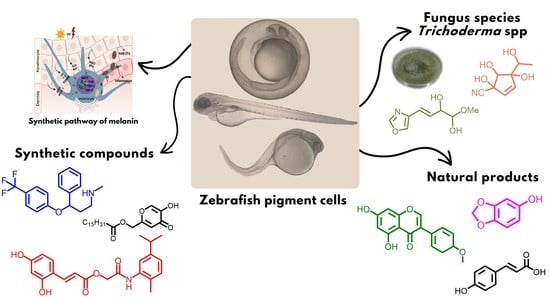
Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model
Abstract
1. Introduction
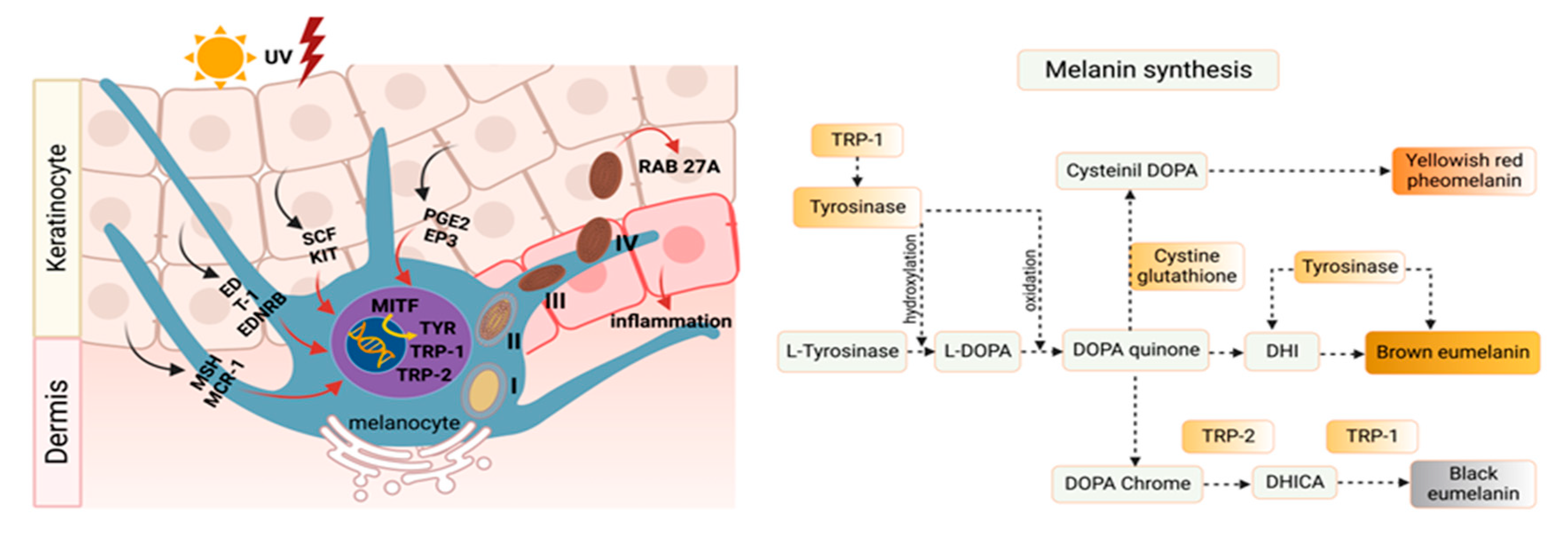
2. Melanin and Tyrosinase Mechanism of Action
3. Inhibitors of Melanogenesis by Fungi of the Genus Trichoderma
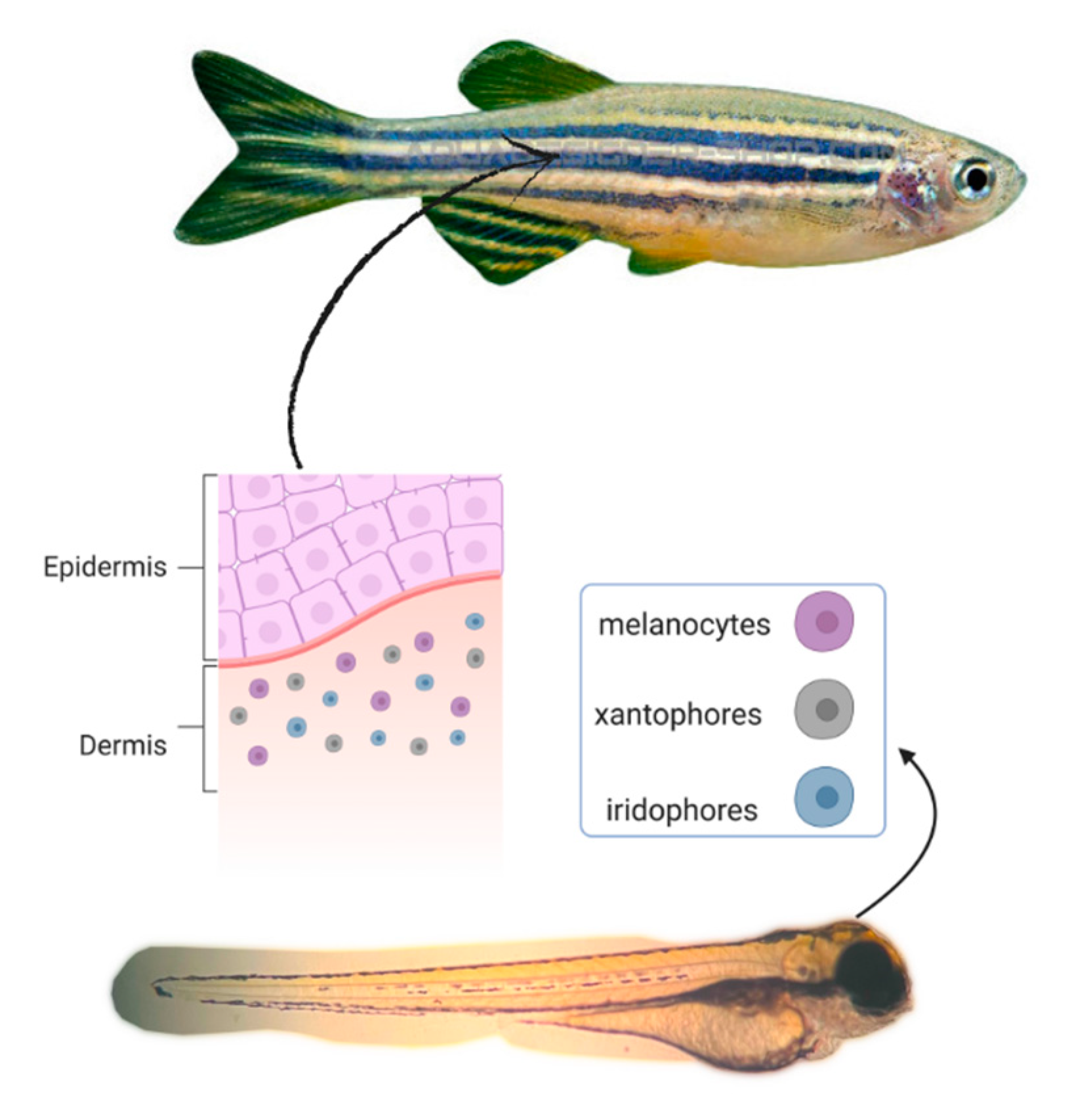
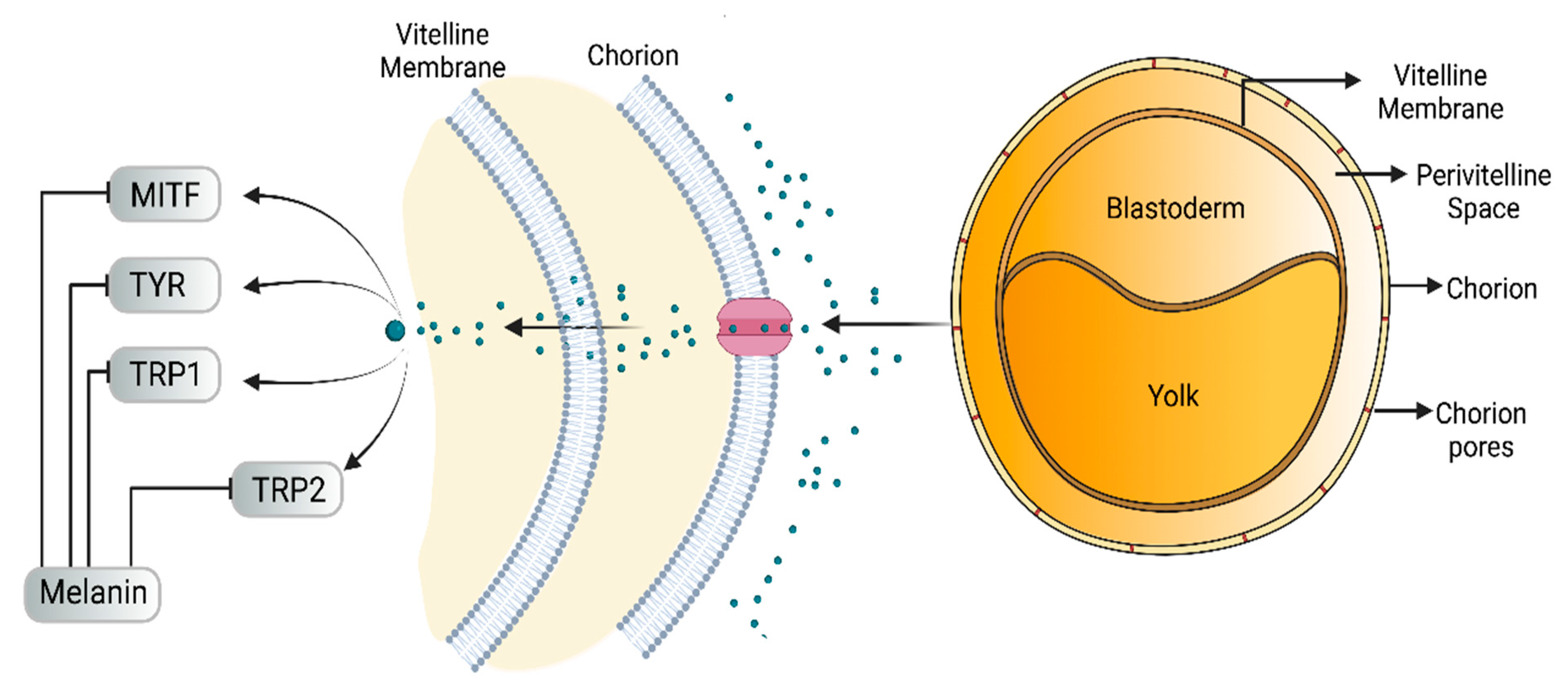
4. Anti-Melanogenic Activity in Zebrafish Embryo
5. Natural Products Used as Melanogenesis Inhibitors in Zebrafish
6. Synthetic Compounds Used as Melanogenesis Inhibitors in Zebrafish
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Ceol, C.J.; Houvras, Y.; Richard, M.; White, R.M.; Zon, L.I. Melanoma biology and the promise of zebrafish. Zebrafish 2008, 5, 247–255. [Google Scholar] [CrossRef]
- Chang, T.S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.L.; Li, K.M.; Balu, N.; Saeed, M.; Devanesan, P.; Higginbotham, S.; Zhao, J.; Gross, M.L.; Rogan, E.G. Catechol orthoquinones: The electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis 2002, 23, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson’s disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Tessari, I.; Bisaglia, M.; Valle, F.; Samori, B.; Bergantino, E.; Mammi, S.; Bubacco, L. The reaction of alpha-synuclein with tyrosinase: Possible implications for Parkinson disease. J. Biol. Chem. 2008, 283, 16808–16817. [Google Scholar] [CrossRef] [PubMed]
- Vontzalidou, A.; Zoidis, G.; Chaita, E.; Makropoulou, M.; Aligiannis, N.; Lambrinidis, G.; Mikros, E.; Skaltsounis, A.L. Design, synthesis and molecular simulation studies of dihydrostilbene derivatives as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5523–5526. [Google Scholar] [CrossRef]
- Wang, N.; Hebert, D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigm. Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef]
- Agarwal, P.; Singh, M.; Singh, J.; Singh, R.P. Microbial Tyrosinases: A Novel Enzyme, Structural Features and Applications. In Applied Microbiology and Bioengineering; Shukla, P., Ed.; Academic Press: London, UK, 2019; pp. 3–19. [Google Scholar]
- Van Gelder, C.W.; Flurkey, W.H.; Wichers, H.J. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 1997, 45, 1309–1323. [Google Scholar] [CrossRef]
- Halaouli, S.; Record, E.; Casalot, L.; Hamdi, M.; Sigoillot, J.C.; Asther, M.; Lomascolo, A. Cloning and characterization of a tyrosinase gene from the white-rot fungus Pycnoporus sanguineus, and overproduction of the recombinant protein in Aspergillus niger. Appl. Microbiol. Biotechnol. 2006, 70, 580–589. [Google Scholar] [CrossRef]
- Yi, Y.J.; Zimmerman, S.W.; Sutovsky, P. Gamete Binding and Fusion. In Cell Fusion: Regulation and Control; Larsson, L.-I., Ed.; Springer Science & Business Media: New York, NY, USA, 2011; pp. 185–201. [Google Scholar]
- Jorde, L.B.; Wooding, S.P. Genetic variation, classification and ‘race’. Nat. Genet. 2004, 36 (Suppl. 11), S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Galiano, S.P. Propuesta metodológica para la revisión de traducciones: Principios generales y parâmetros. Trans. Rev. Trad. 2007, 11, 197–214. [Google Scholar]
- Tishkoff, S.A.; Kidd, K.K. Implications of biogeography of human populations for ‘race’ and medicine. Nat. Genet. 2004, 36, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.-L.; Aligiannis, N. Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Vicanova, J.; Pavel, S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W. Disorders of Hyperpigmentation. In Dermatology, 3rd ed.; Bolognia, J.L., Schaffer, J.V., Cerroni, L., Eds.; Elsevier: London, UK, 2012; pp. 1052–1053. [Google Scholar]
- Khan, T.H.M. Novel Tyrosinase Inhibitors from Natural Resources—Their Computational Studies. Curr. Med. Chem. 2012, 19, 2262–2272. [Google Scholar] [CrossRef]
- Imada, C.; Sugimoto, Y.; Makimura, T.; Kobayashi, T.; Hamada, N.; Watanabe, E. Isolation and characterization of tyrosinase inhibitor producing microorganisms from marine environment. Fish Sci. 2001, 67, 1151–1156. [Google Scholar] [CrossRef]
- Matos, R.; Pinto, V.V.; Ruivo, M.; Lopes, M.F. Study on the dissemination of the bcrABDR cluster in Enterococcus spp. reveals that the BcrAB transporter is sufficient to confer high-level bacitracin resistance. Int. J. Antimicrob. Agents 2009, 34, 142–147. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liu, I.H.; Huang, X.Z.; Chen, H.J.; Chang, S.T.; Chang, M.L.; Ho, Y.T.; Chang, H.T. Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in zebrafish Model. Pharmaceutics 2021, 13, 1059. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Neelkantan, N.; Mikhaylova, A.; Stewart, A.M.; Arnold, R.; Gjeloshi, V.; Kondaveeti, D.; Poudel, M.K.; Kalueff, A.V. Perspectives on Zebrafish Models of Hallucinogenic Drugs and Related Psychotropic Compounds. ACS Chem. Neurosci. 2013, 4, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Rannekamp, A.J.; Peterson, R.T. 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Lee, J.-H.; Yim, S.-H.; Batkhuu, G.J.; Jung, D.-W.; Williams, D.R. Isolation of 4,5-Odicaffeoylquinic acid as a pigmentation inhibitor occurring in Artemisia capillaris Thunberg and its validation in vivo. Evid. Based Complement. Altern. Med. 2016, 2016, 7823541. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.W.; Burn, S.F.; Jackson, I.J. Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res. 2006, 19, 206–213. [Google Scholar] [CrossRef]
- Colanesi, S.; Taylor, K.L.; Temperley, N.D.; Lundegaard, P.R.; Liu, D.; North, T.E.; Ishizaki, H.; Kelsh, R.N.; Patton, E.E. Small molecule screening identifies targetable zebrafish pigmentation pathways. Pigment Cell Melanoma Res. 2012, 25, 131–143. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Souza, A.A.; Ortíz, B.L.S.; Koga, R.C.R.; Sales, P.F.; Cunha, D.B.; Guerra, A.L.M.; Souza, G.C.; Carvalho, J.C.T. Secondary Metabolites Found among the Species Trattinnickia rhoifolia Willd. Molecules 2021, 26, 7661. [Google Scholar] [CrossRef]
- Solano, F. Melanin and Melanin-Related Polymers as Materials with Biomedical and Biotechnological Applications—Cuttlefish Ink and Mussel Foot Proteins as Inspired Biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A. Purification and Characterization of Melanogenic Enzyme Tyrosinase from Button Mushroom. Enzym. Res. 2014, 2014, 120739. [Google Scholar] [CrossRef]
- Chen, W.; He, Q.; Peng, W.; Chen, X.; Liu, K.; Chu, J.; Han, L.; Wang, X. Zebrafish model based biological activity evaluation for melanin inhibition of Vc and Sodium erythorbate. Shandong Sci. 2014, 27, 31–37. [Google Scholar]
- Chang, C.-T.; Chang, W.-L.; Hsu, J.-C.; Shih, Y.; Chou, S.-T. Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Bot. Stud. 2013, 54, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S. Development of Tyrosinase Inhibitors. Ph.D. Thesis, University of Technology Sydney, Sydney, Australia, 2016. [Google Scholar]
- Park, J.; Sung, N.-D. 3D-QSAR analysis and molecular docking of thiosemicarbazone analogues as a potent tyrosinase inhibitor. Bull. Korean Chem. Soc. 2011, 32, 1241–1248. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Bissett, D.L. Latest insights into skin hyperpigmentation. J. Investig. Dermatol. Symp. Proc. 2008, 13, 10–14. [Google Scholar] [CrossRef]
- Miot, L.; Bartoli, D.; Miot, H.A.; Silva, M.G.; Marques, M.E.A. Physiopathology of Melasma: Review. An. Bras. Dermatol. 2009, 84, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Kushimoto, T.; Basrur, V.; Valencia, J.; Matsunaga, J.; Vieira, W.D.; Ferrans, V.J.; Muller, J.; Appella, E.; Hearing, V.J. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc. Natl. Acad. Sci. USA 2001, 98, 10698–10703. [Google Scholar] [CrossRef]
- Berson, J.F.; Harper, D.C.; Tenza, D.; Raposo, G.; Marks, M.S. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol. Cell. Biol. 2001, 12, 3451–3464. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000, 84, 376–393. [Google Scholar] [CrossRef]
- Machado, D.F.M.; Parzianello, F.R.; Silva, A.C.F.; Antoniolli, Z.I. Trichoderma no Brasil: O fungo e o bioagente. Rev. Bras. Cienc. Agrar. 2012, 35, 274–288. [Google Scholar]
- Ramos, M.M.; Morais, E.S.; Sena, I.S.; Lima, A.L.; Oliveira, F.R.; Freitas, C.M.; Fernandes, C.P.; Carvalho, J.C.T.; Ferreira, I.M. Silver nanoparticle from whole cells of the fungi Trichoderma spp. isolated from Brazilian Amazon. Microbiol. Biotechnol. Lett. 2020, 42, 833–843. [Google Scholar] [CrossRef]
- Francisco, C.S.; Ma, X.; Zwyssig, M.M.; McDonald, B.A.; Palma-Guerrero, J. Morphological changes in response to environmental stresses in the fungal plant pathogen Zymoseptoria tritici. Sci. Rep. 2019, 9, 9642. [Google Scholar] [CrossRef] [PubMed]
- Roiger, D.J.; Jeffers, S.N.; Caldwell, R.W. Occurrence of Trichoderma species in apple orchard and woodland soils. Soil Biol. Biochem. 1991, 23, 353–359. [Google Scholar] [CrossRef]
- Tahía, B.; Ana, M.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Fernandes, M.S.; Savita Kerkar, S. Microorganisms as a source of tyrosinase inhibitors: A review. Ann. Microbiol. 2017, 67, 343–358. [Google Scholar] [CrossRef]
- El-Shora, H.M.; El-Sharkawy, R.M. Evaluation of Putative Inducers and Inhibitors toward Tyrosinase from two Trichoderma species. Jordan J. Biol. Sci. 2020, 13, 7–12. [Google Scholar]
- Qun Ren, Q.; Henes, B.; Fairhead, M.; Thöny-Meyer, L. High level production of tyrosinase in recombinant Escherichia coli. BMC Biotechnol. 2013, 13, 18. [Google Scholar]
- Yi, S.; Li, T.; Yong-Fu, H.; Yi, S.; Pei, Y.H. A new cyclotetrapeptide from marine fungus Trichoderma reesei. Pharmazie 2006, 61, 809–810. [Google Scholar]
- Kim, K.; Heo, Y.M.; Jang, S.; Lee, H.; Kwon, S.-L.; Park, M.S.; Lim, Y.W.; Kim, J.-J. Diversity of Trichoderma spp. in Marine Environments and Their Biological Potential for Sustainable Industrial Applications. Sustainability 2020, 12, 4327. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galan, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Takahashi, S.; Hashimoto, R.; Hamano, K.; Suzuki, T.; Nakagawa, A. Melanoxazal, new melanin biosynthesis inhibitor discovered by using the larval haemolymph of the silkworm, Bombyx mori. Production, isolation, structural elucidation, and biological properties. J. Antibiot. 1996, 49, 513–518. [Google Scholar] [CrossRef]
- Lee, C.H.; Chung, M.C.; Lee, H.J.; Kho, Y.H.; Lee, K.H. MR304-1, a melanin synthesis inhibitor produced by Trichoderma harzianum. Korean J. Appl. Microbiol. Biotechnol. 1995, 23, 641–646. [Google Scholar]
- Lee, C.H.; Chung, M.C.; Lee, H.J.; Bae, K.S.; Kho, Y.H. MR566A and MR566B, new melanin synthesis inhibitors produced by Trichoderma harzianum I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1997, 50, 469–473. [Google Scholar] [CrossRef]
- Lee, C.H.; Koshino, H.; Chung, M.C.; Lee, H.J.; Hong, J.K.; Yoon, J.S.; Kho, Y.H. MR566A and MR566B, new melanin synthesis inhibitors produced by Trichoderma harzianum II. Physico-chemical properties and structural elucidation. J. Antibiot. 1997, 50, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Takahiro, T.; Yamada, K.; Minoura, K.; Miyamoto, K.; Usami, Y.; Kobayashi, T.; Hamada-Sato, N.; Imada, C.; Tsujibo, H. Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol. Pharm. Bull. 2008, 31, 1618–1620. [Google Scholar]
- Kang, D.W.; Kim, K.-M.; Kim, Y.-S.; Seo, Y.-J.; Song, D.-Y.; Oh, D.-Y.; Choi, S.-O.; Hwang, J.-H.; Kim, S.W.; Bang, K.H.; et al. Inhibition of Tyrosinase by Metabolites Originating from Thrichoderma atroviride. J. Life Sci. 2021, 31, 47–51. [Google Scholar]
- Hwang, K.-S.; Yang, J.Y.; Lee, J.; Lee, Y.-R.; Kim, S.S.; Kim, G.R.; Chae, J.S.; Ahn, J.H.; Shin, D.-S.; Choi, T.-Y.; et al. A novel anti-melanogenic agent, KDZ-001, inhibits tyrosinase enzymatic activity. J. Dermatol. Sci. 2018, 89, 165–171. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Park, S.R.; Choi, Y.H.; Park, E.K.; Jin, C.-Y.; Yu, H.; Jo, W.S.; Kyoung Tae Lee, K.T.; Kim, G.-Y. GSK-3β-Targeting Fisetin Promotes Melanogenesis in B16F10 Melanoma Cells and Zebrafish Larvae through β-Catenin Activation. Int. J. Mol. Sci. 2020, 21, 312. [Google Scholar] [CrossRef]
- Caioni, G.; D’Angelo, M.; Panella, G.; Merola, C.; Cimini, A.; Amorena, M.; Benedetti, E.; Perugini, M. Environmentally relevant concentrations of triclocarban affect morphological traits and melanogenesis in zebrafish larvae. Aquat. Toxicol. 2021, 236, 105842. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Matthieu Muffato, M.; John, E.; Collins, J.E.; Sean Humphray, S.; Karen Mclaren, K.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Jin, E.-J.; Thibaudeau, G. Effects of lithium on pigmentation in the embryonic zebrafish (Brachydanio rerio). Biochim. Biophys. Acta 1999, 1449, 93–99. [Google Scholar] [CrossRef]
- Camp, E.; Lardell, M. Tyrosinase gene expression in zebrafish embryos. Dev. Genes Evol. 2021, 211, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Sartor, E.; Fagotto, L.; Colombo, A.; Tiso, N.; Alaibac, M. The Zebrafish model in dermatology: An update for clinicians. Discov. Oncol. 2022, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; D’Ischia, M.; Misuraca, G.; Prota, G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim. Biophys. Acta 1991, 1073, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bonsignorio, D.; Perego, L.; Del Giacco, L.; Cotelli, F. Structure and macromolecular composition of the zebrafish egg chorion. Zygote 1996, 4, 101–108. [Google Scholar] [CrossRef]
- Hamm, J.T.; Ceger, P.; Allen, D.; Stout, M.; Maull, E.A.; Baker, G.; Zmarowski, A.; Padilla, S.; Perkins, E.; Planchart, A.; et al. Characterizing Sources of Variability in Zebrafish Embryo Screening Protocols. Altex 2019, 36, 103–120. [Google Scholar] [CrossRef]
- Chen, J.S.; Wei, C.; Marshall, M.R. Inhibition mechanism of kojic acid on polyphenol oxidase. J. Agric. Food Chem. 1991, 39, 1897–1901. [Google Scholar] [CrossRef]
- Kumari, S.; Thng, S.T.G.; Verma, N.K.; Gautam, H.K. Melanogenesis Inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- Pavic, A.; Ilic-Tomic, T.; Clija, J.G. Unravelling Anti-Melanogenic Potency of Edible Mushrooms Laetiporus sulphureus and Agaricus silvaticus In Vivo Using the Zebrafish Model. J. Fungi 2021, 7, 834. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, K.-C.; Wang, H.-T.; Hsieh, C.-W.; Lai, Y.-J. Extracts of Antrodia cinnamomea mycelium as a Highly Potent Tyrosinase Inhibitor. J. Cosmet. Dermatol. 2021, 20, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Azfaralariff, A.; Mohamad, A.; Airianah, O.B.; Law, D.; Dyari, H.R.E.; Lim, Y.C.; Fazry, S.; Shiitake, M. Extracts inhibit melanin-producing neural crest-derived cells in zebrafish embryo. Comp. Biochem. Physiol. 2021, 245 Pt C, 109033. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Hordinsky, M.K.; Wood, J.M. Thioredoxin reductase. Role in free radical reduction in different hypopigmentation disorders. Arch. Dermatol. 1987, 123, 615–619. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Lerner, A.B.; Mcguire, J.S. Effect ofalpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature 1961, 189, 176–179. [Google Scholar] [CrossRef]
- Lerner, A.B.; Mcguire, J.S. Melanocyte-stimulating hormone and adrenocorticotrophic hormone: Their relation to pigmentation. N. Engl. J. Med. 1964, 270, 539–546. [Google Scholar] [CrossRef]
- Kim, M.M.Y.; Lee, H.-E.; Im, M.; Lee, Y.; Kim, C.-D.; Lee, J.-H.; Seo, Y.-J. Effect of Adenosine on Melanogenesis in B16 Cells and Zebrafish. Ann. Dermatol. 2014, 26, 209–213. [Google Scholar] [CrossRef]
- Kang, H.Y.; Yoon, T.J.; Lee, G.J. Whitening Effects of Marine Pseudomonas Extract. Ann. Dermatol. 2011, 23, 144–149. [Google Scholar] [CrossRef]
- Hsu, K.-D.; Chen, H.-I.; Wang, C.-S.; Lum, C.-C.; Wu, S.-P.; Lin, S.P.; Cheng, K.-C. Extract of Ganoderma formosanum Mycelium as a Highly Potent Tyrosinase Inhibitor. Sci. Rep. 2016, 6, 32854. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Segner, H.; Ros, A.; Knapen, D.; Vergauwen, L. Thyroid Hormone Disruptors Interfere with Molecular Pathways of Eye Development and Function in Zebrafish. Int. J. Mol. Sci. 2019, 20, 1543. [Google Scholar] [CrossRef] [PubMed]
- Marelli, F.; Carra, S.; Agostini, M.; Cotelli, F.; Peeters, R.; Chatterjee, K.; Persani, L. Patterns of thyroid hormone receptor expression in zebrafish and generation of a novel model of resistance to thyroid hormone action. Mol. Cell. Endocrinol. 2016, 424, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Lee, S.H. Omeprazole inhibits melanin biosynthesis in melan-a cells and zebrafish. Exp. Dermatol. 2016, 25, 239–241. [Google Scholar] [CrossRef]
- Je, J.-G.; Jiang, Y.; Heo, J.-H.; Li, X.; Jeon, Y.-J.; Ryu, B.-M. Mitigative Effects of PFF-A Isolated from Ecklonia cava on Pigmentation in a Zebrafish Model and Melanogenesis in B16F10 Cells. Mar. Drugs 2022, 20, 123. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xu, J.J.; Chen, X.Y.; Li, X.M.; Chen, J.M.; Pan, Y.T.; Xue, Y. Alcohol extracts of Narcissus bulb inhibits melanogenesis in zebrafish embryos. Acta Lab. Anim. Sci. Sin. 2017, 25, 1005–4847. [Google Scholar]
- Zhou, J.; Ren, T.; Li, Y.; Cheng, A.; Xie, W.; Xu, L.; Peng, L.; Lin, J.; Lian, L.; Diao, Y.; et al. Oleoylethanolamide inhibits α-melanocyte stimulating hormone-stimulated melanogenesis via ERK, Aktand CREB signaling pathways in B16 melanoma cells. Oncotarget 2017, 8, 56868–56879. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, S.H. Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possibleinvolvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 2015, 24, 761–766. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wang, S.-Y.; Liao, J.-W.; Hsu, L.-S.; Yang, H.-L.; Hseu, Y.-C. Depigmenting effects of gallic Acid: A novel skin lightening agent for hyperpigmentary skin diseases. Int. Union Biochem. Mol. Biol. 2013, 39, 259–270. [Google Scholar]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.-C.; Lin, H.-H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-C.; Kim, S.; Hwang, K.-S.; Kim, C.-H. p-Coumaric acid potently down-regulates zebrafish embryo pigmentation: Comparison of in vivo assay and computational molecular modeling with phenylthiourea. Biomed. Sci. Lett. 2017, 23, 8–16. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.M.; Myung, C.H.; Lee, K.R.; Hyun, S.M.; Lee, J.E.; Park, Y.S.; Jeon, S.R.; Park, J.; Chang, S.E.; et al. Melanogenesis inhibition of β-lapachone, a natural product from Tabebuia avellanedae, with effective in vivo lightening potency. Arch. Dermatol. Res. 2015, 307, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Monte, A.P. Skin Lightening Compounds. U.S. Patent WO2016014529A1, 21 July 2014. [Google Scholar]
- Lin, V.C.; Ding, H.-Y.; Tsai, P.-C.; Wu, J.-Y.; Lu, Y.-H.; Chang, T.-S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Chen, C.-Y.; Wen, Z.-H. Compositiion for Inhibiting Melanogenesis and Use Thereof. U.S. Patent 8455023B2, 4 June 2013. [Google Scholar]
- Wang, H.-M.; Chen, C.-Y.; Wen, Z.-H. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Exp. Dermatol. 2011, 20, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Jeong, H.B.; Ro, H.; Lee, J.-H.; Kim, C.D.; Yoon, T.-J. Inhibitory effect of 5-iodotubercidin onpigmentation. Biochem. Biophys. Res. Commun. 2017, 490, 1282–1286. [Google Scholar] [CrossRef]
- Shin, S.-H.; Lee, Y.-M. Glyceollins, a novel class of soybean phytoalexins, inhibit SCF-induced melanogenesis through attenuation of SCF/c-kit downstream signaling pathways. Exp. Mol. Med. 2013, 45, e17. [Google Scholar] [CrossRef]
- Park, H.; Song, K.H.; Jung, P.M.; Kim, J.-E.; Ro, H.; Kim, M.Y.; Ma, J.Y. Inhibitory effect of arctigenin from Fructus arctii extract on melanin synthesis via repression of tyrosinase expression. Evid. Based Complement. Altern. Med. 2013, 2013, 965312. [Google Scholar] [CrossRef]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N Inhibits melanogenesis through regulating the PI3K/Akt and MAPK/ERK signaling pathways in melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, S.H.; Kim, D.H.; Choi, T.Y.; Yoon, T.J.; Hwang, J.S.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Downregulation of melanin synthesis by haginin a and its application to in vivo lightening model. J. Investig. Dermatol. 2008, 128, 1227–1235. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, J.; Jeong, Y.T.; Hee Byun, G.; Kim, J.H. Melanogenesis inhibition activity of floralginsenoside A from Panax ginseng berry. J. Ginseng. Res. 2017, 41, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Jeong, Y.T.; Jeong, S.C.; Lee, M.K.; Min, J.W.; Lee, J.W.; Kim, G.S.; Lee, S.E.; Ahn, Y.S.; Kang, H.C.; et al. Melanin Biosynthesis Inhibition Effects of Ginsenoside Rb2 Isolated from Panax ginseng Berry. J. Microbiol. Biotechnol. 2015, 25, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Yang, H.-M.; Kang, S.-M.; Ahn, G.; Roh, S.W.; Lee, W.; Kim, D.; Jeon, Y.-J. Whitening effect of octaphlorethol A isolated from Ishige foliacea in an in vivo zebrafish model. J. Microbiol. Biotechnol. 2015, 25, 448–451. [Google Scholar] [CrossRef]
- Jang, D.K.; Pham, C.H.; Lee, I.S.; Jung, S.H.; Jeong, J.H.; Shin, H.S.; Yoo, H.M. Anti-Melanogenesis Activity of 6-O-Isobutyrylbritannilactone from Inula britannica on B16F10 Melanocytes and In Vivo Zebrafish Models. Molecules 2020, 25, 3887. [Google Scholar] [CrossRef]
- Henry, T.R.; Spitsbergen, J.M.; Hornung, M.W.; Abnet, C.C.; Peterson, R.E. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 1997, 142, 56–68. [Google Scholar] [CrossRef]
- Lajis, A.F.B. A Zebrafish Embryo as an Animal Model for the Treatment of Hyperpigmentation in Cosmetic Dermatology Medicine. Medicina 2018, 54, 35. [Google Scholar] [CrossRef]
- Ding, H.-Y.; Chang, T.-S.; Chiang, C.-M.; Li, S.-Y.; Tseng, D.-Y. Melanogenesis inhibition by a crude extract of Magnolia officinalis. J. Med. Plants Res. 2011, 5, 237–244. [Google Scholar]
- Veselinović, J.B.; Veselinović, A.M.; Ilic-Tomic, T.; Davis, R.; O’Connor, K.; Pavic, A.; Nikodinovic-Runic, J. Potent anti-melanogenic activity and favorable toxicity profile of selected 4-phenyl hydroxycoumarins in the zebrafish model and the computational molecular modeling studies. Bioorg. Med. Chem. 2017, 25, 6286–6296. [Google Scholar] [CrossRef]
- Lajis, A.F.B.; Hamid, M.; Ahmad, S.; Ariff, A. Lipase-catalyzed synthesis of kojic acid derivative in bioreactors and the analysis of its depigmenting and antioxidant activities. Cosmetics 2017, 4, 22. [Google Scholar] [CrossRef]
- Abbas, Q.; Ashraf, Z.; Hassan, M.; Nadeem, H.; Latif, M.; Afzal, S.; Seo, S.-Y. Development of highly potent melanogenesis inhibitor by in vitro, in vivo and computational studies. Drug Des. Dev. Ther. 2017, 11, 2029–2046. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhou, L.; Wu, H.; Zhou, J.; Jin, Y.; Liao, S. Application of Fluoxetine to Treatment of Depigmentation Disease. U.S. Patent 9,833,424, 5 December 2017. [Google Scholar]
- Thach, B.D.; Vu, Q.; Dao, T.; Thi, L.; Giang, L.; Nguyen, T.; Linh, T.; Thi, B.; Pham, N.; Uyen, A.; et al. Inhibitor effect of flavonoid from Blumea balsamifera (L.) dc. leaves extract on melanin synthesis in cultured B16F10 cell line and zebrafish. Eur. J. Res. Med. Sci. 2017, 5, 31–36. [Google Scholar]
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Germano, M.P.; Luca, L.; Ricci, F.; Corallo, D.; Aveic, S.; Mariotto, E.; et al. Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem. 2022, 231, 114–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Na, D.-S.; Ju, B.-K. Zebrafish Chorion as an Extracellular Matrix for Cell Culture. In World Congress on Medical Physics and Biomedical Engineering 2006; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3379–3381. [Google Scholar]
- Hsu, K.-D.; Chan, Y.-H.; Chen, H.-J.; Lin, S.-P.; Cheng, K.-C. Tyrosinase-based TLC Autography for antimelanogenic drug screening. Sci. Rep. 2018, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Bennion, B.J.; Be, N.A.; Mcnerney, M.W.; Lao, V.; Carlson, E.M.; Valdez, C.A.; Malfatti, M.A.; Enright, H.A.; Nguyen, T.H.; Lightstone, F.C.; et al. Predicting a drug’s membrane permeability: A computational modelvalidated with in vitro permeability assay data. J. Phys. Chem. B 2017, 121, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, X.; Zhu, F. Application of Suloctidil in Preparation of Cosmetics or Medicine. Patent CN 103181860A, 9 April 2013. [Google Scholar]
| Fungus | Molecules and Their Derivatives | Reference |
|---|---|---|
| Trichoderma viride | 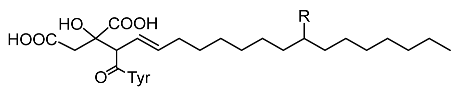 Viridiofungins and derivatives (R = -OH; -H; -C=O) | Reino et al. [53] |
| Trichoderma spp. | 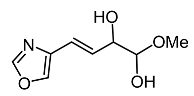 Melanoxazal | Takahashi et al. [54] |
| Trichoderma harzianum | 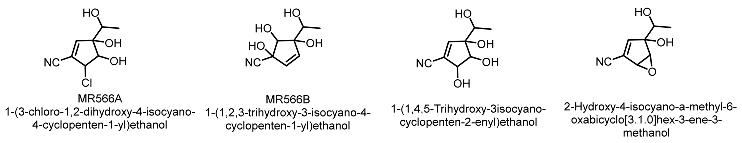 | Lee et al. [55] |
| Entry | Bioactive Compound/Structure | Mechanism | Toxicity/Concentration | Reference |
|---|---|---|---|---|
| 1 | Fisetin | Blocks tyrosinase-induced tyrosine oxidation | Did not show (25 µM, 50 µM, 75 µM, and 100 µM) | Ilandarage et al. [61] |
| 2 | KDZ-001 | TYR active site | Did not show (10 µM) | Kyu-Seok et al. [60] |
| 3 | 1-phenyl-2-thiourea | Unknown | Did not show | Ilandarage et al. [52] |
| 4 | 2-mercaptobenzothiazole | Unknown | Did not show | Ilandarage et al. [61] 2020; Tae-Young et al. [66] |
| 5 | Haginin | Unknown | Did not show | Tae-Young et al. [66] |
| 6 | YT16i | Unknown | Showed toxicity (1 mM) | Tae-Young et al. [66] |
| 7 | triclocarban (3,4,4′-trichlorocarbanilide) | Unknown | Showed toxicity (50 µg/L). | Giulia et al. [62] |
| 8 | Adenosine | Inhibits melanogenesis by down-regulating tyrosinase | Did not show (400 µM) | Mi Yoon et al. [84] |
| 9 | Ecklonia cava seaweed extract | Unknown | Slight toxicity (400 µM) | Kang et al. [85] |
| 10 | Sargassum siliquastrum seaweed extract | Unknown | Did not show (400 µM) | Kang et al. [85] |
| 11 | Ganoderma formosanum mycelium extract | Blocks tyrosinase-induced tyrosine oxidation | Did not show (400 ppm) | Kai et al. [86] |
| Entry | Name | Chemical Structure | Reference |
|---|---|---|---|
| 1. | Mearsetin | 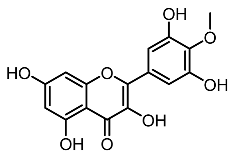 | Huang et al. [22] |
| 2. | Myricetin | 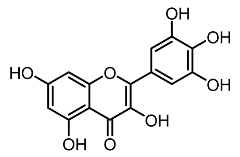 | Huang et al. [22] |
| 3. | Arbutin | 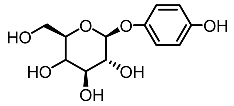 | Ilandarage et al. [61] |
| 4. | Niacinamide | 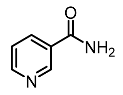 | Hako-Zaki et al. [81] |
| 5. | Sesamol | 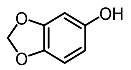 | Baek et al. [93] |
| 6. | Gallic acid | 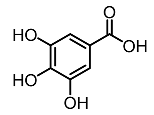 | Kumar et al. [94] |
| 7. | Ascorbic acid | 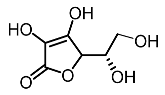 | Kumar et al. [94] |
| 8. | Bis(4-hydroxybenzyl)sulfide | 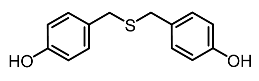 | Wang et al. [95] |
| 9. | Coumaric acid | 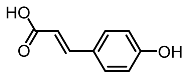 | Kim et al. [96] |
| 10. | β-Lapachone | 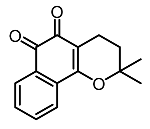 | Kim et al. [97] |
| 11. | Tretinoin |  | Huang et al. [98] |
| 12. | 2-Morpholinobutyl-4-thiophenol | 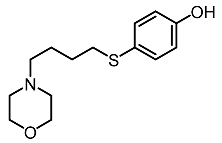 | Huang et al. [98] |
| 13. | Biochanin A | 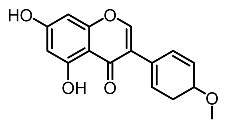 | Lin et al. [99] |
| 14. | Subamolide A | 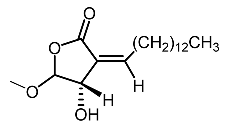 | Hiu et al. [100]; Wang et al. [101] |
| 15. | Linderanolide B |  | Hiu et al. [100]; Wang et al. [101] |
| 16. | 5-Iodotubersidin | 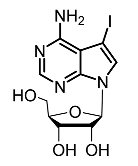 | Kim et al. [102] |
| 17. | Glyceollin I | 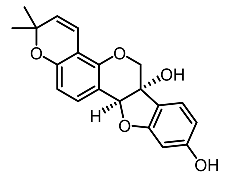 | Shin et al. [103] |
| 18. | Arctigenin | 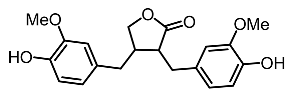 | Park et al. [104] |
| 19. | Gomisin N | 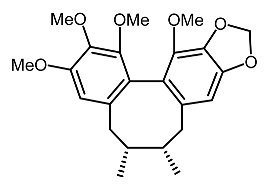 | Chae et al. [105] |
| 20. | Haginin A | 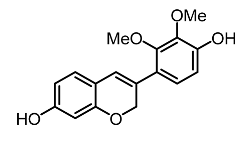 | Kim et al. [106] |
| 21. | Glabridin | 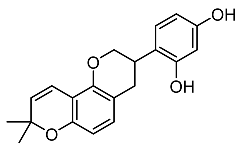 | Chen et al. [107] |
| 22. | Floralginsenoside A | 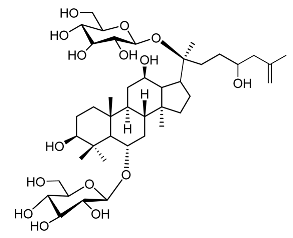 | Lee et al. [108] |
| 23. | Ginsenoside Rb2 |  | Lee et al. [109]. |
| 24. | Octaphlorethol A | 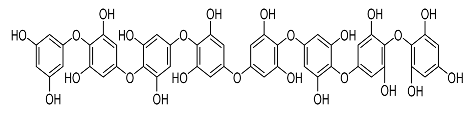 | Kin et al. [110] |
| 25. | 6-O-isobutyrylbritannilactone | 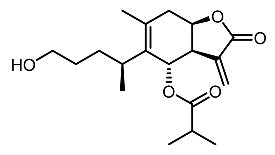 | Dae et al. [111] |
| 26. | 2,3,7,8-tetrachlorodibenzo-p-dioxin | 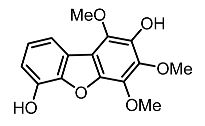 | Henry et al. [112] |
| Entry | Name | Chemical Structure | Reference |
|---|---|---|---|
| 9 | Sodium erythorbate |  | Chen et al. [34] |
| 12 | Omeprazole | 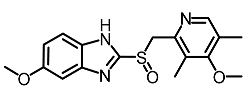 | Baek et al. [89] |
| 7 | 2-Methylphenyl-E-(3-hydroxy- 5-methoxy)-styryl ether |  | Huang et al. [98] |
| 2 | MEK-1 |  | Huang et al. [98] |
| 8 | 4-phenyl hydroxycoumarins | 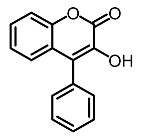 | Veselinovi'c et al. [115] |
| 4 | Kojic acid palmitate |  | Lajis et al. [116] |
| 1 | Suloctidil | 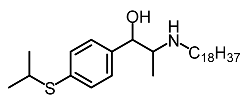 | Li et al. [117] |
| 3 | Compound 6 | 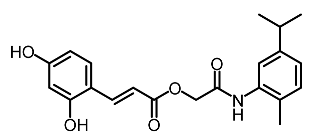 | Abbas et al. [118] |
| 5 | Fluoxetina | 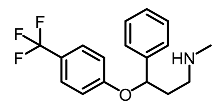 | Shang et al. [119] |
| 6 | Tretinoína | 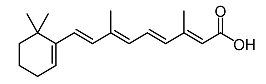 | Shang et al. [119] |
| 11 | (E)-1-(4-(3-chloro-4-fluorophenyl)piperazin-1-yl)-3-(3-nitrophenyl)prop-2-en-1-one | 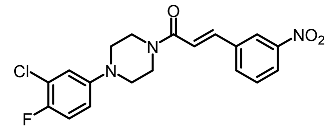 | Shang et al. [119] |
| 10 | Phenylthiourea | 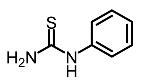 | Kim et al. [96]; Hsu et al. [86]; Thach et al. [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.M.; de Souza, A.A.; Koga, R.d.C.R.; Sena, I.d.S.; Matos, M.d.J.S.; Tomazi, R.; Ferreira, I.M.; Carvalho, J.C.T. Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model. Molecules 2023, 28, 1053. https://doi.org/10.3390/molecules28031053
Ferreira AM, de Souza AA, Koga RdCR, Sena IdS, Matos MdJS, Tomazi R, Ferreira IM, Carvalho JCT. Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model. Molecules. 2023; 28(3):1053. https://doi.org/10.3390/molecules28031053
Chicago/Turabian StyleFerreira, Adriana M., Agerdânio A. de Souza, Rosemary de Carvalho R. Koga, Iracirema da S. Sena, Mateus de Jesus S. Matos, Rosana Tomazi, Irlon M. Ferreira, and José Carlos T. Carvalho. 2023. "Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model" Molecules 28, no. 3: 1053. https://doi.org/10.3390/molecules28031053
APA StyleFerreira, A. M., de Souza, A. A., Koga, R. d. C. R., Sena, I. d. S., Matos, M. d. J. S., Tomazi, R., Ferreira, I. M., & Carvalho, J. C. T. (2023). Anti-Melanogenic Potential of Natural and Synthetic Substances: Application in Zebrafish Model. Molecules, 28(3), 1053. https://doi.org/10.3390/molecules28031053