Effect of Linear Energy Transfer on Cystamine’s Radioprotective Activity: A Study Using the Fricke Dosimeter with 6–500 MeV per Nucleon Carbon Ions—Implication for Carbon Ion Hadrontherapy
Abstract
:1. Introduction
1.1. Radiolysis of Water: Formation of Primary Radical and Molecular Products and Influence of the Quality of the Radiation
1.2. Employing the Aqueous Ferrous Sulfate (Fricke) Dosimeter as an Indicator of Cystamine’s Radioprotective and Antioxidant Properties in the Context of Irradiations by Fast Carbon Ions in the Energy Range of 6–500 MeV per Nucleon
2. Results and Discussion
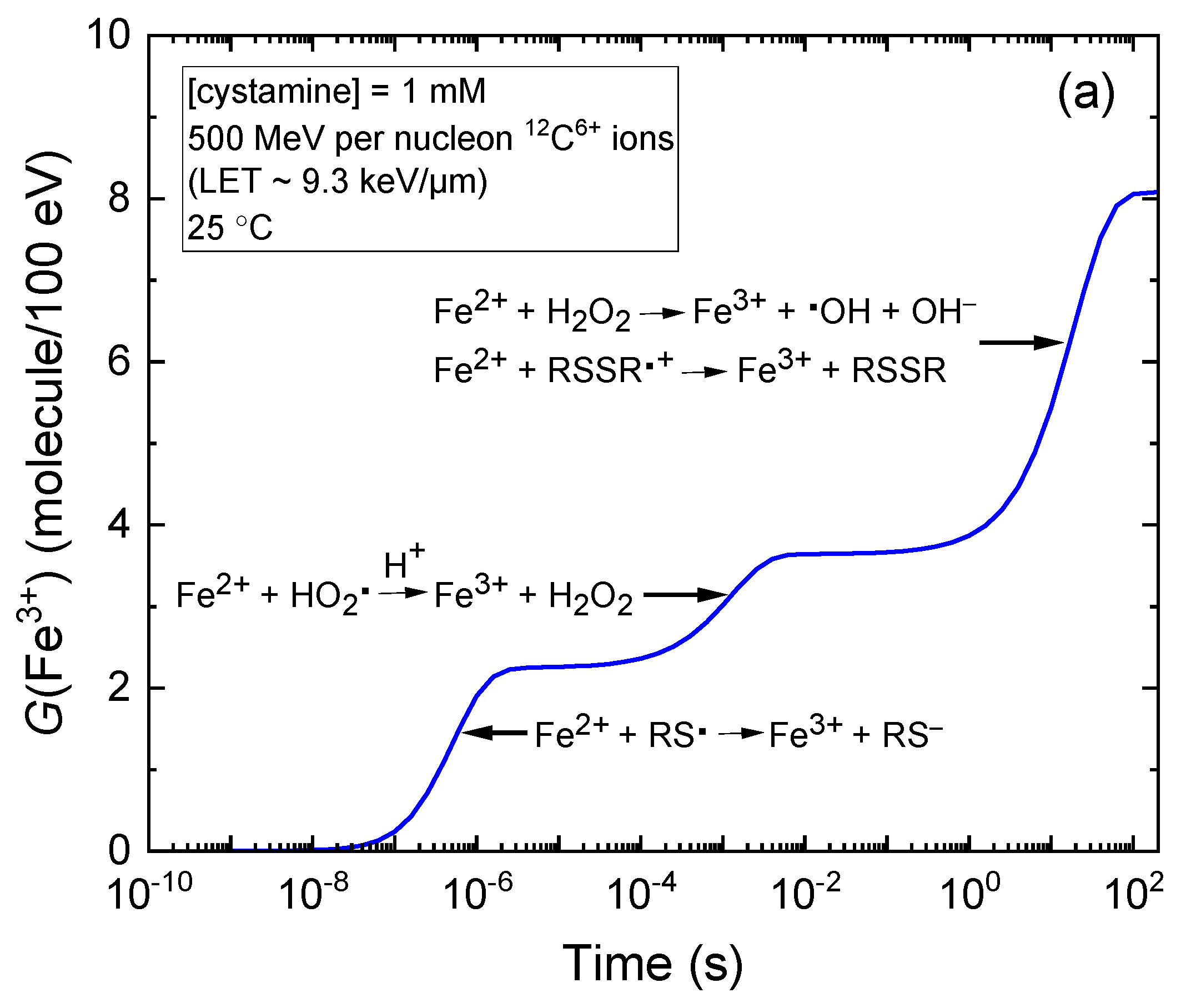
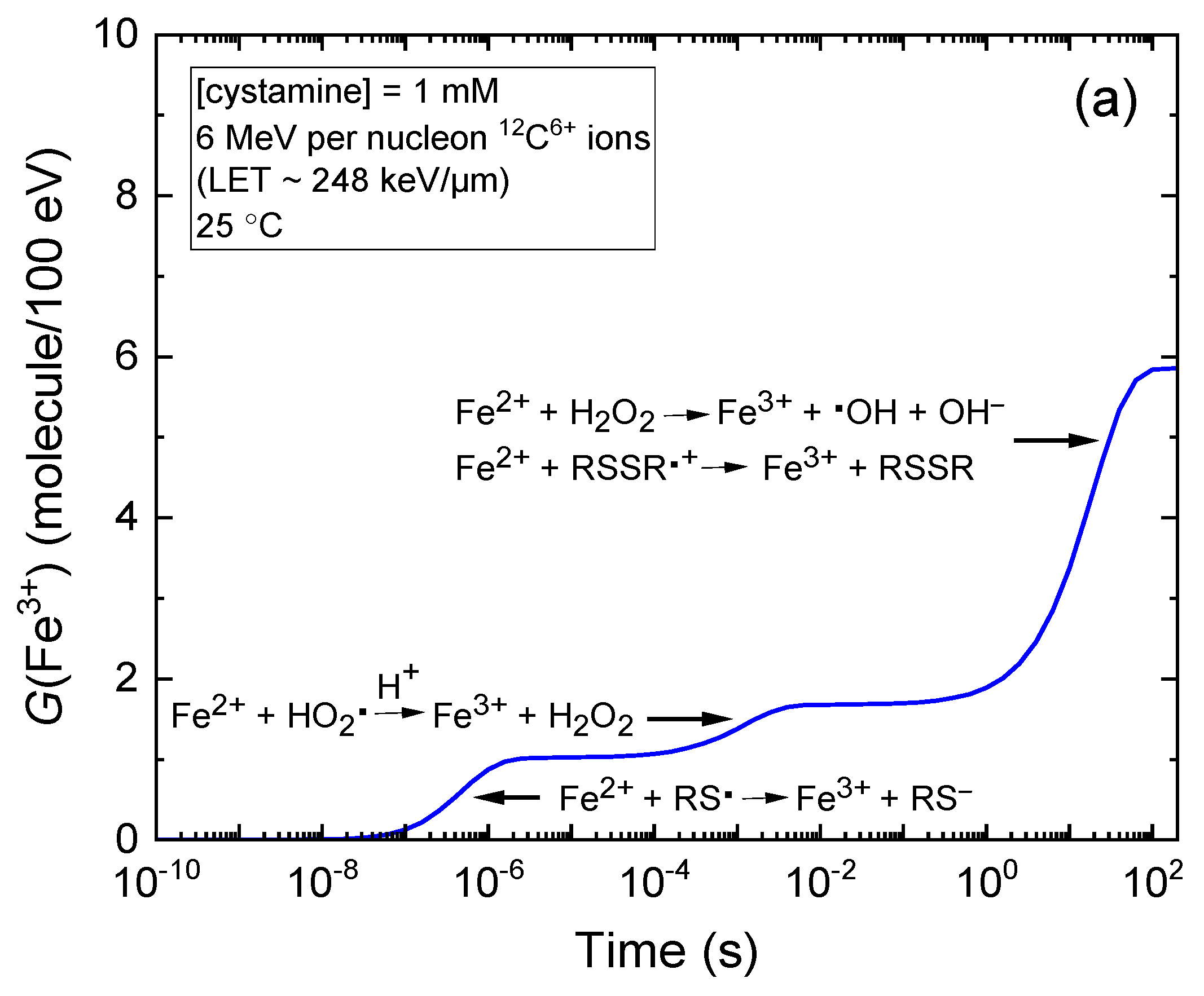
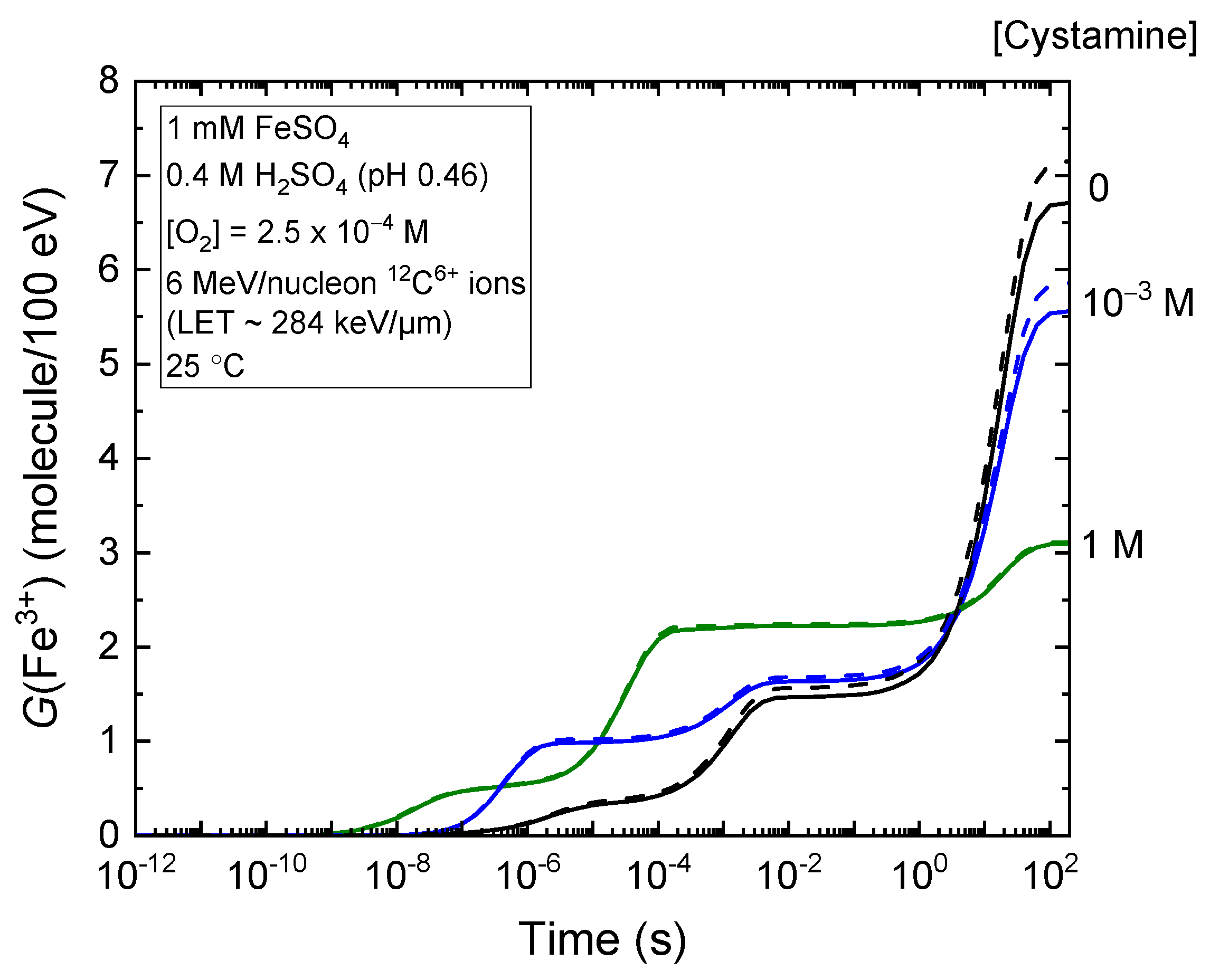
2.1. Kinetics of Fe3+ Formation in Fricke–Cystamine Solutions Subjected to 6–500 MeV per Nucleon Carbon Ion Irradiation
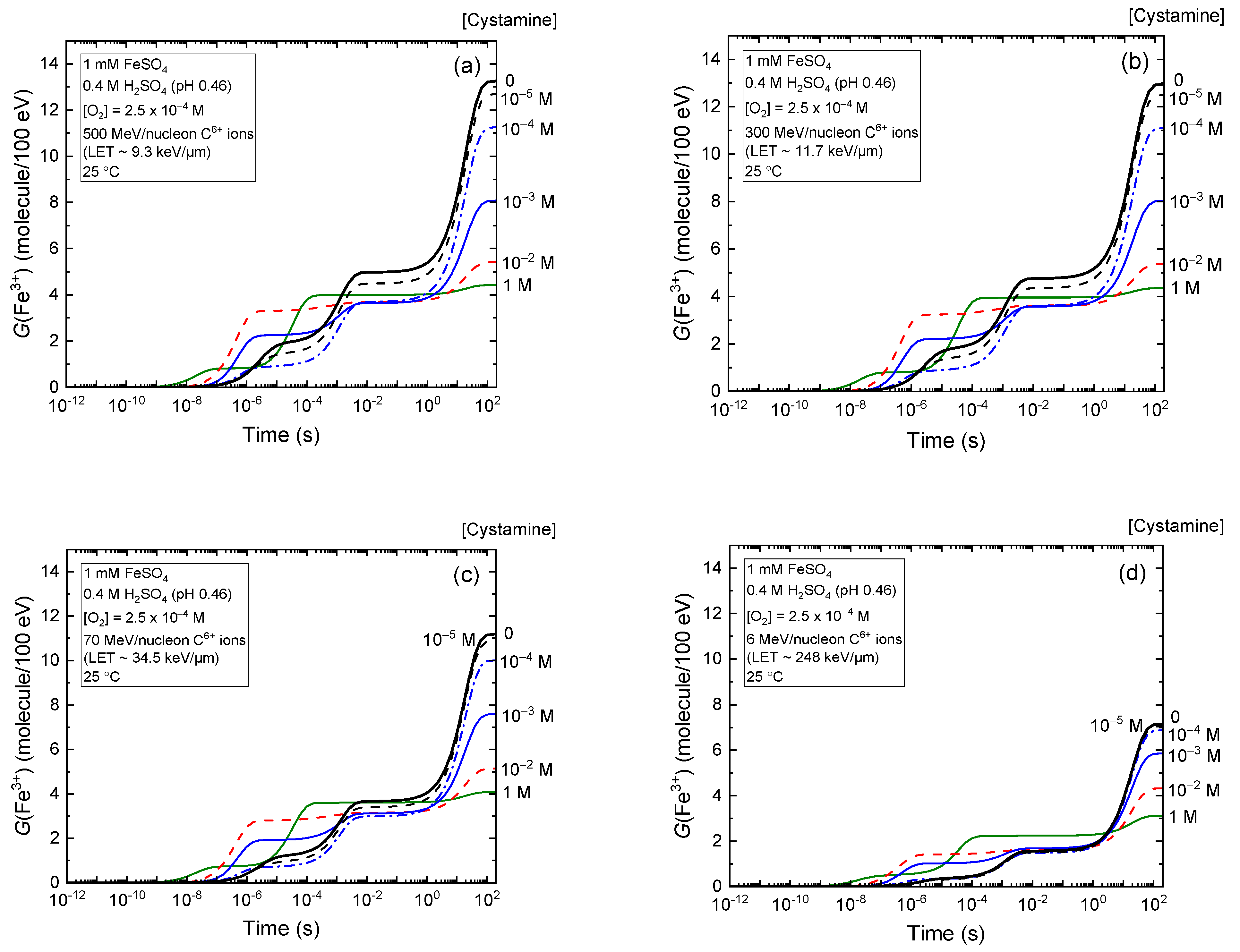
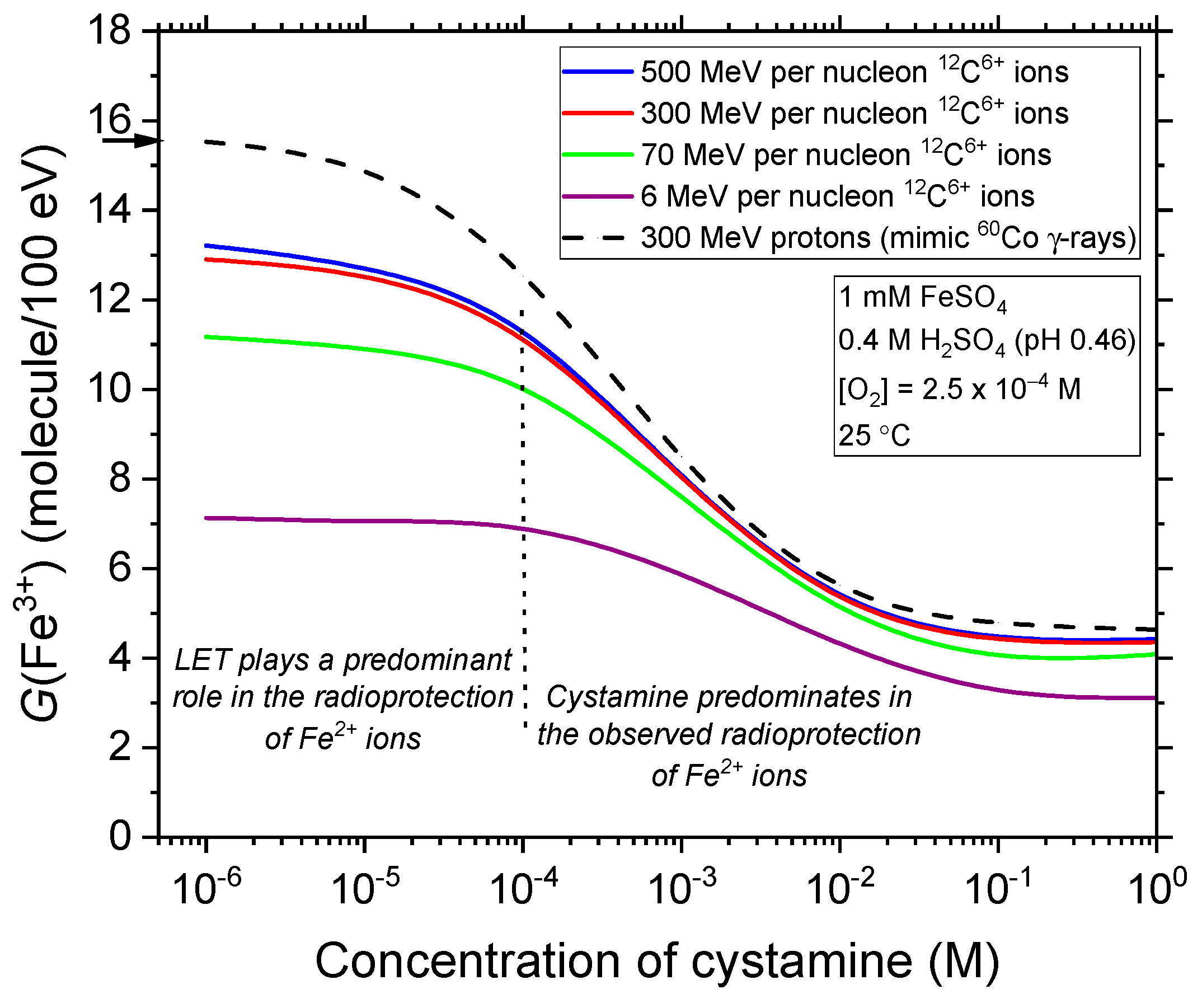
2.2. Effect of Cystamine Concentration on the Fricke Yield
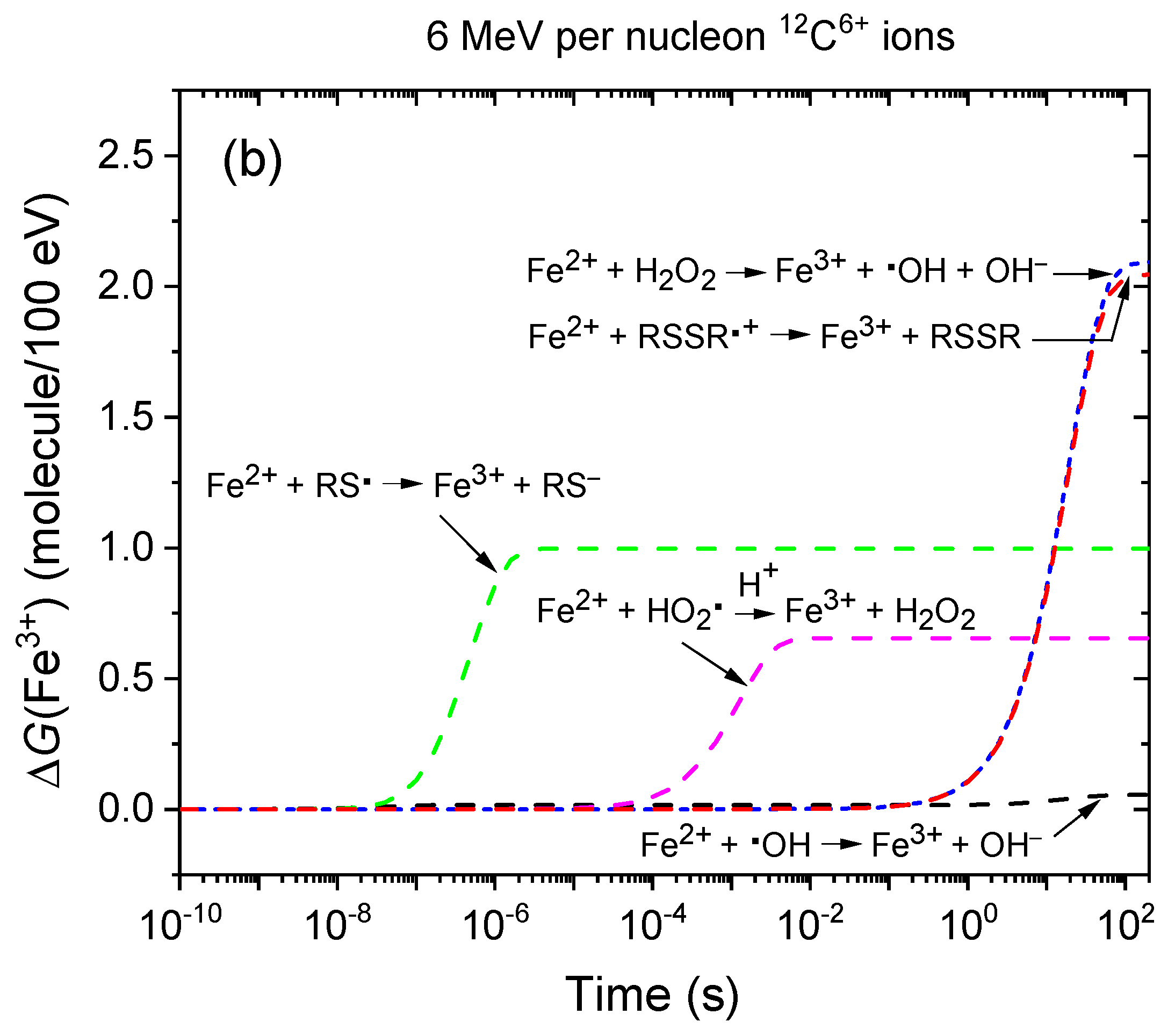
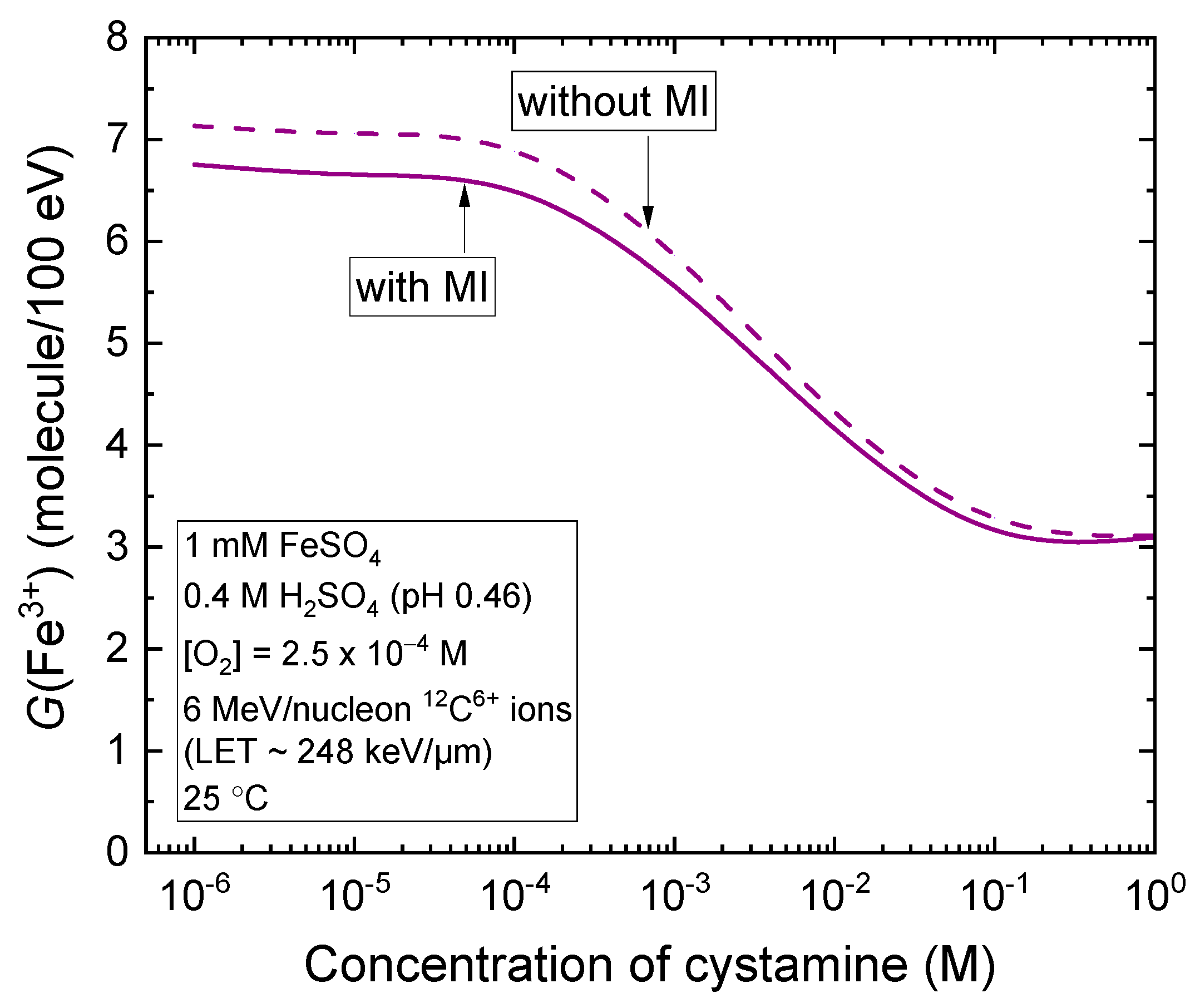
2.3. Monte Carlo Track Chemistry Simulations of the Radiolysis of Fricke–Cystamine Solutions: Effect of Multiple Ionization under 6 MeV per Nucleon (~248 keV/μm) Carbon Ion Irradiation
3. Materials and Methods
3.1. The Ferrous Sulfate, or Fricke, Chemical Dosimeter
3.2. Monte Carlo Track Chemistry Simulations of the Radiolysis of Fricke–Cystamine Solutions Using High-Energy Carbon Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halperin, E.C.; Wazer, D.E.; Perez, C.A.; Brady, L.W. Perez & Brady’s Principles and Practice of Radiation Oncology, 7th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019. [Google Scholar]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 126–134. [Google Scholar]
- Poggi, M.M.; Coleman, C.N.; Mitchell, J.B. Sensitizers and protectors of radiation and chemotherapy. Curr. Probl. Cancer 2001, 25, 334–411. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Ranjan, A.; Kaur, N.; Sur, S.; Tandon, V. Radioprotective agents: Strategies and translational advances. Med. Res. Rev. 2016, 36, 461–493. [Google Scholar] [CrossRef]
- Nair, C.K.K.; Parida, D.K.; Nomura, T. Radioprotectors in radiotherapy. J. Radiat. Res. 2001, 42, 21–37. [Google Scholar] [CrossRef]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Bump, E.A.; Malaker, K. (Eds.) Radioprotectors: Chemical, Biological, and Clinical Perspectives; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar] [CrossRef]
- Conklin, J.J.; Walker, R.I. (Eds.) Military Radiobiology; Academic Press: Orlando, FL, USA, 1987. [Google Scholar] [CrossRef]
- Dziegielewski, J.; Goetz, W.; Baulch, J.E. Heavy ions, radioprotectors and genomic instability: Implications for human space exploration. Radiat. Environ. Biophys. 2010, 49, 303–316. [Google Scholar] [CrossRef]
- Meesat, R.; Sanguanmith, S.; Meesungnoen, J.; Lepage, M.; Khalil, A.; Jay-Gerin, J.-P. Utilization of the ferrous sulfate (Fricke) dosimeter for evaluating the radioprotective potential of cystamine: Experiment and Monte Carlo simulation. Radiat. Res. 2012, 177, 813–826. [Google Scholar] [CrossRef]
- Sepulveda, E.; Sanguanmith, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Evaluation of the radioprotective ability of cystamine for 150 keV–500 MeV proton irradiation: A Monte Carlo track chemistry simulation study. Can. J. Chem. 2019, 97, 100–111. [Google Scholar] [CrossRef]
- Penabeï, S.; Meesungnoen, J.; Jay-Gerin, J.-P. Assessment of cystamine’s radioprotective/antioxidant ability under high-dose-rate irradiation: A Monte Carlo multi-track chemistry simulation study. Antioxidants 2023, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair: A Chemical Perspective; Springer: Berlin, Germany, 2006. [Google Scholar]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V. Radiation chemistry of the liquid state: (1) Water and homogeneous aqueous solutions. In Radiation Chemistry: Principles and Applications; Farhataziz, Rodgers, M.A.J., Eds.; VCH: New York, NY, USA, 1987; pp. 321–349. [Google Scholar]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Ferradini, C.; Jay-Gerin, J.-P. Radiolysis of water and aqueous solutions: History and present state of the science. Can. J. Chem. 1999, 77, 1542–1575. [Google Scholar] [CrossRef]
- Ferradini, C.; Jay-Gerin, J.-P. The effect of pH on water radiolysis: A still open question—A minireview. Res. Chem. Interm. 2000, 26, 549–565. [Google Scholar] [CrossRef]
- Elliot, A.J.; Bartels, D.M. The Reaction Set, Rate Constants and g-Values for the Simulation of the Radiolysis of Light Water over the Range 20 to 350 °C Based on Information Available in 2008; Report No. 153-127160-450-001; Atomic Energy of Canada Limited: Mississauga, ON, Canada, 2009. [Google Scholar]
- Klassen, N.V. Primary species in irradiated water. J. Chim. Phys. 1991, 88, 747–757. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Kuppermann, A. Diffusion kinetics in radiation chemistry. In Actions Chimiques et Biologiques des Radiations; Haïssinsky, M., Ed.; Masson: Paris, France, 1961; Volume 5, pp. 85–166. [Google Scholar]
- Linear Energy Transfer. ICRU Report No. 16; International Commission on Radiation Units and Measurements: Washington, DC, USA, 1970.
- Mozumder, A. Fundamentals of Radiation Chemistry; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- LaVerne, J.A. Radiation chemical effects of heavy ions. In Charged Particle and Photon Interactions with Matter: Chemical, Physicochemical, and Biological Consequences with Applications; Mozumder, A., Hatano, Y., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 403–429. [Google Scholar]
- Meesungnoen, J.; Jay-Gerin, J.-P. High-LET radiolysis of liquid water with 1H+, 4He2+, 12C6+, and 20Ne9+ ions: Effects of multiple ionization. J. Phys. Chem. A 2005, 109, 6406–6419. [Google Scholar] [CrossRef]
- Magee, J.L. Radiation chemistry. Annu. Rev. Nucl. Sci. 1953, 3, 171–192. [Google Scholar] [CrossRef]
- Freeman, G.R. Basics of radiation chemistry. In The Study of Fast Processes and Transient Species by Electron Pulse Radiolysis: Proceedings of the NATO Advanced Study Institute Held at Capri, Italy, 7–18 September 1981; Baxendale, J.H., Busi, F., Eds.; Reidel Publishing: Dordrecht, The Netherlands, 1982; pp. 19–34. [Google Scholar] [CrossRef]
- Chatterjee, A.; Holley, W.R. Computer simulation of initial events in the biochemical mechanisms of DNA damage. Adv. Radiat. Biol. 1993, 17, 181–221. [Google Scholar] [CrossRef]
- Sanguanmith, S.; Meesungnoen, J.; Muroya, Y.; Lin, M.; Katsumura, Y.; Jay-Gerin, J.-P. On the spur lifetime and its temperature dependence in the low linear energy transfer radiolysis of water. Phys. Chem. Chem. Phys. 2012, 14, 16731–16736. [Google Scholar] [CrossRef]
- Muroya, Y.; Plante, I.; Azzam, E.I.; Meesungnoen, J.; Katsumura, Y.; Jay-Gerin, J.-P. High-LET ion radiolysis of water: Visualization of the formation and evolution of ion tracks and relevance to the radiation-induced bystander effect. Radiat. Res. 2006, 165, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, D.; Krämer, M.; Fuss, M.C.; Durante, M.; Scifoni, E. Impact of target oxygenation on the chemical track evolution of ion and electron radiation. Int. J. Mol. Sci. 2020, 21, 424. [Google Scholar] [CrossRef]
- Ward, J.F. Chemical aspects of DNA radioprotection. In Radioprotectors and Anticarcinogens; Nygaard, O.F., Simić, M.G., Eds.; Academic Press: New York, NY, USA, 1983; pp. 73–85. [Google Scholar]
- Bacq, Z.-M.; Alexander, P. Principes de Radiobiologie; Masson: Paris, France, 1955; pp. 361–407. [Google Scholar]
- Bacq, Z.-M.; Beaumariage, M.L. Action radioprotectrice de la cystéamine et de la cystamine chez la souris en fonction du temps séparant l’injection du protecteur du début de l’irradiation par rayons X. Arch. Int. Pharmacodyn. Ther. 1965, 153, 457–459. [Google Scholar] [PubMed]
- Pinto, J.T.; Van Raamsdonk, J.M.; Leavitt, B.R.; Hayden, M.R.; Jeitner, T.M.; Thaler, H.T.; Krasnikov, B.F.; Cooper, A.J. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: Implications for the treatment of Huntington disease. J. Neurochem. 2005, 94, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M.; Pinto, J.T.; Cooper, A.J.L. Cystamine and cysteamine as inhibitors of transglutaminase activity in vivo. Biosci. Rep. 2018, 38, BSR20180691. [Google Scholar] [CrossRef]
- Bousquet, M.; Gibrat, C.; Ouellet, M.; Rouillard, C.; Calon, F.; Cicchetti, F. Cystamine metabolism and brain transport properties: Clinical implications for neurodegenerative diseases. J. Neurochem. 2010, 114, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. Therapeutic applications of cysteamine and cystamine in neurodegenerative and neuropsychiatric diseases. Front. Neurol. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Jayson, G.G.; Owen, T.C.; Wilbraham, A.C. The radiation chemistry of cystamine sulphate. J. Chem. Soc. B Phys. Org. 1967, 944–949. [Google Scholar] [CrossRef]
- Bidzilya, V.A.; Golovkova, L.P.; Beregovskaya, N.N.; Basyuk, V.V.; Korol’, É.N.; Chuiko, A.A.; Znamenskii, V.V.; Barkaya, V.S. Radioprotective effect of immobilized cystamine. Pharm. Chem. J. 1991, 25, 782–786. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Saint-Pierre, M.; Bourhis, E.; Lévesque, D.; Rouillard, C.; Cicchetti, F. Neuroprotective effects of cystamine in aged parkinsonian mice. Neurobiol. Aging 2006, 27, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Borrell-Pagès, M.; Canals, J.M.; Cordelières, F.P.; Parker, J.A.; Pineda, J.R.; Grange, G.; Bryson, E.A.; Guillermier, M.; Hirsch, E.; Hantraye, P.; et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J. Clin. Investig. 2006, 116, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Okauchi, M.; Xi, G.; Keep, R.F.; Hua, Y. Tissue-type transglutaminase and the effects of cystamine on intracerebral hemorrhage-induced brain edema and neurological deficits. Brain Res. 2009, 1249, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Sulfur metabolism in AIDS: Cystamine as an anti-HIV agent. AIDS Res. Hum. Retroviruses 2009, 25, 1057–1060. [Google Scholar] [CrossRef]
- Jayson, G.G.; Wilbraham, A.C. The utilisation of the Fricke dosimeter for evaluating the biological radiation-protective potential of water-soluble organic compounds. Chem. Commun. 1968, 641–642. [Google Scholar] [CrossRef]
- Lalitha, B.; Mittal, J.P. Electron transfer reaction in the radiation chemistry of some biologically important disulphide compounds. Radiat. Eff. 1971, 7, 159–162. [Google Scholar] [CrossRef]
- Fricke, H.; Morse, S. The chemical action of roentgen rays on dilute ferrosulphate solutions as a measure of dose. Am. J. Roentgenol. Radium Ther. 1927, 18, 430–432. [Google Scholar]
- Fricke, H.; Hart, E.J. Chemical dosimetry. In Radiation Dosimetry, 2nd ed.; Attix, F.H., Roesch, W.C., Eds.; Academic Press: New York, NY, USA, 1966; Volume II, pp. 167–239. [Google Scholar]
- Dewhurst, H.A. Effect of organic substances on the γ-ray oxidation of ferrous sulfate. J. Chem. Phys. 1951, 19, 129. [Google Scholar] [CrossRef]
- Guczi, L. Étude de l’effet d’addition de diverses substances sur l’oxydation des ions ferreux en solution aqueuse. J. Chim. Phys. 1962, 59, 795–796. [Google Scholar] [CrossRef]
- Das, R.C. Radiation chemistry of aqueous aerated ferrous sulphate solution. Radiat. Res. Rev. 1971, 3, 121–139. [Google Scholar]
- Matthews, R.W. Aqueous chemical dosimetry. Int. J. Appl. Radiat. Isot. 1982, 33, 1159–1170. [Google Scholar] [CrossRef]
- Cobut, V.; Frongillo, Y.; Patau, J.P.; Goulet, T.; Fraser, M.-J.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water. I. Physical and physicochemical aspects. Radiat. Phys. Chem. 1998, 51, 229–243. [Google Scholar] [CrossRef]
- Frongillo, Y.; Goulet, T.; Fraser, M.-J.; Cobut, V.; Patau, J.P.; Jay-Gerin, J.-P. Monte Carlo simulation of fast electron and proton tracks in liquid water. II. Nonhomogeneous chemistry. Radiat. Phys. Chem. 1998, 51, 245–254. [Google Scholar] [CrossRef]
- Meesungnoen, J.; Jay-Gerin, J.-P. Radiation chemistry of liquid water with heavy ions: Monte Carlo simulation studies. In Charged Particle and Photon Interactions with Matter: Recent Advances, Applications, and Interfaces; Hatano, Y., Katsumura, Y., Mozumder, A., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2011; pp. 355–400. [Google Scholar]
- Alanazi, A.; Meesungnoen, J.; Jay-Gerin, J.-P. A computer modeling study of water radiolysis at high dose rates. Relevance to FLASH radiotherapy. Radiat. Res. 2021, 195, 149–162. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Esplen, N.; Mendonca, M.S.; Bazalova-Carter, M. Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy: A topical review. Phys. Med. Biol. 2020, 65, 23TR03. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Vozenin, M.-C. Reinventing radiobiology in the light of FLASH radiotherapy. Annu. Rev. Cancer Biol. 2023, 7, 1–21. [Google Scholar] [CrossRef]
- Burns, P.C.; Ewing, R.C.; Navrotsky, A. Nuclear fuel in a reactor accident. Science 2012, 335, 1184–1188. [Google Scholar] [CrossRef]
- Sokol, O.; Durante, M. Carbon ions for hypoxic tumors: Are we making the most of them? Cancers 2023, 15, 4494. [Google Scholar] [CrossRef]
- Castro, J.R. Results of heavy ion radiotherapy. Radiat. Environ. Biophys. 1995, 34, 45–48. [Google Scholar] [CrossRef]
- Schlaff, C.D.; Krauze, A.; Belard, A.; O’Connell, J.J.; Camphausen, K.A. Bringing the heavy: Carbon ion therapy in the radiobiological and clinical context. Radiat. Oncol. 2014, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, O.; Makishima, H.; Kamada, T. Evolution of carbon ion radiotherapy at the National Institute of Radiological Sciences in Japan. Cancers 2018, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon ion therapy: A modern review of an emerging technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Pompos, A.; Foote, R.L.; Koong, A.C.; Le, Q.T.; Mohan, R.; Paganetti, H.; Choy, H. National effort to re-establish heavy ion cancer therapy in the United States. Front. Oncol. 2022, 12, 880712. [Google Scholar] [CrossRef] [PubMed]
- Schardt, D.; Elsässer, T.; Schulz-Ertner, D. Heavy-ion tumor therapy: Physical and radiobiological benefits. Rev. Mod. Phys. 2010, 82, 383–425. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M. Carbon ion radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Amaldi, U.; Kraft, G. Radiotherapy with beams of carbon ions. Rep. Prog. Phys. 2005, 68, 1861–1882. [Google Scholar] [CrossRef]
- Christman, E.A.; Appleby, A.; Jayko, M. Radiation chemistry of high-energy carbon, neon, and argon ions: Integral yields from ferrous sulfate solutions. Radiat. Res. 1981, 85, 443–457. [Google Scholar] [CrossRef]
- LaVerne, J.A.; Schuler, R.H. Track effects in water radiolysis: Yields of the Fricke dosimeter for carbon ions with energies up to 1700 MeV. J. Phys. Chem. 1994, 98, 4043–4049. [Google Scholar] [CrossRef]
- LaVerne, J.A. Track effects of heavy ions in liquid water. Radiat. Res. 2000, 153, 487–496. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Effects of track structure on the ion radiolysis of the Fricke dosimeter. J. Phys. Chem. A 2002, 106, 9420–9427. [Google Scholar] [CrossRef]
- Allen, A.O. The Radiation Chemistry of Water and Aqueous Solutions; D. Van Nostrand Co.: Princeton, NJ, USA, 1961. [Google Scholar]
- Barendsen, G.W.; Walter, H.M.D. Effects of different ionizing radiations on human cells in tissue culture. IV. Modification of radiation damage. Radiat. Res. 1964, 21, 314–329. [Google Scholar] [CrossRef]
- Wolff, R.K.; Aldrich, J.E.; Penner, T.L.; Hunt, J.W. Picosecond pulse radiolysis. V. Yield of electrons in irradiated aqueous solution with high concentrations of scavenger. J. Phys. Chem. 1975, 79, 210–219. [Google Scholar] [CrossRef]
- Bevan, P.L.T.; Hamill, W.H. Evidence for very early ionic effects in water radiolysis. Trans. Faraday Soc. 1970, 66, 2533–2540. [Google Scholar] [CrossRef]
- Ražem, D.; Dvornik, I. Scavenging of electrons prior to thermalization in ethanol. In Proceedings of the Third Tihany Symposium on Radiation Chemistry, Tihany, Hungary, 10–15 May 1971; Dobó, J., Hedvig, P., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1972; Volume 1, pp. 223–228. [Google Scholar]
- Sanguanmith, S.; Meesungnoen, J.; Muroya, Y.; Jay-Gerin, J.-P. Scavenging of “dry” electrons prior to hydration by azide ions: Effect on the formation of H2 in the radiolysis of water by 60Co γ-rays and tritium β-electrons. Can. J. Chem. 2021, 99, 881–889. [Google Scholar] [CrossRef]
- Gardès-Albert, M.; Jore, D.; Abedinzadeh, Z.; Rouscilles, A.; Deycard, S.; Bouffard, S. Réduction du tétranitrométhane par les espèces primaires formées lors de la radiolyse de l’eau par des ions lourds Ar18+ (in French). J. Chim. Phys. 1996, 93, 103–110. [Google Scholar] [CrossRef]
- Ferradini, C.; Jay-Gerin, J.-P. Does multiple ionization intervene for the production of HO2• radicals in high-LET liquid water radiolysis? Radiat. Phys. Chem. 1998, 51, 263–267. [Google Scholar]
- Zakaria, A.M.; Colangelo, N.W.; Meesungnoen, J.; Azzam, E.I.; Plourde, M.-É.; Jay-Gerin, J.-P. Ultra-high dose-rate, pulsed (FLASH) radiotherapy with carbon ions: Generation of early, transient, highly oxygenated conditions in the tumor environment. Radiat. Res. 2020, 194, 587–593. [Google Scholar] [CrossRef]
- Meesungnoen, J. Effect of Multiple Ionization on the Radiolysis of Liquid Water Irradiated with Heavy Ions: A Theoretical Study Using Monte Carlo Simulations. Ph.D. Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2007. [Google Scholar]
- Platzman, R.L. On the primary processes in radiation chemistry and biology. In Symposium on Radiobiology. The Basic Aspects of Radiation Effects on Living Systems; Nickson, J.J., Ed.; Wiley: New York, NY, USA, 1952; pp. 97–116. [Google Scholar]
- Tippayamontri, T.; Sanguanmith, S.; Meesungnoen, J.; Sunaryo, G.R.; Jay-Gerin, J.-P. Fast neutron radiolysis of the ferrous sulfate (Fricke) dosimeter: Monte Carlo simulations. In Recent Research Developments in Physical Chemistry; Pandalai, S.G., Ed.; Transworld Research Network: Trivandrum, India, 2009; Volume 10, pp. 143–211. ISBN 978-81-7895-431-8. [Google Scholar]
- Klassen, N.V.; Shortt, K.R.; Seuntjens, J.; Ross, C.K. Fricke dosimetry: The difference between G(Fe3+) for 60Co γ-rays and high-energy X-rays. Phys. Med. Biol. 1999, 44, 1609–1624. [Google Scholar] [CrossRef]
- McEwen, M.; El Gamal, I.; Mainegra-Hing, E.; Cojocaru, C. Determination of the Radiation Chemical Yield (G) for the Fricke Chemical Dosimetry System in Photon and Electron Beams; Report NRC-PIRS-1980; National Research Council Canada: Ottawa, ON, Canada, 2014. [Google Scholar] [CrossRef]
- The Dosimetry of Pulsed Radiation; ICRU Report No. 34; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1982.
- Radiation Dosimetry: X rays and Gamma rays with Maximum Photon Energies Between 0.6 and 50 MeV; ICRU Report No. 14; International Commission on Radiation Units and Measurements: Washington, DC, USA, 1969.
- Sehested, K.; Bjergbakke, E.; Holm, N.W.; Fricke, H. The reaction mechanism of the ferrous sulphate dosimeter at high dose rates. In Dosimetry in Agriculture, Industry, Biology and Medicine; International Atomic Energy Agency Publication STI/PUB/311; International Atomic Energy Agency: Vienna, Austria, 1973; pp. 397–404. [Google Scholar]
- Precek, M.; Kubelik, P.; Vysin, L.; Schimdhammer, U.; Larbre, J.-P.; Demarque, A.; Jeunesse, P.; Mostafavi, M.; Juha, L. Dose rate effects in fluorescence chemical dosimeters exposed to picosecond electron pulses: An accurate measurement of low doses at high dose rates. Radiat. Res. 2022, 197, 131–148. [Google Scholar] [CrossRef]
- Tachiya, M. Theory of diffusion-controlled reactions: Formulation of the bulk reaction rate in terms of the pair probability. Radiat. Phys. Chem. 1983, 21, 167–175. [Google Scholar] [CrossRef]
- Pimblott, S.M.; Pilling, M.J.; Green, N.J.B. Stochastic models of spur kinetics in water. Radiat. Phys. Chem. 1991, 37, 377–388. [Google Scholar] [CrossRef]
- Plante, I. Développement de Codes de Simulation Monte Carlo de la Radiolyse de l’Eau par des Électrons, Ions Lourds, Photons et Neutrons. Applications à Divers Sujets d’Intérêt Expérimental. Ph.D. Thesis, Université de Sherbrooke, Sherbrooke, QC, Canada, 2009. [Google Scholar]
- Bĕgusová, M.; Pimblott, S.M. Stochastic simulation of γ radiolysis of acidic ferrous sulfate solution at elevated temperatures. Radiat. Prot. Dosim. 2002, 99, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.H.; Bartels, D.M. Lack of ionic strength effect in the recombination of hydrated electrons: (e−)aq + (e−)aq → 2(OH−) + H2. Chem. Phys. 1995, 190, 145–152. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penabeï, S.; Sepulveda, E.; Zakaria, A.M.; Meesungnoen, J.; Jay-Gerin, J.-P. Effect of Linear Energy Transfer on Cystamine’s Radioprotective Activity: A Study Using the Fricke Dosimeter with 6–500 MeV per Nucleon Carbon Ions—Implication for Carbon Ion Hadrontherapy. Molecules 2023, 28, 8144. https://doi.org/10.3390/molecules28248144
Penabeï S, Sepulveda E, Zakaria AM, Meesungnoen J, Jay-Gerin J-P. Effect of Linear Energy Transfer on Cystamine’s Radioprotective Activity: A Study Using the Fricke Dosimeter with 6–500 MeV per Nucleon Carbon Ions—Implication for Carbon Ion Hadrontherapy. Molecules. 2023; 28(24):8144. https://doi.org/10.3390/molecules28248144
Chicago/Turabian StylePenabeï, Samafou, Esteban Sepulveda, Abdullah Muhammad Zakaria, Jintana Meesungnoen, and Jean-Paul Jay-Gerin. 2023. "Effect of Linear Energy Transfer on Cystamine’s Radioprotective Activity: A Study Using the Fricke Dosimeter with 6–500 MeV per Nucleon Carbon Ions—Implication for Carbon Ion Hadrontherapy" Molecules 28, no. 24: 8144. https://doi.org/10.3390/molecules28248144
APA StylePenabeï, S., Sepulveda, E., Zakaria, A. M., Meesungnoen, J., & Jay-Gerin, J.-P. (2023). Effect of Linear Energy Transfer on Cystamine’s Radioprotective Activity: A Study Using the Fricke Dosimeter with 6–500 MeV per Nucleon Carbon Ions—Implication for Carbon Ion Hadrontherapy. Molecules, 28(24), 8144. https://doi.org/10.3390/molecules28248144







