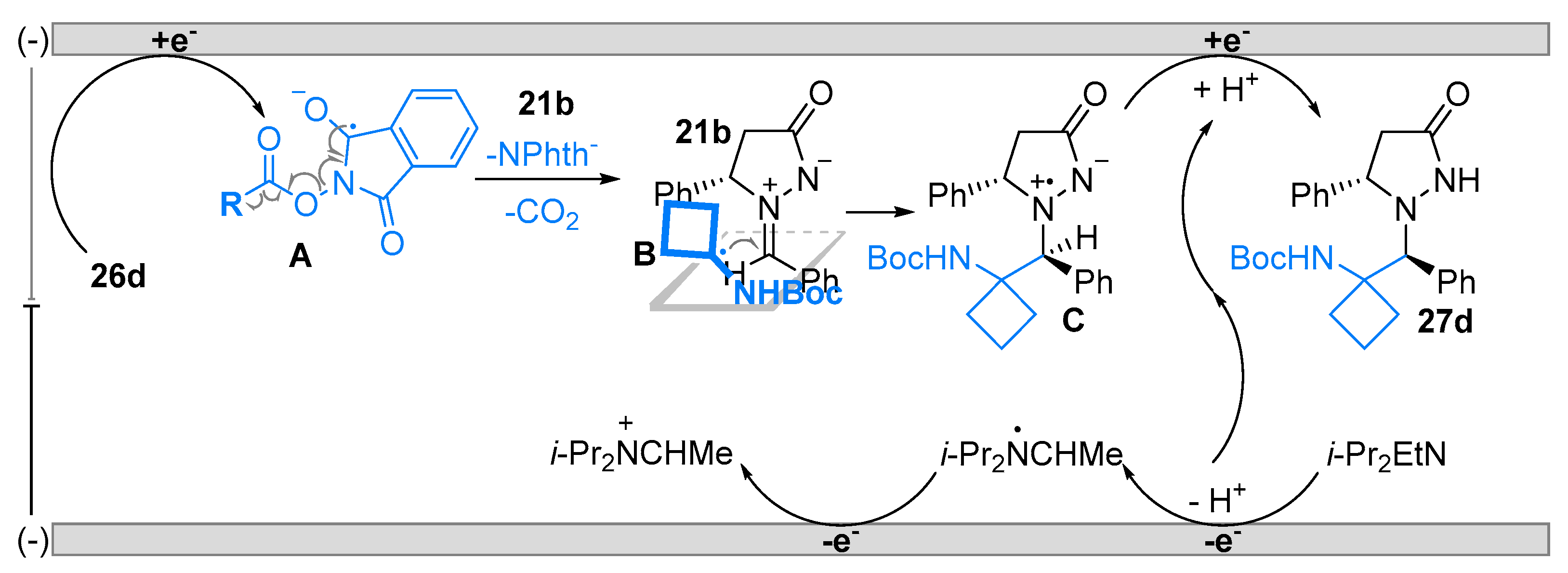
2.2. Azomethine Imines
2.3. Various Dipoles
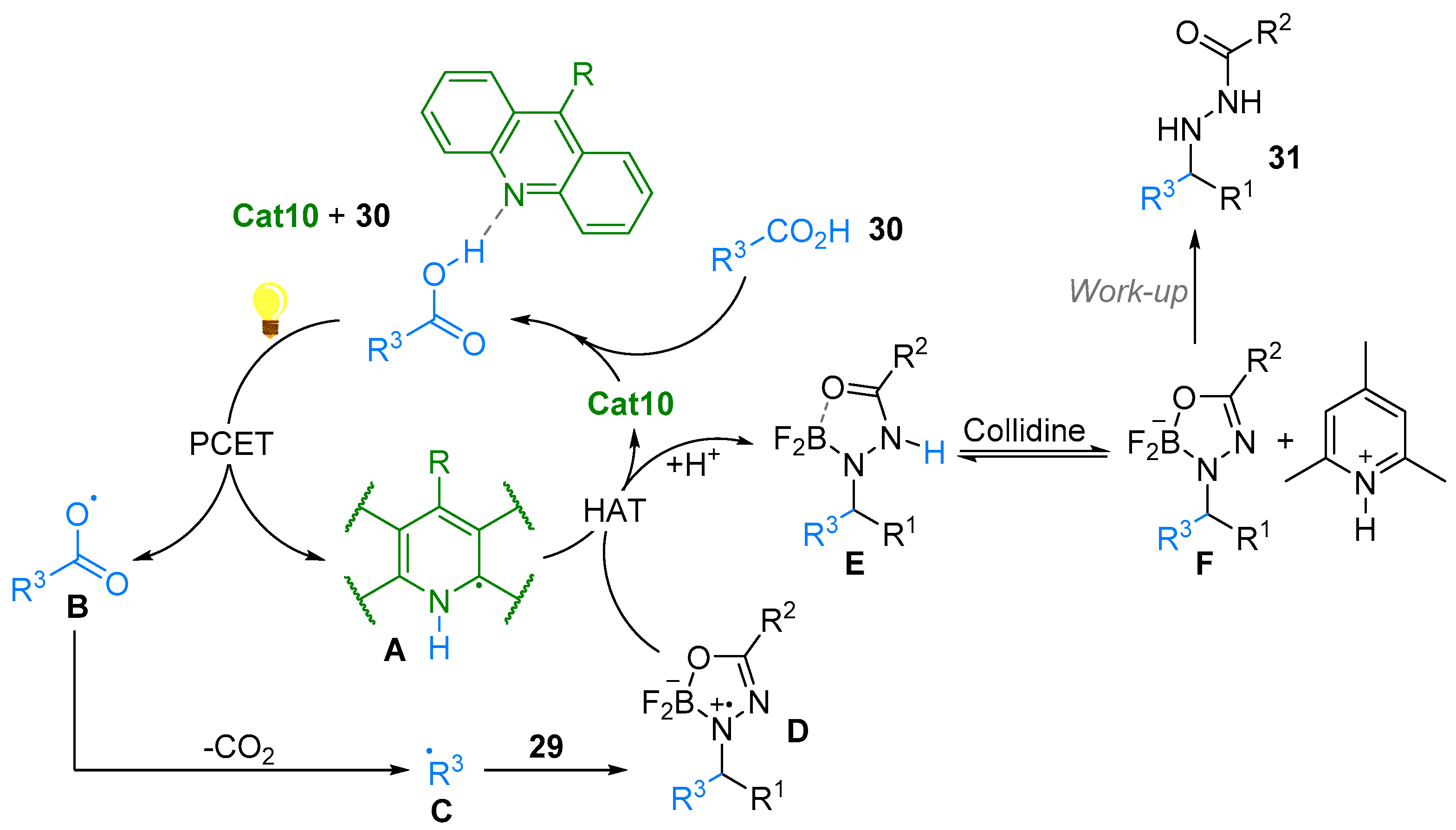
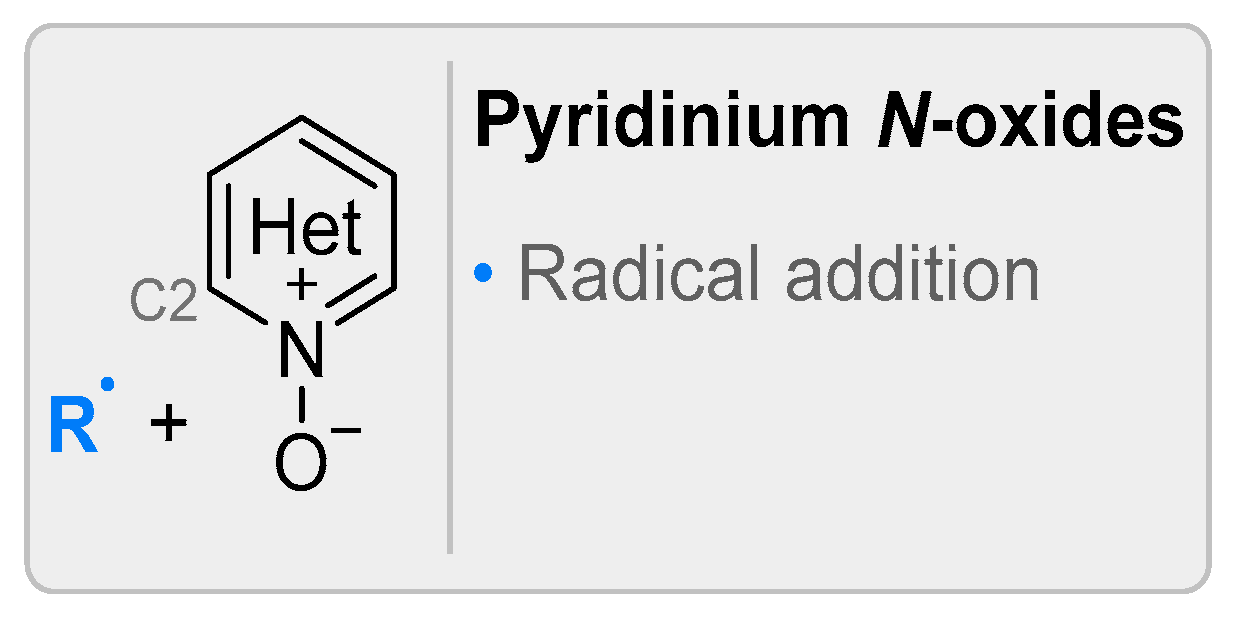
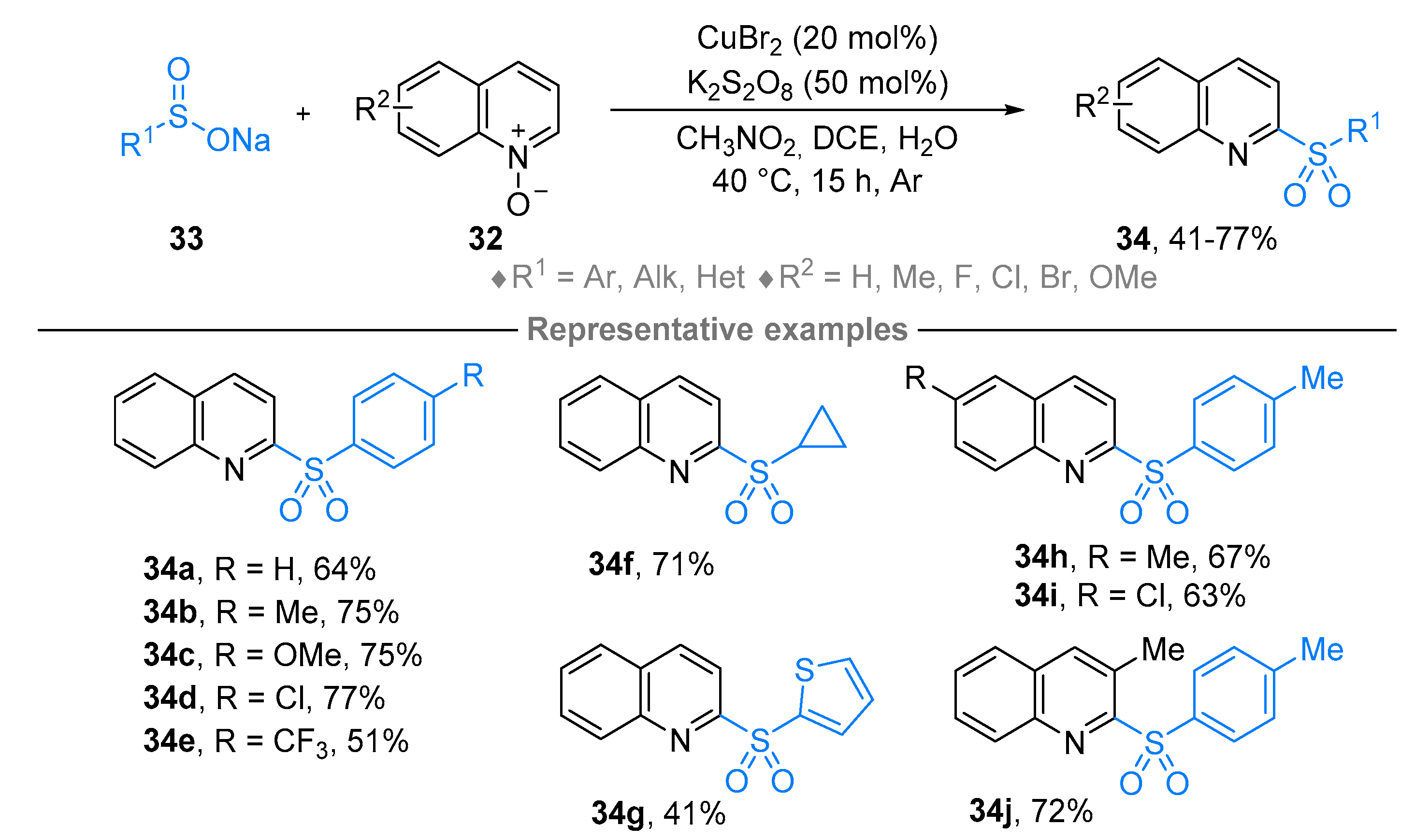
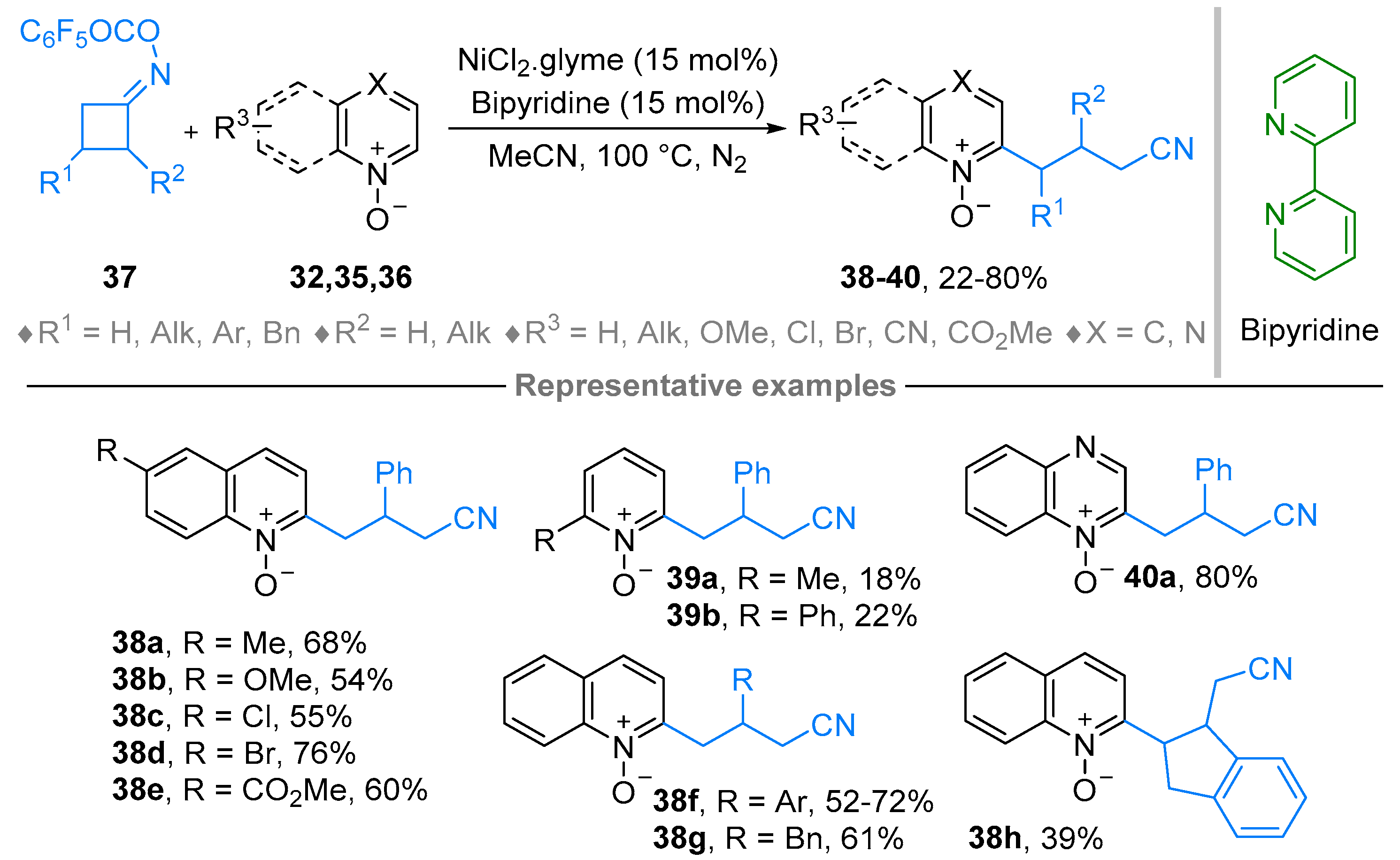
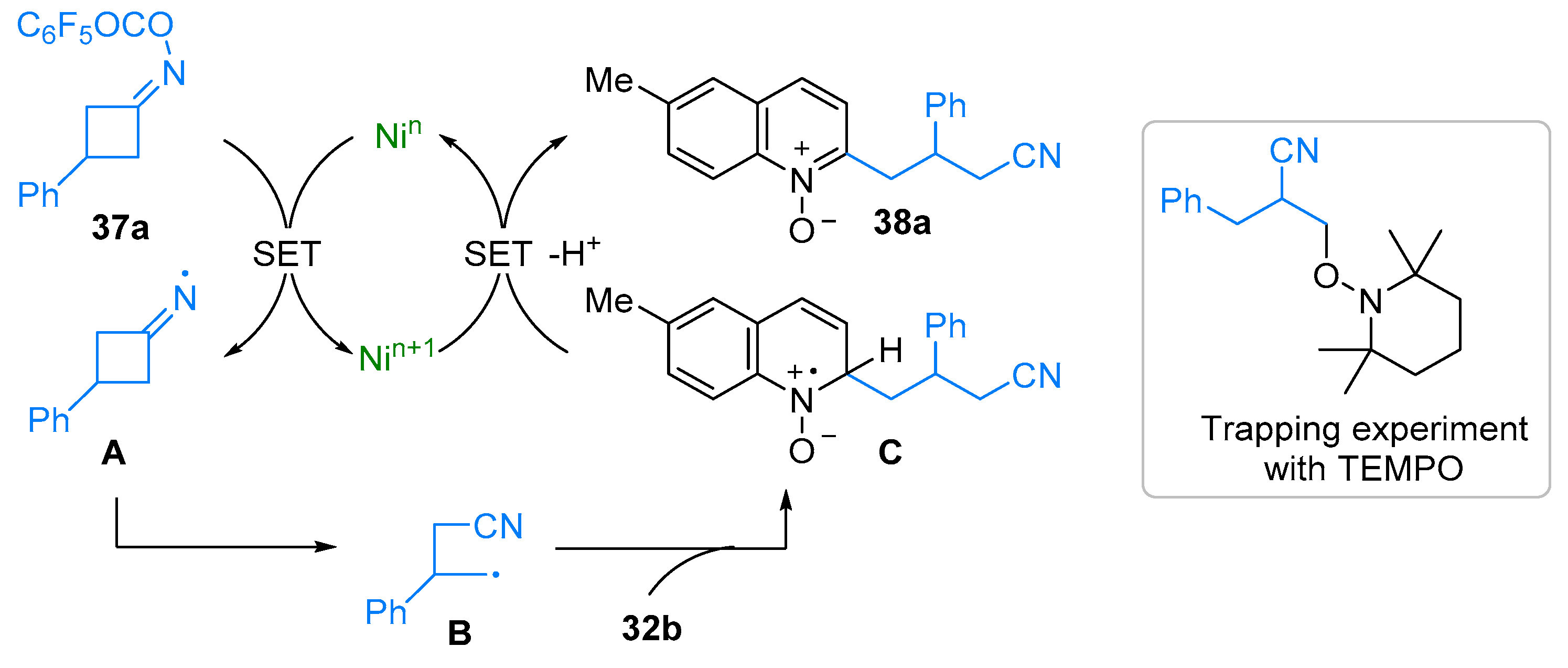
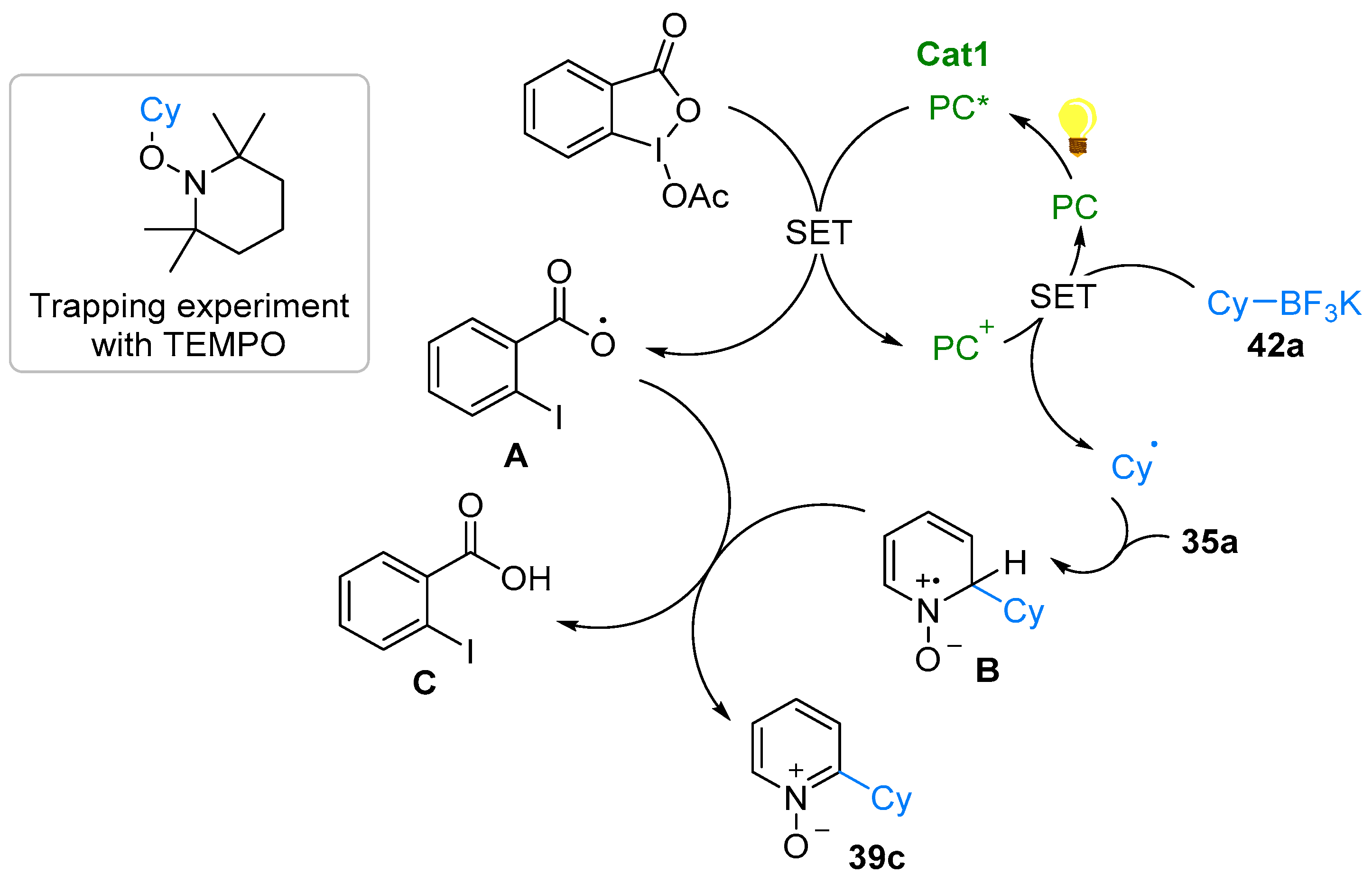
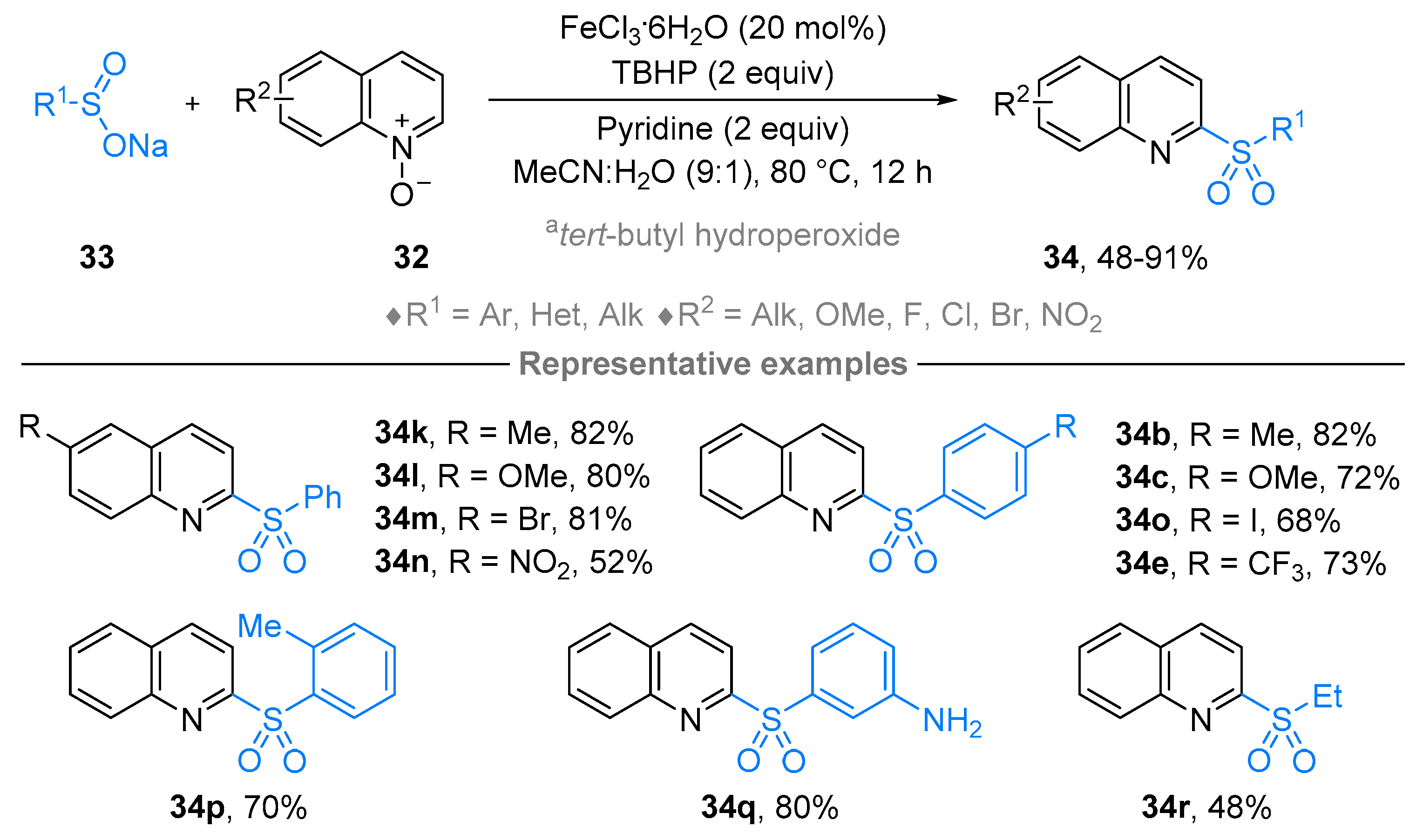
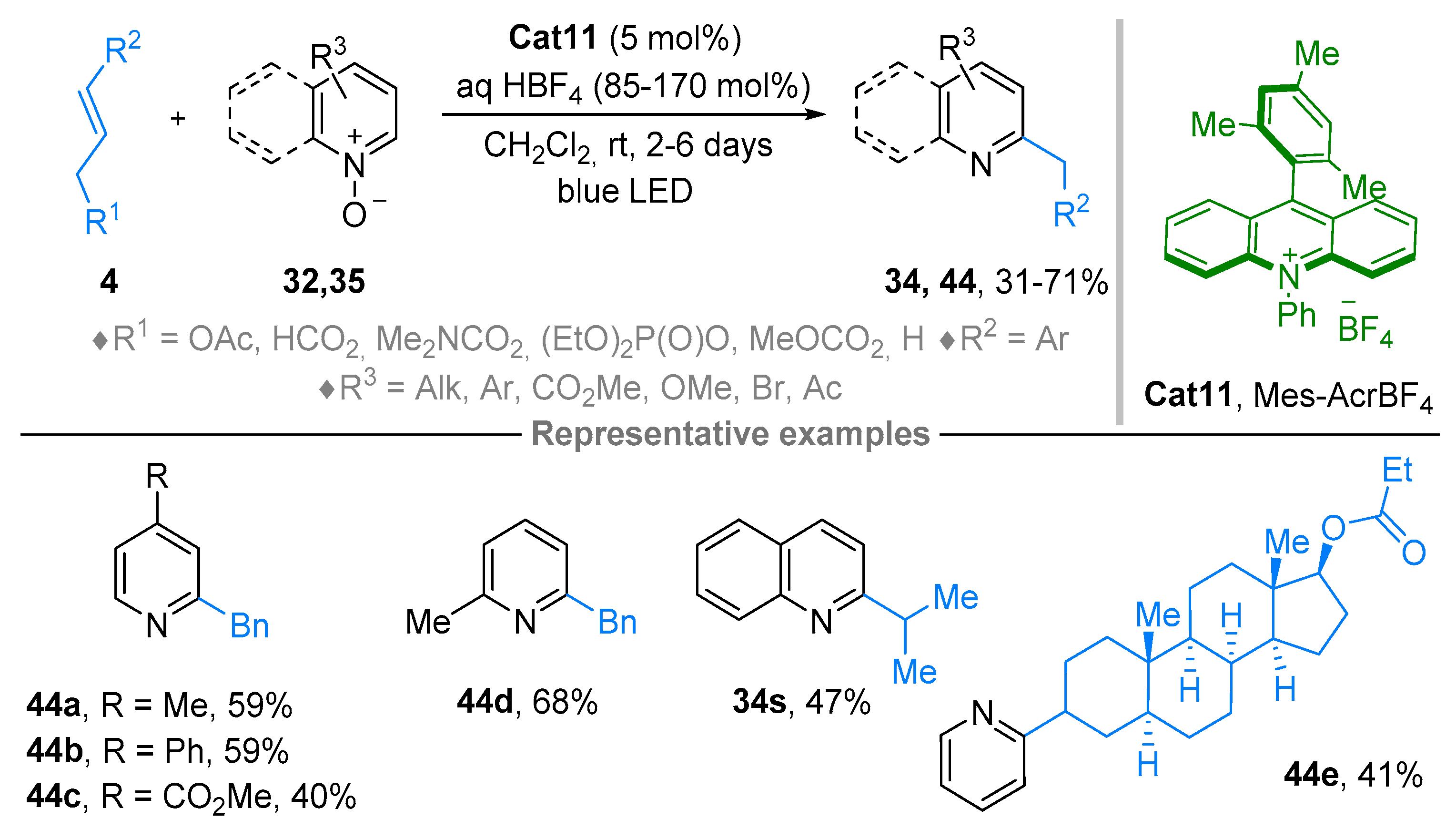
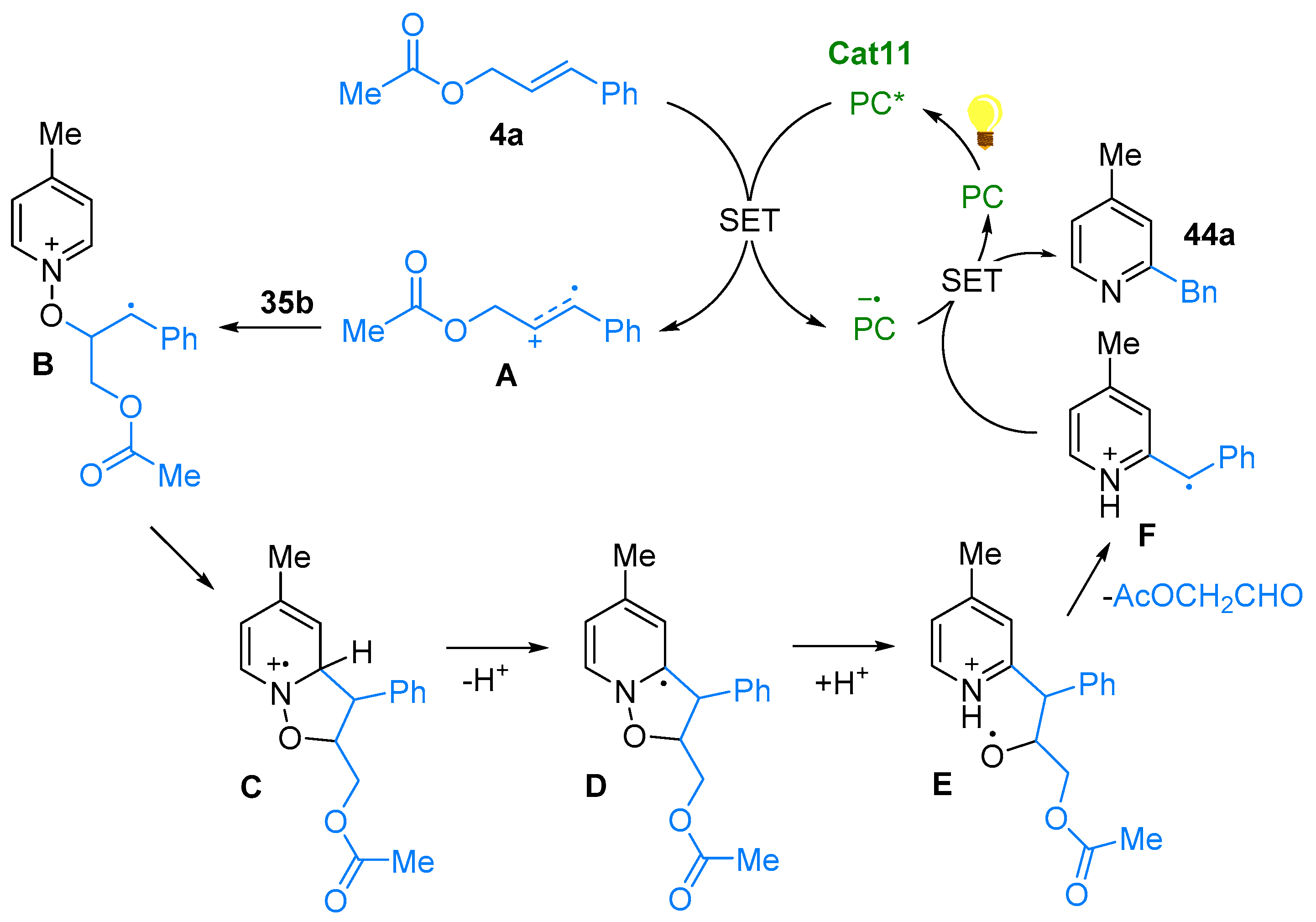
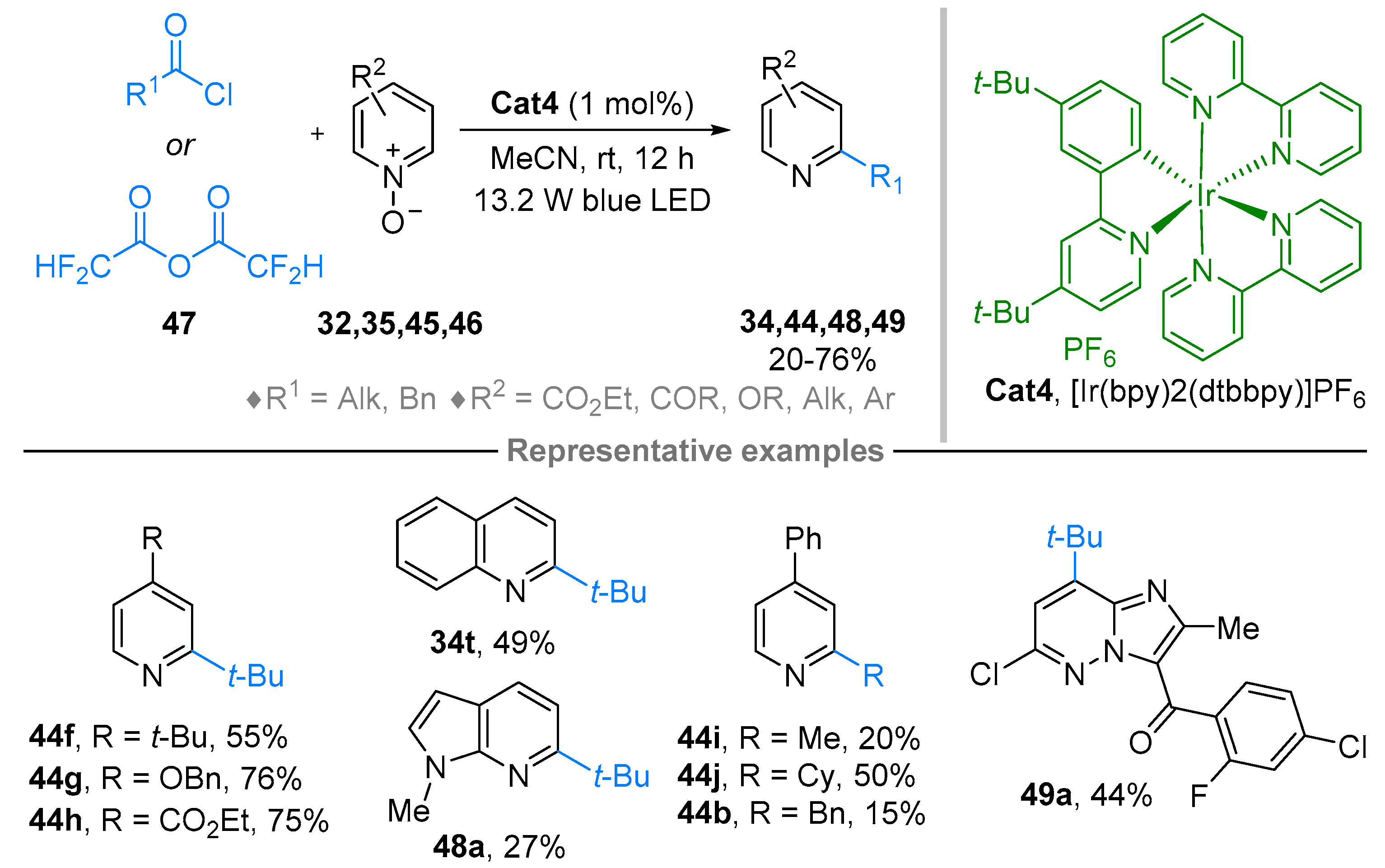
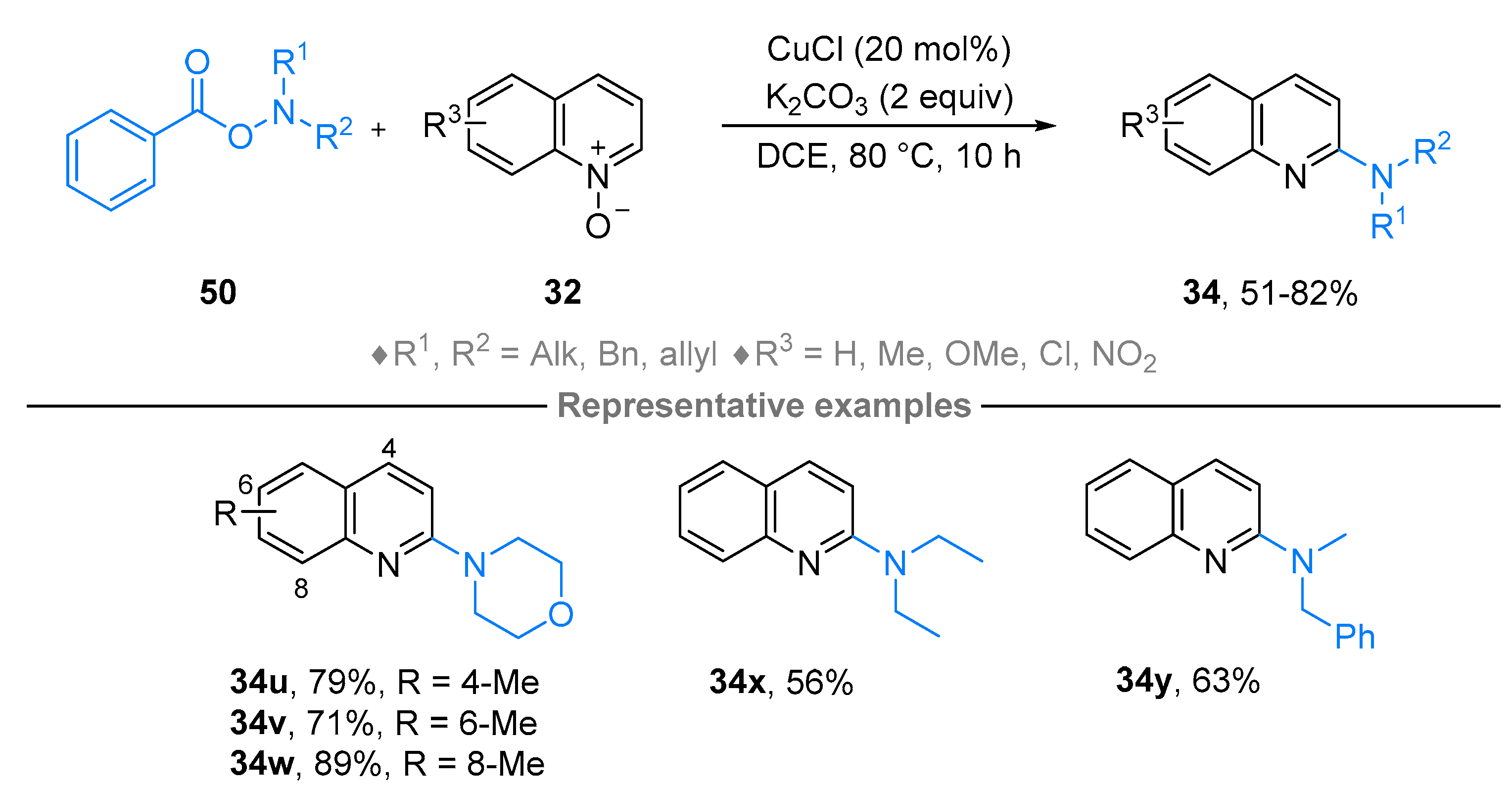
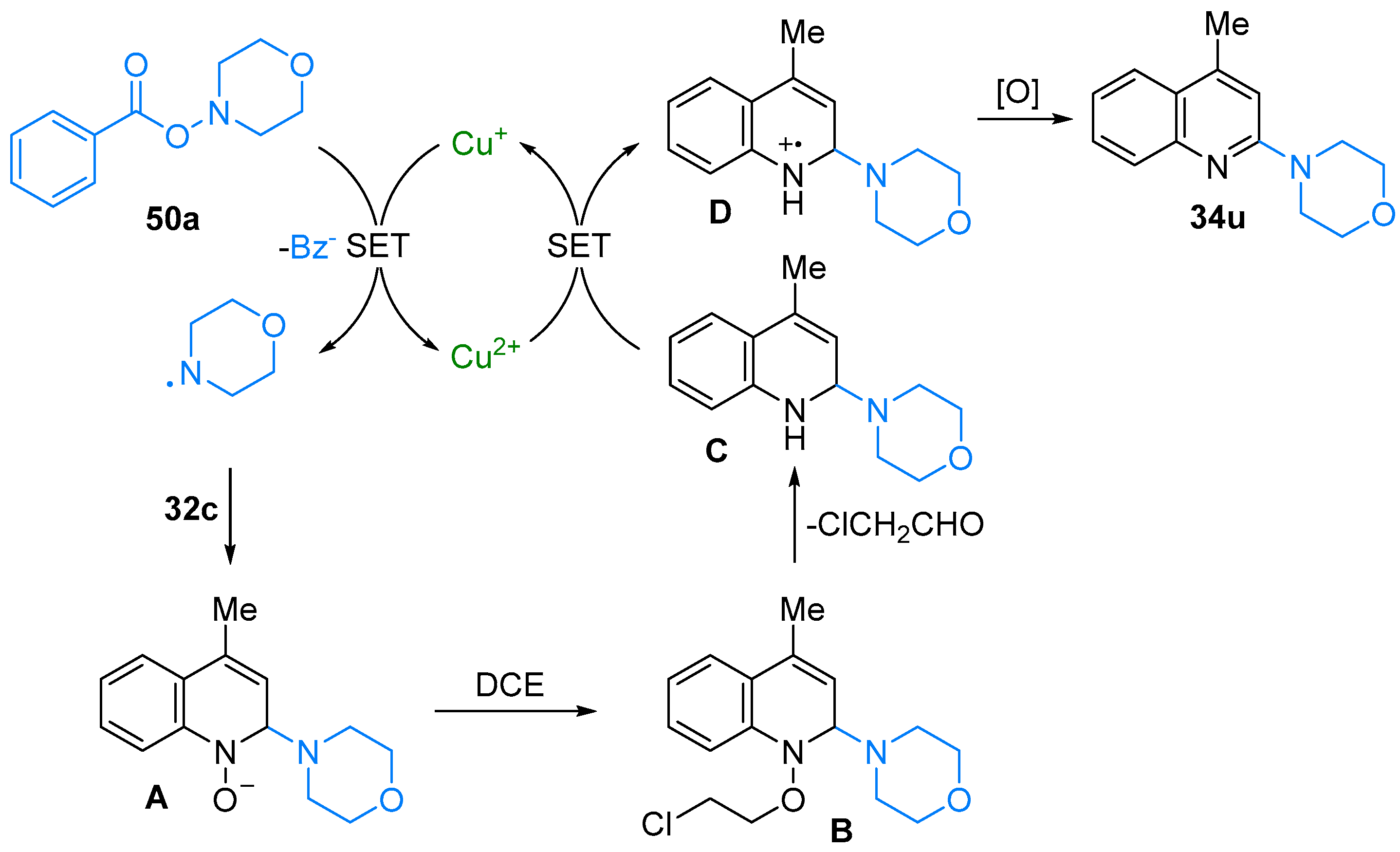
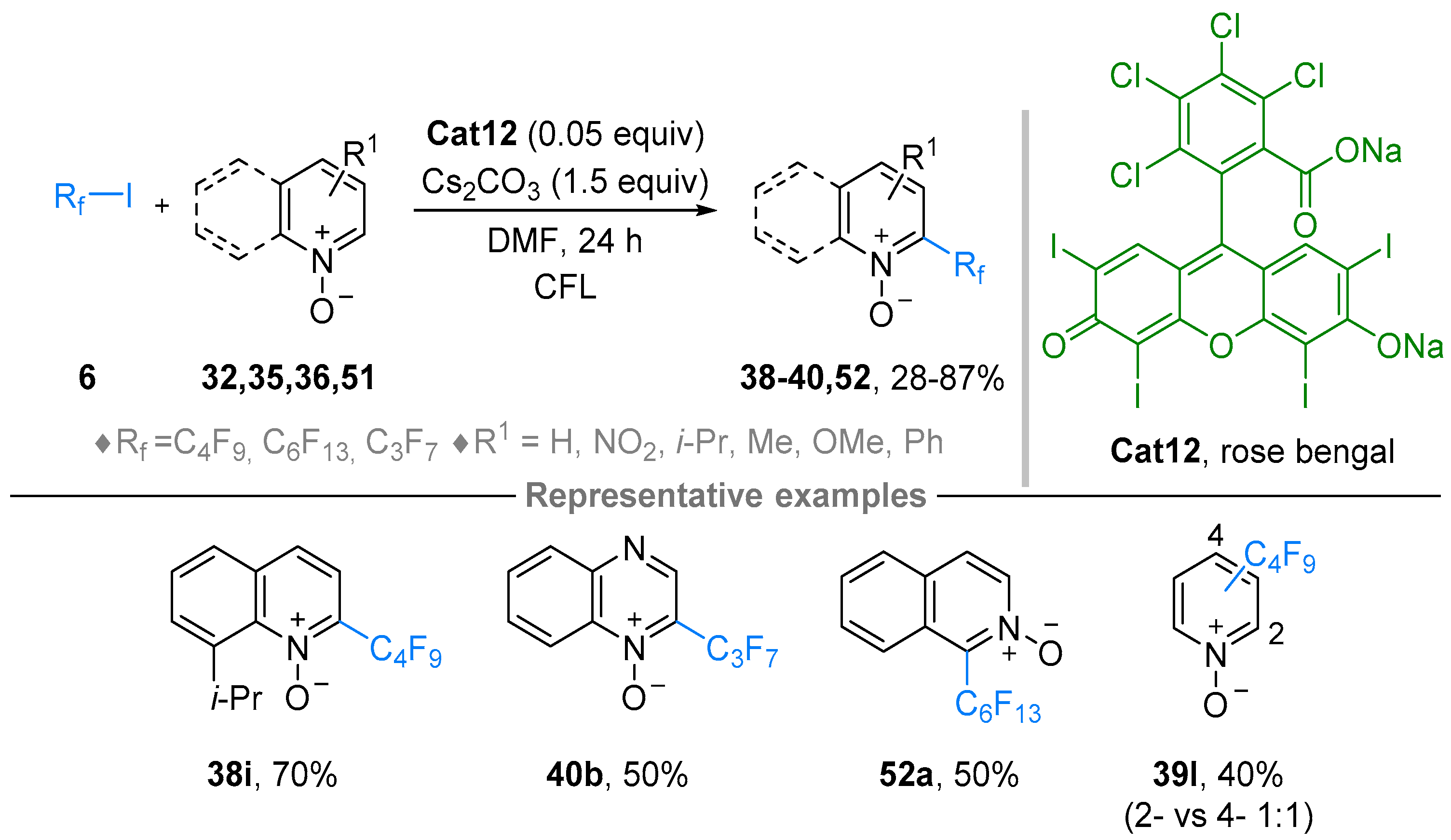
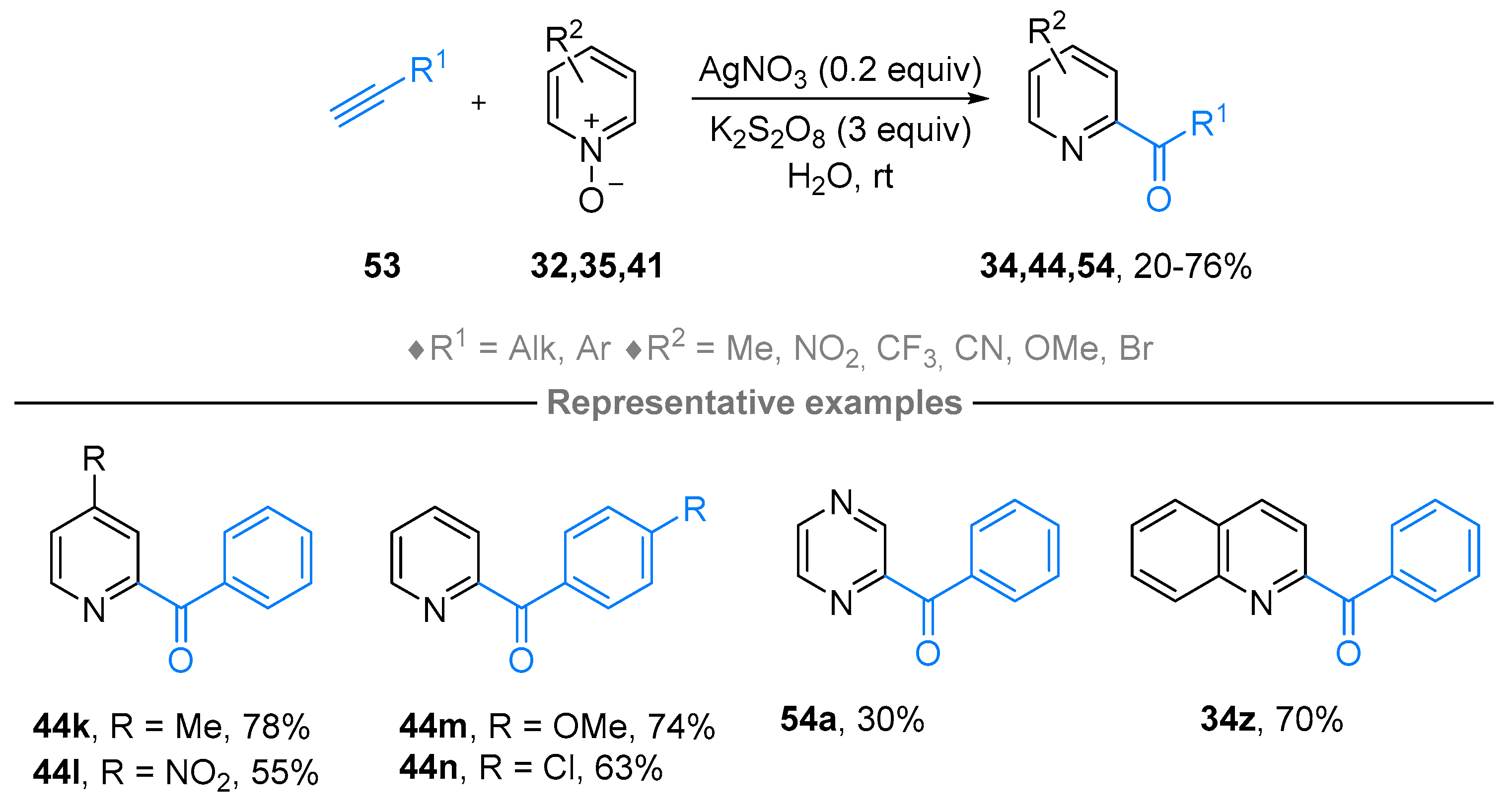
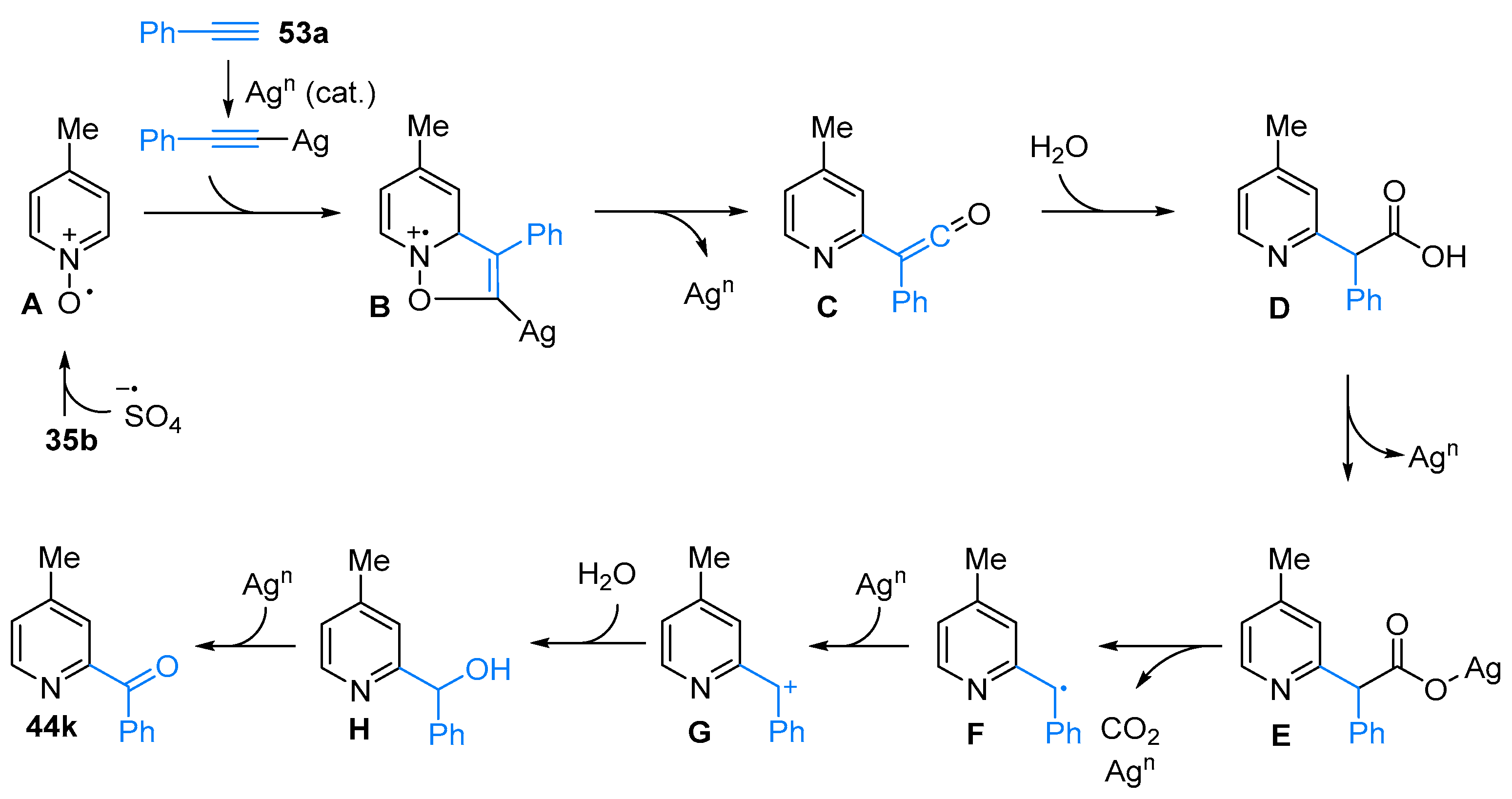
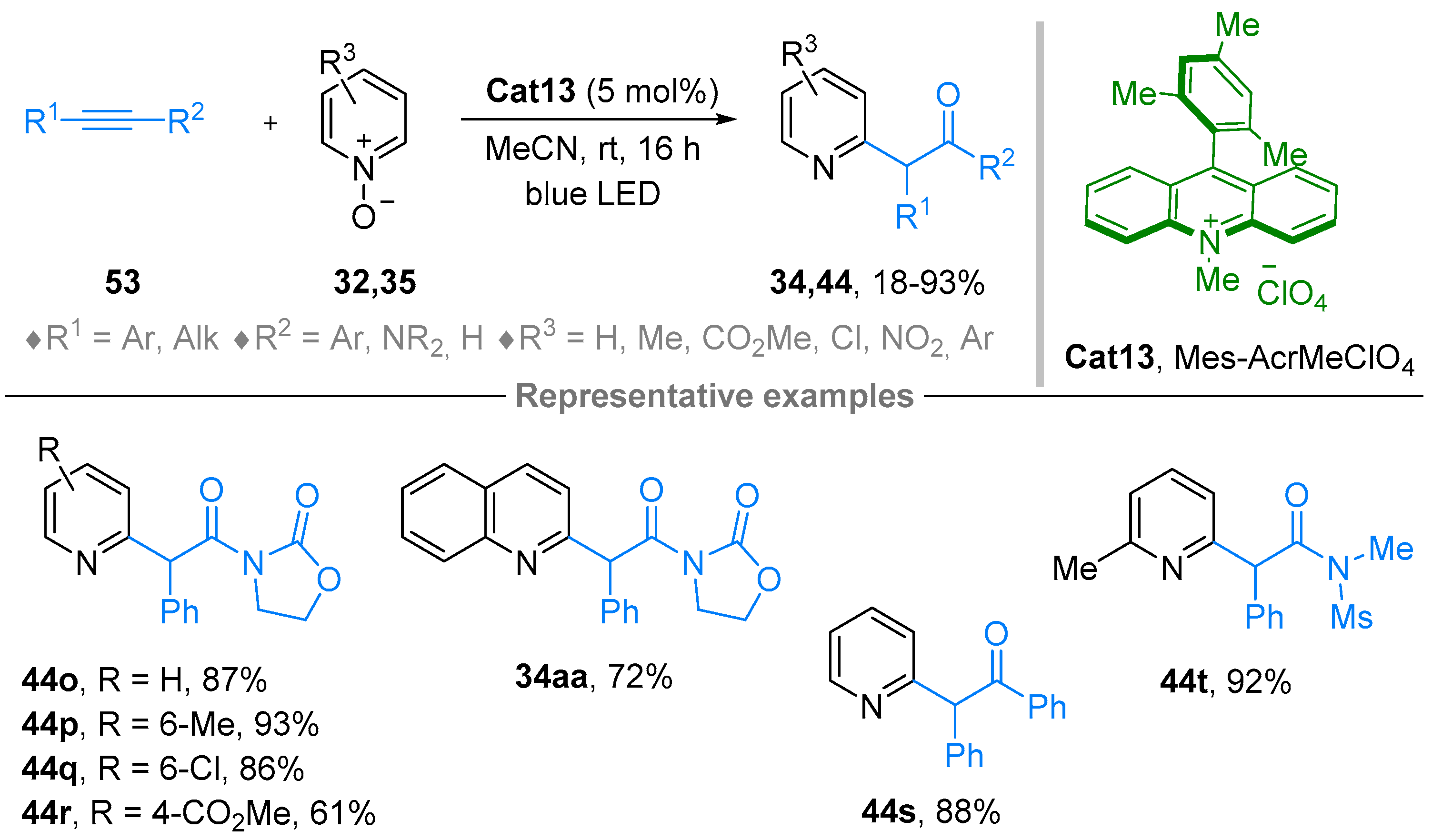
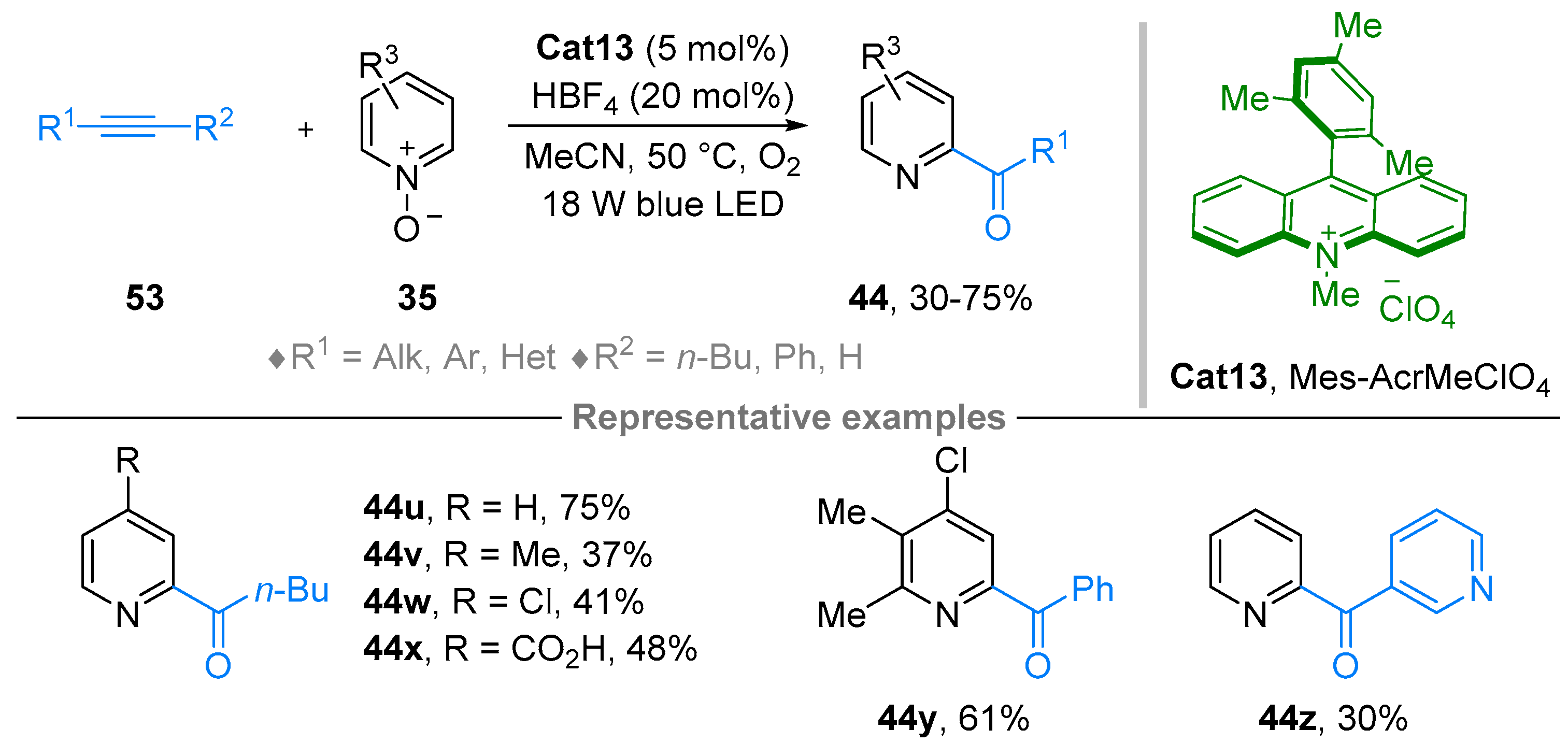
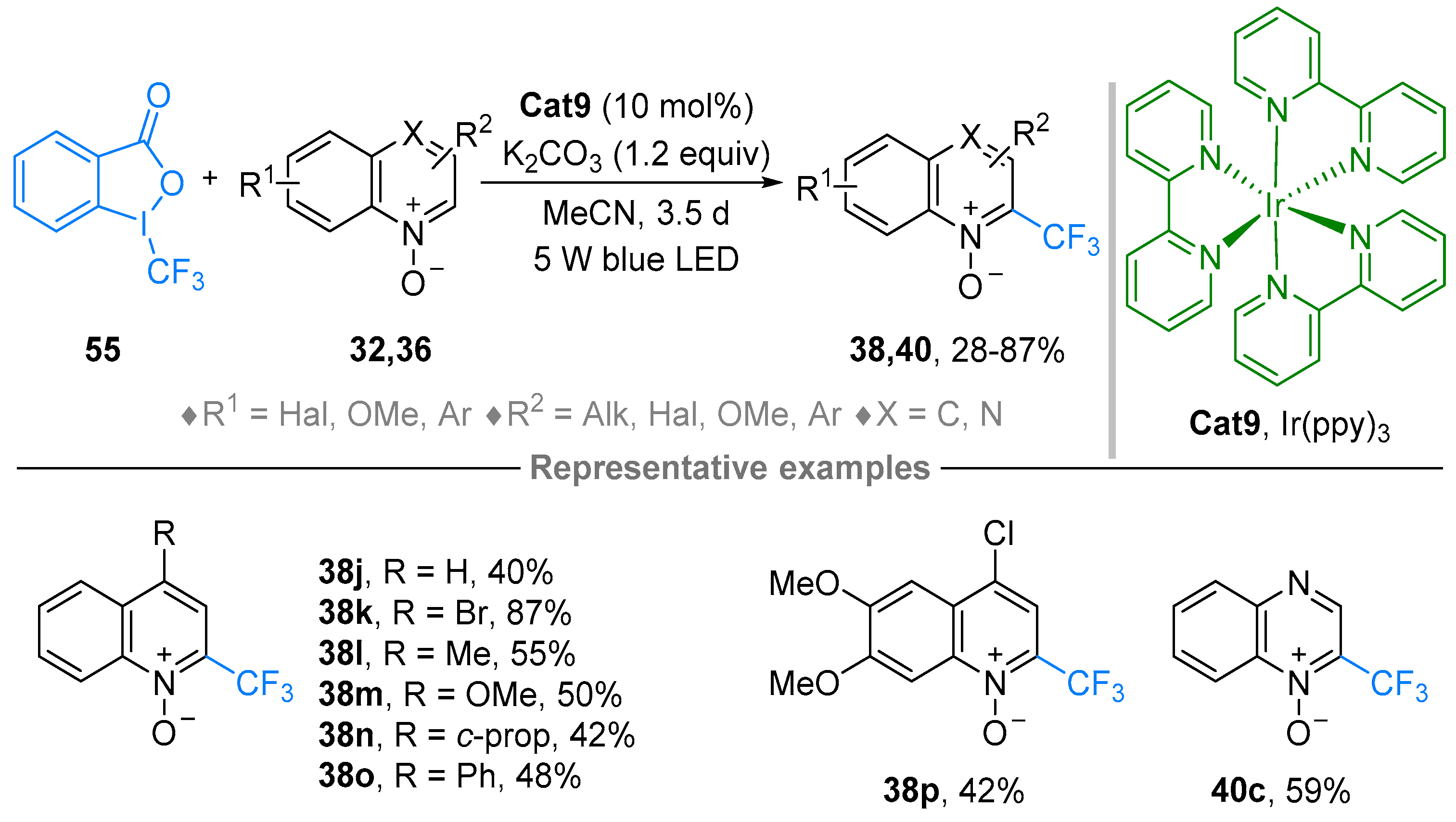
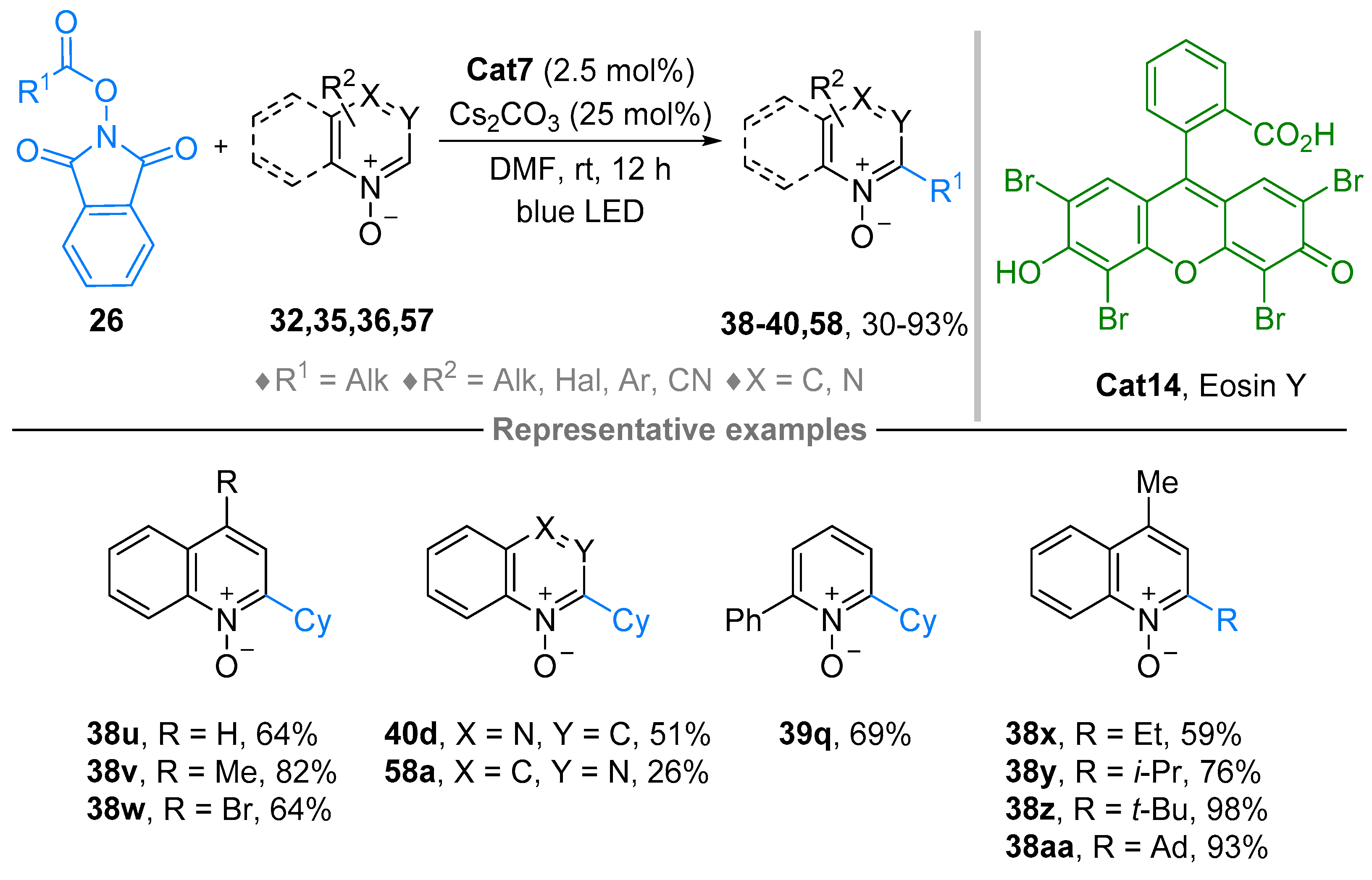
2.4. Pyridinium-N-Oxide
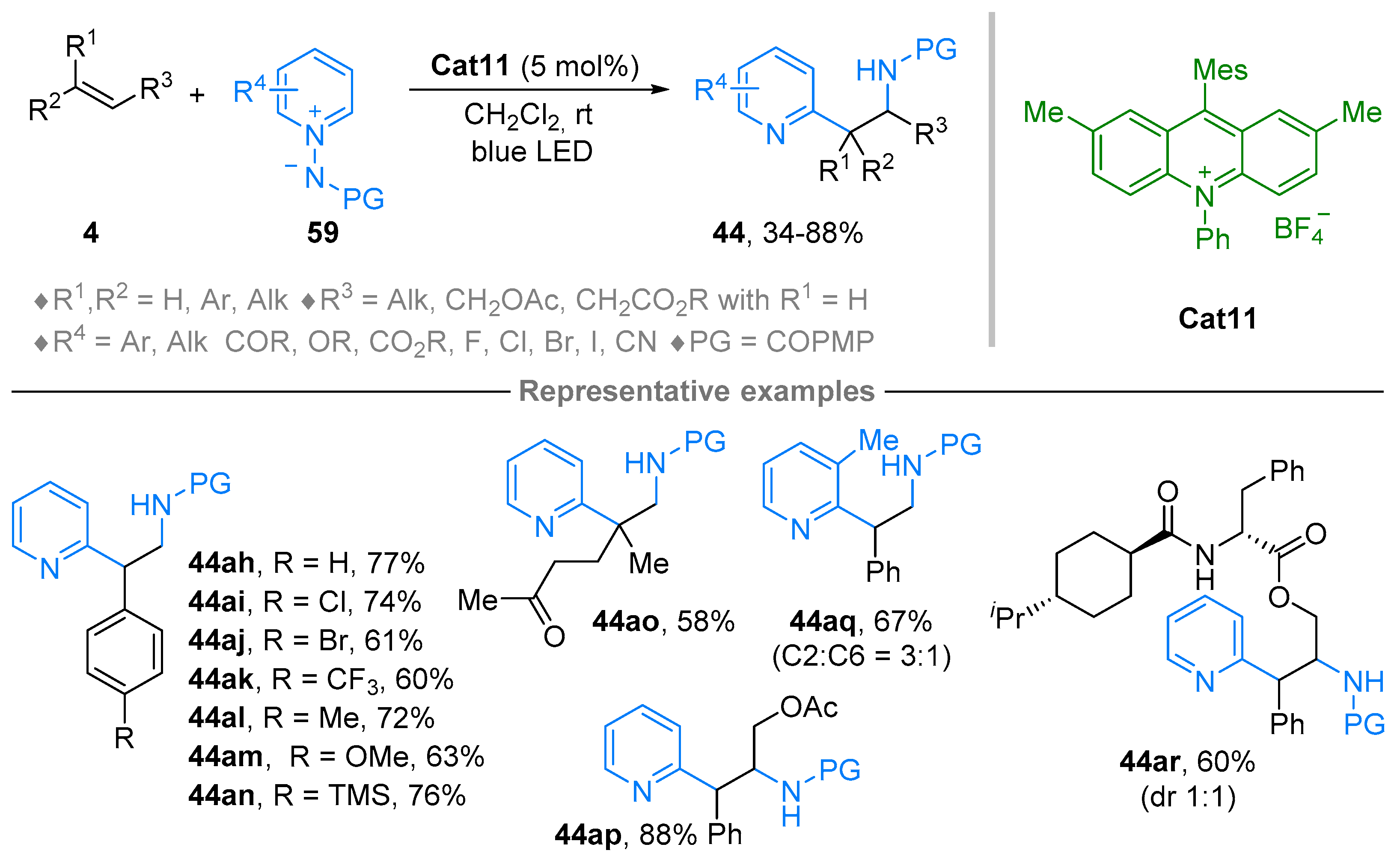
2.5. N-Aminopyridinium Ylides
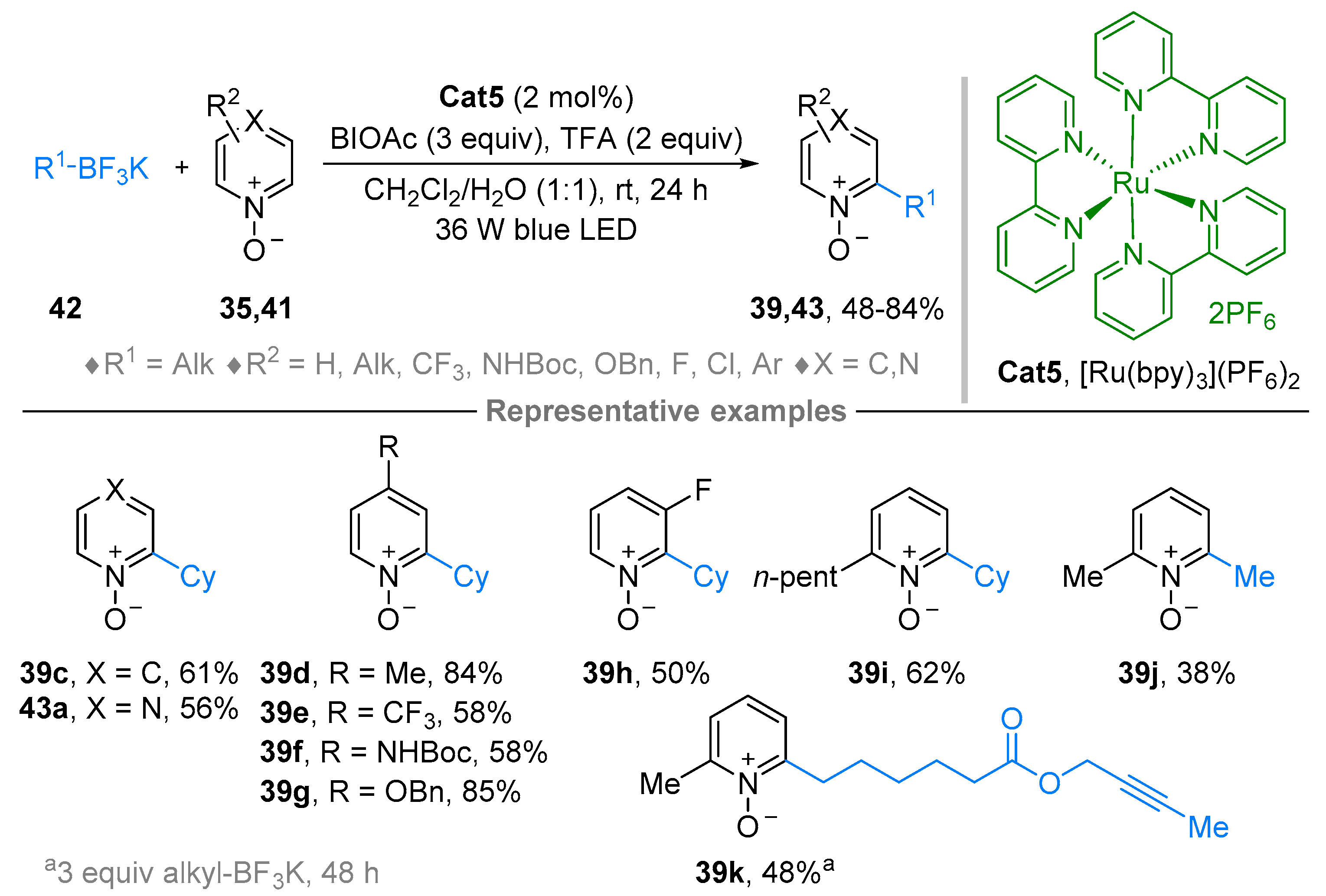
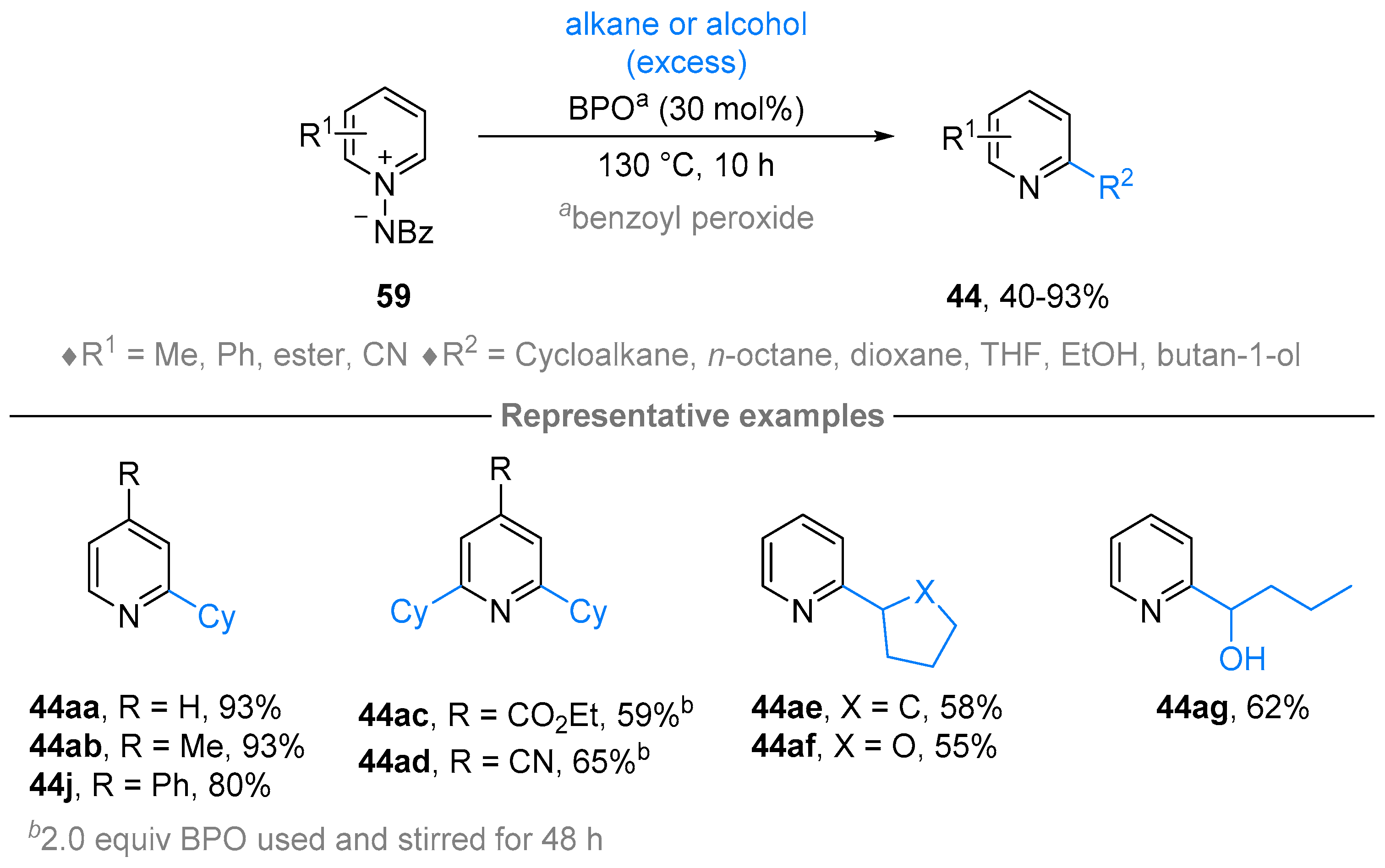
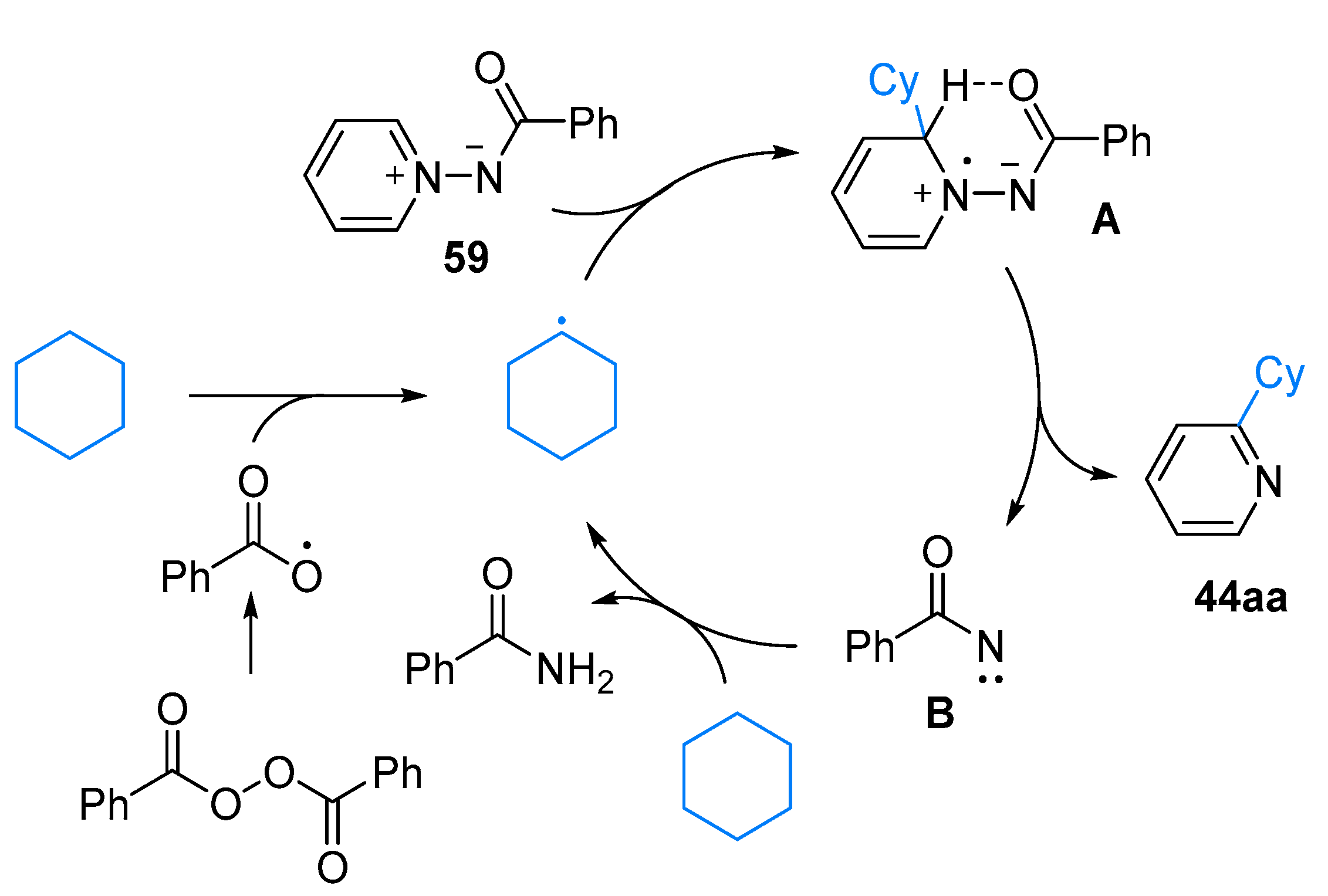
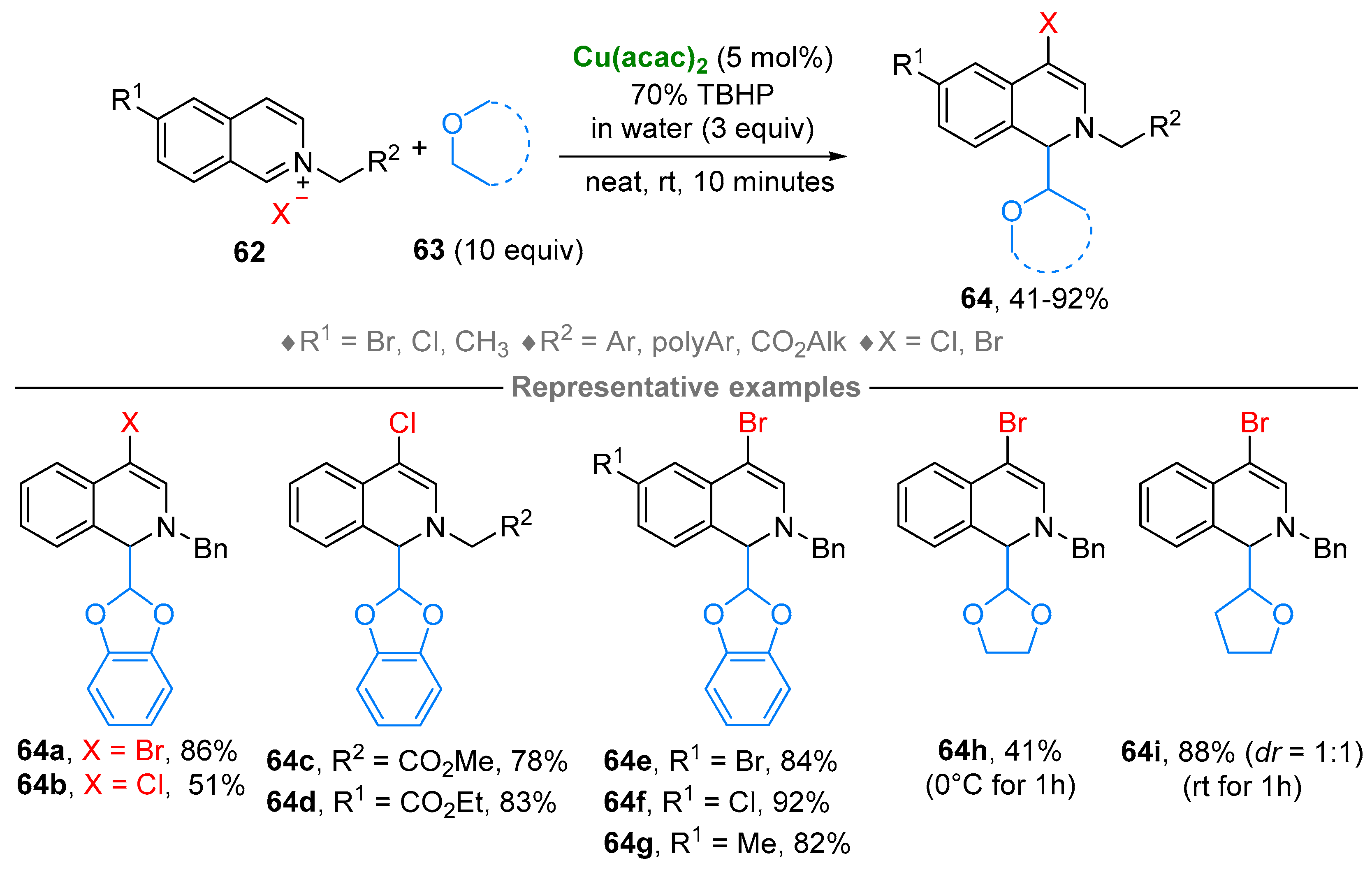
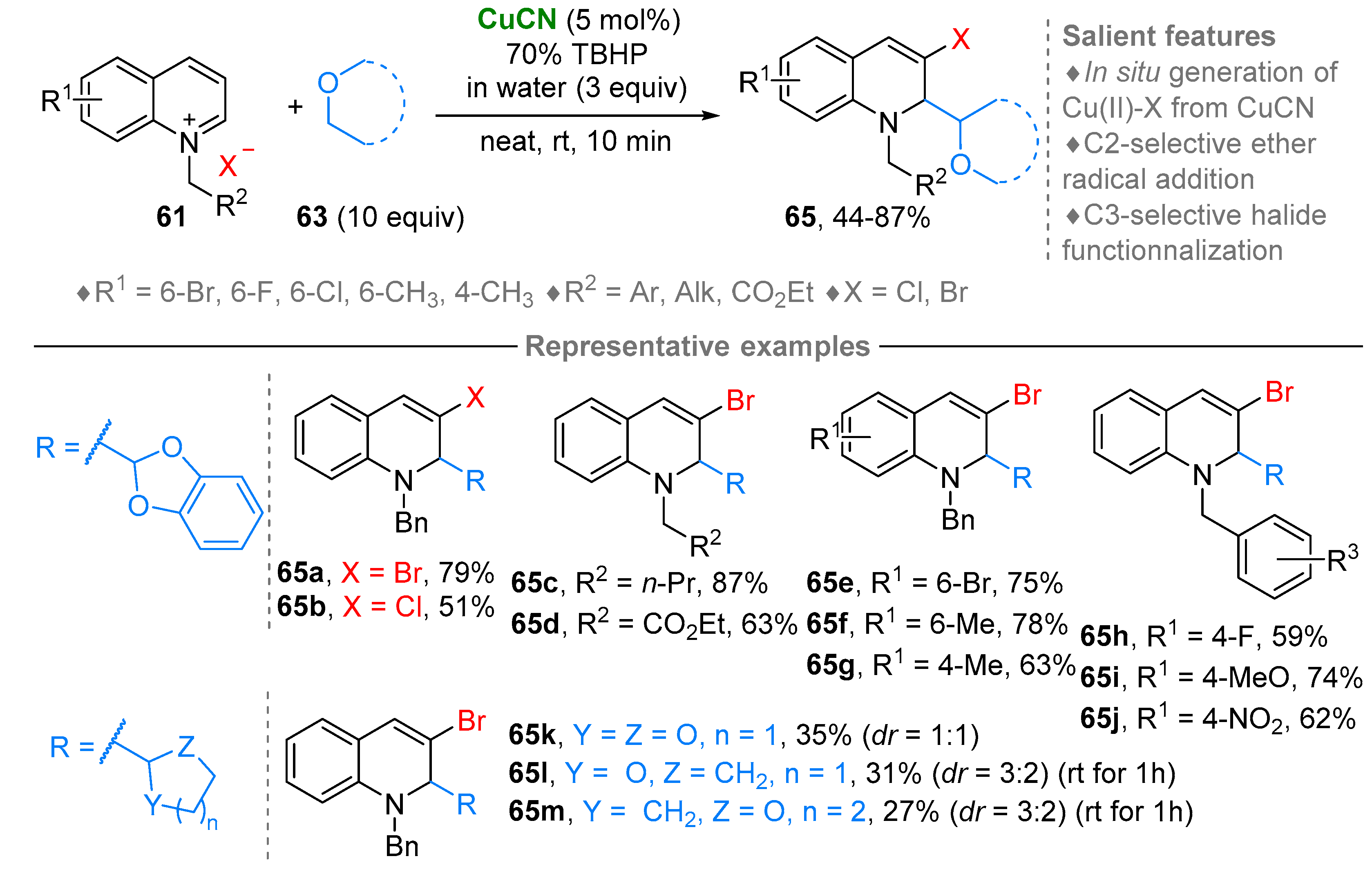
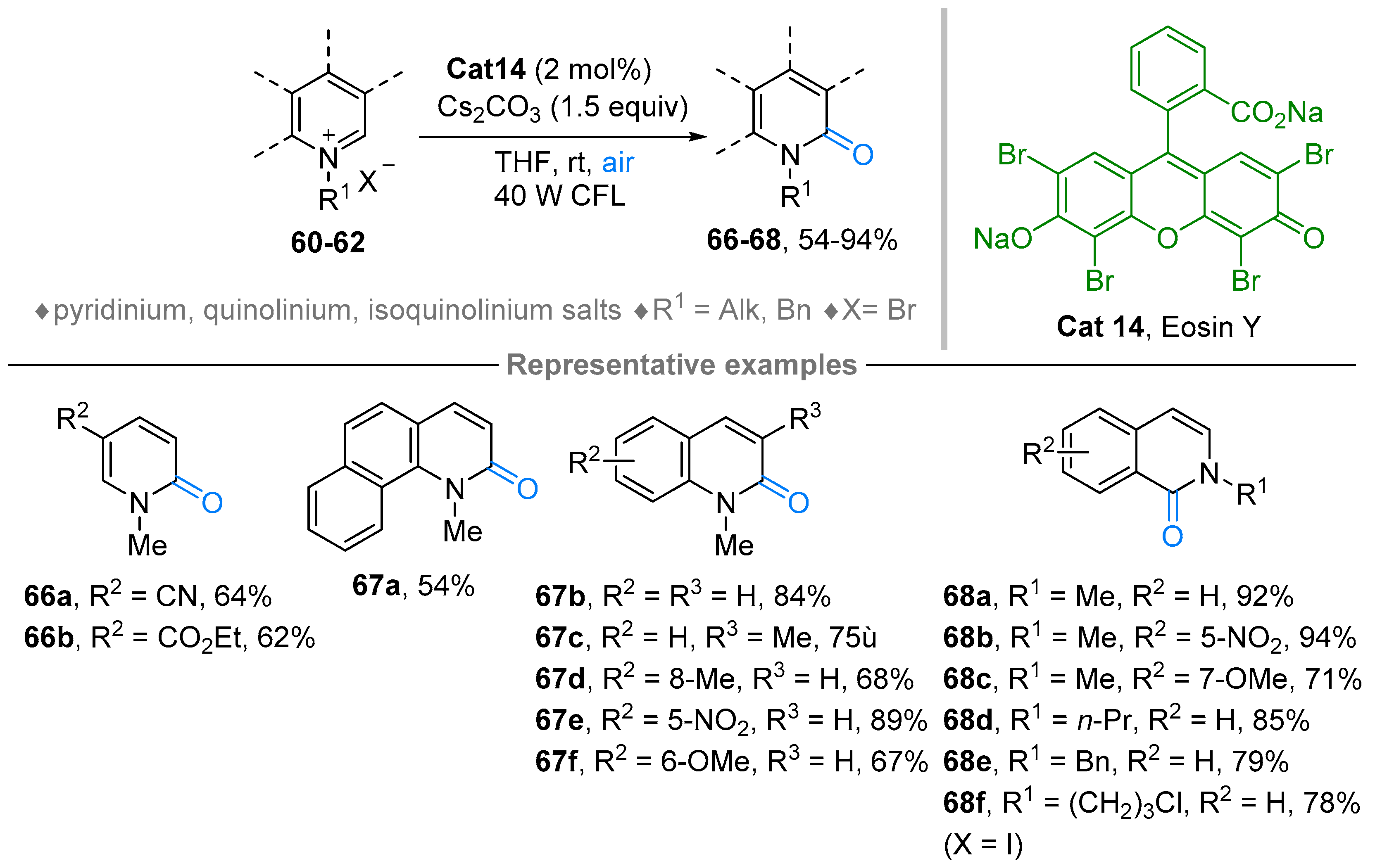
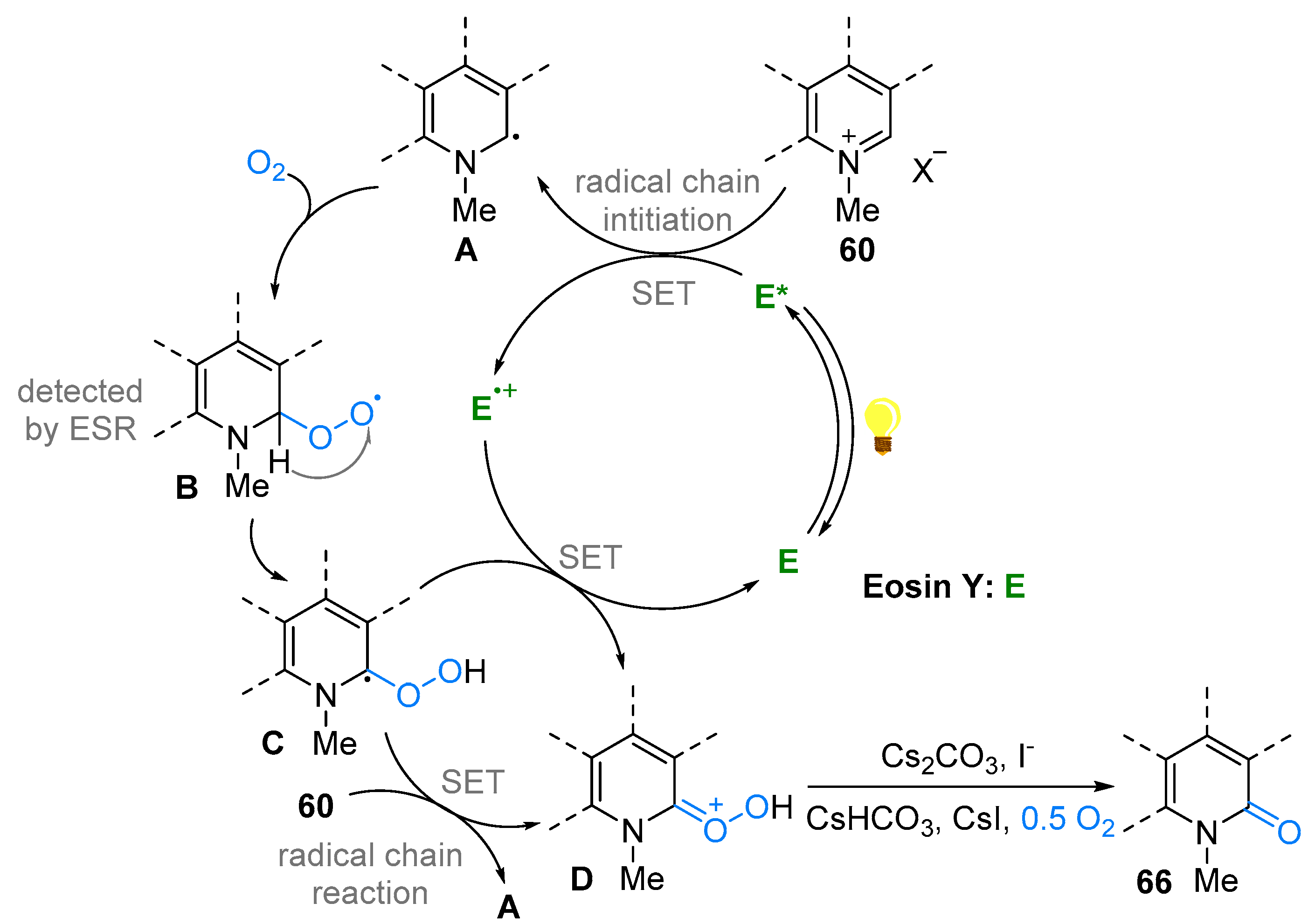
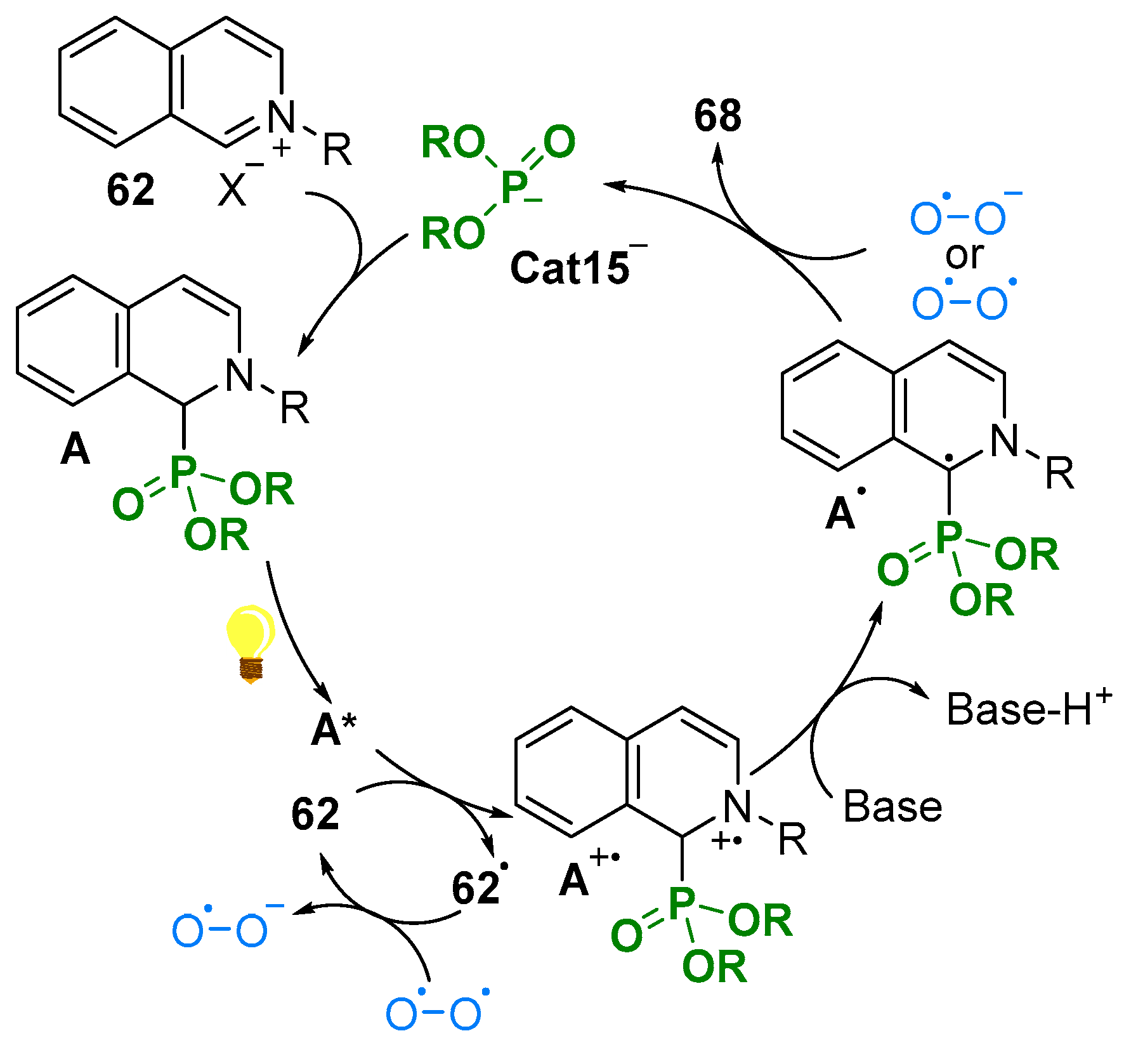
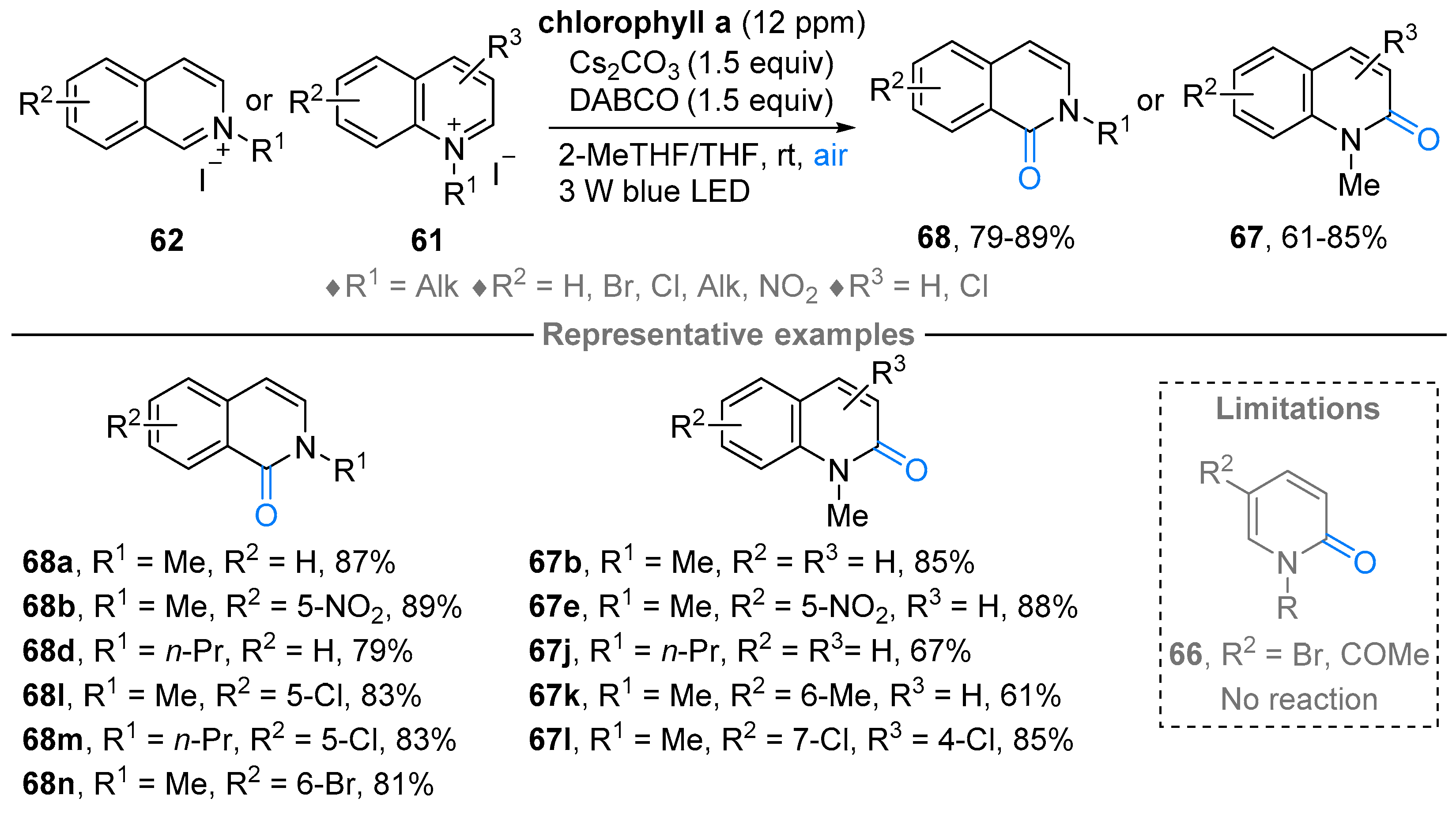
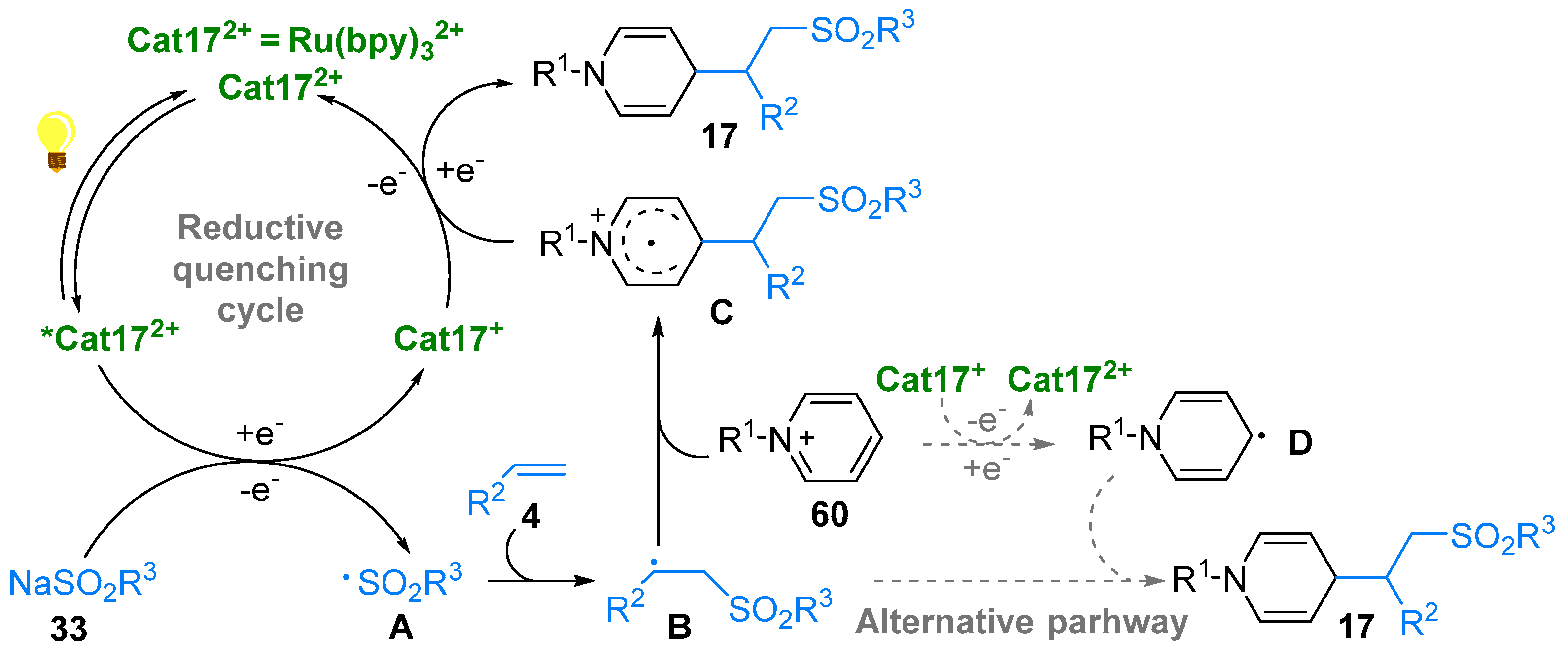
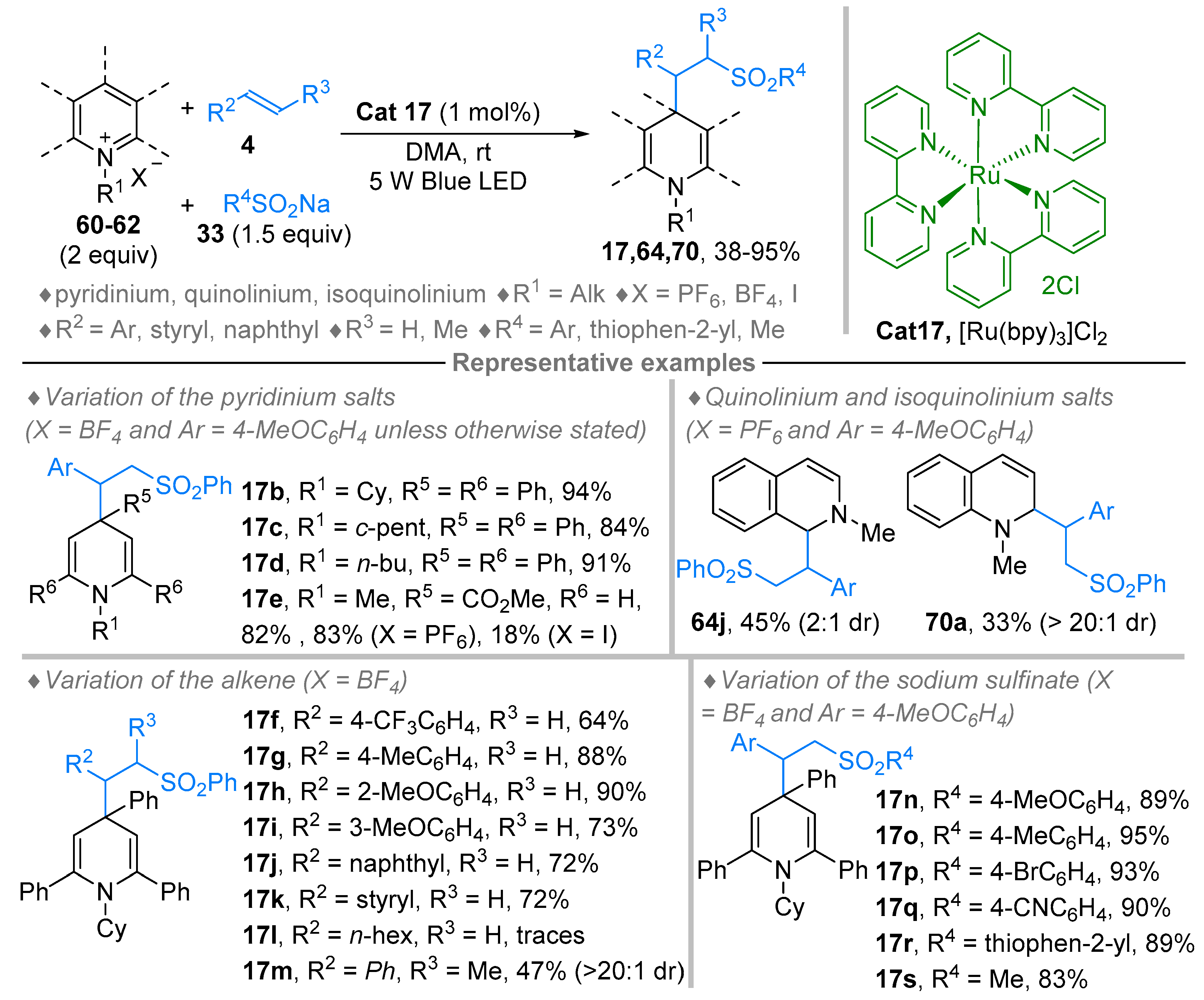
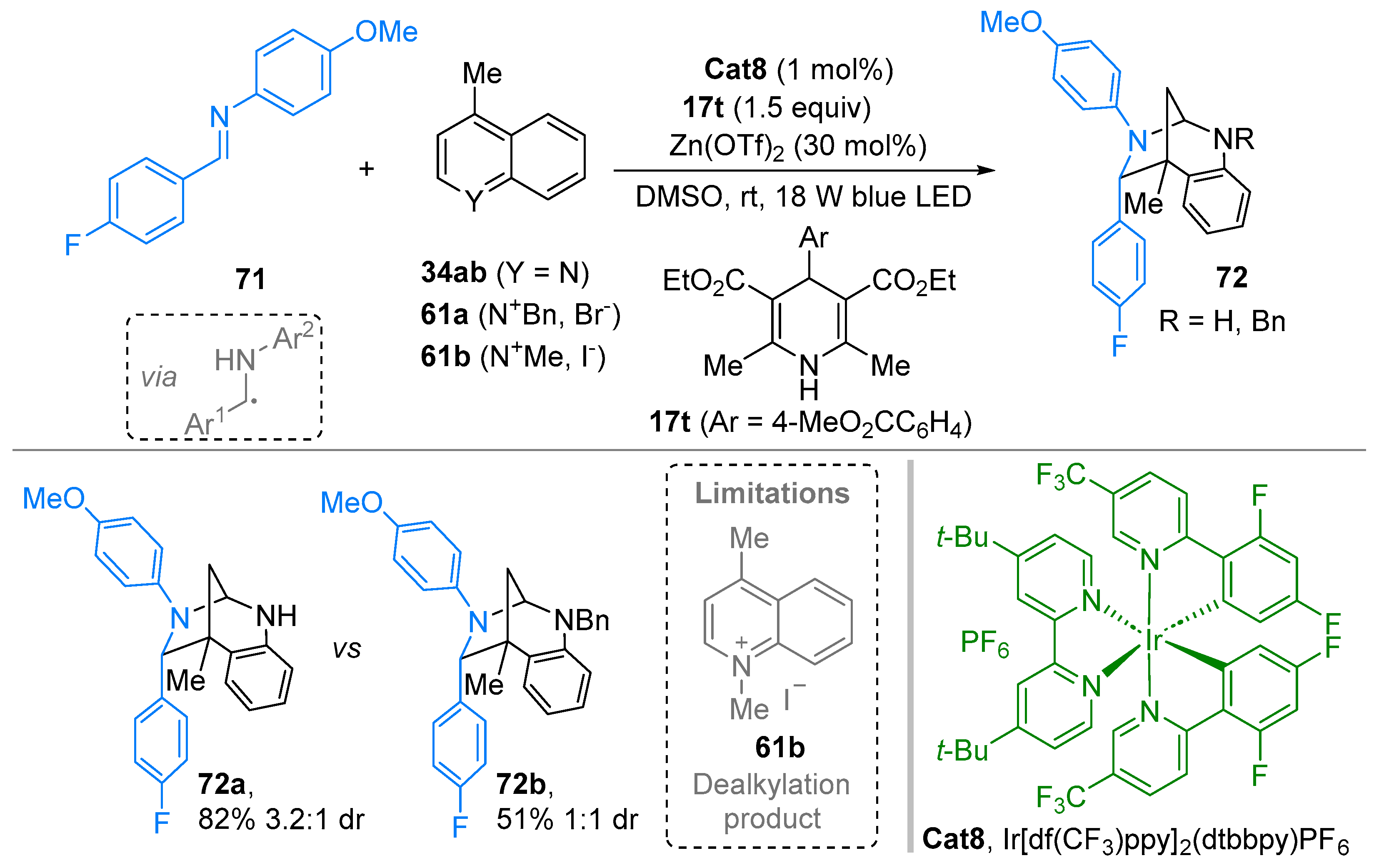
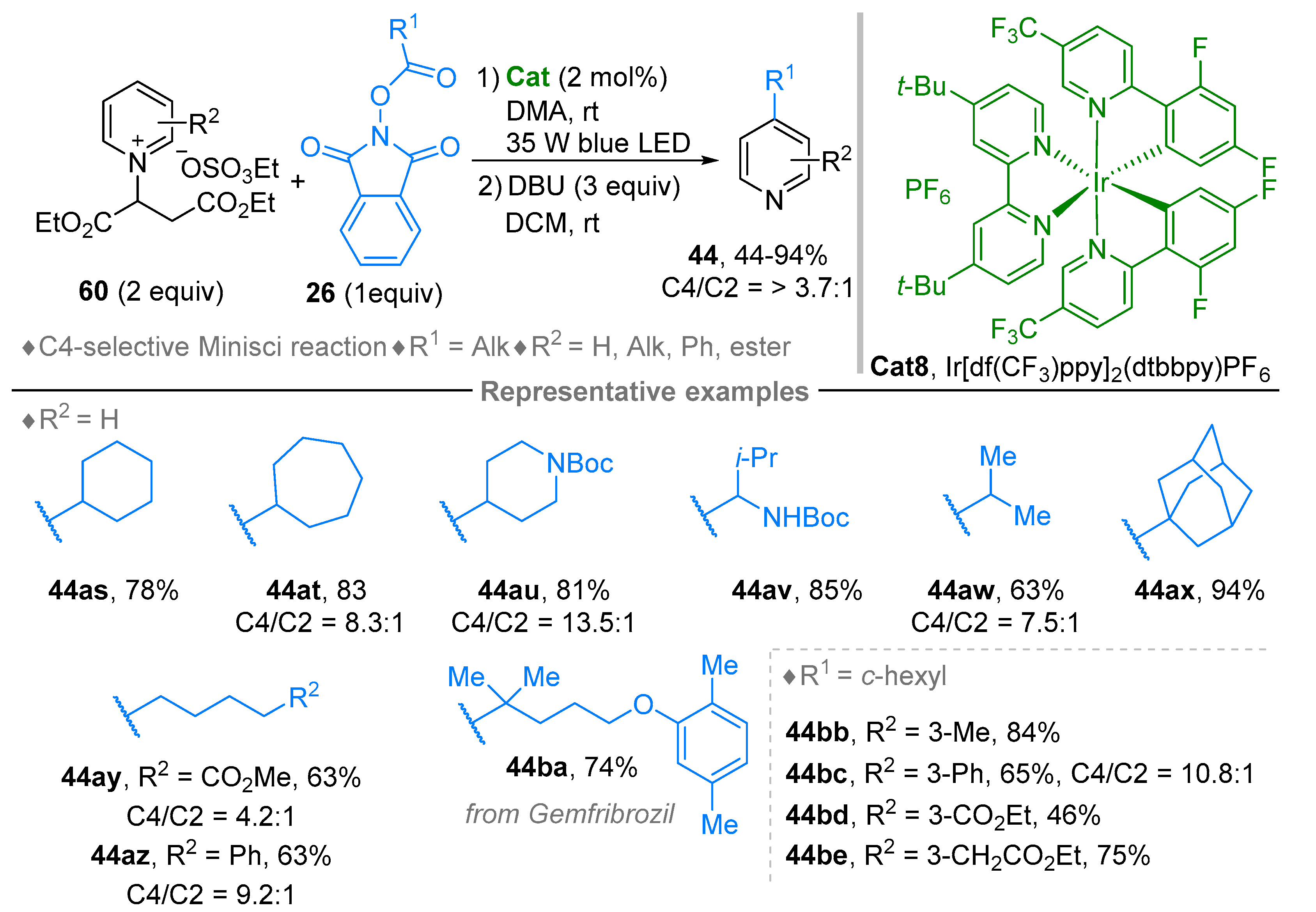
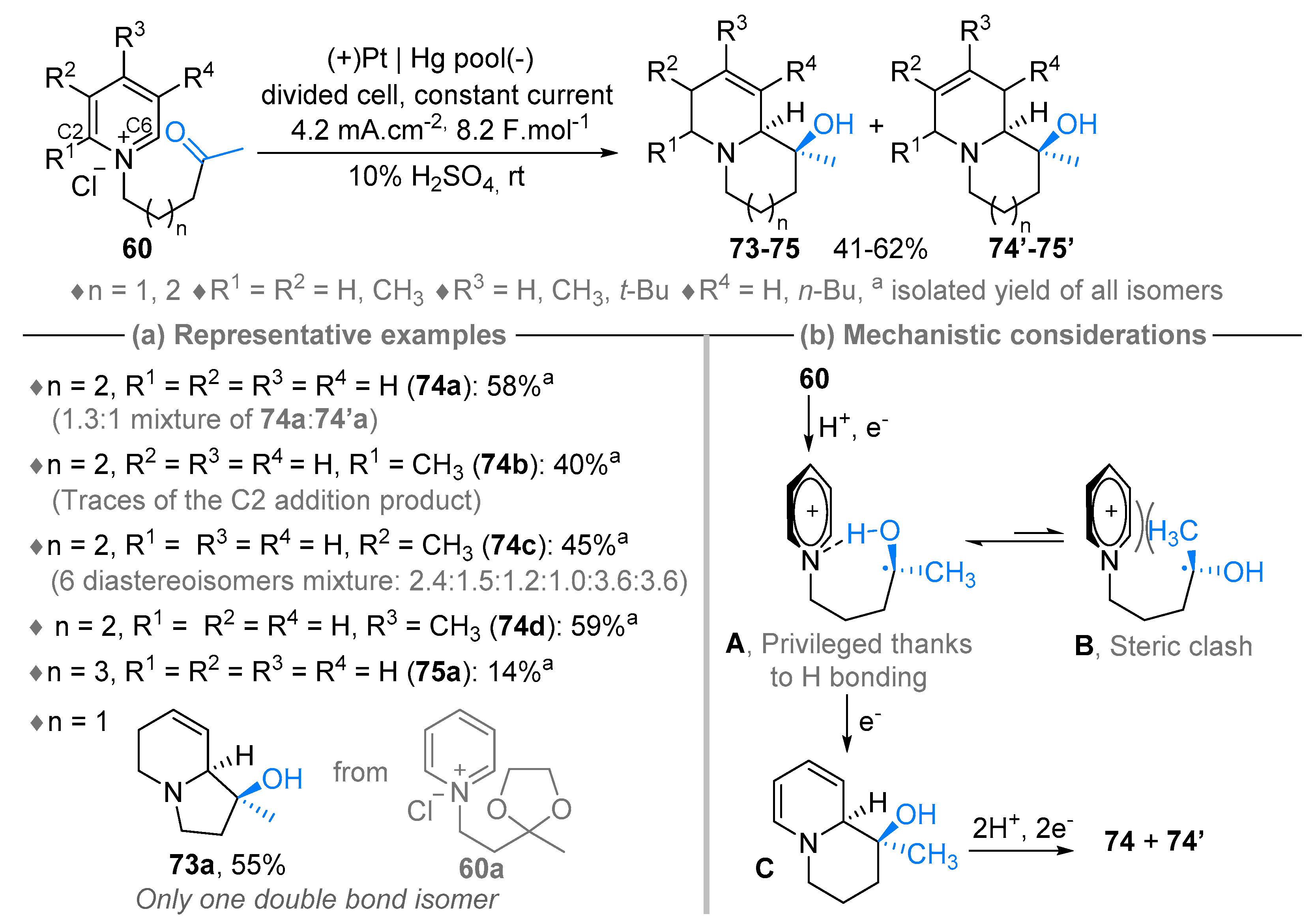
3. N-Alkylazaarenium Salts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Studer, A. From Curiosity to Reliable Methodology—Organic Free Radical Chemistry. Adv. Synth. Catal. 2020, 362, 2073. [Google Scholar] [CrossRef]
- Studer, A.; Curran, D.P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. Int. Ed. 2016, 55, 58–102. [Google Scholar] [CrossRef] [PubMed]
- Tay, N.E.S.; Lehnherr, D.; Rovis, T. Photons or Electrons? A Critical Comparison of Electrochemistry and Photoredox Catalysis for Organic Synthesis. Chem. Rev. 2022, 122, 2487–2649. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Gunnoe, T.B.; Stamenkovic, V.R. Organic Electrosynthesis: When Is It Electrocatalysis? ACS Catal. 2020, 10, 13156–13158. [Google Scholar] [CrossRef]
- Xu, Z.J.; Wang, X. Electrocatalysis: A Core Technique for a Sustainable Future. Chem. Eur. J. 2020, 26, 3897. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Centi, G.; Perathoner, S.; Lanzafame, P. Catalysis for e-Chemistry: Need and Gaps for a Future De-Fossilized Chemical Production, with Focus on the Role of Complex (Direct) Syntheses by Electrocatalysis. ACS Catal. 2022, 12, 2861–2876. [Google Scholar] [CrossRef]
- Kingston, C.; Palkowitz, M.D.; Takahira, Y.; Vantourout, J.C.; Peters, B.K.; Kawamata, Y.; Baran, P.S. A Survival Guide for the “Electro-curious”. Acc. Chem. Res. 2020, 53, 72–83. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Zhu, C.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef]
- Beil, S.B.; Pollok, D.; Waldvogel, S.R. Reproducibility in Electroorganic Synthesis—Myths and Misunderstandings. Angew. Chem. Int. Ed. 2021, 60, 14750–14759. [Google Scholar] [CrossRef]
- Lam, K.; Wheelhouse, K.M.P. Unleashing the Potential to Electrify Process Chemistry: From Bench to Plant. Org. Proc. Res. Dev. 2021, 25, 2579–2580. [Google Scholar] [CrossRef]
- Pastori, N.; Gambarotti, C.; Punta, C. Recent Developments in Nucleophilic Radical Addition to Imines: The Key Role of Transition Metals and the New Porta Radical-Type Version of the Mannich and Strecker Reactions. Mini-Rev. Org. Chem. 2009, 6, 184–195. [Google Scholar] [CrossRef]
- Miyabe, H.; Yoshioka, E.; Kohtani, S. Progress in Intermolecular Carbon Radical Addition to Imine Derivatives. Curr. Org. Chem. 2010, 14, 1254–1264. [Google Scholar] [CrossRef]
- Tauber, J.; Imbri, D.; Opatz, T. Radical Addition to Iminium Ions and Cationic Heterocycles. Molecules 2014, 19, 16190–16222. [Google Scholar] [CrossRef]
- Proctor, R.S.J.; Phipps, R.J. Recent Advances in Minisci-Type Reactions. Angew. Chem. Int. Ed. 2019, 58, 13666–13699. [Google Scholar] [CrossRef]
- Zeng, C.; Guo, B.; Xu, K.; Meng, W. Recent Advances in Minisci Reactions under Electrochemical Conditions. Chin. J. Org. Chem. 2021, 41, 2621–2635. [Google Scholar]
- He, F.-S.; Ye, S.; Wu, J. Recent Advances in Pyridinium Salts as Radical Reservoirs in Organic Synthesis. ACS Catal. 2019, 9, 8943–8960. [Google Scholar] [CrossRef]
- Rössler, S.L.; Jelier, B.J.; Magnier, E.; Dagousset, G.; Carreira, E.M.; Togni, A. Pyridiniumsalze als redoxaktive Reagenzien zur Übertragung funktioneller Gruppen. Angew. Chem. Int. Ed. 2020, 132, 9350–9366. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, F.-Y. Recent Developments in Transition-Metal-Free Functionalization and Derivatization Reactions of Pyridines. Synlett 2020, 32, 159–178. [Google Scholar] [CrossRef]
- Kim, M.; Koo, Y.; Hong, S. N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C-H Functionalization via Radical-Based Processes under Visible Light Irradiation. Acc. Chem. Res. 2022, 55, 3043–3056. [Google Scholar] [CrossRef]
- Xu, J.; Chen, D.; Liu, C. Recent advances of aminoazanium salts as amination reagents in organic synthesis. Org. Biomol. Chem. 2022, 20, 8353–8365. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y. Recent Developments in the Chemistry of Heteroaromatic N-Oxides. Synthesis 2015, 47, 289–305. [Google Scholar] [CrossRef]
- Sowmiah, S.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Pyridinium salts: From synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar] [CrossRef]
- Murahashi, S.-I.; Imada, Y. Synthesis and Transformations of Nitrones for Organic Synthesis. Chem. Rev. 2019, 119, 4684–4716. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Kato, R.; Kinugawa, D.; Kamiya, H.; Kudo, R.; Hasegawa, M.; Fujii, H.; Suga, H. Photochemically-induced C-C bond formation between tertiary amines and nitrones. Org. Biomol. Chem. 2015, 13, 8919–8924. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gao, F.; Yang, C.; Gao, G.L.; Zhao, Y.; Gao, Y.; Xia, W. Visible-Light-Mediated Anti-Regioselective Nitrone 1,3-Dipolar Cycloaddition Reaction and Synthesis of Bisindolylmethanes. Org. Lett. 2017, 19, 5086–5089. [Google Scholar] [CrossRef]
- Supranovich, V.I.; Levin, V.V.; Struchkova, M.I.; Dilman, A.D. Photocatalytic Reductive Fluoroalkylation of Nitrones. Org. Lett. 2018, 20, 840–843. [Google Scholar] [CrossRef]
- Ye, C.X.; Melcamu, Y.Y.; Li, H.H.; Cheng, J.T.; Zhang, T.T.; Ruan, Y.P.; Zheng, X.; Lu, X.; Huang, P.Q. Dual catalysis for enantioselective convergent synthesis of enantiopure vicinal amino alcohols. Nat. Commun. 2018, 9, 410. [Google Scholar] [CrossRef]
- Huang, S.-H.; Hong, R. Pinacol coupling going in a photocatalytic asymmetric manner: Construction of chiral vicinal amino alcohols. Sci. China Chem. 2018, 61, 509–510. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Zheng, X.; Huang, P.-Q. Photoredox Catalysis for the Coupling Reaction of Nitrones with Aromatic Tertiary Amines. Acta. Chim. Sin. 2019, 77, 850–855. [Google Scholar] [CrossRef]
- Haun, G.; Paneque, A.N.; Almond, D.W.; Austin, B.E.; Moura-Letts, G. Synthesis of Chromenoisoxazolidines from Substituted Salicylic Nitrones via Visible-Light Photocatalysis. Org. Lett. 2019, 21, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, B.T.; Correia, J.T.M.; Paixão, M.W. Visible-Light-Mediated α-Amino Alkylation of Azomethine Imines: An Approach to N-(β-Aminoalkyl)pyrazolidinones. Org. Lett. 2020, 22, 7891–7896. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, B.T.; Oliveira, P.H.R.; Correia, J.T.M.; Paixão, M.W. Carbamoylation of Azomethine Imines via Visible-Light Photoredox Catalysis. Org. Lett. 2021, 23, 6775–6779. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mukherjee, A.; Kovalev, I.S.; Kopchuk, D.S.; Zyryanov, G.V.; Tsurkan, M.V.; Majee, A.; Ranu, B.C.; Charushin, V.N.; Chupakhin, O.N.; et al. Recent Advances on Diverse Decarboxylative Reactions of Amino Acids. Adv. Synth. Catal. 2019, 361, 2161–2214. [Google Scholar] [CrossRef]
- Li, H.H.; Li, J.Q.; Zheng, X.; Huang, P.Q. Photoredox-Catalyzed Decarboxylative Cross-Coupling of alpha-Amino Acids with Nitrones. Org. Lett. 2021, 23, 876–880. [Google Scholar] [CrossRef]
- Dorn, H.; Otto, A. Syntheses by Means of 1-Alkylidene- and 1-(Arylalkylidene)-3-pyrazolidone N,N-Betaines, a New Type of Stable Azomethine Imine. Angew. Chem. Int. Ed. 1968, 7, 214–215. [Google Scholar] [CrossRef]
- Qiu, G.; Kuang, Y.; Wu, J. N-Imide Ylide-Based Reactions: C–H Functionalization, Nucleophilic Addition and Cycloaddition. Adv. Synth. Catal. 2014, 356, 3483–3504. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M.; Yus, M. 1,3-Dipolar cycloadditions of azomethine imines. Org. Biomol. Chem. 2015, 13, 8596–8636. [Google Scholar] [CrossRef]
- Grošelj, U.; Požgan, F.; Štefane, B.; Svete, J. Copper-Catalyzed Azomethine Imine–Alkyne Cycloadditions (CuAIAC). Synthesis 2018, 50, 4501–4524. [Google Scholar]
- Deepthi, A.; Thomas, N.V.; Sruthi, S.L. An overview of the reactions involving azomethine imines over half a decade. New J. Chem. 2021, 45, 8847–8873. [Google Scholar] [CrossRef]
- Xia, P.-J.; Ye, Z.-P.; Song, D.; Ren, J.-W.; Wu, H.-W.; Xiao, J.-A.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. Photocatalytic reductive radical–radical coupling of N,N′-cyclicazomethine imines with difluorobromo derivatives. Chem. Commun. 2019, 55, 2712–2715. [Google Scholar] [CrossRef] [PubMed]
- Leleu, L.; Martzel, T.; Fall, A.; Sanselme, M.; Levacher, V.; Oudeyer, S.; Briere, J.F. Diastereoselective addition of redox active esters to azomethine imines by electrosynthesis. Chem. Commun. 2022, 58, 6100–6103. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, I.A.; Levin, V.V.; Dilman, A.D. Boron Chelates Derived from N-Acylhydrazones as Radical Acceptors: Photocatalyzed Coupling of Hydrazones with Carboxylic Acids. Org. Lett. 2021, 23, 8973–8977. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kang, G.; Lee, K.; Park, B.; Kang, D.; Jung, H.; He, Y.T.; Baik, M.H.; Hong, S. Site-Selective Functionalization of Pyridinium Derivatives via Visible-Light-Driven Photocatalysis with Quinolinone. J. Am. Chem. Soc. 2019, 141, 9239–9248. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Qian, P.; Wang, Y.; Mei, H.; Han, J.; Pan, Y. Cu-Catalyzed Deoxygenative C2-Sulfonylation Reaction of Quinoline N-Oxides with Sodium Sulfinate. Org. Lett. 2016, 18, 4144–4147. [Google Scholar] [CrossRef]
- Gu, Y.R.; Duan, X.H.; Yang, L.; Guo, L.N. Direct C-H Cyanoalkylation of Heteroaromatic N-Oxides and Quinones via C-C Bond Cleavage of Cyclobutanone Oximes. Org. Lett. 2017, 19, 5908–5911. [Google Scholar] [CrossRef]
- Zhang, W.M.; Dai, J.J.; Xu, J.; Xu, H.J. Visible-Light-Induced C2 Alkylation of Pyridine N-Oxides. J. Org. Chem. 2017, 82, 2059–2066. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Y.; Li, H.; Dong, W.; Peng, Z.; An, D. Iron-catalyzed deoxygenation and 2-sulfonylation of quinoline N-oxides by sodium sulfinates towards 2-sulfonyl quinolines. Synth. Commun. 2018, 48, 1909–1918. [Google Scholar] [CrossRef]
- Zhou, W.; Miura, T.; Murakami, M. Photocatalyzed ortho-Alkylation of Pyridine N-Oxides through Alkene Cleavage. Angew. Chem. Int. Ed. 2018, 57, 5139–5142. [Google Scholar] [CrossRef]
- Sun, A.C.; McClain, E.J.; Beatty, J.W.; Stephenson, C.R.J. Visible Light-Mediated Decarboxylative Alkylation of Pharmaceutically Relevant Heterocycles. Org. Lett. 2018, 20, 3487–3490. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.-Y.; Li, P.; Wang, L. Copper-Catalyzed Deoxygenative C-2 Amination of Quinoline N-Oxides. Eur. J. Org. Chem. 2018, 2018, 5954–5960. [Google Scholar] [CrossRef]
- Lantano, B.; Barata-Vallejo, S.; Postigo, A. Organic dye-photocatalyzed fluoroalkylation of heteroarene-N-oxide derivatives. Org. Biomol. Chem. 2018, 16, 6718–6727. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, M.; Vishwakarma, R.A.; Verma, M.K.; Singh, P.P. Room Temperature Metal-Catalyzed Oxidative Acylation of Electron-Deficient Heteroarenes with Alkynes, Its Mechanism, and Application Studies. J. Org. Chem. 2018, 83, 12420–12431. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.P.; Wang, B.; Stevens, E.D.; Burris, S.C.; Deng, Y. ortho-Alkylation of Pyridine N-Oxides with Alkynes by Photocatalysis: Pyridine N-Oxides as a Redox Auxiliary. Chemistry 2019, 25, 6638–6644. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Wu, W.B.; Wu, J. Photoinduced Divergent Alkylation/Acylation of Pyridine N-Oxides with Alkynes under Anaerobic and Aerobic Conditions. Org. Lett. 2019, 21, 5321–5325. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhuo, W.-T.; Niu, Y.-N.; Gao, G.-L. Visible-Light-Promoted C2 Trifluoromethylation of Quinoline N-Oxides. Synthesis 2019, 52, 219–226. [Google Scholar] [CrossRef]
- Li, D.; Liang, C.; Jiang, Z.; Zhang, J.; Zhuo, W.T.; Zou, F.Y.; Wang, W.P.; Gao, G.L.; Song, J. Visible-Light-Promoted C2 Selective Arylation of Quinoline and Pyridine N-Oxides with Diaryliodonium Tetrafluoroborate. J. Org. Chem. 2020, 85, 2733–2742. [Google Scholar] [CrossRef]
- Qin, P.T.; Sun, J.; Wang, F.; Wang, J.Y.; Wang, H.; Zhou, M.D. Visible-Light-Induced C2 Alkylation of Heterocyclic N-Oxides with N-Hydroxyphthalimide Esters under Metal-Free Conditions. Adv. Synth. Catal. 2020, 362, 4707–4715. [Google Scholar] [CrossRef]
- Gong, H.; Wang, J.; Peng, Y.; Chen, H.; Deng, H.; Hao, J.; Wan, W. Photocatalyzed difluoroalkylation of pyridine N-oxides. Synth. Commun. 2022, 52, 1727–1741. [Google Scholar] [CrossRef]
- Nuñez, A.; de Viedma, A.G.; Martínez-Barrasa, V.; Burgos, C.; Alvarez-Builla, J. N-Azinylpyridinium N-Aminides: An Approach to Pyrazolopyridines via an Intramolecular Radical Pathway. Synlett 2002, 2002, 1093–1096. [Google Scholar] [CrossRef]
- Fang, L.; Chen, L.; Yu, J.; Wang, L. Benzoyl Peroxide Promoted Radicalortho-Alkylation of Nitrogen Heteroaromatics with Simple Alkanes and Alcohols. Eur. J. Org. Chem. 2015, 2015, 1910–1914. [Google Scholar] [CrossRef]
- Moon, Y.; Lee, W.; Hong, S. Visible-Light-Enabled Ortho-Selective Aminopyridylation of Alkenes with N-Aminopyridinium Ylides. J. Am. Chem. Soc. 2020, 142, 12420–12429. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.A.; Mousseau, J.J.; Pelletier, G.; Charette, A.B. Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N-activated pyridines. Chem. Rev. 2012, 112, 2642–2713. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Hu, F.; Jia, J. Transition-Metal-Catalyzed Nucleophilic Dearomatization of Electron-Deficient Heteroarenes. Synthesis 2022, 54, 92–110. [Google Scholar]
- Segovia, C.; Nocquet, P.-A.; Levacher, V.; Brière, J.-F.; Oudeyer, S. Organocatalysis: A Tool of Choice for the Enantioselective Nucleophilic Dearomatization of Electron-Deficient Six-Membered Ring Azaarenium Salts. Catalysts 2021, 11, 1249. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.Y.; Sun, J.; Han, Y.; Jia, X.; Yan, C.G. Construction of C(sp(2))-X (X = Br, Cl) Bonds through a Copper-Catalyzed Atom-Transfer Radical Process: Application for the 1,4-Difunctionalization of Isoquinolinium Salts. Org. Lett. 2018, 20, 987–990. [Google Scholar] [CrossRef]
- Fang, H.-L.; Sun, Q.; Ye, R.; Sun, J.; Han, Y.; Yan, C.-G. Copper-catalyzed selective difunctionalization of N-heteroarenes through a halogen atom transfer radical process. New J. Chem. 2019, 43, 13832–13836. [Google Scholar] [CrossRef]
- Youte, J.J.; Barbier, D.; Al-Mourabit, A.; Gnecco, D.; Marazano, C. An enantioselective access to 1-alkyl-1,2,3,4-tetrahydroisoquinolines. Application to a new synthesis of (-)-argemonine. J. Org. Chem. 2004, 69, 2737–2740. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, R.; Deng, R.; Lin, S.; Guo, S.; Yan, Z. Copper-Mediated Oxidative Functionalization of C(sp(3))-H Bonds with Isoquinolines: Two-Step Synthesis of 5-Oxaprotoberberinones. J. Org. Chem. 2016, 81, 11162–11167. [Google Scholar] [CrossRef]
- Luo, W.K.; Shi, X.; Zhou, W.; Yang, L. Iodine-Catalyzed Oxidative Functionalization of Azaarenes with Benzylic C(sp(3))-H Bonds via N-Alkylation/Amidation Cascade: Two-Step Synthesis of Isoindolo [2,1-b]isoquinolin-7(5H)-one. Org. Lett. 2016, 18, 2036–2039. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, W.-K.; Yang, L.; Ma, D.-Y. Iodine-catalyzed oxidative multiple C–H bond functionalization of isoquinolines with methylarenes: An efficient synthesis of isoquinoline-1,3,4(2H)-triones. Org. Biomol. Chem. 2017, 15, 7112–7116. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ou, L.; Yang, H.; Fu, H. Visible-Light-Mediated Aerobic Oxidation of N-Alkylpyridinium Salts under Organic Photocatalysis. J. Am. Chem. Soc. 2017, 139, 14237–14243. [Google Scholar] [CrossRef] [PubMed]
- Motaleb, A.; Bera, A.; Maity, P. An organocatalyst bound alpha-aminoalkyl radical intermediate for controlled aerobic oxidation of iminium ions. Org. Biomol. Chem. 2018, 16, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Singh, K.; Tyagi, S.; Yadav, A.; Yadav, P.P. Harnessing selective PET and EnT catalysis by chlorophyll to synthesize N-alkylated quinoline-2(1H)-ones, isoquinoline-1(2H)-ones and 1,2,4-trioxanes. Org. Biomol. Chem. 2021, 19, 9433–9438. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.H.; Teng, F.; Li, J.H. Dearomatization-Enabled Visible-Light-Induced 1,2-Alkylsulfonylation of Alkenes Using Sodium Sulfinates and Pyridinium Salts. Adv. Synth. Catal. 2020, 362, 3369–3373. [Google Scholar] [CrossRef]
- Leitch, J.A.; Rogova, T.; Duarte, F.; Dixon, D.J. Dearomative Photocatalytic Construction of Bridged 1,3-Diazepanes. Angew. Chem. Int. Ed. 2020, 59, 4121–4130. [Google Scholar] [CrossRef]
- Choi, J.; Laudadio, G.; Godineau, E.; Baran, P.S. Practical and Regioselective Synthesis of C-4-Alkylated Pyridines. J. Am. Chem. Soc. 2021, 143, 11927–11933. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Q.; Zhang, X.; Yang, C. Photoredox-catalysed regioselective synthesis of C-4-alkylated pyridines with N-(acyloxy)phthalimides. Org. Biomol. Chem. 2022, 20, 1969–1973. [Google Scholar] [CrossRef]
- Gorny, R.; Schäfer, H.J.; Fröhlich, R. Diastereoselective Cathodic Cyclization of 1-(4- and 1-(3-Oxoalkyl)pyridinium Salts to Quinolizidine and Indolizidine Derivatives. Angew. Chem. Int. Ed. 1995, 34, 2007–2009. [Google Scholar] [CrossRef]























Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudeyer, S.; Levacher, V.; Beucher, H.; Brière, J.-F. Recent Advances in Catalytic and Technology-Driven Radical Addition to N,N-Disubstituted Iminium Species. Molecules 2023, 28, 1071. https://doi.org/10.3390/molecules28031071
Oudeyer S, Levacher V, Beucher H, Brière J-F. Recent Advances in Catalytic and Technology-Driven Radical Addition to N,N-Disubstituted Iminium Species. Molecules. 2023; 28(3):1071. https://doi.org/10.3390/molecules28031071
Chicago/Turabian StyleOudeyer, Sylvain, Vincent Levacher, Hélène Beucher, and Jean-François Brière. 2023. "Recent Advances in Catalytic and Technology-Driven Radical Addition to N,N-Disubstituted Iminium Species" Molecules 28, no. 3: 1071. https://doi.org/10.3390/molecules28031071
APA StyleOudeyer, S., Levacher, V., Beucher, H., & Brière, J.-F. (2023). Recent Advances in Catalytic and Technology-Driven Radical Addition to N,N-Disubstituted Iminium Species. Molecules, 28(3), 1071. https://doi.org/10.3390/molecules28031071







