Characterization of Galectin Fusion Proteins with Glycoprotein Affinity Columns and Binding Assays
Abstract
1. Introduction
2. Results
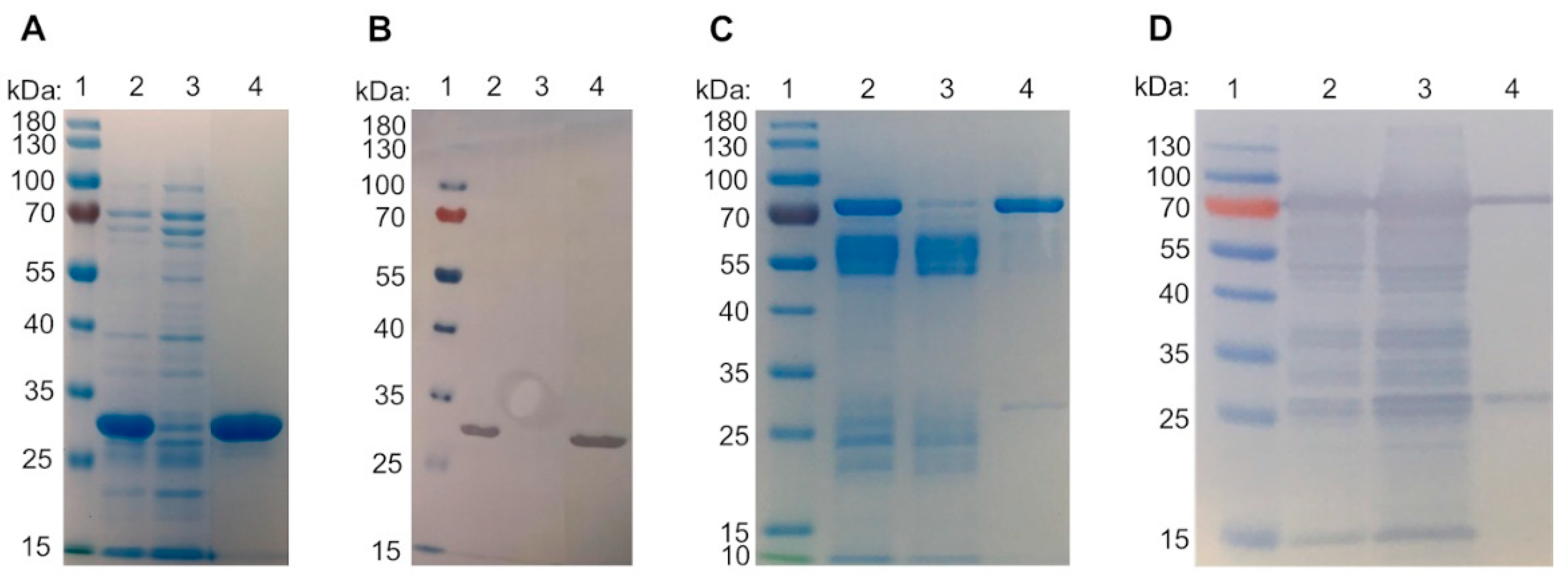
2.1. Galectin Purification
2.2. Binding Characteristics of Gal-3 Constructs on ASF
2.3. Binding of Gal-3 Constructs to ECM Glycoproteins
3. Discussions
3.1. Galectin Purification on Glycoprotein Columns
3.2. Binding of Galectins to ASF and ECM Glycoproteins
4. Materials and Methods
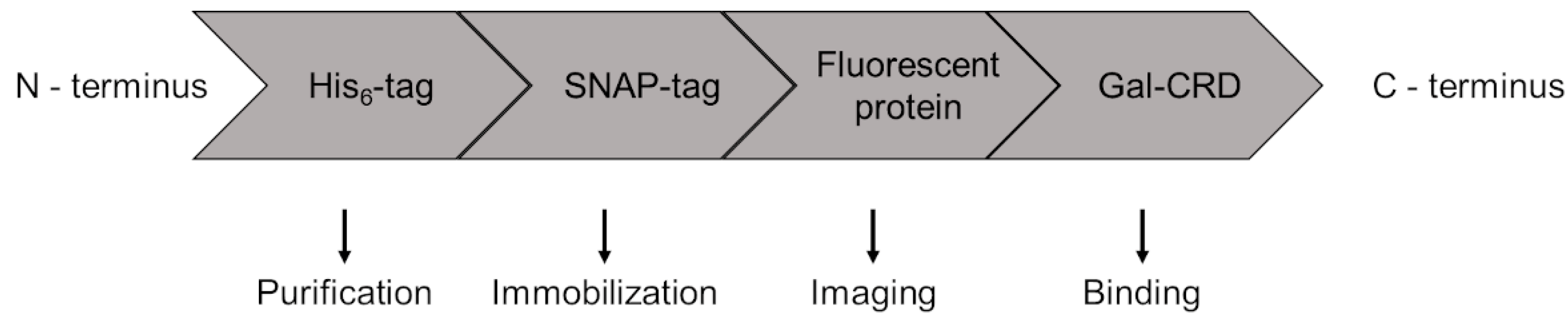
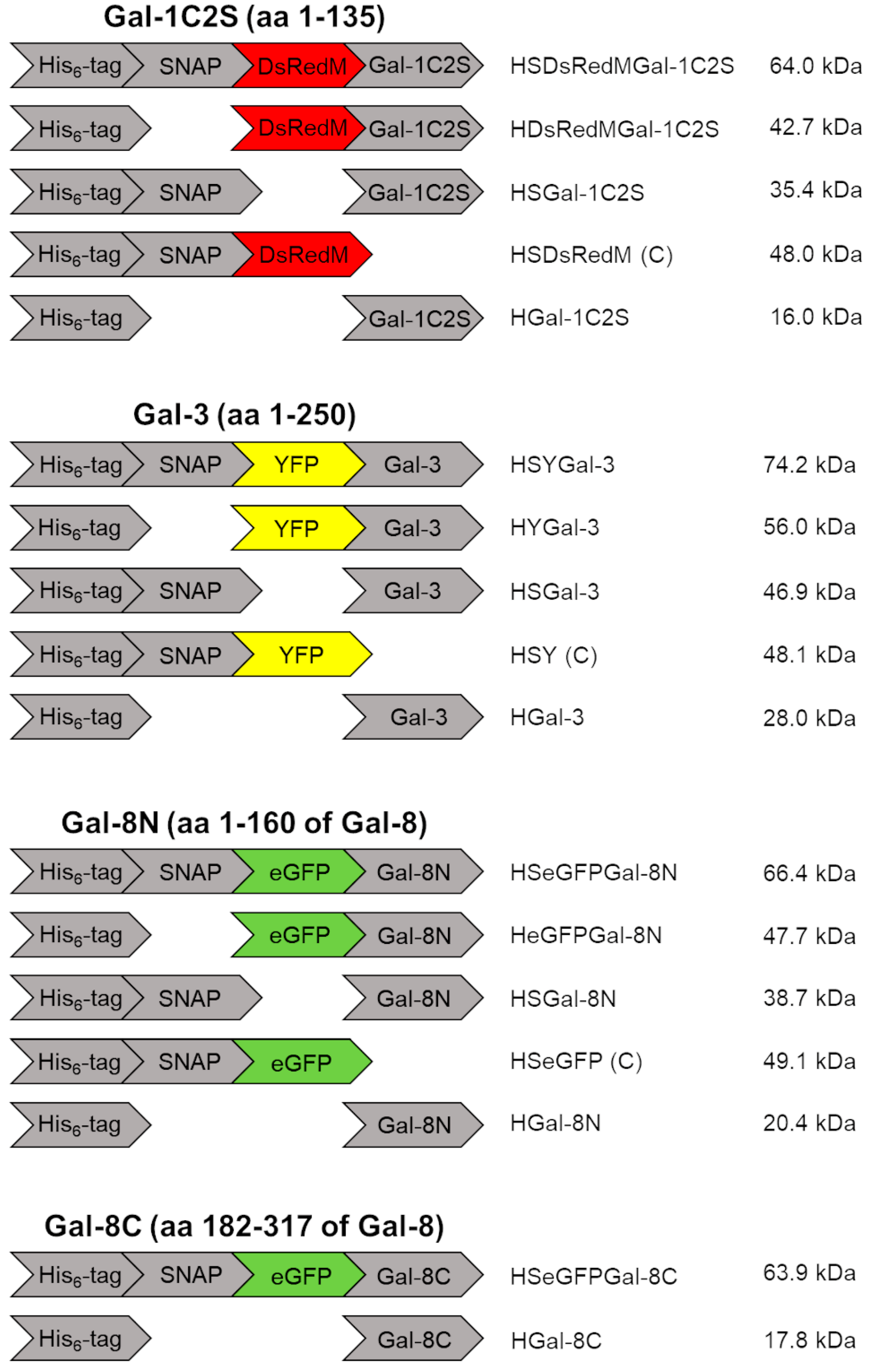
4.1. Galectin Constructs
4.2. Expression and Purification
4.3. Glycoprotein Affinity Resin and Galectin Purification
4.4. SDS-PAGE and Immunoblot
4.5. Size Exclusion Chromatography (SEC)
- Maximum pressure limit pmax
- Pressure limit at a maximum flow rate Δp
- Pressure of the dripping column at max. flow rate (1.8 mL × min−1) Δpcolumn
- Pressure with MQ at max. flow rate (not attached to the system) Δpbefore
- Pressure of column at intended conditions (0.75 mL × min−1, PBS pH 7.5) Δpbefore(2)
- Total system pressure Δptotal
- Average distribution constant KAV
- Total volume of the column VC
- Void volume V0
- Elution volume of standard protein/sample VE
4.6. Galectin Binding on ASF
4.7. Galectin Binding on ECM Glycoproteins
4.8. Galectin-Mediated Crosslinking of Extracellular Matrix Glycoproteins
4.9. Enzyme-Linked Lectin Assay (ELLA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, R.D.; Liu, F.T.; Rabinovich, G.A.; Stowell, S.R.; Vasta, G.R. Galectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Darvill, A.G., Kinoshita, T., Packer, N.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 491–504. [Google Scholar]
- Nakahara, S.; Raz, A. On the role of galectins in signal transduction. Methods Enzymol. 2006, 417, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Viguier, M.; Advedissian, T.; Delacour, D.; Poirier, F.; Deshayes, F. Galectins in epithelial functions. Tissue Barriers 2014, 2, e29103. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Hokama, A.; Mizoguchi, E.; Mizoguchi, A. Roles of galectins in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5133–5137. [Google Scholar] [CrossRef]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the hallmarks of cancer: Galectins as multifunctional mediators of tumor progression. J. Exp. Med. 2020, 217, e20182041. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Martinez-Bosch, N.; Barranco, L.E.; Orozco, C.A.; Moreno, M.; Visa, L.; Iglesias, M.; Oldfield, L.; Neoptolemos, J.P.; Greenhalf, W.; Earl, J.; et al. Increased plasma levels of galectin-1 in pancreatic cancer: Potential use as biomarker. Oncotarget 2018, 9, 32984–32996. [Google Scholar] [CrossRef]
- Masoodi, M.; Shah, Z.A.; Beigh, A.H.; Ahmad, S.Z.; Mir, A.W.; Yasin, B.; Rasool, R.; Masoodi, K.Z.; Bhat, G.M. Galectin-1 as a predictive biomarker in ovarian cancer. J. Ovarian Res. 2021, 14, 123. [Google Scholar] [CrossRef]
- Yi, N.; Zhao, X.; Ji, J.; Xu, M.; Jiao, Y.; Qian, T.; Zhu, S.; Jiang, F.; Chen, J.; Xiao, M. Serum galectin-3 as a biomarker for screening, early diagnosis, prognosis and therapeutic effect evaluation of pancreatic cancer. J. Cell. Mol. Med. 2020, 24, 11583–11591. [Google Scholar] [CrossRef] [PubMed]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefał, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnology 2018, 16, 73. [Google Scholar] [CrossRef]
- Bumba, L.; Laaf, D.; Spiwok, V.; Elling, L.; Křen, V.; Bojarová, P. Poly-N-Acetyllactosamine Neo-Glycoproteins as Nanomolar Ligands of Human Galectin-3: Binding Kinetics and Modeling. Int. J. Mol. Sci. 2018, 19, 372. [Google Scholar] [CrossRef] [PubMed]
- Laaf, D.; Bojarová, P.; Pelantová, H.; Křen, V.; Elling, L. Tailored Multivalent Neo-Glycoproteins: Synthesis, Evaluation, and Application of a Library of Galectin-3-Binding Glycan Ligands. Bioconjug. Chem. 2017, 28, 2832–2840. [Google Scholar] [CrossRef]
- Long, F.; Li, W.; Chen, W.; Liu, D.; Chen, Y.; Zhou, R.; Li, P. An amperometric biosensor based on Cu2O@Au nanocomposites for the detection of galectin-1 via lactose-galectin interactions. Nanotechnology 2019, 30, 485706. [Google Scholar] [CrossRef] [PubMed]
- Martos-Maldonado, M.C.; Quesada-Soriano, I.; García-Fuentes, L.; Vargas-Berenguel, A. Multivalent Lactose-Ferrocene Conjugates Based on Poly (Amido Amine) Dendrimers and Gold Nanoparticles as Electrochemical Probes for Sensing Galectin-3. Nanomaterials 2020, 10, 203. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, L.; Li, Y.; Pan, H.; Zhang, W.; Guan, M.; Li, W.; Chen, Y.; Li, Q.; Li, Z.; et al. Lactose-Functionalized Gold Nanorods for Sensitive and Rapid Serological Diagnosis of Cancer. ACS Appl. Mater. Interfaces 2016, 8, 5813–5820. [Google Scholar] [CrossRef]
- Heine, V.; Dey, C.; Bojarová, P.; Křen, V.; Elling, L. Methods of in vitro study of galectin-glycomaterial interaction. Biotechnol. Adv. 2022, 58, 107928. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Lin, H.-Y.; Tu, Z.; Huang, B.-S.; Wu, S.-C.; Lin, C.-H. Structural Basis Underlying the Binding Preference of Human Galectins-1, -3 and -7 for Galbeta1-3/4GlcNAc. PLoS ONE 2015, 10, e0125946. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xia, B.; Stowell, S.R.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 2009, 16, 36–47. [Google Scholar] [CrossRef]
- Kupper, C.; Böcker, S.; Liu, H.; Adamzyk, C.; van de Kamp, J.; Recker, T.; Lethaus, B.; Jahnen-Dechent, W.; Neuss, S.; Müller-Newen, G.; et al. Fluorescent SNAP-Tag Galectin Fusion Proteins as Novel Tools in Glycobiology. Curr. Pharm. Des. 2013, 19, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Böcker, S.; Elling, L. Binding characteristics of galectin-3 fusion proteins. Glycobiology 2017, 27, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Poland, P.A.; Kinlough, C.L.; Hughey, R.P. Cloning, Expression, and Purification of Galectins for In Vitro Studies. In Galectins; Humana Press: Totowa, NJ, USA, 2022; Volume 2442, pp. 41–54. [Google Scholar] [CrossRef]
- Maller, S.M.; Cagnoni, A.J.; Bannoud, N.; Sigaut, L.; Perez Sáez, J.M.; Pietrasanta, L.I.; Yang, R.-Y.; Liu, F.-T.; Croci, D.O.; Di Lella, S.; et al. An adipose tissue galectin controls endothelial cell function via preferential recognition of 3-fucosylated glycans. FASEB J. 2020, 34, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Li, J.; Mu, N.; Gao, Y.; Huang, T.; Zhang, Y.; Wang, Z.; Li, M.; Hao, Q.; Li, W.; et al. Expression, purification and characterization of galectin-1 in Escherichia coli. Protein Expr. Purif. 2014, 99, 58–63. [Google Scholar] [CrossRef]
- Paul, A.; Wu, S.-C.; Patel, K.R.; Ho, A.D.; Allen, J.W.L.; Verkerke, H.; Arthur, C.M.; Stowell, S.R. Purification of Recombinant Galectins from Different Species Using Distinct Affinity Chromatography Methods. In Galectins; Humana Press: Totowa, NJ, USA, 2022; Volume 2442, pp. 55–74. [Google Scholar] [CrossRef]
- Sakthivel, D.; Littler, D.; Shahine, A.; Troy, S.; Johnson, M.; Rossjohn, J.; Piedrafita, D.; Beddoe, T. Cloning, expression, purification and crystallographic studies of galectin-11 from domestic sheep (Ovis aries). Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 993–997. [Google Scholar] [CrossRef]
- Voss, P.G.; Gray, R.M.; Dickey, S.W.; Wang, W.; Park, J.W.; Kasai, K.-I.; Hirabayashi, J.; Patterson, R.J.; Wang, J.L. Dissociation of the carbohydrate-binding and splicing activities of galectin-1. Arch. Biochem. Biophys. 2008, 478, 18–25. [Google Scholar] [CrossRef]
- Welch, C.J.; Kadav, P.D.; Edwards, J.L.; Krycia, J.; Talaga, M.L.; Bandyopadhyay, P.; Dam, T.K. A Rapid and Facile Purification Method for Glycan-Binding Proteins and Glycoproteins. Curr. Protoc. Protein Sci. 2020, 101, e113. [Google Scholar] [CrossRef]
- Kadav, P.D.; Edwards, J.L.; Krycia, J.; Bandyopadhyay, P.; Dam, T.K. Rapid Detection and Purification of Galectin-3 by the Capture and Release (CaRe) Method. In Galectins; Humana Press: Totowa, NJ, USA, 2022; Volume 2442, pp. 89–103. [Google Scholar] [CrossRef]
- Welch, C.J.; Talaga, M.L.; Kadav, P.D.; Edwards, J.L.; Bandyopadhyay, P.; Dam, T.K. A capture and release method based on non-covalent ligand crosslinking and facile filtration for purification of lectins and glycoproteins. J. Biol. Chem. 2020, 295, 223–236. [Google Scholar] [CrossRef]
- Ahmed, H.; Fink, N.E.; Pohl, J.; Vasta, G.R. Galectin-1 from bovine spleen: Biochemical characterization, carbohydrate specificity and tissue-specific isoform profiles. J. Biochem. 1996, 120, 1007–1019. [Google Scholar] [CrossRef]
- Gitt, M.A.; Colnot, C.; Poirier, F.; Nani, K.J.; Barondes, S.H.; Leffler, H. Galectin-4 and galectin-6 are two closely related lectins expressed in mouse gastrointestinal tract. J. Biol. Chem. 1998, 273, 2954–2960. [Google Scholar] [CrossRef]
- Jones, J.L.; Saraswati, S.; Block, A.S.; Lichti, C.F.; Mahadevan, M.; Diekman, A.B. Galectin-3 is associated with prostasomes in human semen. Glycoconj. J. 2010, 27, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tamura, M.; Ishii, N.; Ishikida, H.; Sugimoto, S.; Suzuki, D.; Nishiyama, K.; Takahashi, H.; Natsugari, H.; Arata, Y. Purification of galectin-1 mutants using an immobilized Galactoseβ1-4Fucose affinity adsorbent. Protein Expr. Purif. 2015, 111, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Salahuddin, A. Isolation and some properties of mammalian hepatic membrane lectins. FEBS Lett. 1989, 246, 163–165. [Google Scholar] [CrossRef]
- Beyer, E.C.; Zweig, S.E.; Barondes, S.H. Two lactose binding lectins from chicken tissues. Purified lectin from intestine is different from those in liver and muscle. J. Biol. Chem. 1980, 255, 4236–4239. [Google Scholar] [CrossRef]
- Cerra, R.F.; Gitt, M.A.; Barondes, S.H. Three soluble rat beta-galactoside-binding lectins. J. Biol. Chem. 1985, 260, 10474–10477. [Google Scholar] [CrossRef] [PubMed]
- Leffler, H.; Barondes, S.H. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides. J. Biol. Chem. 1986, 261, 10119–10126. [Google Scholar] [CrossRef]
- de Waard, A.; Hickman, S.; Kornfeld, S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J. Biol. Chem. 1976, 251, 7581–7587. [Google Scholar] [CrossRef]
- Roff, C.F.; Wang, J.L. Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J. Biol. Chem. 1983, 258, 10657–10663. [Google Scholar] [CrossRef]
- Zlocowski, N.; Grupe, V.; Garay, Y.C.; Nores, G.A.; Lardone, R.D.; Irazoqui, F.J. Purified human anti-Tn and anti-T antibodies specifically recognize carcinoma tissues. Sci. Rep. 2019, 9, 8097. [Google Scholar] [CrossRef]
- Dam, T.K.; Gabius, H.-J.; André, S.; Kaltner, H.; Lensch, M.; Brewer, C.F. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.-J.; André, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous crosslinked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Balan, V.; Raz, A. Galectin-3 binding and metastasis. Methods Mol. Biol. 2012, 878, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, I.; Liu, F.T. Galectin-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 1996, 156, 3939–3944. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Warfield, P. Galectin-3 binding potentials of mouse tumor EHS and human placental laminins. Biochem. Biophys. Res. Commun. 1995, 217, 402–406. [Google Scholar] [CrossRef]
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Böcker, S.; Laaf, D.; Elling, L. Galectin Binding to Neo-Glycoproteins: LacDiNAc Conjugated BSA as Ligand for Human Galectin-3. Biomolecules 2015, 5, 1671–1696. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef]
- Cuatrecasas, P.; Wilchek, M.; Anfinsen, C.B. Selective enzyme purification by affinity chromatography. Proc. Natl. Acad. Sci. USA 1968, 61, 636–643. [Google Scholar] [CrossRef]
- Baenziger, J.U.; Fiete, D. Structure of the complex oligosaccharides of fetuin. J. Biol. Chem. 1979, 254, 789–795. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.-J.; Sabesan, S.; Oscarson, S.; Brewer, C.F. Thermodynamic binding studies of bivalent oligosaccharides to galectin-1, galectin-3, and the carbohydrate recognition domain of galectin-3. Glycobiology 2004, 14, 817–825. [Google Scholar] [CrossRef]
- Brewer, C.F. Thermodynamic binding studies of galectin-1, -3 and -7. Glycoconj. J. 2002, 19, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Öberg, C.T.; Carlsson, M.C.; Sundin, A.; Nilsson, U.J.; Smith, D.; Cummings, R.D.; Almkvist, J.; Karlsson, A.; Leffler, H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology 2007, 17, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Ideo, H.; Seko, A.; Ishizuka, I.; Yamashita, K. The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology 2003, 13, 713–723. [Google Scholar] [CrossRef]
- Kumar, S.; Frank, M.; Schwartz-Albiez, R. Understanding the specificity of human Galectin-8C domain interactions with its glycan ligands based on molecular dynamics simulations. PLoS One 2013, 8, e59761. [Google Scholar] [CrossRef] [PubMed]
- Vokhmyanina, O.A.; Rapoport, E.M.; Ryzhov, I.M.; Korchagina, E.Y.; Pazynina, G.V.; Severov, V.V.; Kaltner, H.; André, S.; Gabius, H.-J.; Bovin, N.V. Carbohydrate specificity of chicken and human tandem-repeat-type galectins-8 in composition of cells. Biochemistry 2011, 76, 1185–1192. [Google Scholar] [CrossRef]
- Vokhmyanina, O.A.; Rapoport, E.M.; André, S.; Severov, V.V.; Ryzhov, I.; Pazynina, G.V.; Korchagina, E.; Gabius, H.-J.; Bovin, N.V. Comparative study of the glycan specificities of cell-bound human tandem-repeat-type galectin-4, -8 and -9. Glycobiology 2012, 22, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Hayes, M.R.; Pietruszka, J.; Elling, L. Synthesis of the Thomsen-Friedenreich-antigen (TF-antigen) and binding of Galectin-3 to TF-antigen presenting neo-glycoproteins. Glycoconj. J. 2020, 37, 457–470. [Google Scholar] [CrossRef]
- Kremers, G.-J.; Goedhart, J.; van Munster, E.B.; Gadella, T.W., Jr. Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET Förster radius. Biochemistry 2006, 45, 6570–6580. [Google Scholar] [CrossRef]
- Zacharias, D.A.; Violin, J.D.; Newton, A.C.; Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 2002, 296, 913–916. [Google Scholar] [CrossRef]
- Stöhr, K.; Siegberg, D.; Ehrhard, T.; Lymperopoulos, K.; Öz, S.; Schulmeister, S.; Pfeifer, A.C.; Bachmann, J.; Klingmüller, U.; Sourjik, V.; et al. Quenched substrates for live-cell labeling of SNAP-tagged fusion proteins with improved fluorescent background. Anal. Chem. 2010, 82, 8186–8193. [Google Scholar] [CrossRef]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003, 21, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Strongin, D.E.; Bevis, B.; Khuong, N.; Downing, M.E.; Strack, R.L.; Sundaram, K.; Glick, B.S.; Keenan, R.J. Structural rearrangements near the chromophore influence the maturation speed and brightness of DsRed variants. Protein Eng. Des. Sel. 2007, 20, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.N., Jr. Structure and dynamics of green fluorescent protein. Curr. Opin. Struct. Biol. 1997, 7, 821–827. [Google Scholar] [CrossRef]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Matarrese, P.; Tinari, N.; Semeraro, M.L.; Natoli, C.; Iacobelli, S.; Malorni, W. Galectin-3 overexpression protects from cell damage and death by influencing mitochondrial homeostasis. FEBS Lett. 2000, 473, 311–315. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 1992, 267, 6983–6990. [Google Scholar] [CrossRef]
- Wang, J.L.; Werner, E.A.; Laing, J.G.; Patterson, R.J. Nuclear and cytoplasmic localization of a lectin-ribonucleoprotein complex. Biochem. Soc. Trans. 1992, 20, 269–274. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef]
- Fujiwara, S.; Shinkai, H.; Deutzmann, R.; Paulsson, M.; Timpl, R. Structure and distribution of N-linked oligosaccharide chains on various domains of mouse tumour laminin. Biochem. J. 1988, 252, 453–461. [Google Scholar] [CrossRef]
- Hughes, R.C. Role of glycosylation in cell interactions with extracellular matrix. Biochem. Soc. Trans. 1992, 20, 279–284. [Google Scholar] [CrossRef]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin--a glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P. Stretching fibronectin. J. Muscle Res. Cell Motil. 2002, 23, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Basak, T.; Vega-Montoto, L.; Zimmerman, L.J.; Tabb, D.L.; Hudson, B.G.; Vanacore, R.M. Comprehensive Characterization of Glycosylation and Hydroxylation of Basement Membrane Collagen IV by High-Resolution Mass Spectrometry. J. Proteome Res. 2016, 15, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Hennet, T. Collagen glycosylation. Curr. Opin. Struct. Biol. 2019, 56, 131–138. [Google Scholar] [CrossRef]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Dias-Baruffi, M.; Zhu, H.; Cho, M.; Karmakar, S.; McEver, R.P.; Cummings, R.D. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 2003, 278, 41282–41293. [Google Scholar] [CrossRef]
- Leppänen, A.; Stowell, S.; Blixt, O.; Cummings, R.D. Dimeric galectin-1 binds with high affinity to α2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J. Biol. Chem. 2005, 280, 5549–5562. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Slanina, K.A.; Horton, J.R.; Smith, D.F.; Cummings, R.D. Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J. Biol. Chem. 2008, 283, 20547–20559. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J. Biol. Chem. 1991, 266, 23648–23653. [Google Scholar] [CrossRef]
- Cormack, B.P.; Valdivia, R.H.; Falkow, S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 1996, 173, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.K.; Potvin, B.; Carlsson, S.; Sturm, D.; Leffler, H.; Stanley, P. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 2006, 16, 305–317. [Google Scholar] [CrossRef] [PubMed]

 ) and HSYGal-3 (■) is shown for IMAC-purified (A) and IMAC/ASF-resin-purified (B) galectins. Galectin binding was detected with a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
) and HSYGal-3 (■) is shown for IMAC-purified (A) and IMAC/ASF-resin-purified (B) galectins. Galectin binding was detected with a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
 ) and HSYGal-3 (■) is shown for IMAC-purified (A) and IMAC/ASF-resin-purified (B) galectins. Galectin binding was detected with a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
) and HSYGal-3 (■) is shown for IMAC-purified (A) and IMAC/ASF-resin-purified (B) galectins. Galectin binding was detected with a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.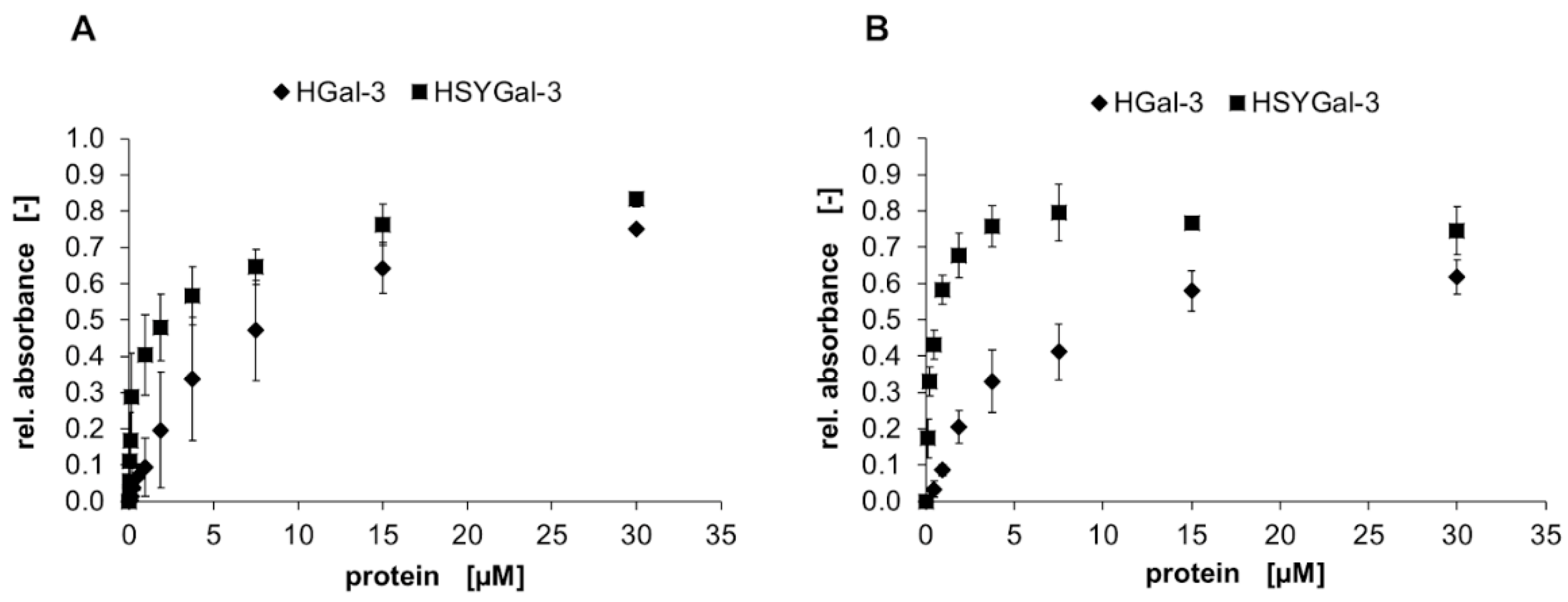
 ), HYGal-3 (●), and HSY (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
), HYGal-3 (●), and HSY (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
 ), HYGal-3 (●), and HSY (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
), HYGal-3 (●), and HSY (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations.
 ), HDsRedMGal-1C2S and HeGFPGal-8N (●), HSDsRedM and HSeGFP (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations. The apparent binding behavior of SNAP-tagged galectins is higher compared to non-SNAP-tagged galectins.
), HDsRedMGal-1C2S and HeGFPGal-8N (●), HSDsRedM and HSeGFP (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations. The apparent binding behavior of SNAP-tagged galectins is higher compared to non-SNAP-tagged galectins.
 ), HDsRedMGal-1C2S and HeGFPGal-8N (●), HSDsRedM and HSeGFP (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations. The apparent binding behavior of SNAP-tagged galectins is higher compared to non-SNAP-tagged galectins.
), HDsRedMGal-1C2S and HeGFPGal-8N (●), HSDsRedM and HSeGFP (▲) (control) in a solid-phase binding assay on ASF. Bound galectins were detected using a peroxidase-conjugated anti-His6-antibody and conversion of TMB as the mean signal of three data points. Errors indicate standard deviations. The apparent binding behavior of SNAP-tagged galectins is higher compared to non-SNAP-tagged galectins.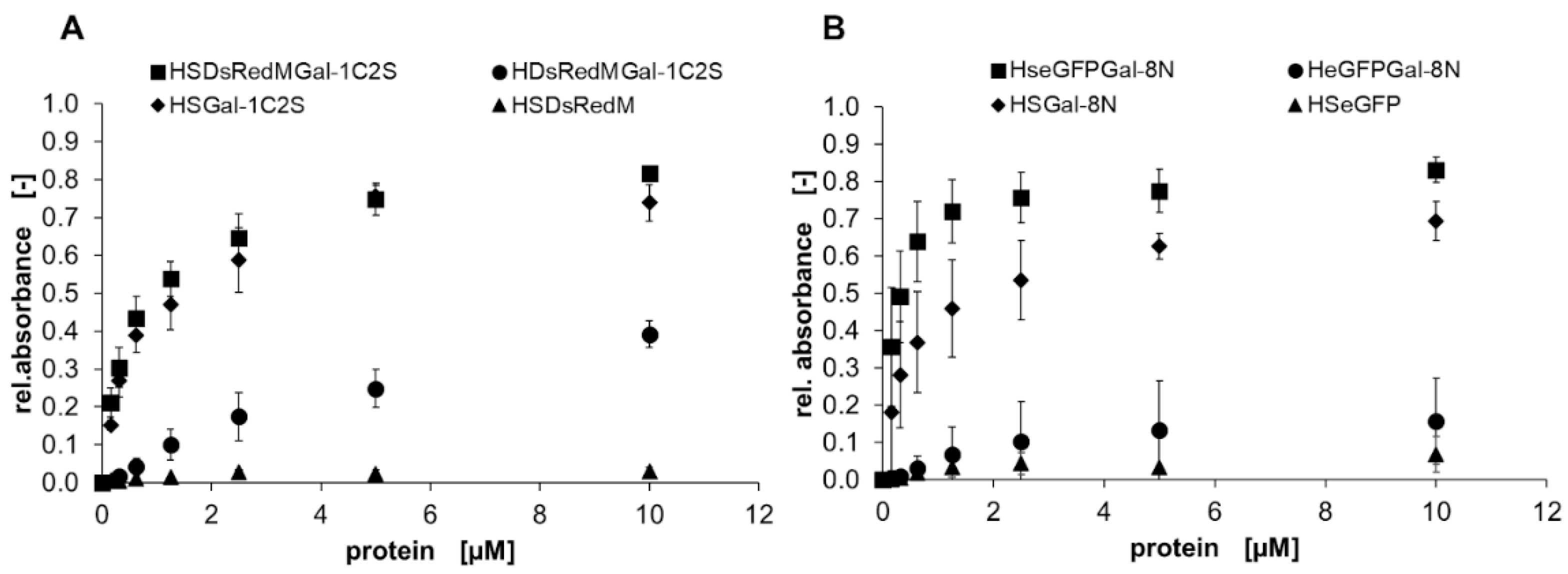
 ), and collagen IV (▲) is shown in a solid-phase assay. Galectin binding was detected by an anti-His6-antibody, and conversion of TMB was measured using three data points. Error bars indicate standard deviations.
), and collagen IV (▲) is shown in a solid-phase assay. Galectin binding was detected by an anti-His6-antibody, and conversion of TMB was measured using three data points. Error bars indicate standard deviations.
 ), and collagen IV (▲) is shown in a solid-phase assay. Galectin binding was detected by an anti-His6-antibody, and conversion of TMB was measured using three data points. Error bars indicate standard deviations.
), and collagen IV (▲) is shown in a solid-phase assay. Galectin binding was detected by an anti-His6-antibody, and conversion of TMB was measured using three data points. Error bars indicate standard deviations.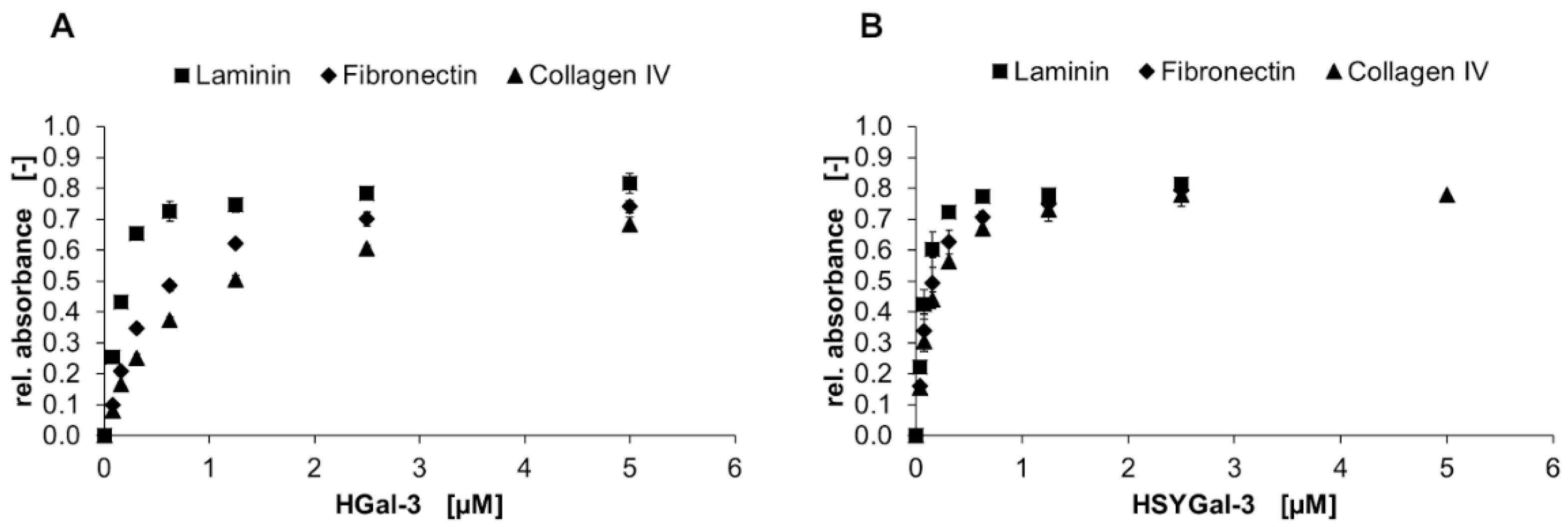
 ) and galectin fusion proteins (■) of (A) Gal-1C2S (proto-type), (B) Gal-3 (chimera-type) and (C) Gal-8N (tandem-repeat-type) in a solid-phase binding assay. ECM glycoprotein crosslinking was detected by primary anti-ECM protein-antibodies and corresponding secondary peroxidase-conjugated antibodies. Conversion of TMB was measured using three data points. Error bars indicate standard deviations.
) and galectin fusion proteins (■) of (A) Gal-1C2S (proto-type), (B) Gal-3 (chimera-type) and (C) Gal-8N (tandem-repeat-type) in a solid-phase binding assay. ECM glycoprotein crosslinking was detected by primary anti-ECM protein-antibodies and corresponding secondary peroxidase-conjugated antibodies. Conversion of TMB was measured using three data points. Error bars indicate standard deviations.
 ) and galectin fusion proteins (■) of (A) Gal-1C2S (proto-type), (B) Gal-3 (chimera-type) and (C) Gal-8N (tandem-repeat-type) in a solid-phase binding assay. ECM glycoprotein crosslinking was detected by primary anti-ECM protein-antibodies and corresponding secondary peroxidase-conjugated antibodies. Conversion of TMB was measured using three data points. Error bars indicate standard deviations.
) and galectin fusion proteins (■) of (A) Gal-1C2S (proto-type), (B) Gal-3 (chimera-type) and (C) Gal-8N (tandem-repeat-type) in a solid-phase binding assay. ECM glycoprotein crosslinking was detected by primary anti-ECM protein-antibodies and corresponding secondary peroxidase-conjugated antibodies. Conversion of TMB was measured using three data points. Error bars indicate standard deviations.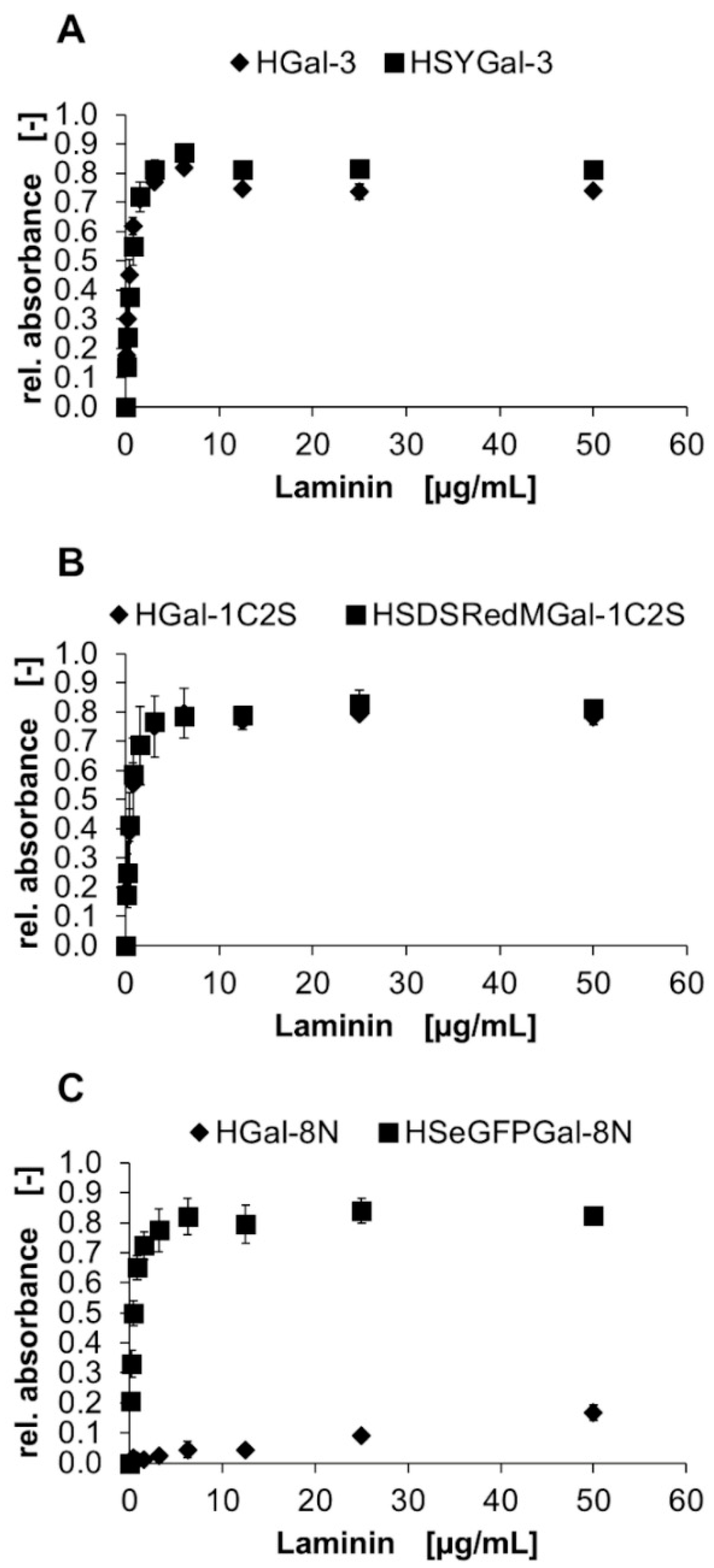
| IMAC | IMAC + ASF Affinity Resin | |||
|---|---|---|---|---|
| Elution Peak | MW (Calc.) | Elution Peak | MW (Calc.) | |
| HSYGal-3 (74.2 kDa) | 1 | 86.5 | 1 | 84.9 |
| 2 | 68 | 2 | 15.1 | |
| 3 | 31.7 | 3 | 1.8 | |
| HYGal-3 (56 kDa) | 1 | 47.8 | 1 | 50.6 |
| 2 | 30.3 | 2 | 2.0 | |
| HSGal-3 (46.9 kDa) | 1 | 48.5 | 1 | 48.3 |
| 2 | 28.7 | |||
| 3 | 6.2 | |||
| HGal-3 (28 kDa) | 1 | 21.0 | 1 | 28.7 |
| HSY (48.1 kDa) | 1 | 88.9 | ||
| Protein | Apparent KD [µM] | Apparent Binding Efficiency [µM−1] |
|---|---|---|
| IMAC | ||
| HGal-3 | 6.9 ± 1.6 | 0.1 |
| HSYGal-3 | 1.5 ± 0.8 | 0.6 |
| HSGal-3 | 0.3 ± 0.02 | 2.7 |
| HYGal-3 | 2.3 ± 0.4 | 0.3 |
| IMAC and ASF affinity chromatography | ||
| HGal-3 | 5.3 ± 1.0 | 0.1 |
| HSYGal-3 | 0.5 ± 0.1 | 1.7 |
| HSGal-3 | 0.3 ± 0.02 | 3.1 |
| HYGal-3 | 4.1 ± 1.2 | 0.1 |
| Protein | Apparent KD [µM] | Apparent Binding Efficiency [µM−1] |
|---|---|---|
| HSDsRedMGal-1C2S | 0.6 ± 0.1 | 1.4 |
| HSGal1-C2S | 0.8 ± 0.1 | 1.1 |
| HDsRedMGal-1C2S | 8.8 ± 3.0 | 0.1 |
| HGal-1C2S | 0.5 ± 0.1 | 1.6 |
| HSeGFPGal-8N | 0.2 ± 0.1 | 4.0 |
| HSGal-8N | 0.5 ± 0.2 | 1.3 |
| HeGFPGal-8N | 2.9 ± 3.3 | 0.1 |
| HGal-8N | 0.37 ± 0.02 | 2.2 |
| Apparent KD [µM] | |||
|---|---|---|---|
| Laminin | Fibronectin | Collagen IV | |
| HGal-3 | 0.13 ± 0.01 | 0.43 ± 0.02 | 0.64 ± 0.03 |
| HSYGal-3 | 0.07 ± 0.01 | 0.11 ± 0.01 | 0.13 ± 0.01 |
| HGal-1C2S | 0.42 ± 0.02 | 1.97 ± 0.38 | 1.34 ± 0.15 |
| HSDsRedMGal-1C2S | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.14 ± 0.03 |
| HGal-8N | 0.18 ± 0.02 | 0.52 ± 0.03 | 0.60 ± 0.03 |
| HSeGFPGal-8N | 0.04 ± 0.04 | 0.06 ± 0.01 | 0.08 ± 0.01 |
| ASF-Bound Galectin | Apparent KD [µM] of Laminin |
|---|---|
| HGal-3 | 0.27 ± 0.03 |
| HSYGal-3 | 0.45 ± 0.04 |
| HGal-1C2S | 0.38 ± 0.07 |
| HSDsRedMGal-1C2S | 0.37 ± 0.03 |
| HGal-8N | 69.5 ± 22.7 |
| HSeGFPGal-8N | 0.27 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, C.; Palm, P.; Elling, L. Characterization of Galectin Fusion Proteins with Glycoprotein Affinity Columns and Binding Assays. Molecules 2023, 28, 1054. https://doi.org/10.3390/molecules28031054
Dey C, Palm P, Elling L. Characterization of Galectin Fusion Proteins with Glycoprotein Affinity Columns and Binding Assays. Molecules. 2023; 28(3):1054. https://doi.org/10.3390/molecules28031054
Chicago/Turabian StyleDey, Carina, Philip Palm, and Lothar Elling. 2023. "Characterization of Galectin Fusion Proteins with Glycoprotein Affinity Columns and Binding Assays" Molecules 28, no. 3: 1054. https://doi.org/10.3390/molecules28031054
APA StyleDey, C., Palm, P., & Elling, L. (2023). Characterization of Galectin Fusion Proteins with Glycoprotein Affinity Columns and Binding Assays. Molecules, 28(3), 1054. https://doi.org/10.3390/molecules28031054







