Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly
Abstract
1. Introduction
2. Results
2.1. Expression and Purification of Gal-4 and Its Binding Domains
2.2. Carbohydrate Ligands of Gal-4
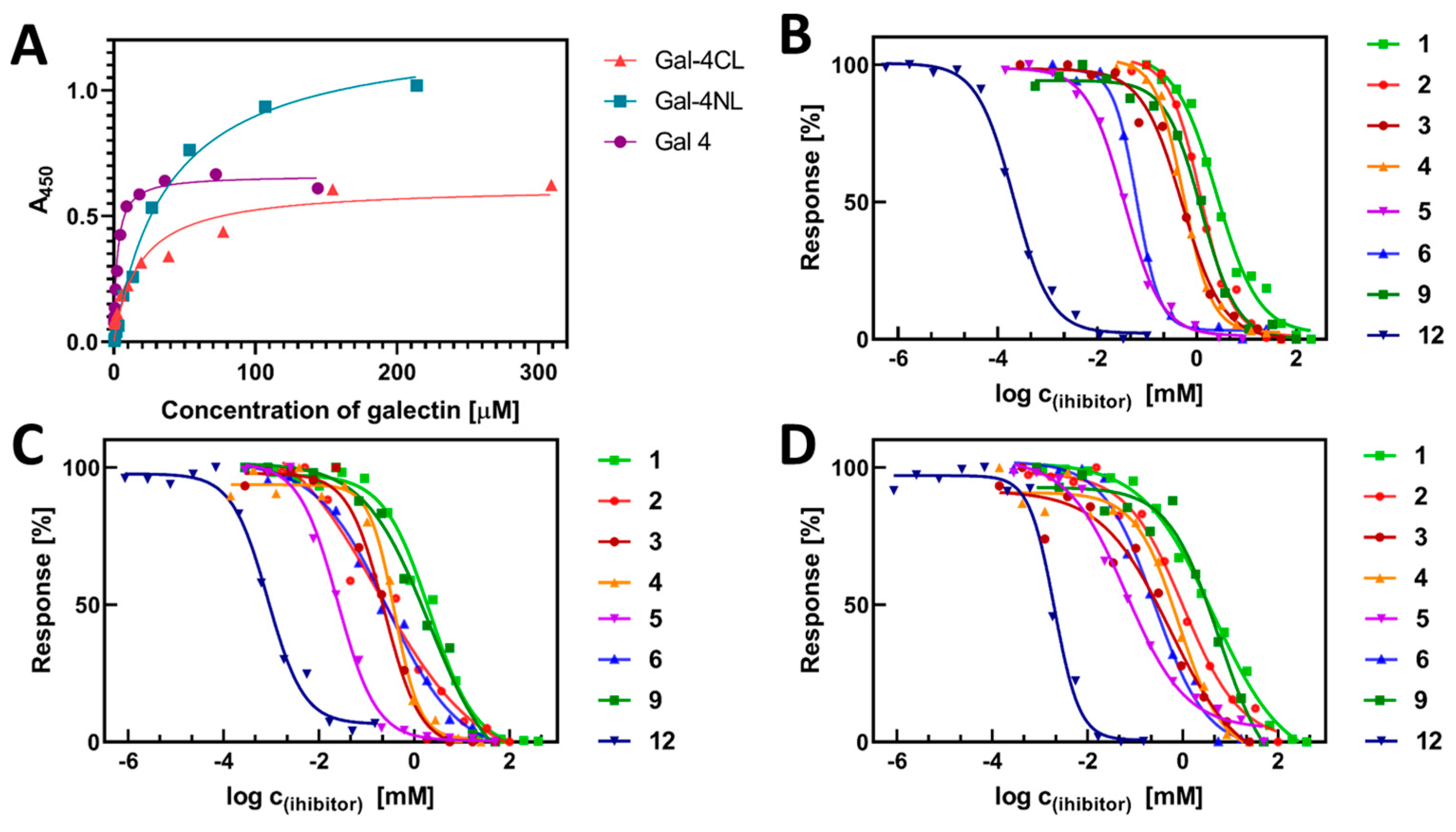
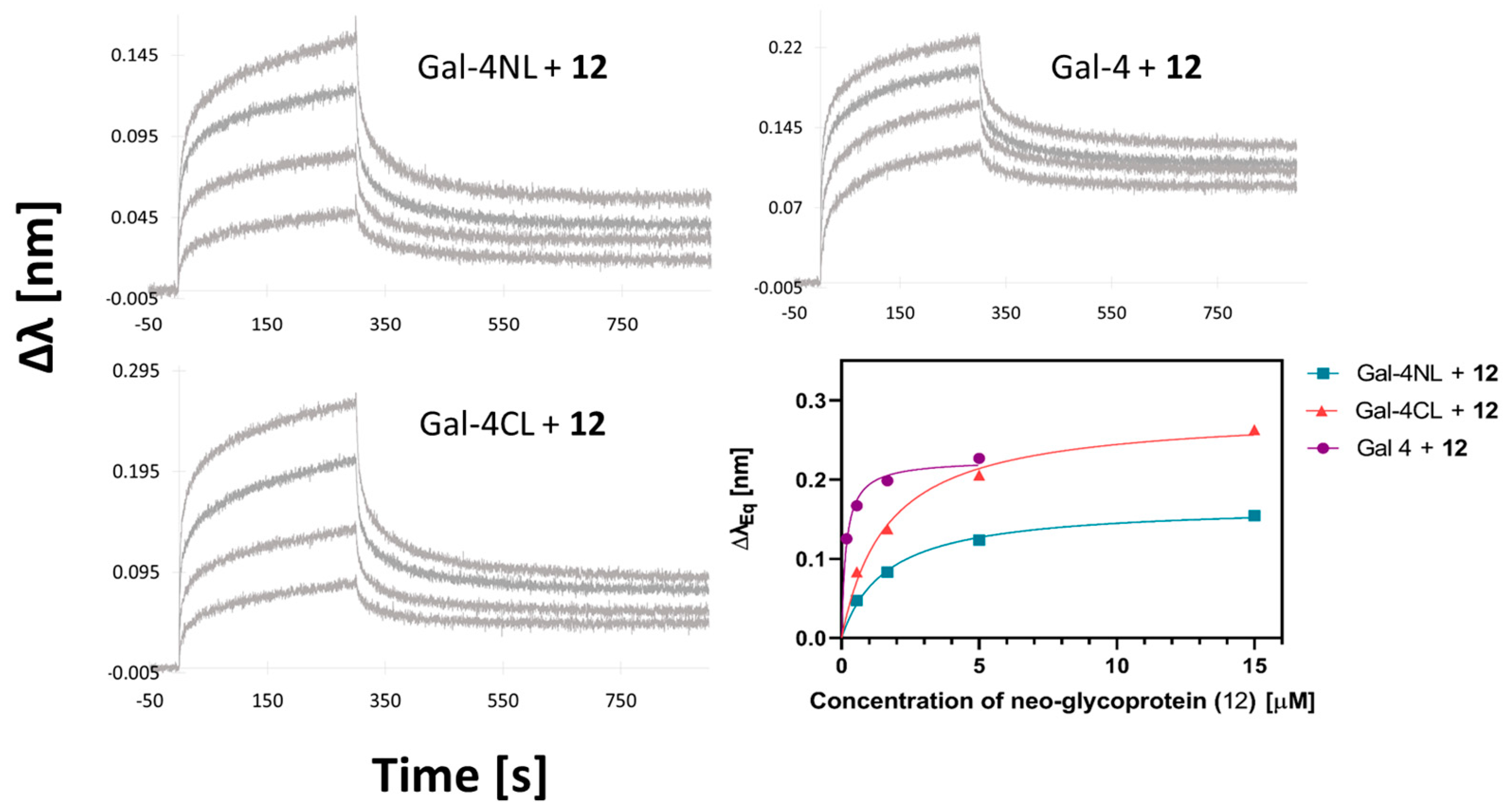
2.3. Binding Affinity of Gal-4 and Its Subunits to a Library of Carbohydrate Ligands
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Carbohydrate Ligands of Gal-4
4.2.1. Enzymatic Synthesis of Thomsen–Friedenreich Antigen (9; TF antigen; β-d-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-d-galactopyranose)
4.2.2. Chemo-Enzymatic Synthesis of Lactosyl-Decorated Neo-Glycoprotein 12
4.3. Expression and Purification of Gal-4
4.4. Expression and Purification of the N- and C-Terminal Subunits of Gal-4
4.5. ELISA Assays with Gal-4 and Its Subunits
4.6. Biolayer Interferometry (BLI)
4.7. Dynamic Light Scattering (DLS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Elola, M.T.; Blindner, A.G.; Ferragut, F.; Bracalente, C.; Rabinovich, G.A. Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes. Biochem. J. 2015, 469, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cousin, J.M.; Cloninger, M.J. The role of galectin-1 in cancer progression, and synthetic multivalent systems for the study of galectin-1. Int. J. Mol. Sci. 2016, 17, 1566. [Google Scholar] [CrossRef] [PubMed]
- Heine, V.; Dey, C.; Bojarová, P.; Křen, V.; Elling, L. Methods of in vitro study of galectin-glycomaterial interaction. Biotechnol. Adv. 2022, 58, 107928. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-Q.; Guo, X.-L. The role of galectin-4 in physiology and diseases. Protein Cell 2016, 7, 314–324. [Google Scholar] [CrossRef]
- Xiao, Q.; Ludwig, A.-K.; Romano, C.; Buzzacchera, I.; Sherma, S.E.; Vetro, M.; Vértesy, S.; Kaltner, H.; Reed, E.H.; Möller, M.; et al. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc. Natl. Acad. Sci. USA 2018, 115, E2509–E2518. [Google Scholar] [CrossRef]
- Bum-Erdene, K.; Leffler, H.; Nilsson, U.J.; Blanchard, H. Structural characterization of human galectin-4 N-terminal carbohydrate recognition domain in complex with glycerol, lactose, 3′-sulfolactose and 2′-fucosyllactose. Sci. Rep. 2016, 6, 20289. [Google Scholar] [CrossRef]
- Bum-Erdene, K.; Leffler, H.; Nilsson, U.J.; Blanchard, H. Structural characterization of human galectin-4 C-terminal domain: Elucidating the molecular basis for recognition of glycosphingolipids, sulfated saccharides and blood group antigens. FEBS J. 2015, 282, 3348–3367. [Google Scholar] [CrossRef]
- Rustiguel, J.K.; Soares, R.O.S.; Meisburger, S.P.; Davis, K.M.; Malzbender, K.L.; Ando, N.; Dias-Baruffi, M.; Nonato, M.C. Full-length model of the human galectin-4 and insights into dynamics of inter-domain communication. Sci. Rep. 2016, 6, 33633. [Google Scholar] [CrossRef]
- Danielsen, E.M.; Hansen, G.H. Lipid raft organization and function in the small intestinal brush border. J. Physiol. Biochem. 2008, 64, 377–382. [Google Scholar] [CrossRef]
- Delacour, D.; Koch, A.; Jakob, R. The role of galectins in protein trafficking. Traffic 2009, 10, 1405–1413. [Google Scholar] [CrossRef]
- Delacour, D.; Gouyer, V.; Zanetta, J.-P.; Drobecq, H.; Leteurtre, E.; Grard, G.; Moreau-Hannedouche, O.; Maes, E.; Pons, A.; André, S.; et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J. Cell Biol. 2005, 169, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Morelle, W.; Stechly, L.; André, S.; Van Seuningen, I.; Porchet, N.; Gabius, H.J.; Michalski, J.-C.; Huet, G. Glycosylation pattern of brush border-associated glycoproteins in enterocyte-like cells: Involvement of complex-type N-glycans in apical trafficking. Biol. Chem. 2009, 390, 529–544. [Google Scholar]
- Satelli, A.; Rao, P.S.; Thirumala, S.; Rao, U.S. Galectin-4 functions as a tumour suppressor of human colorectal cancer. Int. J. Cancer 2011, 129, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, K.C.; Jeon, S.M.; Ohn, T.B.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Abrogation of galectin-4 expression promotes tumorigenesis in colorectal cancer. Cell Oncol. 2013, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.-G. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 2011, 17, 7035–7046. [Google Scholar] [CrossRef] [PubMed]
- Barrow, H.; Rhodes, J.M.; Yu, L.-G. Simultaneous determination of serum galectin-3 and -4 levels detects metastases in colorectal cancer patients. Cell Oncol. 2013, 36, 9–13. [Google Scholar] [CrossRef]
- Huflejt, M.E.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconjug. J. 2003, 20, 247–255. [Google Scholar] [CrossRef]
- Hayashi, T.; Saito, T.; Fujimura, T.; Hara, K.; Takamochi, K.; Mitani, K.; Mineki, R.; Kazuno, S.; Oh, S.; Ueno, T.; et al. Galectin-4, a novel predictor for lymph node metastasis in lung adenocarcinoma. PLoS ONE 2013, 8, e81883. [Google Scholar] [CrossRef]
- Ideo, H.; Seko, A.; Ohkura, T.; Matta, K.L.; Yamashita, K. High-affinity binding of recombinant human galectin-4 to SO3−→3Galβ1→3GalNAc pyranoside. Glycobiology 2002, 12, 199–208. [Google Scholar] [CrossRef]
- Vokhmyanina, O.A.; Rapoport, E.M.; André, S.; Severov, V.V.; Ryzhov, I.; Pazynina, G.V.; Korchagina, E.; Gabius, H.-J.; Bovin, N.V. Comparative study of the glycan specificities of cell-bound human tandem-repeat-type galectin-4, -8 and -9. Glycobiology 2012, 22, 1207–1217. [Google Scholar] [CrossRef]
- Quintana, J.I.; Delgado, S.; Núñez-Franco, R.; Cañada, F.J.; Jiménez-Osés, G.; Jiménez-Barbero, J.; Ardá, A. Galectin-4 N-terminal domain: Binding preferences toward A and B antigens with different peripheral core presentations. Front. Chem. 2021, 9, 664097. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.I.; Stegmayr, J.; Grant, O.C.; Yang, Z.; Nilsson, U.J.; Boos, I.; Carlsson, M.C.; Woods, R.J.; Unverzagt, C.; Leffler, H.; et al. Galectin binding to cells and glycoproteins with genetically modified glycosylation reveals galectin-glycan specificities in a natural context. J. Biol. Chem. 2018, 293, 20249–20262. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Liu, J.-H.; Singh, T.; André, S.; Kaltner, H.; Gabius, H.-J. Effects of polyvalency of glycotopes and natural modifications of human blood group ABH/Lewis sugars at the Galbeta1-terminated core saccharides on the binding of domain-I of recombinant tandem-repeat-type galectin-4 from rat gastrointestinal tract (G4-N). Biochimie 2004, 86, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.-F.; Zhang, Y.; Sun, H.; Li, D.-F.; Wang, D.-C. Structural basis for distinct binding properties of the human galectins to Thomsen-Friedenreich antigen. PLoS ONE 2011, 6, e25007. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Sasaki, T. Cloning and characterization of the gene encoding a novel β-galactosidase from Bacillus circulans. Biosci. Biotechnol. Biochem. 1997, 61, 1270–1276. [Google Scholar] [CrossRef]
- Naundorf, A.; Caussette, M.; Ajisaka, K. Characterization of the immobilized β-galactosidase C from Bacillus circulans and the production of β(1→3)-linked disaccharides. Biosci. Biotechnol. Biochem. 1998, 62, 1313–1317. [Google Scholar] [CrossRef]
- Ohnuma, T.; Taku, T.; Nagatani, T.; Horii, A.; Imaoka, S.; Tanaka, T. Chemo-enzymatic synthesis of lacto-N-biose I catalyzed by β-1,3-galactosidase from Bacillus circulans using 4,6-dimethoxy-1,3,5-triazin-2-yl β-galactopyranoside as a glycosyl donor. Biosci. Biotechnol. Biochem. 2021, 85, 1716–1719. [Google Scholar] [CrossRef]
- Drozdová, A.; Bojarová, P.; Křenek, K.; Weignerová, L.; Henßen, B.; Elling, L.; Christensen, H.; Jensen, H.H.; Pelantová, H.; Kuzma, M.; et al. Enzymatic synthesis of dimeric glycomimetic ligands of NK cell activation receptors. Carbohydr. Res. 2011, 346, 1599–1609. [Google Scholar] [CrossRef]
- Laaf, D.; Bojarová, P.; Pelantová, H.; Křen, V.; Elling, L. Tailored multivalent neo-glycoproteins: Synthesis, evaluation, and application of a library of galectin-3-binding glycan ligands. Bioconjug. Chem. 2017, 28, 2832–2840. [Google Scholar] [CrossRef]
- Šimonová, A.; Kupper, C.E.; Böcker, S.; Müller, A.; Hofbauerová, K.; Pelantová, H.; Elling, L.; Křen, V.; Bojarová, P. Chemo-enzymatic synthesis of LacdiNAc dimers of varying length as novel galectin ligands. J. Mol. Catal. B Enzym. 2014, 101, 47–55. [Google Scholar] [CrossRef]
- Bojarová, P.; Kulik, N.; Hovorková, M.; Slámová, K.; Pelantová, H.; Křen, V. The β-N-acetylhexosaminidase in the synthesis of bioactive glycans: Protein and reaction engineering. Molecules 2019, 24, 599. [Google Scholar] [CrossRef] [PubMed]
- Konvalinková, D.; Dolníček, F.; Hovorková, M.; Červený, J.; Kundrát, O.; Pelantová, H.; Petrásková, L.; Cvačka, J.; Faizulina, M.; Varghese, B.; et al. Glycocalix[4]arenes and their affinity to a library of galectins: The linker matters. Org. Biomol. Chem. 2023, 21, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Bumba, L.; Laaf, D.; Spiwok, V.; Elling, L.; Křen, V.; Bojarová, P. Poly-N-acetyllactosamine neo-glycoproteins as nanomolar ligands of human galectin-3: Binding kinetics and modeling. Int. J. Mol. Sci. 2018, 19, 372. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Ayona, D.; Fournier, P.E.; Henrissat, B.; Desnues, B. Utilization of galectins by pathogens for infection. Front. Immunol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Hovorková, M.; Červený, J.; Bumba, L.; Pelantová, H.; Cvačka, J.; Křen, V.; Renaudet, O.; Goyard, D.; Bojarová, P. Advanced high-affinity glycoconjugate ligands of galectins. Bioorg. Chem. 2023, 131, 106279. [Google Scholar] [CrossRef]
- Bojarová, P.; Tavares, M.R.; Laaf, D.; Bumba, L.; Petrásková, L.; Konefal, R.; Bláhová, M.; Pelantová, H.; Elling, L.; Etrych, T.; et al. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnol. 2018, 16, 73. [Google Scholar] [CrossRef]
- Compain, P.; Bodlenner, A. The multivalent effect in glycosidase inhibition: A new, rapidly emerging topic in glycoscience. ChemBioChem 2014, 15, 1239–1251. [Google Scholar] [CrossRef]
- Kamerke, C.; Pattky, M.; Huhn, C.; Elling, L. Synthesis of nucleotide-activated disaccharides with recombinant β3-galactosidase from Bacillus circulans. J. Mol. Catal. B Enzym. 2013, 89, 73–81. [Google Scholar] [CrossRef]
- Nekvasilová, P.; Hovorková, M.; Mészáros, Z.; Petrásková, L.; Pelantová, H.; Křen, V.; Slámová, K.; Bojarová, P. Engineered glycosidases for the synthesis of human milk oligosaccharides. Int. J. Mol. Sci. 2022, 23, 4106. [Google Scholar] [CrossRef]
- Heine, V.; Hovorková, M.; Vlachová, M.; Filipová, M.; Bumba, L.; Janoušková, O.; Hubálek, M.; Cvačka, J.; Petrásková, L.; Pelantová, H.; et al. Immunoprotective neo-glycoproteins: Chemoenzymatic synthesis of multivalent glycomimetics for inhibition of cancer-related galectin-3. Eur. J. Med. Chem. 2021, 220, 113500. [Google Scholar] [CrossRef] [PubMed]
- Huflejt, M.E.; Jordan, E.T.; Gitt, M.A.; Barrondes, S.H.; Leffler, H. Strikingly different localization of galectin-3 and galectin-4 in human colon adenocarcinoma T84 cells. Galectin-4 is localized at sites of cell adhesion. J. Biol. Chem. 1997, 272, 14294–14303. [Google Scholar] [CrossRef] [PubMed]
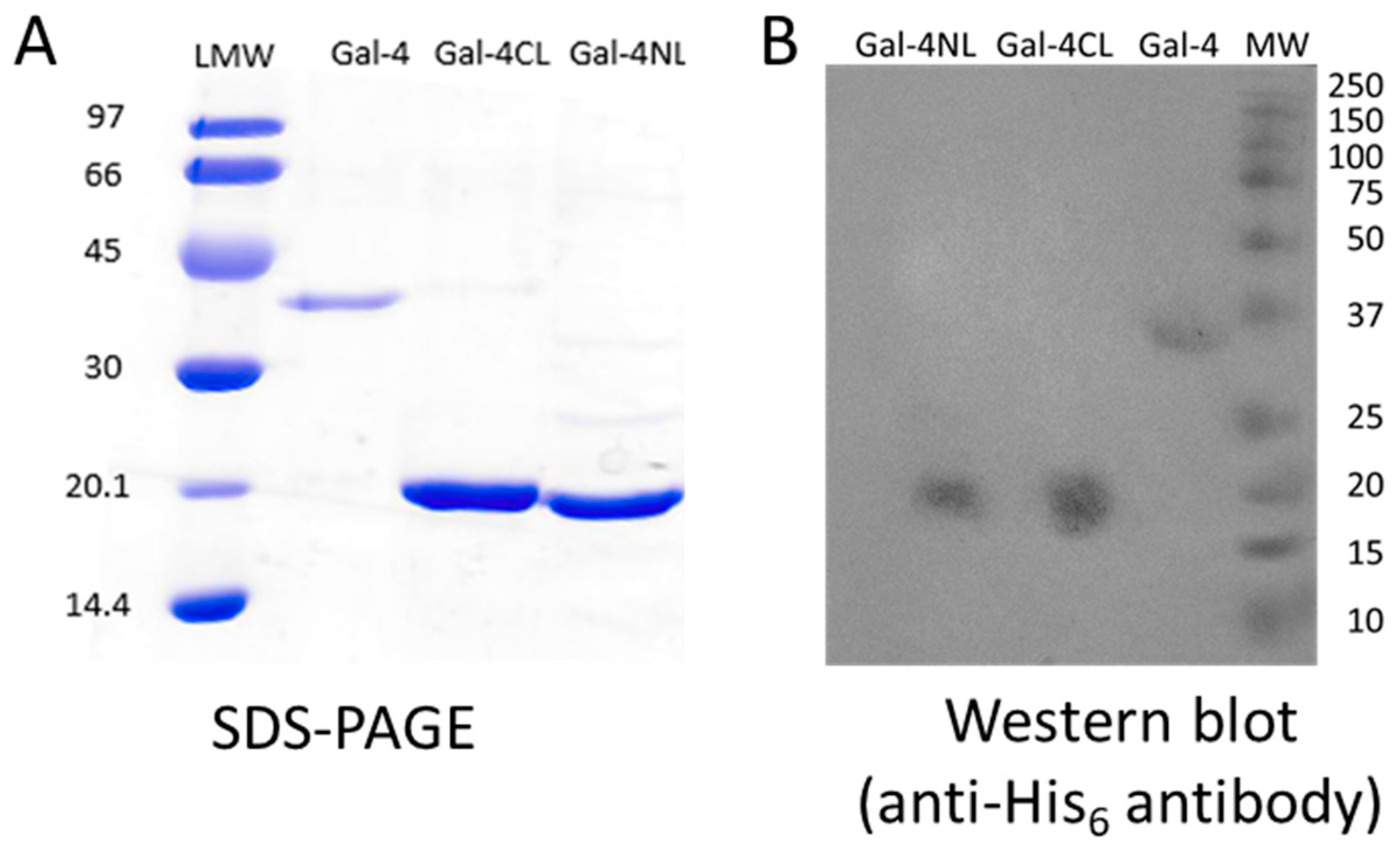

| Protein | Cells/L Medium [g/L] | Protein/Cells [mg/g] |
|---|---|---|
| Gal-4 | 1.8 | 4.2 |
| Gal-4NL | 2.2 | 2.1 |
| Gal-4CL | 3.1 | 2.4 |
| Ligand | IC50 [mM] | rp a |
|---|---|---|
| lactose (1) b | 2.5 ± 0.2 | 1.0 |
| 2′-fucosyllactose (2) | 1.5 ± 0.2 | 1.7 |
| lacto-N-tetraose (3) | 0.43 ± 0.07 | 5.9 |
| lacto-N-neotetraose (4) | 0.6 ± 0.1 | 4.1 |
| blood-group antigen A (5) | 0.05 ± 0.02 | 48 |
| blood-group antigen B (6) | 0.08 ± 0.03 | 31 |
| TF antigen motif (9) | 1.18 ± 0.05 | 2.1 |
| neo-glycoprotein 12 | 0.00019 ± 0.00002 | 13,000 (1400) c |
| Ligand | IC50 [mM] | rp a | ||
|---|---|---|---|---|
| Gal-4NL | Gal-4CL | Gal-4NL | Gal-4CL | |
| lactose (1) | 2.9 ± 0.4 | 8.6 ± 1.1 | 1.0 | 1.0 |
| 2′-fucosyllactose (2) | 0.60 ± 0.06 | 1.1 ± 0.1 | 4.8 | 7.8 |
| lacto-N-tetraose (3) | 0.21 ± 0.03 | 0.47 ± 0.9 | 14 | 18.3 |
| lacto-N-neotetraose (4) | 0.4 ± 0.1 | 0.8 ± 0.3 | 7.3 | 10 |
| Blood-group antigen A (5) | 0.022 ± 0.001 | 0.027 ± 0.005 | 130 | 320 |
| Blood-group antigen B (6) | 0.26 ± 0.01 | 0.30 ± 0.03 | 11 | 28 |
| TF antigen motif (9) | 3.5 ± 0.2 b | 5.8 ± 0.5 b | 0.8 | 1.5 |
| neo-glycoprotein 12 | 0.0009 ± 0.0001 | 0.0017 ± 0.0004 | 3200 (340) c | 5100 (530) c |
| Protein | Intensity-Weighed Size Distribution | Volume-Weighted Size Distribution | PDI a | ||||
|---|---|---|---|---|---|---|---|
| Size [nm] | Area [%] | Size [nm] | Area [%] | Size [nm] | Area [%] | ||
| Gal-4 | 11 ± 3 | 58 | 1200 ± 600 | 39 | 9 ± 2 | 100 | 0.2 |
| Gal-4NL | 1.3 ± 0.3 | 13 | 16 ± 7 | 50 | 3 ± 1 | 100 | 0.4 |
| Gal-4CL | 8 ± 3 | 63 | 400 ± 100 | 33 | 7 ± 2 | 100 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slámová, K.; Červený, J.; Mészáros, Z.; Friede, T.; Vrbata, D.; Křen, V.; Bojarová, P. Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly. Molecules 2023, 28, 4039. https://doi.org/10.3390/molecules28104039
Slámová K, Červený J, Mészáros Z, Friede T, Vrbata D, Křen V, Bojarová P. Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly. Molecules. 2023; 28(10):4039. https://doi.org/10.3390/molecules28104039
Chicago/Turabian StyleSlámová, Kristýna, Jakub Červený, Zuzana Mészáros, Tereza Friede, David Vrbata, Vladimír Křen, and Pavla Bojarová. 2023. "Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly" Molecules 28, no. 10: 4039. https://doi.org/10.3390/molecules28104039
APA StyleSlámová, K., Červený, J., Mészáros, Z., Friede, T., Vrbata, D., Křen, V., & Bojarová, P. (2023). Oligosaccharide Ligands of Galectin-4 and Its Subunits: Multivalency Scores Highly. Molecules, 28(10), 4039. https://doi.org/10.3390/molecules28104039








