Interleaved Electroactive Molecules into LDH Working on Both Electrodes of an Aqueous Battery-Type Device
Abstract
1. Introduction
2. Results
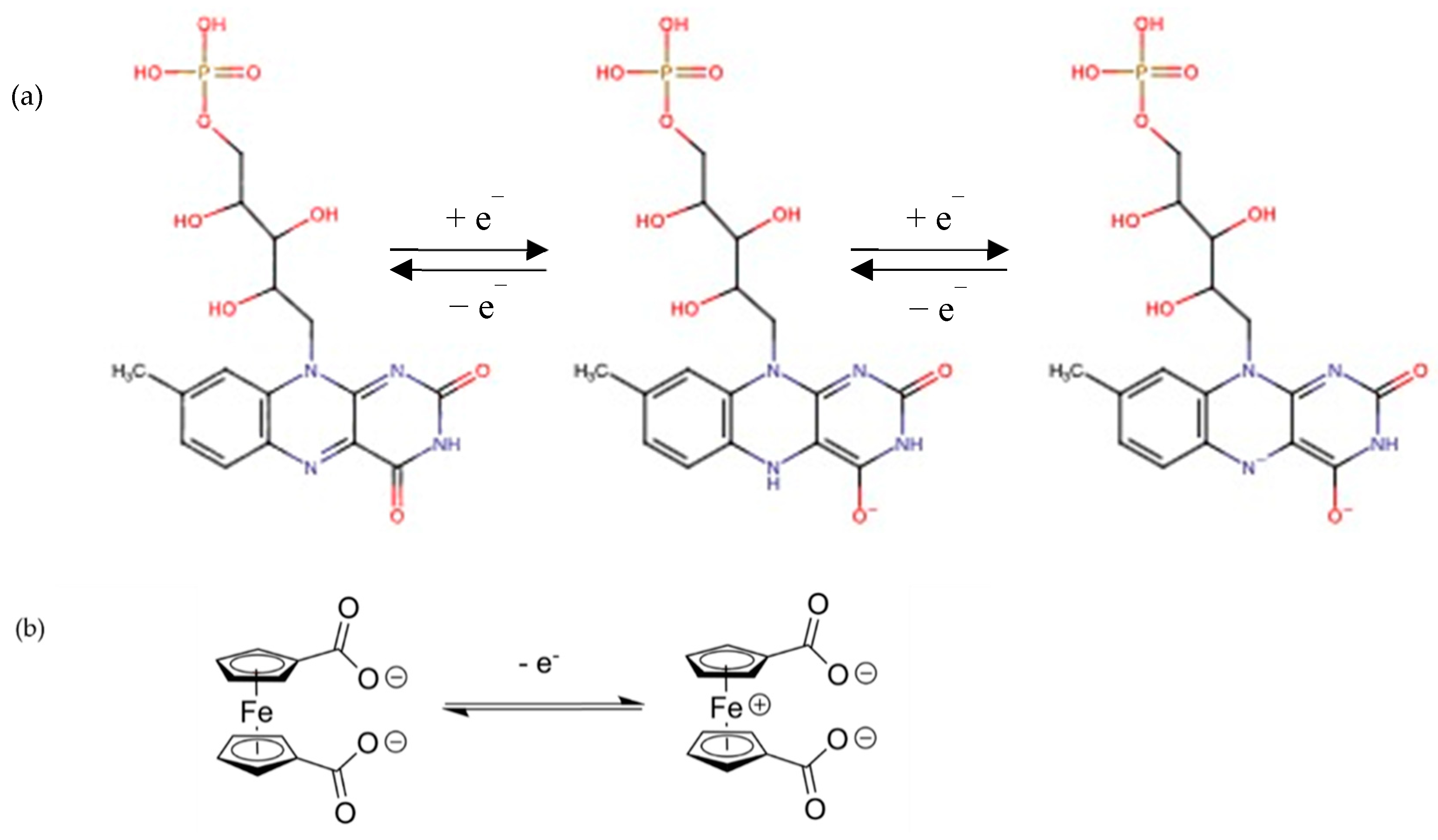
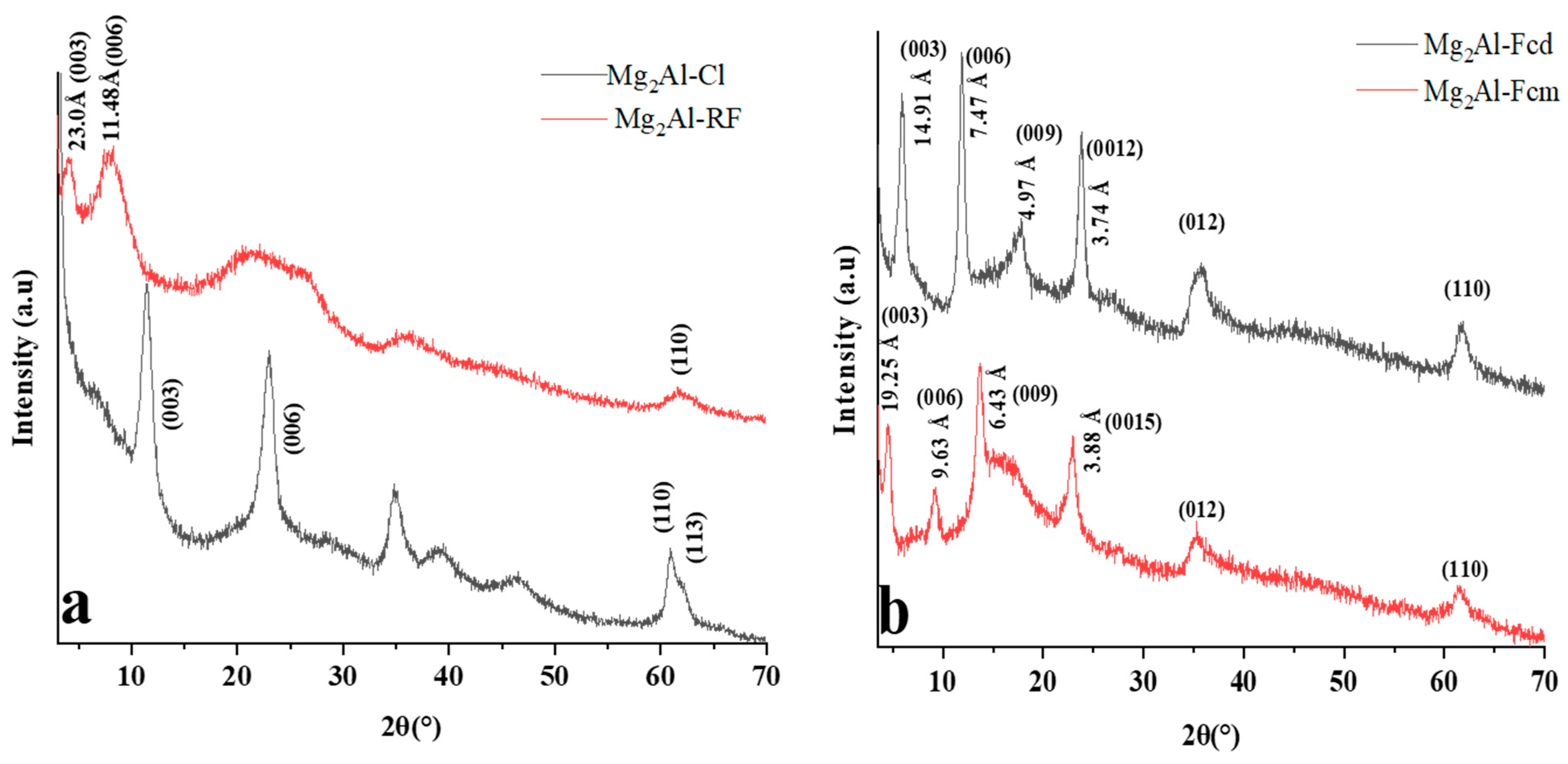
2.1. Intercalation of Electroactive Molecules in LDH Matrix
2.2. Electrochemical Behavior of LDH-RF
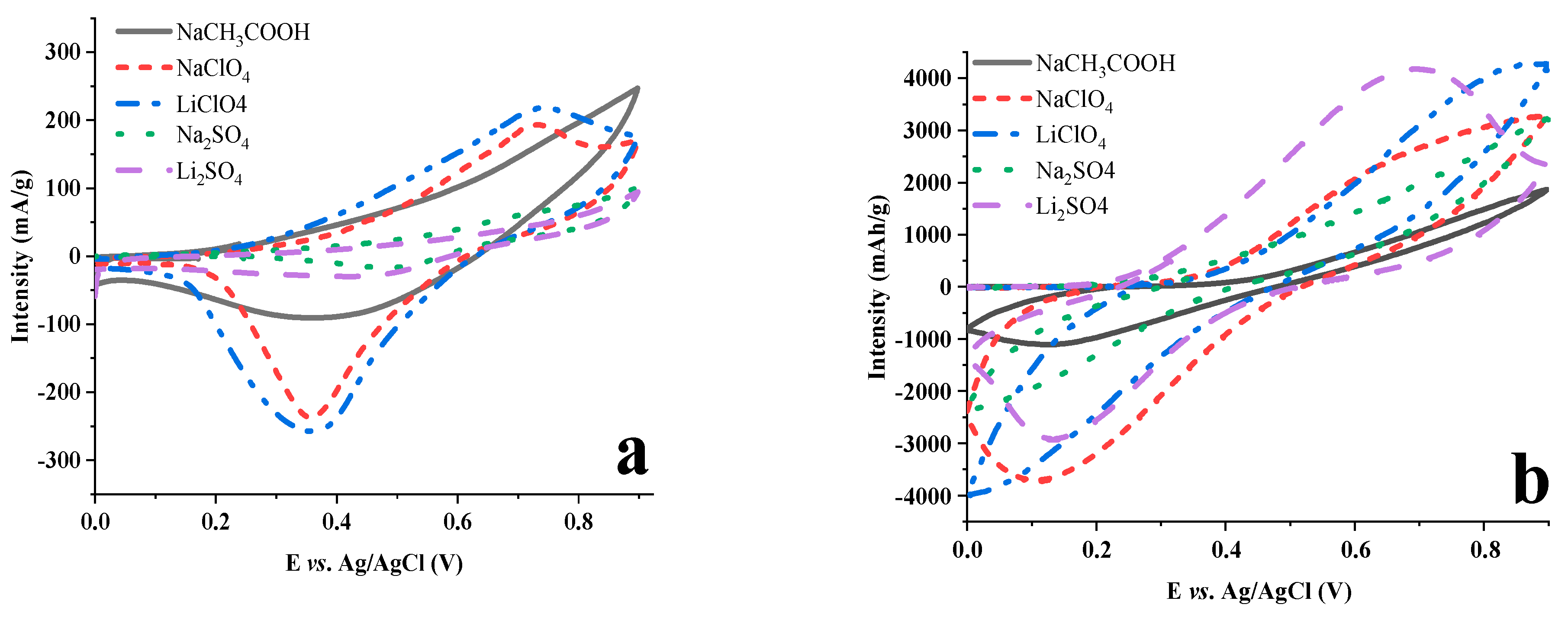
2.3. Electrochemical Behavior of LDH-FCm and LDH-FCd
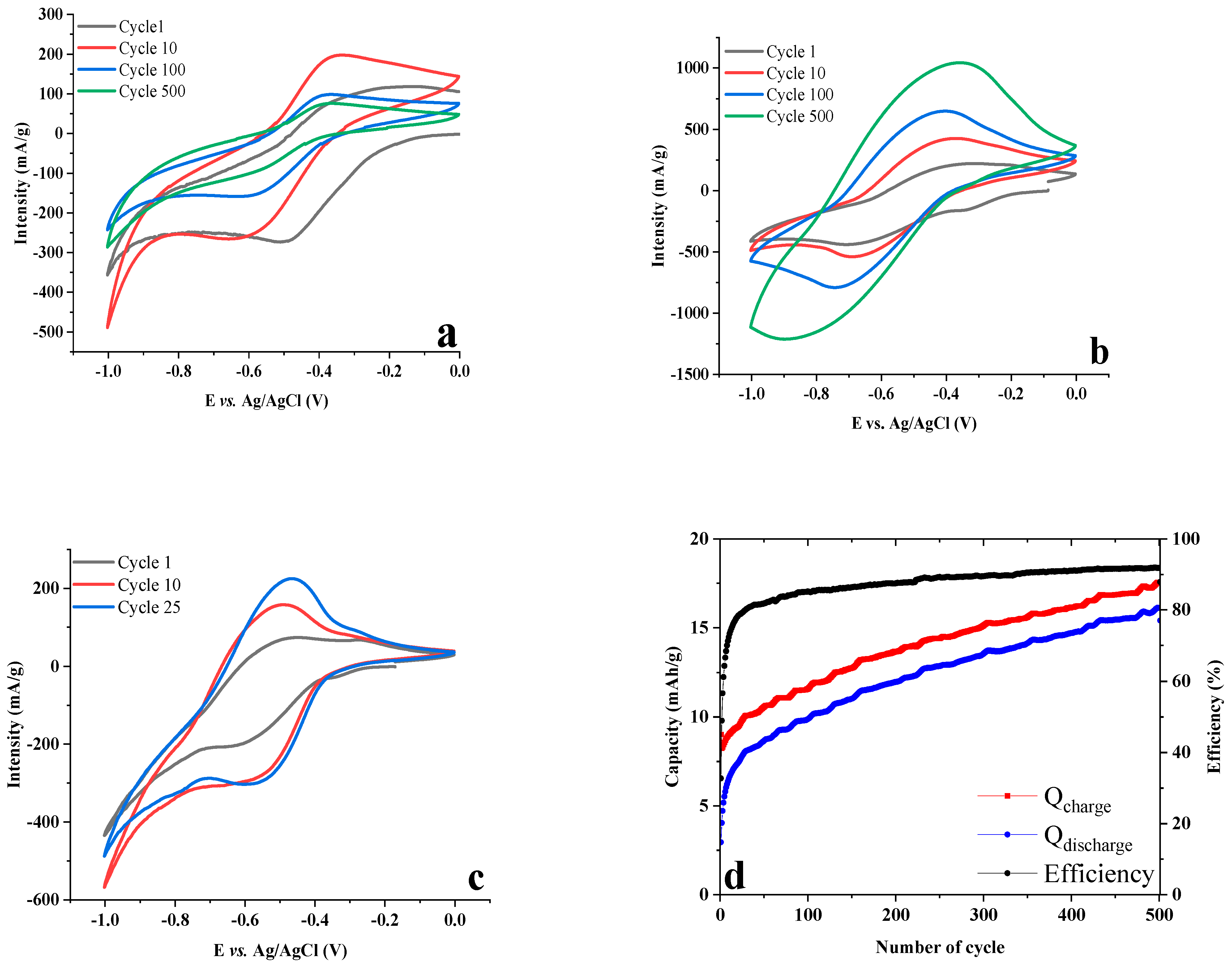
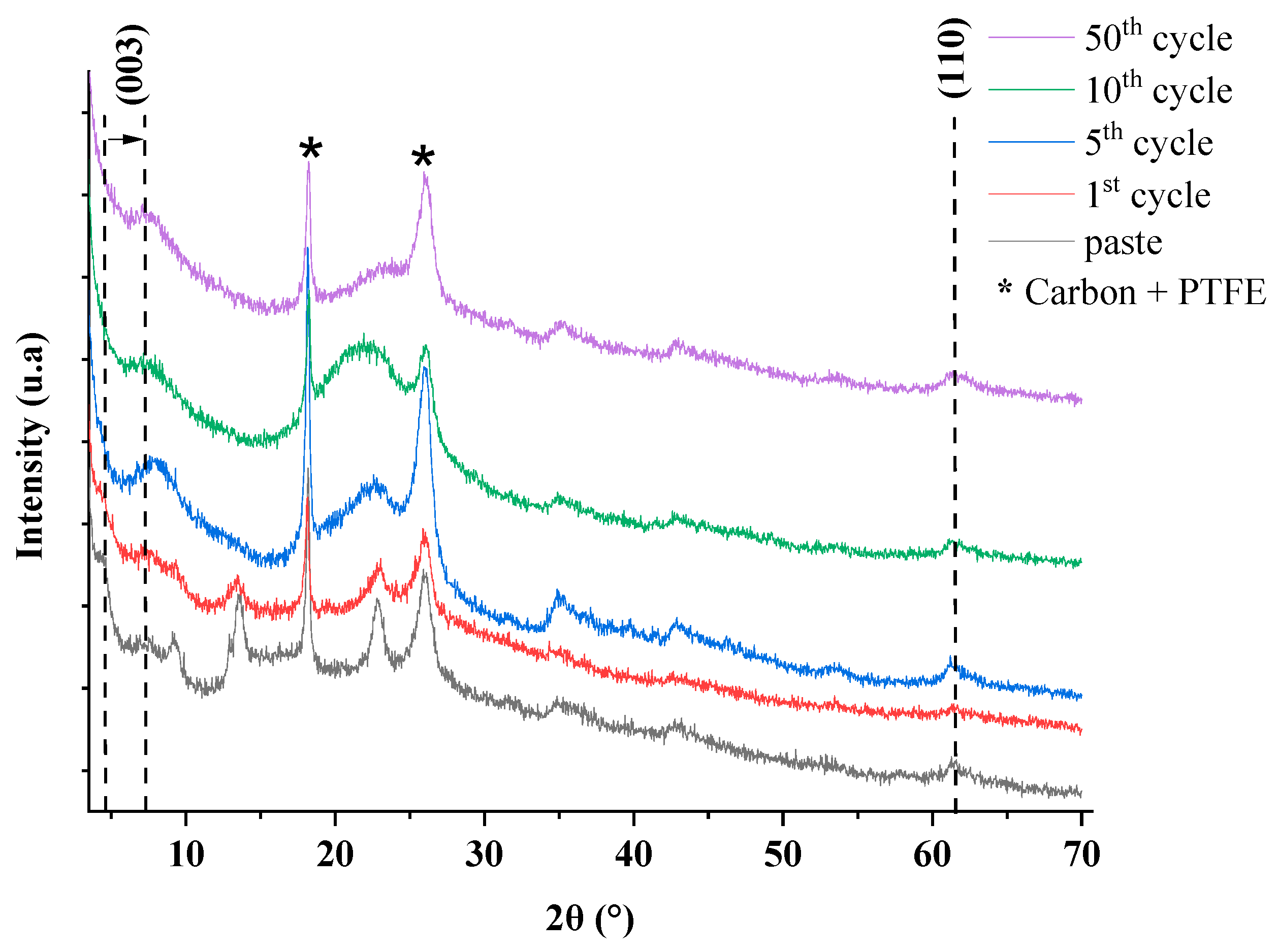
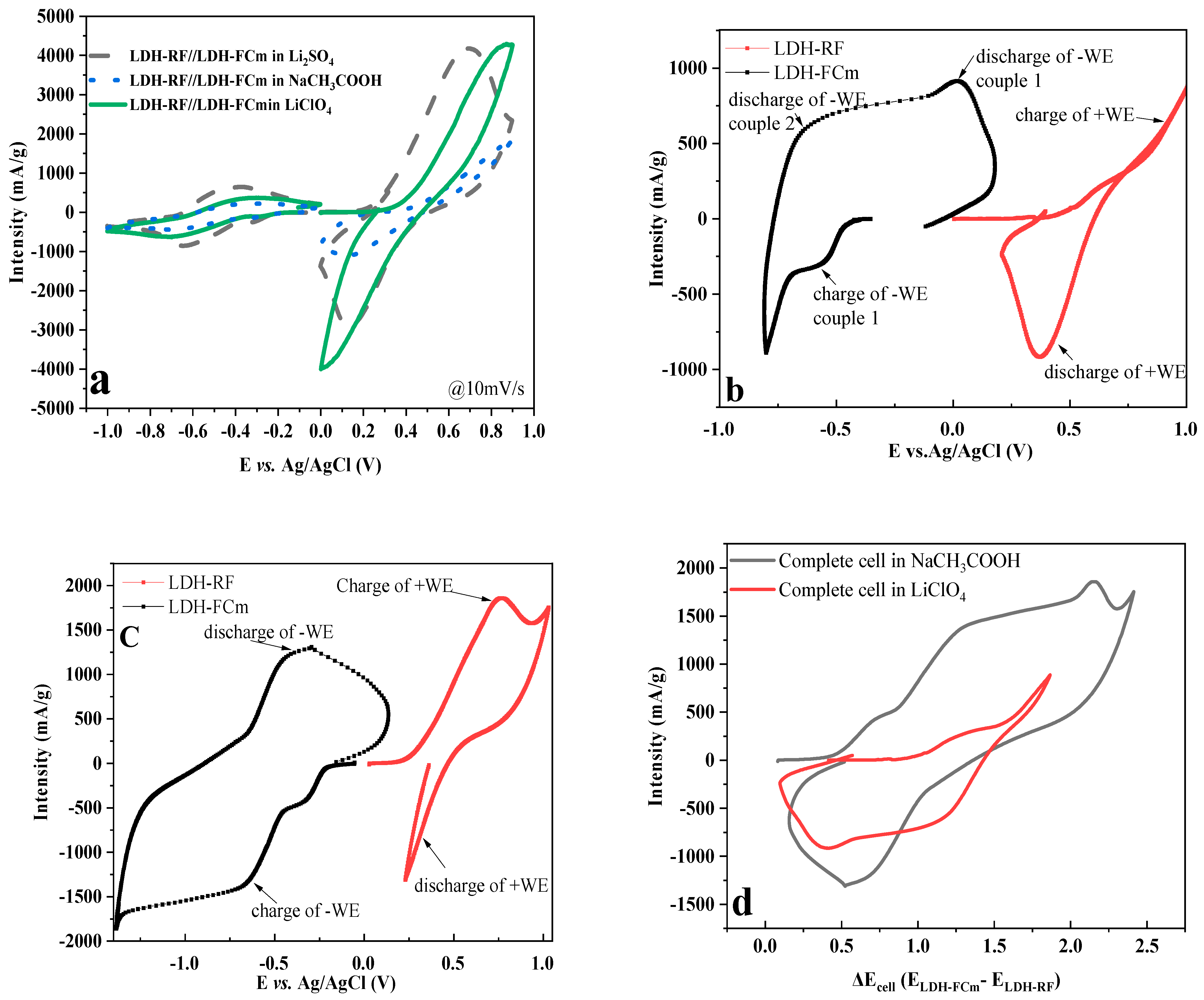
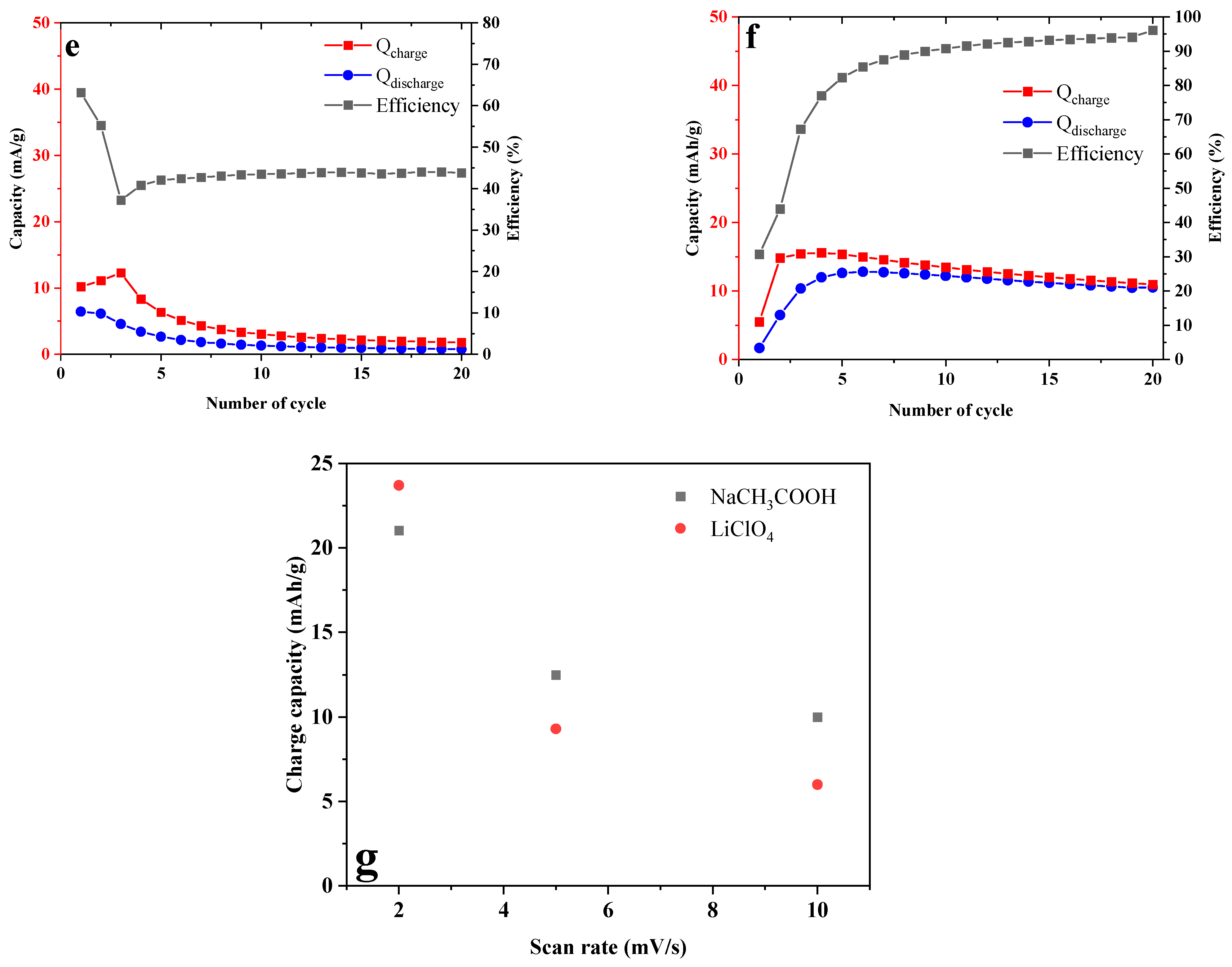
2.4. Test in Aqueous Battery
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Analysis
3.3. Material Synthesis
3.4. Electrode Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Y.Y.; Lu, S.F.; Chen, S.; Wang, H.N.; Zhang, J.; Xiang, A. Sustainable Redox Flow Battery with Alizarin-Based Aqueous Organic Electrolyte. Acs Appl. Energy Mater. 2020, 2, 2469. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.H.; Hasa, I.; Passerini, S. Challenges and Strategies for High-Energy Aqueous Electrolyte Rechargeable Batteries. Angew. Chem.-Int. Ed. 2021, 60, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.N.; Zhao, Q.; Chao, D.L.; Hou, Y.; Pan, H.G.; Sun, W.P.; Yuan, Z.Y.; Li, H.; Ma, T.Y.; Su, D.W. Energetic Aqueous Batteries. Adv. Energ. Maetr. 2022, 12, 2201074. [Google Scholar] [CrossRef]
- Zhong, L.Q.; Lu, Y.; Li, H.X.; Tao, Z.L.; Cheng, J. High-Performance Aqueous Sodium-Ion Batteries with Hydrogel Electrolyte and Alloxazine/CMK-3 Anode. ACS Sustain. Chem. Eng. 2018, 6, 7761–7768. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Yin, J.; Emwas, A.H.; Mohammed, O.F.; Alshareef, H.N. An Aqueous Mg2+-Based Dual-Ion Battery with High Power Density. Adv. Funct. Mater. 2021, 31, 2107523. [Google Scholar] [CrossRef]
- Holland, A.; Mckerracher, R.D.; Cruden, A.; Wills, R.G.A. An aluminium battery operating with an aqueous electrolyte. J. Appl. Electrochem. 2018, 48, 243–250. [Google Scholar] [CrossRef]
- Meng, J.M.; Song, Y.; Qin, Z.M.; Wang, Z.H.; Mu, X.J.; Wang, J.; Liu, X.X. Cobalt-Nickel Double Hydroxide toward Mild Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 202204026. [Google Scholar] [CrossRef]
- Shi, M.M.; Zhao, M.S.; Jiao, L.D.; Su, Z.; Li, M.; Song, X.P. Novel Mo-doped nickel sulfide thin sheets decorated with Ni-Co layered double hydroxide sheets as an advanced electrode for aqueous asymmetric super-capacitor battery. J. Power Sources 2021, 509, 230333. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.Y.; Lv, Z.H.; Yang, H.; Cheng, X.; Zhang, S.Z.; Ye, M.H.; Zhang, Y.F.; Chen, L.B.; Zhao, J.B. Redistributing Zn-ion flux by interlayer ion channels in Mg-Al layered double hydroxide-based artificial solid electrolyte interface for ultra-stable and dendrite-free Zn metal anodes. Energy Strorage Mater. 2021, 41, 230–239. [Google Scholar] [CrossRef]
- Ma, C.H.; Wang, X.; Lu, W.Q.; Wang, C.Z.; Yue, H.J.; Sun, G.; Zhang, D.; Du, F. Achieving stable Zn metal anode via a simple NiCo layered double hydroxides artificial coating for high performance aqueous Zn-ion batteries. Chem. Eng. J. 2022, 429, 132576. [Google Scholar] [CrossRef]
- Bailmare, D.B.; Subramaniyam, C.M.; Dhoble, S.J.; Deshmukh, A.D. Synergistic interaction between magnesium and iron layered hydroxide with enhanced supercapacitor performance. J. Alloys Compd. 2021, 892, 161986. [Google Scholar] [CrossRef]
- Wu, N.N.; Bai, X.; Pan, D.; Dong, B.B.; Wei, R.B.; Naik, N.; Patil, R.R.; Guo, Z.H. Recent Advances of Asymmetric Supercapacitors. Adv. Mater. Interfaces 2021, 8, 202001710. [Google Scholar] [CrossRef]
- Rajkumar, S.R.; Subha, S.; Gowri, S.; Bella, A.; PrincyMerlin, J. Enhanced electrochemical performance of aminophenol-modified ZnO as electrode material for supercapacitors. Ionics 2022, 28, 859–869. [Google Scholar] [CrossRef]
- Javeesh, A.; Rajkumar, S.; PrincyMerlin, J.; Aravind, A.; Sajana, D.; Praveen, C.S. Single step auto-igniting combustion technique grown CeO2 and Ni-doped CeO2 nanostructures for multifunctional applications S. Praveen. J. Alloys Compd. 2021, 882, 160409. [Google Scholar]
- Mousty, C.; Leroux, F. LDHs as Electrode Materials for Electrochemical Detection and Energy Storage: Supercapacitor, Battery and (Bio)-Sensor. Recent Pat. Nanotechnol. 2012, 6, 174–192. [Google Scholar] [CrossRef]
- Zhong, F.; Yang, M.; Ding, M.; Jia, C. Organic Electroactive Molecule-Based Electrolytes for Redox Flow Batteries: Status and Challenges of Molecular Design. Front. Chem. 2020, 8, 451. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Wang, W.; Cho, J. Material Design and Engineering of Next-Generation Flow-Battery Technologies. Nat. Rev. Mater. 2016, 2, 1–18. [Google Scholar] [CrossRef]
- Quan, M.; Sanchez, D.; Wasylkiw, M.F.; Smith, D.K. Voltammetry of Quinones in Unbuffered Aqueous Solution: Reassessing the Roles of Proton Transfer and Hydrogen Bonding in the Aqueous Electrochemistry of Quinones. J. Am. Chem. Soc. 2007, 129, 12847–12856. [Google Scholar] [CrossRef]
- Ega, S.P.; Srinivasan, P. Quinone Materials for Supercapacitor: Current Status, Approaches, and Future Directions. J. Energy Storage 2022, 47, 103700. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Liu, S.; Huang, K.; Fang, D.; Wang, F.; Peng, S. Electrochemical Properties of an All-Organic Redox Flow Battery Using 2,2,6,6-Tetramethyl-1-Piperidinyloxy and N-Methylphthalimide. Electrochem. Solid-State Lett. 2011, 14, A171. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Megiel, E.; Romański, J.; Algarni, H.; Chong, K.F. A Wide Potential Window Symmetric Supercapacitor by TEMPO Functionalized MWCNTs. J. Mol. Liq. 2018, 271, 31–39. [Google Scholar] [CrossRef]
- Tan, S.L.J.; Webster, R.D. Electrochemically Induced Chemically Reversible Proton-Coupled Electron Transfer Reactions of Riboflavin (Vitamin B2). J. Am. Chem. Soc. 2012, 134, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Guo, L. Renewable-Biomolecule-Based Electrochemical Energy-Storage Materials. Adv. Energy Mater. 2017, 7, 1700663. [Google Scholar] [CrossRef]
- Cosimbescu, L.; Wei, X.; Vijayakumar, M.; Xu, W.; Helm, M.L.; Burton, S.D.; Sorensen, C.M.; Liu, J.; Sprenkle, V.; Wang, W. Anion-Tunable Properties and Electrochemical Performance of Functionalized Ferrocene Compounds. Sci. Rep. 2015, 5, 14117. [Google Scholar] [CrossRef]
- Schrage, B.R.; Zhao, Z.; Boika, A.; Ziegler, C.J. Evaluating Ferrocene Ions and All-Ferrocene Salts for Electrochemical Applications. J. Organomet. Chem. 2019, 897, 23–31. [Google Scholar] [CrossRef]
- Fabrizzi, L. Evaluating Ferrocene Ions and All-Ferrocene Salts for Electrochemical Applications; Elsevier Enhanced Reader: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Singh, R.; Rathore, D.; Pandey, C.M.; Geetanjali; Srivastava, R. Electrochemical and Spectroscopic Studies of Riboflavin. Anal. Chem. Lett. 2018, 8, 653–664. [Google Scholar] [CrossRef]
- Orita, A.; Verde, M.G.; Sakai, M.; Meng, Y.S. A Biomimetic Redox Flow Battery Based on Flavin Mononucleotide. Nat. Commun. 2016, 7, 13230. [Google Scholar] [CrossRef]
- Pereira, A.C.; de Santos, A.S.; Kubota, L.T. Electrochemical Behavior of Riboflavin Immobilized on Different Matrices. J. Colloid Interface Sci. 2003, 265, 351–358. [Google Scholar] [CrossRef]
- Wang, S.P.; Qiu, P.M.; Xue, Z.H.; Zhang, C.Y.; Li, Y.; Dai, Y.; Yu, J.X. Mitochondrial Bioenergy Transformation: The Discharge/Charge Reaction of Vitamin B2 in Lithium-Ion Batteries. J. Chem. Educ. 2022, 99, 3613–3622. [Google Scholar] [CrossRef]
- Sekar, P.; Vasanthakumar, P.; Shanmugam, R.; Kumar, S.S.; Agnoli, S.; Deepak, R.J.; Murugan, K. Green synthesis of a redox-active riboflavin-integrated Ni-MOF and its versatile electrocatalytic applications towards oxygen evolution and reduction, and HMF oxidation reactions. Green Chem. 2022, 24, 9233–9244. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, F.M.; Wang, S.F.; Ru, Q.; He, Q.Y.; Hou, X.H.; Su, C.Y.; Shi, Y.M. An organic flow desalination battery. Energy Storage Mater. 2013, 20, 203–207. [Google Scholar] [CrossRef]
- Malinauskas, A. Electrochemical Study of Riboflavin Adsorbed on a Graphite Electrode. Chemija 2008, 19, 3. [Google Scholar]
- Malinauskas, A.; Ruzgas, T.; Gorton, L. Tuning the Redox Potential of Riboflavin by Zirconium Phosphate in Carbon Paste Electrodes. Bioelectrochem. Bioenerg. 1999, 49, 21–27. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Megiel, E.; Cieciórski, P.; Thalji, M.R.; Romański, J.; Algarni, H.; Chong, K.F. Ferrocene Functionalized Multi-Walled Carbon Nanotubes as Supercapacitor Electrodes. J. Mol. Liq. 2020, 318, 114064. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Hadi, R.; Abbasi, H. Synthesis and Characterization of Ferrocene-Functionalized Reduced Graphene Oxide Nanocomposite as a Supercapacitor Electrode Material. J. Organomet. Chem. 2019, 880, 355–362. [Google Scholar] [CrossRef]
- Hu, M.-L.; Abbasi-Azad, M.; Habibi, B.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Liu, K.-G.; Morsali, A. Electrochemical Applications of Ferrocene-Based Coordination Polymers. ChemPlusChem 2020, 85, 2397–2418. [Google Scholar] [CrossRef]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Advances in Applications of Voltammetric Sensors Modified with Ferrocene and Its Derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef]
- Natarajan, A.; Ramasamy, T.; Arivanandhan, M.; Gnanamani, A.; Jayavel, R. A Facile Synthesis of Ferrocene Functionalized Graphene Oxide Nanocomposite for Electrochemical Sensing of Lead. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1021–1028. [Google Scholar]
- Borenstein, A.; Strauss, V.; Kowal, M.D.; Yoonessi, M.; Muni, M.; Anderson, M.; Kaner, R.B. Laser-Reduced Graphene-Oxide/Ferrocene: A 3-D Redox-Active Composite for Supercapacitor Electrodes. J. Mater. Chem. A 2018, 6, 20463–20472. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Valleix, R.; Zhang, Q.; Boyer, D.; Boutinaud, P.; Chadeyron, G.; Feng, Y.; Okuno, H.; Réveret, F.; Hintze-Bruening, H.; Leroux, F. A First Wide-Open LDH Structure Hosting InP/ZnS QDs: A New Route Toward Efficient and Photostable Red-Emitting Phosphor. Adv. Mater. 2021, 33, 2103411. [Google Scholar] [CrossRef] [PubMed]
- Mousty, C.; Forano, C.; Fleutot, S.; Dupin, J.-C. Electrochemical Study of Anionic Ferrocene Derivatives Intercalated in Layered Double Hydroxides: Application to Glucose Amperometric Biosensors. Electroanalysis 2009, 21, 399–408. [Google Scholar] [CrossRef]
- Therias, S.; Lacroix, B.; Schöllhorn, B.; Mousty, C.; Palvadeau, P. Electrochemical Study of Ferrocene and Nitroxide Derivatives Intercalated in Zn–Cr and Zn–Al Layered Double Hydroxides. J. Electroanal. Chem. 1998, 454, 91–97. [Google Scholar] [CrossRef]
- Morlat-Thérias, S.; Mousty, C.; Palvadeau, P.; Molinié, P.; Léone, P.; Rouxel, J.; Taviot-Guého, C.; Ennaqui, A.; de Roy, A.; Besse, J.P. Concomitant Intercalation and Decomplexation of Ferrocene Sulfonates in Layered Double Hydroxides. J. Solid State Chem. 1999, 144, 143–151. [Google Scholar] [CrossRef]
- Gago, S.; Pillinger, M.; Santos, T.M.; Rocha, J.; Gonçalves, I.S. Synthesis and Properties of Zn−Al Layered Double Hydroxides Containing Ferrocenecarboxylate Anions. Eur. J. Inorg. Chem. 2004, 2004, 1389–1395. [Google Scholar] [CrossRef]
- Mohanambe, L.; Vasudevan, S. Inclusion of Ferrocene in a Cyclodextrin-Functionalized Layered Metal Hydroxide: A New Organometallic-Organic-LDH Nanohybrid. Inorg. Chem. 2005, 44, 2128–2130. [Google Scholar] [CrossRef]
- Shan, D.; Yao, W.; Xue, H. Electrochemical Study of Ferrocenemethanol-Modified Layered Double Hydroxides Composite Matrix: Application to Glucose Amperometric Biosensor. Biosens. Bioelectron. 2007, 23, 432–437. [Google Scholar] [CrossRef]
- Mousty, C.; Therias, S.; Forano, C.; Besse, J.-P. Anion-Exchanging Clay-Modified Electrodes: Synthetic Layered Double Hydroxides Intercalated with Electroactive Organic Anions. J. Electroanal. Chem. 1994, 374, 63–69. [Google Scholar] [CrossRef]
- Vialat, P.; Leroux, F.; Mousty, C. Hybrid Co2Al-ABTS/Reduced Graphene Oxide Layered Double Hydroxide: Towards O2 Biocathode Development. Electrochim. Acta 2015, 158, 113–120. [Google Scholar] [CrossRef]
| Sample | %Ntheo | %Nexp | ΔN(%) | %Ctheo | %Cexp | ΔC(%) | %Htheo | %Hexp | ΔH(%) |
|---|---|---|---|---|---|---|---|---|---|
| LDH-RF | 4.52 | 2.73 | 1.74% | 30.52 | 19.07 | 0.46% | 4.52 | 4.37 | 0.63% |
| LDH-FCm | / | / | / | 20.60 | 20.44 | 0.15% | 4.32 | 4.13 | 2.66% |
| LDH-FCd | / | / | / | 29.84 | 29.48 | 0.27% | 4.02 | 4.18 | 1.92% |
| Electrolyte | Eox (V) | Ered (V) | Qc1st cycle (mAh/g)/ Qd1st cycle (mAh/g) * | C. Efficiency * (%) | Qc50th cycle (mAh/g)/ Qd50th cycle (mAh/g) * | C. Efficiency * (%) | Qc500th cycle (mAh/g)/ Qd500th cycle (mAh/g) * | C. Efficiency * (%) |
|---|---|---|---|---|---|---|---|---|
| NaCH3COOH | −0.37 | −0.69 | 2.9/9.0 | 32 | 7.3/10.3 | 71 | 16/18 | 89 |
| NaClO4 | −0.41 | −0.68 | 4.3/9.5 | 45 | 11.5/12.9 | 89 | 10.6/12.3 | 88 |
| LiClO4 | −0.33 | −0.69 | 4.9/11.7 | 42 | 11.9/17.5 | 68 | 14.8/23.7 | 62 |
| NaNO3 | −0.44 | −0.76 | 6.8/31.4 | 22 | 9.1/23.3 | 39 | 9.7/23.2 | 42 |
| Na2SO4 | −0.46 | −0.69 | 5.8/13.2 | 44 | 6.2/17.2 | 36 | 6.6/20.3 | 33 |
| Li2SO4 | −0.41 | −0.65 | 7.6/12.3 | 62 | 9.3/10.1 | 92 | 11.1/12.0 | 93 |
| Electrolyte | Eox (V) | Ered (V) | Qc1st Cycle | Qd1st Cycle | Efficiency (%) | Qc100th Cycle | Qd100th Cycle | Efficiency (%) | |
|---|---|---|---|---|---|---|---|---|---|
| LDH-FCd | NaCH3COOH | 0.58 | 0.33 | 2.7 | 0.9 | 33 | 0.8 | 0.6 | 75 |
| NaClO4 | 0.67 | 0.36 | 2.3 | 1.3 | 57 | 1.1 | 1.0 | 91 | |
| LiClO4 | 0.61 | 0.36 | 2.9 | 1.9 | 66 | 1.0 | 1.0 | 100 | |
| Na2SO4 | 0.63 | 0.48 | 1.1 | 0.1 | 9 | 0.1 | 0.1 | 100 | |
| Li2SO4 | 0.67 | 0.36 | 1.0 | 0.4 | 40 | 0.6 | 0.5 | 83 | |
| LDH-FCm | NaCH3COOH | 0.57 | 0.25 | 19.1 | 14.7 | 60 | 3.2 | 2.3 | 71 |
| NaClO4 | 0.72 | 0.11 | 44.1 | 26.3 | 60 | 11.2 | 10.6 | 94 | |
| LiClO4 | 0.81 | 0.12 | 57.2 | 37.3 | 65 | 6.5 | 5.9 | 91 | |
| Na2SO4 | 0.74 | 0.10 | 41.0 | 11.0 | 27 | 1.1 | 0.1 | 9 | |
| Li2SO4 | 0.70 | 0.13 | 40.5 | 22.1 | 41 | 0.7 | 0.4 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmet, J.; Leroux, F.; Taviot-Gueho, C.; Gerlach, P.; Douard, C.; Brousse, T.; Toussaint, G.; Stevens, P. Interleaved Electroactive Molecules into LDH Working on Both Electrodes of an Aqueous Battery-Type Device. Molecules 2023, 28, 1006. https://doi.org/10.3390/molecules28031006
Sarmet J, Leroux F, Taviot-Gueho C, Gerlach P, Douard C, Brousse T, Toussaint G, Stevens P. Interleaved Electroactive Molecules into LDH Working on Both Electrodes of an Aqueous Battery-Type Device. Molecules. 2023; 28(3):1006. https://doi.org/10.3390/molecules28031006
Chicago/Turabian StyleSarmet, Julien, Fabrice Leroux, Christine Taviot-Gueho, Patrick Gerlach, Camille Douard, Thierry Brousse, Gwenaëlle Toussaint, and Philippe Stevens. 2023. "Interleaved Electroactive Molecules into LDH Working on Both Electrodes of an Aqueous Battery-Type Device" Molecules 28, no. 3: 1006. https://doi.org/10.3390/molecules28031006
APA StyleSarmet, J., Leroux, F., Taviot-Gueho, C., Gerlach, P., Douard, C., Brousse, T., Toussaint, G., & Stevens, P. (2023). Interleaved Electroactive Molecules into LDH Working on Both Electrodes of an Aqueous Battery-Type Device. Molecules, 28(3), 1006. https://doi.org/10.3390/molecules28031006








