Abstract
Acetylated triterpenoids betulin, oleanolic acid, ursolic acid, and glycyrrhetinic acid were converted into their succinyl-spacered acetazolamide conjugates. These conjugates were screened for their inhibitory activity onto carbonic anhydrase II and their cytotoxicity employing several human tumor cell lines and non-malignant fibroblasts. As a result, the best inhibitors were derived from betulin and glycyrrhetinic acid while those derived from ursolic or oleanolic acid were significantly weaker inhibitors but also of diminished cytotoxicity. A betulin-derived conjugate held a Ki = 0.129 μM and an EC50 = 8.5 μM for human A375 melanoma cells.
1. Introduction
The ubiquitous metalloenzymes carbonic anhydrases (CAs) [1,2,3,4,5,6] are present in bacteria [7,8] and fungi [9,10,11,12], plants and animals. Inhibitors of these enzymes have been clinically exploited for decades, and the discovery of multiple human isoforms [13,14,15,16,17,18] has led to many new applications and the development of new therapeutic principles, among them antiglaucoma [19,20,21] and antitumor drugs but also antiepileptic [22,23,24,25,26] and antiobesity drugs [27,28,29] as well as agents for the management of Alzheimer’s disease [30,31], neuropathic pain, cerebral ischemia, and some forms of arthritis [32,33,34]. Furthermore, the development of inhibitors for bacterial carbonic anhydrases is thought as a new concept to develop antibacterial drugs [35,36,37,38,39,40,41,42]. In addition, drug conjugates were investigated for their ability to treat a variety of disorders in a multitargeting approach [43,44,45,46,47]. The most investigated compound, however, is SLC-0111 (U-104, Figure 1) [48,49,50,51,52,53] for the management of advanced, metastatic solid tumors; this compound is now in Phase Ib/II clinical trials [54].
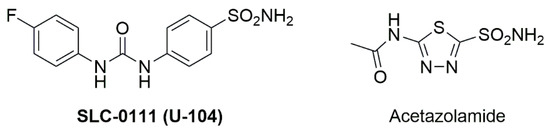
Figure 1.
Structure of well-established CA inhibitors SLC-0111 and acetazolamide.
For many years, especially CA IX and CA XII were in the focus of scientific interest to combat cancer. Recently, CA II in the endothelium of glial tumors became a potential target for therapy [55,56,57,58,59,60,61,62,63,64]. Furthermore, the CA II inhibitor acetazolamide was suggested as a chemosensitizer for treating temozolomide resistant gliomas [65,66,67,68,69]. In addition, CA II was found among up-regulated genes and as a candidate gene correlated with glioma malignancy and patient survival. [70]
The association of CA II with several pathological diseases prompted us to synthesize novel CA II inhibitors. Recently, it was revealed that methyl sulfamates, [71] derived from methyl triterpenoates such as oleanolic acid, ursolic acid, and glycyrrhetinic acid or betulinic acid, are effective and competitive inhibitors of CA II and several cycloartane derivatives [72] held significant activity for CA II, too. Euphol [73] was also shown to induce autophagy and to sensitize temozolomide cytotoxicity in glioblastoma cells. Several 1H-1,2,3-triazoles derived from pentacyclic triterpenoid 3-O-acetyl-(11-keto)-boswellic acids [74] were inhibitors CA II. Furthermore, hybrids of bile acids [75,76,77,78] have been shown to act as inhibitors of CAs.
Consequently, we became interested in the design, synthesis and CA II screening of conjugates derived from pentacyclic triterpenes such as betulin, oleanolic acid, ursolic acid, and glycyrrhetinic acid (Figure 2) with well-known inhibitor acetazolamide.
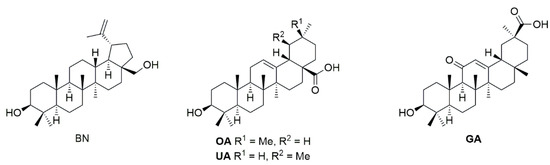
Figure 2.
Structures of starting materials betulin (BN), oleanolic acid (OA), ursolic acid (UA), and glycyrrhetinic acid (GA).
2. Results
Based on our previous results [71], triterpenoids (Figure 2) betulin (BN), oleanolic acid (OA), ursolic acid (UA), and glycyrrhetinic acid (GA) appeared to us as suitable and representative starting materials. BN was converted (Scheme 1) to the diacetate 1 according to known procedures, the selective mono-deacylation of which with CaH2 in a methanol/water mixture gave the 3-O-acetate 2. Reaction of 2 with succinic anhydride in pyridine in the presence of DMAP (cat.) furnished the succinyl derivative 3.
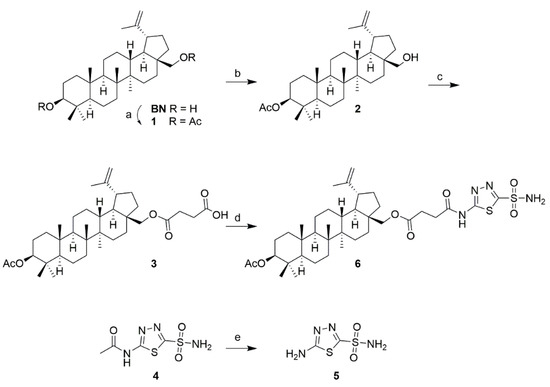
Scheme 1.
Reactions and conditions: (a) Ac2O, TEA, DMAP (cat.), DCM, 20 °C, 12 h, 90%; (b) CaH2, MeOH/THF, 20 °C, 12 h, 83%; (c) pyridine, DMAP, succinic anhydride, reflux, 15 h, 71%; (d) THF, 4-methyl-morpholine, ethyl chloroformate, 20 °C, 15 min, then 5, reflux, 48 h, 88%; (e) conc. HCl, reflux, 3 h, 94%.
Commercial acetazolamide (4) was deacetylated with conc. HCl under reflux and compound 5 was obtained. The reaction of 3 with 5 proved somewhat difficult in the beginning, as both an in situ generation of the corresponding carboxylic acid chloride and its coupling with 5 failed, as was the use of coupling reagents such as EDC, DCC, PyBOP, or T3P. However, a good yield of 6 was obtained by first reacting 3 with ethyl chloroformate in the presence of 4-methyl-morpholine in THF to yield a mixed anhydride in situ, whose reaction with 5 then gave 88% of 6.
Compound 6 shows the basic carbon skeleton unchanged from the starting material BN, and it is further characterized by the presence of the acetyl group at position C-3 (1H NMR δ = 1.99 ppm, and in 13C NMR δ = 171.9 and 21.0 ppm). The succinyl spacer is characterized by the two CH2 groups (in 13C NMR at δ = 30.0 and 30.9 ppm). The heterocycle shows in its 13C NMR spectrum the characteristic signals at δ = 161.0 and 164.3 ppm; the sulfamate group held in the 1H NMR spectrum the signal for the NH2 group at δ = 8.30 ppm.
For the preparation of the corresponding analogous compounds derived from OA, UA, or GA, the commercially relatively inexpensive triterpene carboxylic acids OA, UA first had to be reduced using LiAlH4 (Scheme 2). This allowed the corresponding diols 7 and 8 to be obtained in good yields.
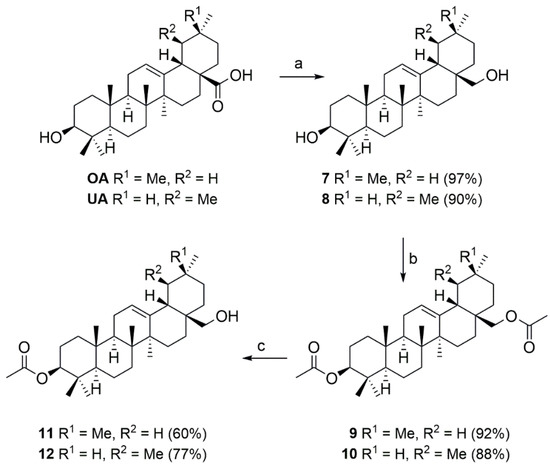
Scheme 2.
Reactions and conditions: (a) LiAlH4, THF, reflux, 2 h; (b) Ac2O, pyridine, 20 °C, 15 h; (c) Al(iPrO)3, iPrOH, reflux, 4 h.
Compounds 7 and 8 were converted into the corresponding diacetates 9 and 10, respectively, whose selective de-acetylation gave compounds 11 and 12. Analogous conditions as described above could now be carried out for the subsequent reactions to yield the target compounds.
Thus, the mono-acetates 11 and 12 were converted (Scheme 3) to the succinyl derivatives 13 and 14.
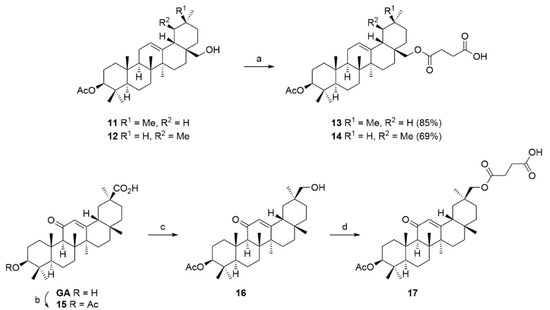
Scheme 3.
Reactions and conditions: (a) Pyridine, DMAP, succinic anhydride, reflux, 24 h; (b) Ac2O, pyridine, 20 °C, 15 h; (c) ethyl chloroformate, TEA, THF, −12 °C, 15 min, then sodium borohydride in water, 15 min; (d) pyridine, DMAP, succinic anhydride, reflux, 24 h.
Since the reduction of GA by LiAlH4 failed to give good yields, GA was first converted into acetate 15, the reaction of which with ethyl chloroformate/TEA gave an un-isolated mixed anhydride, the reduction of which with NaBH4 at room temperature afforded compound 16 in good yields within a few minutes. Its reaction with succinyl anhydride yielded 17.
The coupling of 13, 14, and 17 with 5 (Scheme 4) gave the products 18–20, respectively.
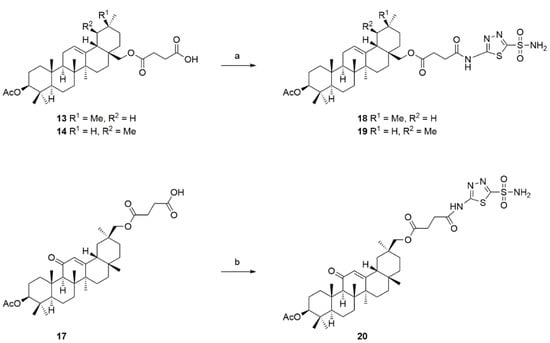
Scheme 4.
Reactions and conditions: (a,b) THF, 4-methyl-morpholine, ethyl chloroformate, 20 °C, 15 min, then 5, reflux, 48 h.
Screening of compounds 6, 18–20 for their activity was performed with CA II as previously described; the results from the assays are compiled in Table 1. Acetazolamide (4) was used as a positive control.

Table 1.
Inhibition percentage of conjugates (at 10 µM concentration) and of standard acetazolamide (4).
These assays showed glycyrrhetinic acid-derived conjugate 20 as the best inhibitor for this enzyme followed by betulin-derived 6. These compounds were even better inhibitors than gold standard acetazolamide (4). Oleanolic and ursolic-derived conjugates showed a diminished ability to inhibit CA II. Parent compounds, i.e., betulin, betulinic acid, ursolic acid, oleanolic acid, and glycyrrhetinic acid did not inhibit the enzyme under the conditions of the assay at all. Compounds 2, 3, 7–17 showed inhibition rates less than 10%.
For compounds with the highest inhibition percentage, i.e., 6 and 19 and 20, some extra measurements were performed to determine their respective inhibition constants Ki values. The results from these experiments are summarized in Table 2; Figure 3 shows the Dixon plot for compound 6; this compound acts as a competitive inhibitor for the enzyme and holds a rather low Ki = 0.129 μM.

Table 2.
Ki values (in μM) for conjugates 6, 19, and 20.
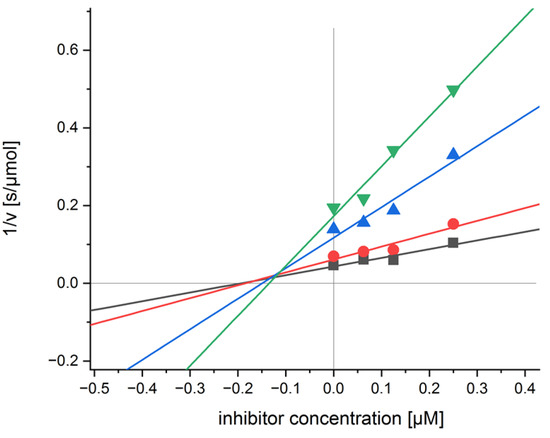
Figure 3.
Dixon plot for compound 6 and CA II.
Initial molecular modelling calculations were performed to get some insights in the mode of action of the conjugates. These calculations, however, did not provide any reasonable explanation for the different ability of the conjugates to inhibit the enzyme. While it seems plausible that the acetazolamide moiety interacts with the active site of the enzyme in a manner like parent acetazolamide, it cannot be excluded; however, that the conjugates also act as non-zinc binding inhibitors, thus paralleling previous findings for structurally similar pentacyclic triterpenoid arjunolic acid [79].
Previously especially CA IX was extensively studied in the process of tumorigenesis, [15,80] and several derivatives of pentacyclic triterpenoids have been revealed as inhibitors of this isoform, too [81]. The selectivity of the triterpenoid investigated so far toward individual isoforms of CA, however, was not particularly pronounced.
Compounds 6 and 18–20 were screened for their cytotoxic activity in sulforhodamine B assays (SRB), employing several human tumor cell lines. The results from these assays are summarized in Table 3. Expression of CA II and its involvement cancer has previously been established for A375 [82], HT29 [83] as well as for MCF-7 cells [84]. Cell line A2780 and non-malignant fibroblasts (NIH 3T3) were employed for comparison.

Table 3.
Cytotoxicity of acetazolamide (4) and conjugates 6, 18-20 assessed from SRB-assays (EC50 values [µM] after 72 h of treatment). Human cancer cell lines: A375 (epithelial melanoma), HT29 (colorectal adenocarcinoma), MCF-7 (breast adenocarcinoma), A2780 (ovarian carcinoma); non-malignant: NIH 3T3 (fibroblasts); n.d. not determined; positive control: doxorubicin (DX).
As a result, the highest cytotoxicity was established for botulin-derived 6 followed by glycyrrhetinic acid-derived 20. This parallels the finding for the inhibition rates for CA II established for these compounds. A significantly lower cytotoxicity was determined for oleanolic or ursolic acid-derived compounds 18 and 19, respectively. The malignant/non-malignant cell selectivity, however, was low for all compounds. No cytotoxicity (EC50 > 30 μM; cut-off of the assay) was found for parent triterpenoic acids.
3. Conclusions
Pentacyclic triterpenoids betulin, oleanolic acid, ursolic acid, and glycyrrhetinic acid were acetylated at position C-3 and converted into their succinyl-spacered acetazolamide conjugates. Their screening for their inhibitory activity onto carbonic anhydrase II and screening for their cytotoxicity in SRB assays employing several human tumor cell lines and non-malignant fibroblasts showed the conjugates derived from betulin and glycyrrhetinic acid to be the best inhibitors while those derived from ursolic or oleanolic acid were significantly weaker inhibitors but also of diminished cytotoxicity. A botulin-derived conjugate held a Ki = 0.129 μM and an EC50 = 8.5 μM for human A375 melanoma cells.
4. Experimental
NMR spectra were recorded using the Varian spectrometers (Darmstadt, Germany) DD2 and VNMRS (400 and 500 MHz, respectively). MS spectra were taken on a Advion expressionL CMS mass spectrometer (Ithaca, USA; positive ion polarity mode, solvent: methanol, solvent flow: 0.2 mL/min, spray voltage: 5.17 kV, source voltage: 77 V, APCI corona discharge: 4.2 μA, capillary temperature: 250 °C, capillary voltage: 180 V, sheath gas: N2). Thin-layer chromatography was performed on pre-coated silica gel plates supplied by Macherey-Nagel (Düren, Germany). IR spectra were recorded on a Spectrum 1000 FT-IR-spectrometer from Perkin Elmer (Rodgau, Germany). The UV/Vis-spectra were recorded on a Lambda 14 spectrometer from Perkin Elmer (Rodgau, Germany); optical rotations were measured using a JASCO-P2000 instrument (JASCO Germany GmbH, Pfungstadt, Germany) The melting points were determined using the Leica hot stage microscope Galen III (Leica Biosystems, Nussloch, Germany) and are uncorrected. The solvents were dried according to usual procedures. Microanalyses were performed with an Elementar Vario EL (CHNS) instrument (Elementar Analysensysteme GmbH, Elementar-Straße 1, D-63505 Langenselbold, Germany). All dry solvents were distilled over respective drying agents except for DMF which was distilled and stored under argon and molecular sieve. Reactions using air- or moisture-sensitive reagents were carried out under argon atmosphere in dried glassware. Triethylamine was stored over potassium hydroxide. Biological assays were performed as previously reported employing cell lines obtained from the Department of Oncology [Martin-Luther-University Halle Wittenberg; they were bought from ATCC: malignant: A 375, HT29, MCF7, and A2780; non-malignant: NIH 3T3]. Oleanolic and ursolic acid were obtained from Betulinines (Strbrna Skalice, Czech Republic) and used as received. Glycyrrhetinic acid was bought from Orgentis Chemicals GmbH (Gatersleben).
For the SRB assay: cells were seeded into 96-well plates on day zero at appropriate cell densities to prevent confluence of the cells during the period of the experiment. After 24 h, the cells were treated with different concentrations (1, 3, 7, 12, 20, and 30 μM), but the final concentration of DMSO/DMF never exceeded 0.5%, which was non-toxic to the cells. After 72 h of treatment, the supernatant media from the 96-well plates were discarded, then the cells were fixed with 10% trichloroacetic acid and allowed to rest at 4 °C. After 24 h of fixation, the cells were washed in a strip washer and then dyed with SRB solution (200 μL, 10 mM) for 20 min. Then the plates were washed four times with 1% acetic acid to remove the excess of the dye and allowed to air-dry overnight. Tris base solution (200 μL, 10 mM) was added to each well. The absorbance was measured with a 96-well plate reader from Tecan Spectra.
For the CA II assay: Carbonic anhydrase II (bCA II, ≥3000 W-A units/mg from bovine erythrocytes) as well as 4-nitrophenyl acetate (4-NA) were purchased from Sigma.
A 96-well microplate spectrometer BMG Labtech Spectrostar Omega working in the slow kinetics mode and measuring the absorbance at a distinct wavelength of λ = 415 nm with center scanning was used for the enzymatic studies. In short: A mixture of 4-NA solution (125 µL, 6 mM in 50 mM Tris-HCl buffer, pH 8), enzyme solution (25 µL, 0.3 mg/mL), and compounds solutions (25 µL, 3 different concentrations and water as a blank) was incubated at 37 °C for 20 min. The substrate (25 µL, [4-NA] = 0.75 mM, 0.50 mM, 0.25 mM, 0.15 mM) was added to start the enzymatic reaction. The absorbance data was recorded under a controlled temperature of 37 °C for 30 min at 1 min intervals at λ = 415 nm. The relative inhibition was determined as the quotient of the slopes (compound divided by blank) of the linear ranges.
4.1. Di-O-Acetyl-betulin
Compound 1 was prepared from BN (15.0 g, 34 mmol) by acetylation with acetic anhydride as previously described; re-crystallization from ethanol gave 1 (15.8 g, 90%) as a white solid; RF = 0.73 (silica gel, hexanes/ethyl acetate, 8:2); m.p.: 221 °C (lit.: [85] 216–218 °C); [α]D = +16.6° (c = 0.061, MeOH), [lit.: [85] [α]D = +19.7° (CHCl3)]; MS (ESI, MeOH): m/z = 467.5 (100%, [M + H-HOAc]+).
4.2. 3-O-Acetyl-betulin
Selective deacetylation of 1 (8.0 g, 15.2 mmol) with cat. amounts of CaH2 in MeOH/THF (100 mL, 1:1 v:v) for 12 h at 20 °C followed by usual aqueous workup and chromatography (silica gel, hexanes/ethyl acetate, 8:2) gave 2 (6.1 g, 83%) as a colorless solid; RF = 0.40 (silica gel, hexanes/ethyl acetate, 8:2); m.p.: 256–259 °C (lit.: [85] 258–260 °C); [α]D = +28.8° (c = 0.039, CHCl3), [lit.: [85] [α]D = +25.7° (CHCl3)]; MS (ESI, MeOH): m/z = 992,0 (100%, [2M+Na]+).
4.3. 4-{[(3β)-3-(Acetyloxy)lup-20(29)-en-28-yl]oxy}-4-oxobutanoic Acid
To a solution of 2 (4.0 g, 8.2 mmol) in dry pyridine (50 mL), DMAP (cat.) and succinic anhydride (1.64 g, 16.4 mmol) were added. The reaction mixture was stirred under reflux for 15 h. Usual aqueous work up and chromatography (silica gel, hexanes/ethyl acetate, 7:3) gave 3 [86,87,88,89,90,91] (3.4 g, 71%) as a white solid; m.p. 189–191 °C (lit.: [86,87,88] 190–191 °C); [α]D = +12.1° (c = 0.198, MeOH); RF = 0.15 (silica gel, hexanes/ethyl acetate, 8:2); IR (ATR): ν = 2943m, 2871w, 1732s, 1713s, 1455w, 1361w, 1366m, 1244s, 1159m, 1027w, 979m, 883w, 754m cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.68 (s, 1H, 29-Ha), 4.58 (s, 1H, 29-Hb), 4.46 (dd, J = 10.6, 5.6 Hz, 1H, 3-Ha), 4.30 (d, J = 11.0 Hz, 1H, 28-Ha), 3.88 (d, J = 11.1 Hz, 1H, 28-Hb), 2.75–2.54 (m, 4H, 34-H, 35-H), 2.42 (td, J = 11.0, 5.8 Hz, 1H, 19-H), 2.03 (s, 3H, 32-H), 2.01–1.88 (m, 1H, 21-Ha), 1.85–1.79 (m, 1H, 16-Ha), 1.75 (dd, J = 12.5, 7.9 Hz, 1H, 22-Ha), 1.68 (s, 3H, 30-H), 1.72–1.54 (m, 7H, 1-Ha, 13-H, 15-Ha, 12-Ha, 2-H, 9-H), 1.50 (s, 1H, 6-Ha), 1.44–1.35 (m, 5H, 6-Hb, 11-Ha, 21-Hb, 7-H), 1.34–1.14 (m, 3H, 16-Hb, 18-H, 11-Hb), 1.02 (s, 3H, 23-H), 1.12–0.90 (m, 4H, 22-Hb, 12-Hb, 15-Hb, 1-Hb), 0.96 (s, 3H, 27-H), 0.84 (s, 3H, 24-H), 0.84 (s, 3H, 26-H), 0.83 (s, 3H, 25-H), 0.78 (m, 1H, 5-H). ppm; 13C NMR (126 MHz, CDCl3): δ = 177.8 (C-36), 172.6 (C-33), 171.3 (C-31), 150.2 (C-20), 110.0 (C-29), 81.1 (C-3), 63.3 (C-28), 55.5 (C-5), 50.4 (C-18), 48.9 (C-9), 47.9 (C-19), 46.6 (C-17), 42.8 (C-14), 41.0 (C-8), 38.5 (C-1), 37.9 (C-4), 37.7 (C-13), 37.2 (C-10), 34.2 (C-22), 29.9 (C-16), 29.1 (C-35), 28.1 (C-24), 27.2 (C-15), 25.3 (C-12), 23.8 (C-2), 21.4 (C-32), 20.9 (C-11), 19.3 (C-30), 18.3 (C-6), 16.6 (C-25), 16.2 (C-23), 14.9 (C-27) ppm; MS (ESI, MeOH): m/z = 583.6 (100%, [M-H]-); analysis calcd for C36H56O6 (584.83): C 73.93, H 9.65; found: C 73.67, H 9.88.
4.4. 5-Amino-1,3,4-thiadiazole-2-sulfonamide
A solution of acetazolamide (4, 9.0 g, 40.7 mmol) in conc. HCl (60 mL) was heated under reflux for 3 h. After neutralization with NaOH, saturation with NaCl and extraction with THF (4 × 100 mL) followed by removal of the organic solvent, 5 (6.9 g, 94 %) was obtained as a white solid; m.p. 195 °C decomp. (lit.: [92] 215.5–216); RF = 0.3 (silica gel, CHCl3/MeOH, 9:1); UV-Vis (MeOH): λmax (log ε) = 278 nm (3.80) IR (ATR): ν = 3427w, 3321m, 3173w, 2870w, 2636w, 1601s, 1496s, 1448m, 1338s, 1172m, 1139m, 1098w, 1058w, 941m, 647s, 581s, 484w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 8.04 (s, 2H, NH2), 7.84 (s, 2H, NH2) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 171.7, 157.9. ppm; MS (ESI, MeOH): m/z = 179.0 (100%, [M-H]-).
4.5. (3β)-3-(Acetyloxy)lup-20(29)-en-28-yl 4-{[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]amino}-4-oxobutanoate
Compound 3 (500 mg, 0.85 mmol) was dissolved in dry THF (50 mL), 4-methylmorpholine (172 mg, 1.7 mmol) and ethyl chloroformate (185 mg, 1.7 mmol.) were added. The reaction mixture was stirred at 20 °C for 15 min. Compound 5 (184 mg, 1.02 mmol) was added, and the mixture was heated under reflux for another 48 h. The solvent was removed, the residue dissolved in CHCl3, washed with 2 M NaOH, water and brine and dried (MgSO4). Chromatography (silica gel, CHCl3/MeOH, 9:1) gave 4 (560 mg, 88%) as a white solid; m.p. 161–164°C; RF = 0.55 (silica gel, hexanes/ethyl acetate, 7:3); UV-Vis (CHCl3): λmax (log ε) = 264 nm (3.92) IR (ATR): ν = 2944m, 1733m, 1701m, 1531w, 1371m, 1245s, 1173s, 1018w, 979m, 882w, 609m, 504w cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 8.30 (s, 2H, NH2), 4.69 (s, 1H, 29-Ha), 4.55 (s, 1H, 29-Hb), 4.36 (dd, J = 11.4, 4.7 Hz, 1H, 3-H), 4.23 (d, J = 10.9 Hz, 1H, 28-Ha), 3.78 (d, J = 11.1 Hz, 1H, 28-Hb), 2.87–2.78 (m, 2H, 35-H), 2.76–2.65 (m, 2H, 34-H), 2.43 (s, 1H, 19-H), 1.99 (s, 3H, 32-H), 1.85 (m, 1H, 21-Ha), 1.63 (s, 3H, 30-H), 1.76–1.43 (m, 9H, 16-Ha, 22-Ha, 12-Ha, 13-H, 1-Ha, 9-H, 15-Ha, 2-H), 1.42–1.12 (m, 9H, 6-H, 11-Ha, 21-Hb, 7-H, 18-H, 16-Hb, 11-Hb), 0.95 (s, 3H, 23-H), 0.93 (s, 3H, 27-H), 1.09–0.73 (m, 5H, 22-Hb, 12-Hb, 1Hb, 15-Hb, 5-H), 0.79 (s, 9H, 24-H, 25-H, 26-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 171.9 (C-31), 171.3 (C-33), 170.1 (C-36), 164.3 (C-38), 161.0 (C-37), 149.8 (C-20), 110.0 (C-29), 79.9 (C-3), 61.9 (C-28), 54.6 (C-5), 49.5 (C-18), 48.1 (C-9), 47.0 (C-19), 46.0 (C-17), 42.2 (C-14), 40.4 (C-8), 37.4 (C-4), 37.0 (C-13), 36.6 (10), 34.2, 34.0 (22), 33.5 (7), 30.9 (34), 30.0 (35), 29.1 (16), 28.9, 28.5 (21), 27.6 (24), 26.6 (C-15), (C-12), 23.4 (C-2), 21.0 (C-32), 20.3 (C-11), 18.7 (C-30), 17.7 (C-6), 16.4 (C-25), 15.8 (C-26), 15.5 (C-23), 14.5 (C-27) ppm; MS (ESI, MeOH): m/z = 745.7 (100%, [M-H]-); analysis calcd for C38H58N4O7S2 (747.03): C 61.10, H 7.83, N 7.50; found: C 60.85, H 8.03, N 7.33.
4.6. (3β) Olean-12-en3-3,28-diol (Erythrodiol)
To a solution of OA (5.0 g, 10.7 mmol, 1.00 eq.) in dry THF (150 mL), LiAlH4 (2.0 g, 53.6 mmol, 5.00 eq.,) was slowly added. Stirring under reflux was continued for another 2 h. After cooling to 20 °C, the reaction was quenched (slow addition of 20 mL MeOH), and aq. HCl (6 M, 50 mL) was added. The reaction mixture was extracted with ethyl acetate (3 × 75 mL); the combined organic phases were washed with aq. NaOH (1 M, 2 × 50 mL), brine (50 mL) and dried (MgSO4). The solvent was removed under reduced pressure, and the residue was subjected to chromatography (silica gel, chloroform/hexanes/ethyl acetate, 10:8:2) to yield 7 (4.61 g, 97%) as a colorless solid; m.p. 218–219 °C (lit.: [93] 217–219 °C); RF = 0.17 (silica gel, chloroform/hexanes/ethyl acetate, 10:8:2); [α]D = +72.1° (c = 0.113, MeOH) (lit.: [94] [α]D = +75.0° (c = 0.325, CHCl3)).
4.7. (3β) Urs-12-ene-3,28-diol (Uvaol, 8)
Following the procedure given for the synthesis of 7, from UA (5.00 g, 10.7 mmol), dry THF (150 mL) and LiAlH4 (2.0 g, 53.6 mmol) followed by chromatography (silica gel, (chloroform/hexanes/ethyl acetate, 10:8:2) 8 (4.36 g, 90%) was obtained as a colorless solid; m.p. 227–229 °C (lit.: [93] 225–227 °C); RF = 0.18 (silica gel, chloroform/hexanes/ethyl acetate, 10:8:2); [α]D = +60.5° (c = 0.109, MeOH); (lit.: [95] [α]D = +62.6° (c = 0.62, CHCl3)).
4.8. (3β)-Olean-12-ene-3,28-diyl Diacetate
Acetylation of 7 (4.00 g, 9.04 mmol) in dry pyridine (16 mL) with acetic anhydride (2.6 mL, 27.1 mmol) for 15 h at 20 °C followed by usual aqueous work up and chromatography (silica gel, (hexanes/ethyl acetate, 9:1) gave 9 (4.37 g, 92%) as a colorless solid; m.p. 184-186 °C (lit.: [96] 184-186 °C); RF = 0.43 (silica gel, hexanes/ethyl acetate, 9:1); [α]D = +62.2° (c = 0.124, CHCl3); (lit.: [97] [α]D = +56.0° (c = 1.0CHCl3)).
4.9. (3β)-Urs-12-ene-3,28-diyl Diacetate
Acetylation of 8 (3.48 g, 7.86 mmol) as described above for the synthesis of 9 gave 10 (3.66 g, 88%) as a colorless solid; m.p. 151–153 °C (lit.: [98] 150-151 °C); RF = 0.41 (silica gel, hexanes/ethyl acetate, 9:1); [α]D = +51.4° (c = 0.115, CHCl3).
4.10. (3β)-28-Hydroxyolean-12-en-3-yl Acetate
A solution of 9 (3.1 g, 5.89 mmol) and aluminum isopropoxide (12.3 g, 58.8 mmol) in isopropanol (150 mL) was heated under reflux for 4 h. Usual aqu. work-up followed by chromatography (silica gel, hexanes/ethyl acetate, 8:2) gave 11 (1.71 g, 60%) as a colorless solid; m.p. 234–236 °C (lit.: [99] 233–234 °C); RF = 0.55 (silica gel, hexanes/ethyl acetate, 8:2); [α]D = +67.4° (c = 0.122, CHCl3) (lit.: [100] [α]D = +71° (c = 0.70, CHCl3)).
4.11. (3β)-28-Hydroxyolean-12-en-3-yl Acetate
As described above for the synthesis of 11, from 10 (2.7 g, 5.13 mmol) and aluminum propoxide (10.7 g, 51.3 mmol) in isopropanol (150 mL) followed by chromatography (silica gel, hexanes/ethyl acetate, 8:2) 12 (1.92 g, 77%) was obtained as a colorless solid; m.p. 265–267 °C (lit.: [101] 258–261 °C); RF = 0.51 (silica gel, hexanes/ethyl acetate, 8:2); [α]D = +63.1° (c = 0.139, CHCl3) (lit.: [102] [α]D = +70.5° (c = 0.145, CHCl3)).
4.12. 4-{[(3β)-3-(Acetyloxy)-olean-12-en-28-yl]oxy}-4-oxobutanoic Acid
To a solution of 11 (0.45 g, 0.928 mmol) in dry pyridine (15 mL) succinic anhydride (0.188 g, 1.86 mmol) and cat. DMAP were added, and the mixture was stirred for 1 day under reflux. Usual aq. work-up followed by chromatography (silica gel, hexanes/ethyl acetate (1% HCOOH), 8:2 → 7:3) gave 13 (0.460 g, 85%) as a colorless solid; m.p. 124–126 °C; RF = 0.47 (silica gel, hexanes/ethyl acetate, 7:3); [α]D = +49.6° (c = 0.126, CHCl3); IR (ATR): ν = 2946m, 2864 w, 1734s, 1712s, 1463w, 1432w, 1387m, 1364m, 1244s, 1160s, 1095w, 1027m, 1004m, 986m, 967m cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.01 (brs, 1H, COO-H), 5.19 (t, J = 3.6 Hz, 1H, 12-H), 4.55–4.44 (m, 1H, 3-H), 4.07 (d, J = 11.0 Hz, 1H, 28-Ha), 3.72 (d, J = 11.0 Hz, 1H, 28-Hb), 2.71–2.59 (m, 4H, 34-H + 35-H), 2.04 (s, 3H, 32-H), 2.01–1.99 (m, 1H, 18-H), 1.97–1.78 (m, 3H, 16-Ha + 11-H), 1.77–1.46 (m, 9H, 19-Ha + 15-Ha + 2-H + 1-Ha + 9-H + 6-Ha + 7-Ha + 22-Ha), 1.45–1.21 (m, 4H, 6-Hb + 22-Hb + 7-Hb + 21-Ha), 1.19–1.10 (m, 5H, 16-Hb + 21-Hb + 27-H), 1.12–0.95 (m, 3H, 19-Hb + 1-Hb + 15-Hb), 0.94 (s, 3H, 25-H), 0.93 (s, 3H, 26-H), 0.88 (s, 3H, 30-H), 0.86 (s, 6H, 29-H + 24-H), 0.85 (s, 3H, 23-H), 0.84–0.80 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 177.9 (C-36), 172.2 (C-33), 171.3 (C-31), 143.7 (C-13), 123.0 (C-12), 81.1 (C-3), 71.3 (C-28), 55.4 (C-5), 47.6 (C-9), 46.3 (C-19), 42.7 (C-18), 41.8 (C-14), 39.9 (C-8), 38.4 (C-1), 37.8 (C-4), 36.9 (C-10), 36.0 (C-17), 34.1 (C-21), 33.3 (C-30), 32.6 (C-7), 31.6 (C-22), 31.0 (C-20), 29.1 (C-34), 29.1 (C-35), 28.2 (C-24), 26.1 (C-27), 25.7 (C-15), 23.7 (C-29), 23.7 (C-11), 23.7 (C-2), 22.3 (C-16), 21.4 (C-32), 18.4 (C-6), 16.8 (C-26), 16.8 (C-23), 15.7 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z = 583.9 (100%, [M-H]-); analysis calcd for C36H56O6 (584.84): C 73.93, H 9.65; found: C 73.71, H 9.86.
4.13. 4-{[(3β)-3-(Acetyloxy)urs-12-en-28-yl]oxy}-4-oxobutanoic Acid
Following the procedure given for 11, from 12 (1.35 g, 2.79 mmol), 14 (1.12 g, 69%) was obtained as a colorless solid; m.p. 112–114 °C; RF = 0.50 (silica gel, hexanes/ethyl acetate, 7:3); [α]D = +42.7° (c = 0.131, CHCl3); IR (ATR): ν = 2948m, 2925m, 1734s, 1712s, 1456m, 1432w, 1388m, 1370m, 1269m, 1244s, 1158s, 1095w, 1025m, 1006m, 985m, 967m cm−1; 1H NMR (500 MHz, CDCl3): δ = 8.01 (brs, 1H, COO-H), 5.13 (dd, J = 3.7 Hz, 1H, 12-H), 4.53–4.44 (m, 1H, 3-H), 4.10 (d, J = 11.0 Hz, 1H, 28-Ha), 3.64 (d, J = 11.0 Hz, 1H, 28-Hb), 2.70–2.60 (m, 4H, 34-H + 35-H), 2.04 (s, 3H, 32-H), 1.98–1.88 (m, 2H, 16-Ha + 11-H), 1.76 – 1.68 (m, 1H, 15-Ha), 1.67–1.59 (m, 2H, 1-Ha + 2-H), 1.59–1.48 (m, 4H, 22-Ha + 7-Ha + 9-H + 6-Ha), 1.47–1.28 (m, 6H, 21-Ha + 18-H + 6-Hb + 19-H + 7-Hb + 22-Hb), 1.27–1.13 (m, 2H, 21-Hb + 16-Hb), 1.08 (s, 3H, 27-H), 1.08–1.06 (m, 1H, 1-Hb), 0.99–0.96 (m, 1H, 15-Hb), 0.97 (s, 3H, 26-H), 0.96 (s, 3H, 25-H), 0.93 (d, J = 5.8 Hz, 3H, 29-H), 0.91–0.88 (m, 1H, 20-H), 0.86 (s, 3H, 24-H), 0.86 (s, 3H, 23-H), 0.85–0.83 (m, 1H, 5-H), 0.80 (d, J = 5.3 Hz, 3H, 30-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 178.0 (C-36), 172.2 (C-33), 171.3 (C-31), 138.3 (C-13), 125.7 (C-12), 81.1 (C-3), 71.8 (C-28), 55.4 (C-5), 54.4 (C-18), 47.7 (C-9), 42.1 (C-14), 40.1 (C-8), 39.5 (C-20), 39.3 (C-19), 38.6 (C-1), 37.8 (C-17), 37.1 (C-4), 36.9 (C-10), 35.8 (C-22), 32.8 (C-7), 30.6 (C-21), 29.2 (C-34), 29.2 (C-35), 28.2 (C-24), 26.1 (C-15), 23.7 (C-2), 23.5 (C-11), 23.5 (C-16), 23.5 (C-27), 21.4 (C-32), 21.4 (C-29), 18.3 (C-6), 17.4 (C-30), 16.9 (C-26), 16.8 (C-23), 15.9 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z = 607.9 (100%, [M + Na]+); analysis calcd for C36H56O6 (584.84): C 73.93, H 9.65; found: C 73.68, H 9.91.
4.14. (3β, 20β)-3-Acetyloxy-11-oxoolean-12-en-29-oic Acid
Acetylation of GA (2.50 g, 5.31 mmol) as described above followed by chromatography (silica gel, hexanes/ethyl acetate, 8:2) gave 15 (2.15 g, 79%) as a colorless solid; m.p. 305–307 °C (lit.: [96] 316–318 °C); RF = 0.41 (silica gel, hexanes/ethyl acetate, 9:1); [α]D = +163.3° (c = 0.142, CHCl3); (lit.: [103] [α]D = +165.1° (c = 0.7, CHCl3)).
4.15. (3β, 20β) 3-Acetyloxy-29-hydroxyolean-12-en-11-one
To a solution of 15 (1.3 g, 2.76 mmol) and triethylamine (1.1 mL, 7.60 mmol) in dry THF (15 mL), at −12 °C, ethyl chloroformate (1.1 mL, 11.1 mmol) was added, and the mixture was stirred for 15 min. The precipitate was filtered off, and the filtrate was slowly added to a freshly prepared solution of sodium borohydride (0.522 g, 13.8 mol) in water (2.5 mL). Stirring at room temperature was continued for another 15 min followed by usual aq. work-up and chromatography (silica gel, CHCl3/Et2O/hexanes/HCOOH, 25:25:43:7) to yield 16 (1.09 g, 82%) as a colorless solid; m.p. 264–266 °C; RF = 0.46 (silica gel, CHCl3/Et2O/hexanes/HCOOH, 25:25:43:7); [α]D = +91.6° (c = 0.129, CHCl3); UV-Vis (CHCl3): λmax (log ε) = 249 nm (4.07); IR (ATR): ν = 3569w, 3550 w, 2925m, 2862w, 1725s, 1695m, 1651s, 1626m, 1465w, 1455m,1386m, 1366m, 1325w, 1279w, 1246s, 1209m, 1173s, 1143m, 1095w, 1048m, 1025s, 1001m, 985m cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.58 (s, 1H, 12-H), 4.50 (dd, J = 11.8, 4.7 Hz, 1H, 3-H), 4.13 (d, J = 11.0 Hz, 1H, 30-Ha), 4.03 (d, J = 11.0 Hz, 1H, 30-Hb (30)), 2.78 (dt, J = 13.6, 3.6 Hz, 1H, 1-Ha), 2.35 (s, 1H, 9-H), 2.13–2.06 (m, 2H, 18-H + 16-Ha), 2.04 (s, 3H, 32-H), 1.86–1.76 (m, 1H, 15-Ha), 1.74–1.53 (m, 7H, 2-Ha + 22-Ha + 7-Ha + 19-Ha + 2-Hb + 21-Ha + 6-Ha) 1.50–1.36 (m, 3H, 6-Hb + 7-Hb + 22-Hb + 19-Hb), 1.36 (s, 3H, 27-H), 1.21– 1.16 (m, 1H, 15-Hb), 1.15 (s, 3H, 25-H), 1.12 (s, 3H, 26-H), 1.07–0.98 (m, 2H, 1-Hb + 16-Hb), 0.95 (s, 3H, 29-H), 0.87 (s, 6H, 28-H + 23-H), 0.86 (s, 3H, 24-H), 0.81–0.77 (m, 1H, 5-H), 0.80 (d, J = 5.7 Hz, 3H, 30-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 200.4 (C-11), 171.4 (C-31), 169.8 (C-13), 128.5 (C-12), 80.9 (C-3), 67.0 (C-30), 61.8 (C-9), 55.2 (C-5), 47.0 (C-18), 45.6 (C-8), 43.5 (C-14), 40.2 (C-19), 38.9 (C-1), 38.2 (C-4), 37.1 (C-10), 36.0 (C-22), 34.3 (C-20), 32.8 (C-7), 32.4 (C-17), 30.2 (C-21), 28.6 (C-28), 28.2 (C-24), 27.9 (C-29), 26.7 (C-16), 26.5 (C-15), 23.7 (C-2), 23.5 (C-27), 21.4 (C-32), 18.8 (C-26), 17.5 (C-6), 16.8 (C-23), 16.5 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z = 497.9 (100%, [M-H]-); analysis calcd for C32H50O4 (498.74): C 77.06, H 10.10; found: C 76.81, H 10.35.
4.16. 4-{[(3β,20β)3-(Acetyloxy)-11-oxoolean-12-en-30-yl]oxy}-4-oxobutanoic Acid
Following the procedure described above, from 16 (0.270 g, 0.541 mmol) 17 (0.315 g, 97%) was obtained as a colorless solid; m.p. 109–111 °C; RF = 0.44 (silica gel, hexanes/ethyl acetate, 1:1); [α]D = +110.5° (c = 0.118, CHCl3); UV-Vis (CHCl3): λmax (log ε) = 254 nm (4.05); IR (ATR): ν = 2949m, 2928m, 2871w, 1730s, 1657m, 1465w, 1456w, 1388m, 1365m, 1322w, 1244s, 1209m, 1158m, 1091w, 1049w, 1028m, 1001m, 986 m cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.01 (brs, 1H, COO-H), 5.64 (s, 1H, 12-H), 4.70 (d, J = 10.9 Hz, 1H, 30-Ha), 4.51 (dd, J = 11.5, 4.9 Hz, 1H, 3-H), 3.46 (d, J = 10.9 Hz, 1H, 30-Hb), 2.85 (dt, J = 13.6, 3.5 Hz, 1H, 1-Ha), 2.79–2.71 (m, 2H, 34-H), 2.58–2.49 (m, 2H, 35-H), 2.37 (s, 1H, 9-H), 2.36–2.25 (m, 1H, 18-H), 2.12–2.06 (m, 1H, 16-Ha), 2.04 (s, 3H, 32-H), 1.87–1.75 (m, 1H, 15-Ha), 1.74–1.36 (m, 9H, 2-H + 7-Ha + 6-Ha + 19-Ha + 6-Hb + 22-Ha + 7-Hb + 21-Ha), 1.35 (s, 3H, 27-H), 1.33–1.14 (m, 4H, 21-Hb + 22-Hb + 19-Hb + 16-Hb), 1.12 (s, 6H, 25-H + 26-H), 1.10– 0.98 (m, 2H, 1-Hb + 15-Hb), 0.95 (s, 3H, 29-H), 0.87 (s, 9H, 28-H + 23-H + 24-H), 0.83–0.77 (m, 1H, 5-H) ppm; 13C NMR (101 MHz, CDCl3): δ = 202.2 (C-11), 174.8 (C-36), 172.9 (C-33), 171.8 (C-13), 171.1 (C-31), 128.1 (C-12), 80.7 (C-3), 67.7 (C-30), 61.9 (C-9), 55.2 (C-5), 46.7 (C-18), 45.5 (C-8), 43.4 (C-14), 39.2 (C-19), 39.0 (C-1), 38.3 (C-4), 37.4 (C-10), 36.1 (C-22), 34.9 (C-20), 32.7 (C-7), 32.3 (C-17), 31.3 (C-21), 29.5 (C-35), 28.9 (C-34), 28.9 (C-28), 28.2 (C-24), 28.2 (C-29), 26.6 (C-16), 26.6 (C-15), 23.7 (C-2), 23.3 (C-27), 21.4 (C-32), 19.0 (C-26), 17.5 (C-6), 16.8 (C-23), 16.7 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1) m/z = 600.0 (96%, [M + H]+); analysis calcd for C36H54O7 (598.82): C 72.21, H 9.09; found: C 71.97, H 9.32.
4.17. (3β) 3-(Acetyloxy)olean-12-en-28-yl-4-{[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]amino}-4-oxobutanoate
To a solution of 13 (0.250 g, 0.427 mmol) in dry THF (20 mL) at −15 °C, 4-methylmorpholine (0.1 mL, 0641 mmol) and ethyl chloroformate (0.05 mL, 0.513 mmol) were added, and the mixture was stirred for 10 min at this temperature. Then 5-amino-1,3,4-thiadiazole-2-sulfonamide 5 (0.092 g, 0.513 mmol) was added, and the mixture was heated under reflux for 4 h. The solvents were removed under diminished pressure, and the residue was subjected to chromatography (silica gel, (CHCl3/MeOH, 95:5) to yield 18 (0.166 g, 52%) as a colorless solid; m.p. 198–200 °C; RF = 0.36 (silica gel, CHCl3/MeOH, 9:1); [α]D = +19.2° (c = 0.122, CHCl3); UV-Vis (CHCl3): λmax (log ε) = 262 nm (4.45); IR (ATR): ν = 3332w, 3234w, 2947m, 2864m, 1734m, 1704s, 1545m, 1536m, 1463w, 1432w, 1422w, 1371s, 1329m, 1245s, 1216m, 1173s, 1092m, 1027m, 1004m, 985m, 967m, 755m, 651m, 637m, 606 s, 504m cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 13.07 (brs, 1H, CON-H), 8.30 (d, J = 6.2 Hz, 2H, SN-H), 5.15 (t, J = 3.7 Hz, 1H, 12-H), 4.38 (dd, J = 11.7, 4.5 Hz, 1H, 3-H), 3.96 (d, J = 10.9 Hz, 1H, 28-Ha), 3.60 (d, J = 10.9 Hz, 1H, 28-Hb), 2.89–2.76 (m, 2H, 34-H), 2.72–2.65 (m, 2H, 35-H), 2.02–1.97 (m, 1H, 18-H), 1.99 (s, 3H, 32-H), 1.86 (td, J = 13.9, 4.6 Hz, 1H, 16-Ha), 1.82–1.75 (m, 2H, 11-H), 1.70 (dd, J = 13.4, 13.4 Hz, 1H, 19-Ha), 1.62–1.13 (m, 11H, 15-Ha + 2-H + 1-Ha + 9-H + 6-Ha + 21-Ha + 22-Ha, 6-Hb + 22-Hb + 7-Hb), 1.11 (s, 3H, 27-H), 1.09–0.93 (m, 4H, 16-Hb + 7-Hb + 19-Hb + 1-Hb), 0.88 (s, 3H, 25-H), 0.85 (d, 3H, 30-H), 0.84 (d, 3H, 29-H), 0.83 (s, 3H, 24-H), 0.83–0.80 (m, 7H, 26-H + 23-H + 5-H); ppm; 13C NMR (126 MHz, DMSO-d6): δ = 171.6 (C-33), 171.2 (C-36), 170.1 (C-31), 164.3 (C-38), 161.0 (C-37), 143.4 (C-13), 122.2 (C-12), 79.9 (C-3), 70.0 (C-28), 54.5 (C-5), 46.7 (C-9), 45.7 (C-19), 41.8 (C-18), 41.1 (C-14), 39.2 (C-8), 37.7 (C-1), 37.3 (C-4), 36.3 (C-10), 35.4 (C-17), 33.4 (C-7), 32.9 (C-30), 31.8 (C-21), 31.0 (C-22), 30.5 (C-20), 30.0 (C-34), 28.4 (C-35), 27.7 (C-24), 25.7 (C-27), 25.1 (C-15), 23.4 (C-29), 23.2 (C-2), 23.0 (C-11), 21.5 (C-16), 20.9 (C-32), 17.7 (C-6), 16.6 (C-26), 16.2 (C-23), 15.2 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z = 770.1 (100%, [M + Na]+); analysis calcd for C38H58S2N4 (747.02): C 61.10, H 7.83, N 7.50; found: C 60.81, H 8.03, N 7.39.
4.18. (3β)-3-(Acetyloxy)urs-12-en-28-yl-4-{[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]amino}4-oxobutanoate
Following the procedure given above for the synthesis of 18, from 14 (0.250 g, 0.427 mmol) 21 (0.217 g, 68%) was obtained as a colorless solid; m.p. 190–192 °C; RF = 0.33 (silica gel, CHCl3/MeOH, 9:1); [α]D = +27.4° (c = 0.129, CHCl3); UV-Vis (CHCl3): λmax (log ε) = 263 nm (4.98); IR (ATR): ν = 3244w, 2948m, 2925m, 2871m, 1733m, 1705s, 1531m, 1457w, 1431w; 1414w, 1367s, 1245s, 1174s, 1094m, 1027m, 1006m, 985m, 967m, 655m, 603s, 508m cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 13.07 (brs, 1H, CON-H), 8.30 (s, J = 2H, SN-H), 5.10 (t, J = 3.2 Hz, 1H, H-12), 4.38 (dd, J = 11.5, 4.7 Hz, 1H, H-3), 3.94 (d, J = 10.8 Hz, 1H, 28-Ha), 3.54 (d, J = 10.9 Hz, 1H, 28-Hb), 2.84–2.78 (m, 2H, 34-H), 2.71–2.65 (m, 2H, 35-H), 1.99 (s, 3H, 32-H), 1.92–1.80 (m, 3H, 16-Ha + 11-H), 1.65–1.53 (m, 3H, 15-Ha + 2-Ha + 1-Ha), 1.53–1.42 (m, 5H, 2-Ha + 9-H + 7-Ha + 6-Ha + 22-Ha), 1.40–1.06 (m, 7H, 18-H + 6-Hb + 21-Ha + 22-Hb + 7-Hb + 21-Hb + 16-Hb), 1.04 (s, 3H, 27-H), 1.01–0.96 (m, 1H, 1-Hb), 0.90 (s, 3H, 23-H), 0.87 (d, J = 6.7, 3H, 29-H), 0.86 (s, 3H, 26-H), 0.85–0.83 (m, 2H, 15-Hb + 1-Hb), 0.83 (s, 6H, 24-H + 25-H), 0.83–0.80 (m, 1H, 20-H), 0.87 (d, J = 5.3 Hz, 3H, 30-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 171.4 (C-33), 171.2 (C-36), 170.1 (C-31), 164.3 (C-38), 161.0 (C-37), 138.0 (C-13), 124.8 (C-12), 79.9 (C-3), 70.4 (C-28), 54.5 (C-5), 53.4 (C-18), 46.8 (C-9), 41.4 (C-14), 39.4 (C-8), 38.7 (C-20), 38.5 (C-19), 37.9 (C-1), 37.3 (C-4), 36.4 (C-17), 36.3 (C-10), 35.1 (C-2), 32.1 (C-7), 30.0 (C-34), 29.9 (C-21), 28.4 (C-35), 27.7 (C-24), 25.5 (C-15), 23.3 (C-2), 23.0 (C-27), 22.9 (C-11), 22.8 (C-16), 21.0 (C-29), 20.9 (C-32), 17.1 (C-6), 17.1 (C-30), 16.6 (C-23), 16.3 (C-26), 15.2 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z = 770.1 (100%, [M + Na]+); analysis calcd for C38H58S2N4O7 (747.02): C 61.10, H 7.83, N 7.50; found: C 60.89, H 8.07, N 7.36.
4.19. (3β, 20β) 3-(Acetyloxy)-11-oxoolean-12-en-30-yl-4-{[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl] amino}-4-oxobutanoate
Following the procedure given above for the synthesis of 18, from 17 (0.250 g, 0.417 mmol) 20 (0.215 g, 68%) was obtained as a colorless solid; m.p. 179–181 °C; RF = 0.41 (silica gel, CHCl3/MeOH, 9:1); [α]D = +83.5° (c = 0.114, CHCl3); UV-Vis (CHCl3): λmax (log ε) = 256 nm (4.25); IR (ATR): ν = 3252w, 2950m, 2872w, 1727m, 1709m, 1643m, 1528m, 1466w, 1455w, 1365s, 1323m, 1247s, 1215m, 1173s, 1088w, 1049w, 1028m, 1001m, 985m, 754s, 667m, 654m, 604s, 509m cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 13.08 (brs, 1H, CON-H), 8.30 (s, J = 2H, SN-H), 5.48 (s, 1H, 12-H), 4.42 (dd, J = 11.8, 4.5 Hz, 1H, 3-H), 4.05 (d, J = 11.0 Hz, 1H, 30-Ha), 3.92 (d, J = 11.0 Hz, 1H, 30-Hb), 2.82 (dd, J = 7.5, 5.5 Hz 2H, 35-H), 2.71 (dd, J = 7.6, 5.5 Hz, 2H, 34-H), 2.61 (dt, J = 13.4, 3.6 Hz, 1H, 1-Ha), 2.38 (s, 1H, 9-H), 2.16–2.04 (m, 2H, 18-H + 16-Ha), 2.00 (s, 3H, 32-H), 1.79–1.35 (m, 7H, 16-Hb + 7-Ha + 19-Ha + 2-Ha + 6-Ha + 2-Hb + 6-Hb). 1.34 (s, 3H, 27-H), 1.34–1.31 (m, 3H, 7-Hb + 21-Hb + 22-Hb), 1.24–1.06 (m, 4H, 19-Hb + 22-Hb + 15-Hb + 1-Hb), 1.06 (s, 1H, 25-H) 1.04 (s, 1H, 26-H)), 0.86– 0.98 (m, 2H, 15-Hb + 5-H), 0.85 (s, 3H, 29-H), 0.82 (s, 6H, 23-H + 24-H), 0.80 (s, 3H, 28-H) ppm; 13C NMR (126 MHz, DMSO-d6): δ = 198.9 (C-11), 171.8 (C-36), 171.1 (C-33), 170.1 (C-31), 170.0 (C-13), 127.4 (C-12), 79.7 (C-3), 67.0 (C-30), 60.8 (C-9), 53.7 (C-5), 46.0 (C-18), 44.9 (C-8), 43.0 (C-14), 39.7 (C-19), 37.8 (C-1), 37.5 (C-4), 36.5 (C-10), 35.4 (C-22), 34.0 (C-20), 31.9 (C-7), 31.8 (C-17), 29.9 (C-35), 29.3 (C-21), 28.3 (C-34), 28.1 (C-28), 27.7 (C-24), 27.2 (C-29), 25.9 (C-16), 25.9 (C-15), 23.2 (C-2), 23.1 (C-27), 20.9 (C-32), 18.3 (C-26), 16.9 (C-6), 16.6 (C-23), 16.1 (C-25) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z = 784.0 (100%, [M + Na]+; analysis calcd for C38H56S2N4O8 (761.00): C 59.97, H 7.42, N 7.36; found: C 59.71, H 7.71, N 7.09.
Author Contributions
Conceptualization, R.C.; validation, R.C.; investigation, T.C.D., N.H., O.K., J.Z., and S.H.; writing—original draft preparation, R.C. writing—review and editing, T.C.D., N.H., J.Z. and S.H R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We like to thank D. Ströhl, Y. Schiller and S. Ludwig for the NMR spectra; IR spectra, micro-analyses, and optical rotations were measured by M. Schneider.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Supuran, C.T. Carbonic anhydrases—An overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Carta, F.; Nocentini, A.; Winum, J.-Y.; Zalubovskis, R.; Onnis, V.; Eldehna, W.M.; Capasso, C.; Carradori, S.; Donald, W.A.; et al. Response to Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors. Biophys. J. 2021, 120, 178–181. [Google Scholar] [CrossRef]
- Berrino, E.; Michelet, B.; Martin-Mingot, A.; Carta, F.; Supuran, C.T.; Thibaudeau, S. Modulating the Efficacy of Carbonic Anhydrase Inhibitors through Fluorine Substitution. Angew. Chem. Int. Ed. 2021, 60, 23068–23082. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2021, 209, 112923. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef]
- Amedei, A.; Capasso, C.; Nannini, G.; Supuran, C.T. Microbiota, bacterial carbonic anhydrases, and modulators of their activity: Links to human diseases? Mediat. Inflamm. 2021, 926082. [Google Scholar] [CrossRef]
- Campestre, C.; De Luca, V.; Carradori, S.; Grande, R.; Carginale, V.; Scaloni, A.; Supuran, C.T.; Capasso, C. Carbonic Anhydrases: New Perspectives on Protein Functional Role and Inhibition in Helicobacter pylori. Front. Microbiol. 2021, 12, 629163. [Google Scholar] [CrossRef]
- Hines, K.M.; Chaudhari, V.; Edgeworth, K.N.; Owens, T.G.; Hanson, M.R. Absence of carbonic anhydrase in chloroplasts affects C3 plant development but not photosynthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107425118. [Google Scholar] [CrossRef]
- Polishchuk, O.V. Stress-Related Changes in the Expression and Activity of Plant Carbonic Anhydrases. Planta 2021, 253, 58. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Ignatova, L.K.; Nadeeva-Zhurikova, E.M.; Fedorchuk, T.P.; Ivanov, B.N.; Borisova-Mubarakshina, M.M. Advances in understanding the physiological role and locations of carbonic anhydrases in C3 plant cells. Protoplasma 2021, 258, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Weerasooriya, H.N.; DiMario, R.J.; Rosati, V.C.; Rai, A.K.; LaPlace, L.M.; Filloon, V.D.; Longstreth, D.J.; Moroney, J.V. Arabidopsis plastid carbonic anhydrase βCA5 is important for normal plant growth. Plant Physiol. 2022, 190, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Buabeng, E.R.; Henary, M. Developments of small molecules as inhibitors for carbonic anhydrase isoforms. Bioorg. Med. Chem. 2021, 39, 116140. [Google Scholar] [CrossRef] [PubMed]
- Elimam, D.M.; Elgazar, A.A.; Bonardi, A.; Abdelfadil, M.; Nocentini, A.; El-Domany, R.A.; Abdel-Aziz, H.A.; Badria, F.A.; Supuran, C.T.; Eldehna, W.M. Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 225, 113800. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.; Malkova, A.; Sharonova, T.; Sharoyko, V.; Bunev, A.; Supuran, C.T.; Krasavin, M. Carbonic Anhydrase IX Inhibitors as Candidates for Combination Therapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 13405. [Google Scholar] [CrossRef]
- Nerella, S.G.; Singh, P.; Arifuddin, M.; Supuran, C.T. Anticancer carbonic anhydrase inhibitors: A patent and literature update 2018–2022. Expert Opin. Ther. Pat. 2022, 32, 833–847. [Google Scholar] [CrossRef]
- Shaldam, M.; Eldehna, W.M.; Nocentini, A.; Elsayed, Z.M.; Ibrahim, T.M.; Salem, R.; El-Domany, R.A.; Capasso, C.; Abdel-Aziz, H.A.; Supuran, C.T. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur. J. Med. Chem. 2021, 216, 113283. [Google Scholar] [CrossRef]
- Testa, C.; Papini, A.M.; Zeidler, R.; Vullo, D.; Carta, F.; Supuran, C.T.; Rovero, P. First studies on tumor associated carbonic anhydrases IX and XII monoclonal antibodies conjugated to small molecule inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 592–596. [Google Scholar] [CrossRef]
- Mincione, F.; Nocentini, A.; Supuran, C.T. Advances in the discovery of novel agents for the treatment of glaucoma. Expert Opin. Drug Discov. 2021, 16, 1209–1225. [Google Scholar] [CrossRef]
- Ozsoy, H.Z. Anticonvulsant Effects of Carbonic Anhydrase Inhibitors: The Enigmatic Link Between Carbonic Anhydrases and Electrical Activity of the Brain. Neurochem. Res. 2021, 46, 2783–2799. [Google Scholar] [CrossRef]
- Supuran, C.T. Novel carbonic anhydrase inhibitors. Future Med. Chem. 2021, 13, 1935–1937. [Google Scholar] [CrossRef] [PubMed]
- Akgul, O.; Lucarini, E.; Mannelli, L.D.C.; Ghelardini, C.; D’Ambrosio, K.; Buonanno, M.; Monti, S.M.; De Simone, G.; Angeli, A.; Supuran, C.T.; et al. Sultam based Carbonic Anhydrase VII inhibitors for the management of neuropathic pain. Eur. J. Med. Chem. 2022, 227, 113956. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, P.; Rathi, E.; Kini, S.G. Computer-aided identification of human carbonic anhydrase isoenzyme VII inhibitors as potential antiepileptic agents. J. Biomol. Struct. Dyn. 2022, 40, 4850–4865. [Google Scholar] [CrossRef] [PubMed]
- Magheru, C.; Magheru, S.; Coltau, M.; Hoza, A.; Moldovan, C.; Sachelarie, L.; Gradinaru, I.; Hurjui, L.L.; Marc, F.; Farcas, D.M. Antiepileptic Drugs and Their Dual Mechanism of Action on Carbonic Anhydrase. J. Clin. Med. 2022, 11, 2614. [Google Scholar] [CrossRef] [PubMed]
- Ozaslan, M.S.; Saglamtas, R.; Demir, Y.; Genc, Y.; Saracoglu, I.; Guelcin, I. Isolation of Some Phenolic Compounds from Plantago subulata L. and Determination of Their Antidiabetic, Anticholinesterase, Antiepileptic and Antioxidant Activity. Chem. Biodivers. 2022, 19, e202200280. [Google Scholar] [CrossRef] [PubMed]
- Shukralla, A.A.; Dolan, E.; Delanty, N. Acetazolamide: Old drug, new evidence? Epilepsia Open 2022, 7, 378–392. [Google Scholar] [CrossRef]
- Das Mahapatra, A.; Queen, A.; Yousuf, M.; Khan, P.; Hussain, A.; Rehman, T.M.; Alajmi, M.F.; Datta, B.; Hassan, I.M. Design and development of 5-(4H)-oxazolones as potential inhibitors of human carbonic anhydrase VA: Towards therapeutic management of diabetes and obesity. J. Biomol. Struct. Dyn. 2022, 40, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Supuran, C.T. Acipimox inhibits human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2022, 37, 672–679. [Google Scholar] [CrossRef]
- Supuran, C.T. Anti-obesity carbonic anhydrase inhibitors: Challenges and opportunities. J. Enzym. Inhib. Med. Chem. 2022, 37, 2478–2488. [Google Scholar] [CrossRef]
- Lemon, N.; Canepa, E.; Ilies, M.A.; Fossati, S. Carbonic anhydrases as potential targets against neurovascular unit dysfunction in Alzheimer’s disease and stroke. Front. Aging Neurosci. 2021, 13, 772278. [Google Scholar] [CrossRef]
- Poggetti, V.; Salerno, S.; Baglini, E.; Barresi, E.; Da Settimo, F.; Taliani, S. Carbonic Anhydrase Activators for Neurodegeneration: An Overview. Molecules 2022, 27, 2544. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, A.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Gratteri, P.; Nocentini, A.; Supuran, C.T. Development of Hydrogen Sulfide-Releasing Carbonic Anhydrases IX- and XII-Selective Inhibitors with Enhanced Antihyperalgesic Action in a Rat Model of Arthritis. J. Med. Chem. 2022, 65, 13143–13157. [Google Scholar] [CrossRef] [PubMed]
- Bulli, I.; Dettori, I.; Coppi, E.; Cherchi, F.; Venturini, M.; Mannelli, L.D.C.; Ghelardini, C.; Nocentini, A.; Supuran, C.T.; Pugliese, A.M.; et al. Role of carbonic anhydrase in cerebral ischemia and carbonic anhydrase inhibitors as putative protective agents. Int. J. Mol. Sci. 2021, 22, 5029. [Google Scholar] [CrossRef] [PubMed]
- Dettori, I.; Fusco, I.; Bulli, I.; Gaviano, L.; Coppi, E.; Cherchi, F.; Venturini, M.; Di Cesare Mannelli, L.; Ghelardini, C.; Nocentini, A.; et al. Protective effects of carbonic anhydrase inhibition in brain ischaemia in vitro and in vivo models. J. Enzym. Inhib. Med. Chem. 2021, 36, 964–976. [Google Scholar] [CrossRef]
- D’Agostino, I.; Mathew, G.E.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Angeli, A.; Carradori, S.; Marinacci, B.; Menghini, L.; Abdelgawad, M.A.; et al. Biological investigation of N-methyl thiosemicarbazones as antimicrobial agents and bacterial carbonic anhydrases inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 986–993. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Carginale, V.; Supuran, C.T.; Capasso, C. The gram-negative bacterium Escherichia coli as a model for testing the effect of carbonic anhydrase inhibition on bacterial growth. J. Enzym. Inhib. Med. Chem. 2022, 37, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Giovannuzzi, S.; Hewitt, C.S.; Nocentini, A.; Capasso, C.; Costantino, G.; Flaherty, D.P.; Supuran, C.T. Inhibition studies of bacterial α-carbonic anhydrases with phenols. J. Enzym. Inhib. Med. Chem. 2022, 37, 666–671. [Google Scholar] [CrossRef]
- Giovannuzzi, S.; Hewitt, C.S.; Nocentini, A.; Capasso, C.; Flaherty, D.P.; Supuran, C.T. Coumarins effectively inhibit bacterial α-carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2022, 37, 333–338. [Google Scholar] [CrossRef]
- Gueller, P.; Atmaca, U.; Gueller, U.; Calisir, U.; Dursun, F. Antibacterial properties and carbonic anhydrase inhibition profiles of azido sulfonyl carbamate derivatives. Future Med. Chem. 2021, 13, 1285–1299. [Google Scholar] [CrossRef]
- Hewitt, C.S.; Abutaleb, N.S.; Elhassanny, A.E.M.; Nocentini, A.; Cao, X.; Amos, D.P.; Youse, M.S.; Holly, K.J.; Marapaka, A.; An, W.; et al. Structure-Activity Relationship Studies of Acetazolamide-Based Carbonic Anhydrase Inhibitors with Activity against Neisseria gonorrhoeae. ACS Infect. Dis. 2021, 7, 1969–1984. [Google Scholar] [CrossRef]
- Nocentini, A.; Hewitt, C.S.; Mastrolorenzo, M.D.; Flaherty, D.P.; Supuran, C.T. Anion inhibition studies of the α-carbonic anhydrases from Neisseria gonorrhoeae. J. Enzym. Inhib. Med. Chem. 2021, 36, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, D.P.; Seleem, M.N.; Supuran, C.T. Bacterial carbonic anhydrases: Underexploited antibacterial therapeutic targets. Future Med. Chem. 2021, 13, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Artasensi, A.; Angeli, A.; Lammi, C.; Bollati, C.; Gervasoni, S.; Baron, G.; Matucci, R.; Supuran, C.T.; Vistoli, G.; Fumagalli, L. Discovery of a Potent and Highly Selective Dipeptidyl Peptidase IV and Carbonic Anhydrase Inhibitor as “Antidiabesity” Agents Based on Repurposing and Morphing of WB-4101. J. Med. Chem. 2022, 65, 13946–13966. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; Fantacuzzi, M.; Ammazzalorso, A.; Angeli, A.; De Filippis, B.; Galati, S.; Petzer, A.; Petzer, J.P.; Poli, G.; Tuccinardi, T.; et al. Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases? Molecules 2022, 27, 7816. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, M.; Tuerkes, C.; Arslan, M.; Demir, Y.; Beydemir, S. Novel benzoic acid derivatives: Synthesis and biological evaluation as multitarget acetylcholinesterase and carbonic anhydrase inhibitors. Arch. Pharm. 2021, 354, 2000282. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Angeli, A.; Calugi, L.; Innocenti, R.; Carta, F.; Supuran, C.T.; Trabocchi, A. Multitargeting application of proline-derived peptidomimetics addressing cancer-related human matrix metalloproteinase 9 and carbonic anhydrase II. Eur. J. Med. Chem. 2021, 214, 113260. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Multitargeting approaches involving carbonic anhydrase inhibitors: Hybrid drugs against a variety of disorders. J. Enzym. Inhib. Med. Chem. 2021, 36, 1702–1714. [Google Scholar] [CrossRef]
- Elbadawi, M.M.; Eldehna, W.M.; Nocentini, A.; Abo-Ashour, M.F.; Elkaeed, E.B.; Abdelgawad, M.A.; Alharbi, K.S.; Abdel-Aziz, H.A.; Supuran, C.T.; Gratteri, P.; et al. Identification of N-phenyl-2-(phenylsulfonyl)acetamides/propanamides as new SLC-0111 analogues: Synthesis and evaluation of the carbonic anhydrase inhibitory activities. Eur. J. Med. Chem. 2021, 218, 113360. [Google Scholar] [CrossRef]
- Huo, Z.; Bilang, R.; Supuran, C.T.; von der Weid, N.; Bruder, E.; Holland-Cunz, S.; Martin, I.; Muraro, M.G.; Gros, S.J. Perfusion-Based Bioreactor Culture and Isothermal Microcalorimetry for Preclinical Drug Testing with the Carbonic Anhydrase Inhibitor SLC-0111 in Patient-Derived Neuroblastoma. Int. J. Mol. Sci. 2022, 23, 3128. [Google Scholar] [CrossRef]
- Kumar, A.; Siwach, K.; Supuran, C.T.; Sharma, P.K. A decade of tail-approach based design of selective as well as potent tumor associated carbonic anhydrase inhibitors. Bioorg. Chem. 2022, 126, 105920. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Combs, J.; Singh, S.; Andring, J.; Wolff, A.; Tu, C.; Zhang, Z.; McKenna, R.; Frost, S.C. Inhibition of Carbonic Anhydrase Using SLC-149: Support for a Noncatalytic Function of CAIX in Breast Cancer. J. Med. Chem. 2021, 64, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.; Rezzola, S.; Chiodelli, P.; Nocentini, A.; Supuran, C.T.; Ronca, R. Antiproliferative effects of sulphonamide carbonic anhydrase inhibitors C18, SLC-0111 and acetazolamide on bladder, glioblastoma and pancreatic cancer cell lines. J. Enzym. Inhib. Med. Chem. 2022, 37, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors: An update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin. Invest. Drugs 2021, 30, 1197–1208. [Google Scholar] [CrossRef]
- McDonald, P.C.; Chia, S.; Bedard, P.L.; Chu, Q.; Lyle, M.; Tang, L.; Singh, M.; Zhang, Z.; Supuran, C.T.; Renouf, D.J.; et al. A Phase 1 Study of SLC-0111, a Novel Inhibitor of Carbonic Anhydrase IX, in Patients With Advanced Solid Tumors. Am. J. Clin. Oncol. 2020, 43, 484–490. [Google Scholar] [CrossRef]
- Amiri, A.; Le, P.U.; Moquin, A.; Machkalyan, G.; Petrecca, K.; Gillard, J.W.; Yoganathan, N.; Maysinger, D. Inhibition of carbonic anhydrase IX in glioblastoma multiforme. Eur. J. Pharm. Biopharm. 2016, 109, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, J.; Hilvo, M.; Nordfors, K.; Haapasalo, H.; Parkkila, S.; Hyrskyluoto, A.; Rantala, I.; Waheed, A.; Sly, W.S.; Pastorekova, S.; et al. Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro Oncol. 2008, 10, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, J.; Nordfors, K.; Jarvela, S.; Bragge, H.; Rantala, I.; Parkkila, A.-K.; Haapasalo, H.; Parkkila, S. Carbonic anhydrase II in the endothelium of glial tumors: A potential target for therapy. Neuro Oncol. 2007, 9, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ihnatko, R.; Kubes, M.; Takacova, M.; Sedlakova, O.; Sedlak, J.; Pastorek, J.; Kopacek, J.; Pastorekova, S. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int. J. Oncol. 2006, 29, 1025–1033. [Google Scholar] [CrossRef]
- Ivanov, S.; Liao, S.-Y.; Ivanova, A.; Danilkovitch-Miagkova, A.; Tarasova, N.; Weirich, G.; Merrill, M.J.; Proescholdt, M.A.; Oldfield, E.H.; Lee, J.; et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 2001, 158, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Korkolopoulou, P.; Perdiki, M.; Thymara, I.; Boviatsis, E.; Agrogiannis, G.; Kotsiakis, X.; Angelidakis, D.; Rologis, D.; Diamantopoulou, K.; Thomas-Tsagli, E.; et al. Expression of hypoxia-related tissue factors in astrocytic gliomas. A multivariate survival study with emphasis upon carbonic anhydrase IX. Hum. Pathol. 2007, 38, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Nordfors, K.; Haapasalo, J.; Korja, M.; Niemela, A.; Laine, J.; Parkkila, A.-K.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: An association of CA IX with poor prognosis. BMC Cancer 2010, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Proescholdt, M.A.; Mayer, C.; Kubitza, M.; Schubert, T.; Liao, S.-Y.; Stanbridge, E.J.; Ivanov, S.; Oldfield, E.H.; Brawanski, A.; Merrill, M.J. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro Oncol. 2005, 7, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Proescholdt, M.A.; Merrill, M.J.; Stoerr, E.-M.; Lohmeier, A.; Pohl, F.; Brawanski, A. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol. 2012, 14, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Supuran, C.T.; Hageman, C.; Staab, A.; Polat, B.; Katzer, A.; Scozzafava, A.; Anacker, J.; Flentje, M.; Vordermark, D. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr. Pharm. Des. 2010, 16, 3288–3299. [Google Scholar] [CrossRef]
- Boyd, N.H.; Walker, K.; Fried, J.; Hackney, J.R.; McDonald, P.C.; Benavides, G.A.; Spina, R.; Audia, A.; Scott, S.E.; Landis, C.J.; et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight 2017, 2, e92928. [Google Scholar] [CrossRef]
- Mujumdar, P.; Kopecka, J.; Bua, S.; Supuran, C.T.; Riganti, C.; Poulsen, S.-A. Carbonic Anhydrase XII Inhibitors Overcome Temozolomide Resistance in Glioblastoma. J. Med. Chem. 2019, 62, 4174–4192. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Mujumdar, P.; Annovazzi, L.; Kopecka, J.; Mellai, M.; Schiffer, D.; Poulsen, S.-A.; Riganti, C. Carbonic anhydrase XII inhibitors overcome P-glycoprotein-mediated resistance to temozolomide in glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef]
- Tann, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S. Khasraw, M. Mangement of glioblastoma: State of the art andfuture directions. S. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Zhao, K.; Schaefer, A.; Zhang, Z.; Elsaesser, K.; Culmsee, C.; Zhong, L.; Pagenstecher, A.; Nimsky, C.; Bartsch, J.W. Inhibition of Carbonic Anhydrase 2 Overcomes Temozolomide Resistance in Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 157. [Google Scholar] [CrossRef]
- Hannen, R.; Selmansberger, M.; Hauswald, M.; Pagenstecher, A.; Nist, A.; Stiewe, T.; Acker, T.; Carl, B.; Nimsky, C.; Bartsch, J.W. Comparative transcriptomic analysis of temozolomide resistant primary GBM stem-like cells and recurrent GBM identifies upo-regulation of carbonic anhydrase CA2 gene as resistance factor. Cancers 2019, 11, 921. [Google Scholar] [CrossRef]
- Schwarz, S.; Sommerwerk, S.; Lucas, S.D.; Heller, L.; Csuk, R. Sulfamates of methyl triterpenoates are effective and competitive inhibitors of carbonic anhydrase II. Eur. J. Med. Chem. 2014, 86, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Halim, S.A.; Khan, A.; Khan, M.; Al-Hatmi, S.; Al-Harrasi, A. Commikuanoids A-C: New cycloartane triterpenoids with exploration of carbonic anhydrase-II inhibition from the resins of Commiphora kua by in vitro and in silico molecular docking. Fitoterapia 2022, 157, 105125. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.O.; Rosa, M.N.; Miranda-Goncalves, V.; Costa, A.M.; Tansini, A.; Evangelista, A.F.; Martinho, O.; Carloni, A.C.; Jones, C.; Lima, J.P.; et al. Euphol, a tetracyclic triterpene, from Euphorbia tirucalli induces autophagy and sensitizes temozolomide cytotoxicity on glioblastoma cells. Invest. New Drugs 2019, 37, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.K.; Rehman, N.U.; Khan, M.; Halim, S.A.; Khan, A.; Rafiq, K.; Csuk, R.; Das, B.; Al-Harrasi, A. New synthetic 1H-1,2,3-triazole derivatives of 3-O-acetyl-β-boswellic acid and 3-O-acetyl-11-keto-β-boswellic acid from Boswellia sacra inhibit carbonic anhydrase II in vitro. Med. Chem. Res. 2021, 30, 1185–1198. [Google Scholar] [CrossRef]
- Bulbul, M.; Saracoglu, N.; Irfan Kufrevioglu, O.; Ciftci, M. Bile acid derivatives of 5-amino-1,3,4-thiadiazole-2-sulfonamide as new carbonic anhydrase inhibitors: Synthesis and investigation of inhibition effects. Bioorg. Med. Chem. 2002, 10, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Battle, C.H.; Zhang, N.; Aryal, G.H.; Mottamal, M.; Jayawickramarajah, J. Bile Acid Conjugated DNA Chimera that Conditionally Inhibits Carbonic Anhydrase-II in the Presence of MicroRNA-21. Bioconjugate Chem. 2015, 26, 1606–1612. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Preparation of potent sulfonamides inhibitors incorporating bile acid tails. Bioorg. Med. Chem. Lett. 2002, 12, 1551–1557. [Google Scholar] [CrossRef]
- Trifunovic, J.; Borcic, V.; Mikov, M. Bile acids and their oxo derivatives: Potential inhibitors of carbonic anhydrase I and II, androgen receptor antagonists and CYP3A4 substrates. Biomed. Chromatogr. 2017, 31, 3870. [Google Scholar] [CrossRef]
- Kalyanavenkatapaman, S.; Nanjen, P.; Banerji, A.; Nair, B.C.; Kumar, G.B. Discovery of arjunolic acid as a novel non-zinc binding carbonic anhydrase II inhibitor. Bioorg. Chem. 2016, 66, 72–79. [Google Scholar] [CrossRef]
- Pastorekova, S.; Gillies, R.J.M. The role of carbonic anhydrase IX in cancer development: Links to hypxia, acidosis and beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef]
- Vanchanagiri, K.; Emmerich, D.; Brusche, M.; Bache, M.; Seifert, F.; Csuk, R.; Vordermark, D.; Paschke, R. Synthesis and biological investigation of new carbonic anhydrase IX (CAIX) inhibitors. Chem. Biol. Interact. 2018, 284, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Annan, D.A.; Maishi, N.; Soga, T.; Bawood, R.; Li, C.; Kikuchi, H.K.; Hajo, T.; Morimoto, M.; Kitamura, T.; Alam, M.T.; et al. Carbonic anhydrase 2 (CA II) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun. Signal. 2019, 17, 169. [Google Scholar] [CrossRef]
- Bekku, S.; Mochizuki, H.; Takayama, E.; Shinomiya, N.; Fukamachi, H.; Ichinose, M.; Tadakuma, T.; Yamamoto, T. Carbonic anhydrase I and II as a differntiation marker of human and rat colonic enterocytes. Res. Exp. Med. 1998, 198, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.I.; Jamali, S.; Ames, S.; Langer, S.; Deitmer, J.W.; Becker, H.M. A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells. eLife 2018, 7, e35176. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and structure–activity relationship study of cytotoxic germanicane- and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorganic Med. Chem. 2007, 15, 6144–6157. [Google Scholar] [CrossRef] [PubMed]
- Flekhter, O.B.; Karachurina, L.T.; Poroikov, V.V.; Nigmatullina, L.P.; Baltina, L.A.; Zarudii, F.S.; Davydova, V.A.; Spirikhin, L.V.; Baikova, I.P.; Galin, F.Z.; et al. The synthesis and hepatoprotective activity of esters of the lupane group triterpenoids. Russ. J. Bioorg. Chem. 2000, 26, 192–200. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Medvedeva, N.I.; Karachurina, L.T.; Baltina, L.A.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A. Synthesis and Pharmacological Activity of Betulin, Betulinic Acid, and Allobetulin Esters. Pharm. Chem. J. 2005, 39, 401–404. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Smirnova, I.E.; Baltina, L.A.; Boreko, E.I.; Savinova, O.V.; Pokrovskii, A.G. Antiviral Activity of Acyl Derivatives of Betulin and Betulinic and Dihydroquinopimaric Acids. Russ. J. Bioorg. Chem. 2018, 44, 740–744. [Google Scholar] [CrossRef]
- Komissarova, N.G.; Dubovitskii, S.N.; Orlov, A.V.; Shitikova, O.V. New Conjugates of Betulin with 2-Aminoethanesulfonic Acid. Chem. Nat. Compd. 2019, 55, 300–304. [Google Scholar] [CrossRef]
- Pokrovskii, A.G.; Plyasunvoa, O.A.; Il’icheva, T.N.; Borisova, O.A.; Fedyuk, N.V.; Petrenko, N.I.; Petukhova, V.Z.; Shul’ts, E.E.; Tolstikov, G.A. Synthesis of derivatives of plant triterpenes and study of their antiviral and immunostimulating activity. Khim. Interes. Ustoich. Razvit. 2001, 9, 485–491. [Google Scholar]
- Shintyapina, A.B.; Shults, E.E.; Petrenko, N.I.; Uzenkova, N.V.; Tolstikov, G.A.; Pronkina, N.V.; Kozhevnikov, V.S.; Pokrovsky, A.G. Effect of nitrogen-containing derivatives of the plant triterpenes betulin and glycyrrhetic acid on the growth of MT-4, MOLT-4, CEM, and Hep G2 tumor cells. Russ. J. Bioorg. Chem. 2007, 33, 579–583. [Google Scholar] [CrossRef]
- Roblin, R.O., Jr.; Clapp, J.W. The preparation of heterocyclic sulfonamides. J. Am. Chem. Soc. 1950, 72, 4890–4892. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Tolstikov, G.A.; Baikova, I.P.; Zaprutko, L.; Apryshko, G.N. Synthesis and antitumor activity of aminopropoxy derivatives of betulin, erythrodiol, and uvaol. Russ. J. Bioorg. Chem. 2011, 37, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J. A monostearic ester of a triterpenediol from the fruits of Erythroxylon novogranatense. Recl. Trav. Chim. Pays-Bas Belg. 1932, 51, 1200–1203. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Kratschmar, D.V.; Schuster, D.; Pfisterer, P.H.; Gumy, C.; Aubry, E.M.; Brandstoetter, S.; Stuppner, H.; Wolber, G.; Odermatt, A. 11β-Hydroxysteroid dehydrogenase 1 inhibiting constituents from Eriobotrya japonica revealed by bioactivity-guided isolation and computational approaches. Bioorg. Med. Chem. 2010, 18, 1507–1515. [Google Scholar] [CrossRef]
- Agarwal, K.P.; Roy, A.C.; Dhar, M.L. Triterpenes from the bark of Myrica esculenta. Indian J. Chem. 1963, 1, 28–30. [Google Scholar]
- Garcia-Granados, A.; Lopez, P.E.; Melguizo, E.; Parra, A.; Simeo, Y. Partial synthesis of C-ring derivatives from oleanolic and maslinic acids. Formation of several triene systems by chemical and photochemical isomerization processes. Tetrahedron 2004, 60, 1491–1503. [Google Scholar] [CrossRef]
- El-Seedi, H.R. Antimicrobial triterpenes from Poulsenia armata Mif. Standl. Nat. Prod. Res. 2005, 19, 197–202. [Google Scholar]
- Kim, M.-R.; Lee, H.-H.; Hahm, K.-S.; Moon, Y.-H.; Woo, E.-R. Pentacyclic triterpenoids and their cytotoxicity from the stem bark of Styrax japonica S. et Z. Arch. Pharmacal Res. 2004, 27, 283–286. [Google Scholar] [CrossRef]
- Alves, H.M.; Arndt, V.H.; Ollis, W.D.; Eyton, W.B.; Gottlieb, O.R.; Magalhaes, M.T. Chemistry of Brazilian leguminosae. VIII. β-Amyrin constituents of Machaerium incorruptibile. An. Acad. Bras. Cienc. 1965, 37, 49–50. [Google Scholar]
- Sengupta, P.; Dey, A.K.; Mukherjee, J.; Ghosh, S.; Das, K.G. Terpenoids and related compounds. XVI. Terpenoids of the bark of Rhododendron falconeri. J. Indian Chem. Soc. 1969, 66, 775–778. [Google Scholar]
- Chen, D.; Xu, F.; Zhang, P.; Deng, J.; Sun, H.; Wen, X.; Liu, J. Practical Synthesis of α-Amyrin, β-Amyrin, and Lupeol: The Potential Natural Inhibitors of Human Oxidosqualene Cyclase. Arch. Pharm. 2017, 350, 1700178. [Google Scholar] [CrossRef] [PubMed]
- Beseda, I.; Czollner, L.; Shah, P.S.; Khunt, R.; Gaware, R.; Kosma, P.; Stanetty, C.; del Ruiz-Ruiz, M.C.; Amer, H.; Mereiter, K.; et al. Synthesis of glycyrrhetinic acid derivatives for the treatment of metabolic diseases. Bioorg. Med. Chem. 2010, 18, 433–454. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).