Therapeutic Potential of Ginsenoside Rb1-PLGA Nanoparticles for Heart Failure Treatment via the ROS/PPARα/PGC1α Pathway
Abstract
:1. Introduction
2. Results
2.1. Preparation of GRb1@PLGA@NPs
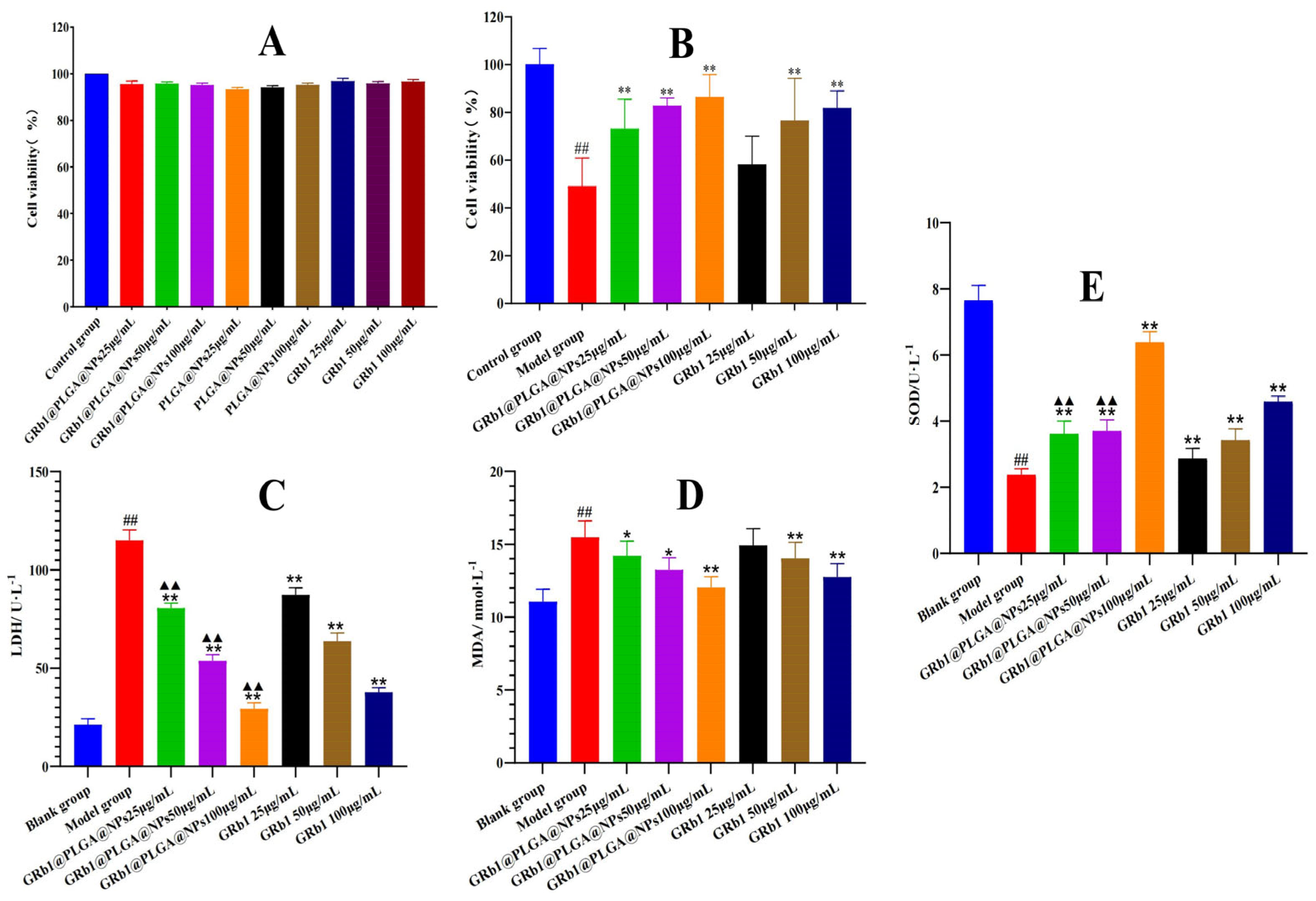
2.2. In Vitro Cytotoxicity
2.3. GRb1@PLGA@NPs Alleviate Hypoxia–Reoxygenation Injury in H9c2 Cells
2.4. GRb1@PLGA@NPs Reduced the Intracellular Levels of LDH, MDA and Increased the Content of SOD in Hypoxia–Reoxygenation-Treated H9c2 Cells
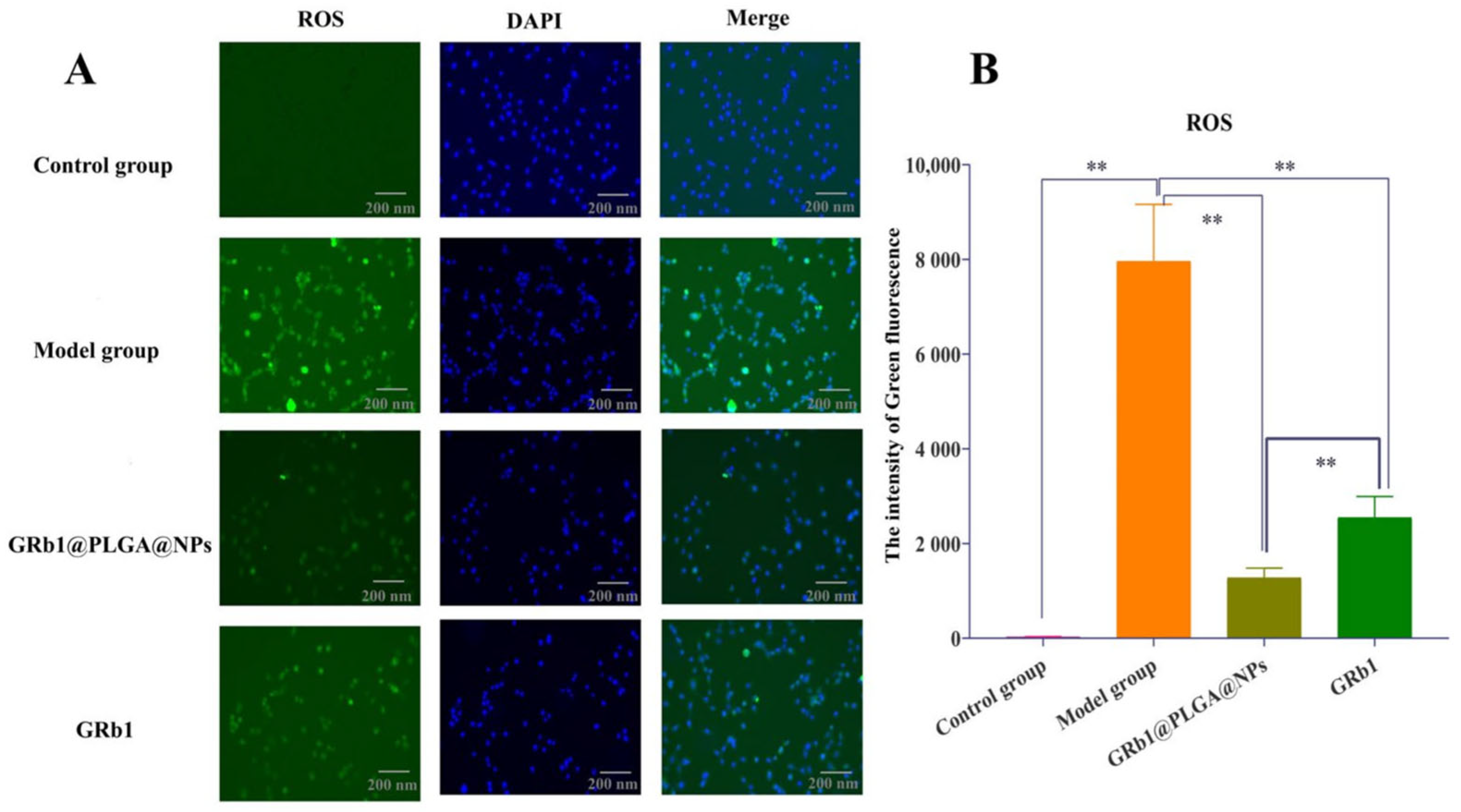
2.5. GRb1@PLGA@NPs Reduced Intracellular ROS Levels in Hypoxia–Reoxygenation-Treated H9c2 Cells
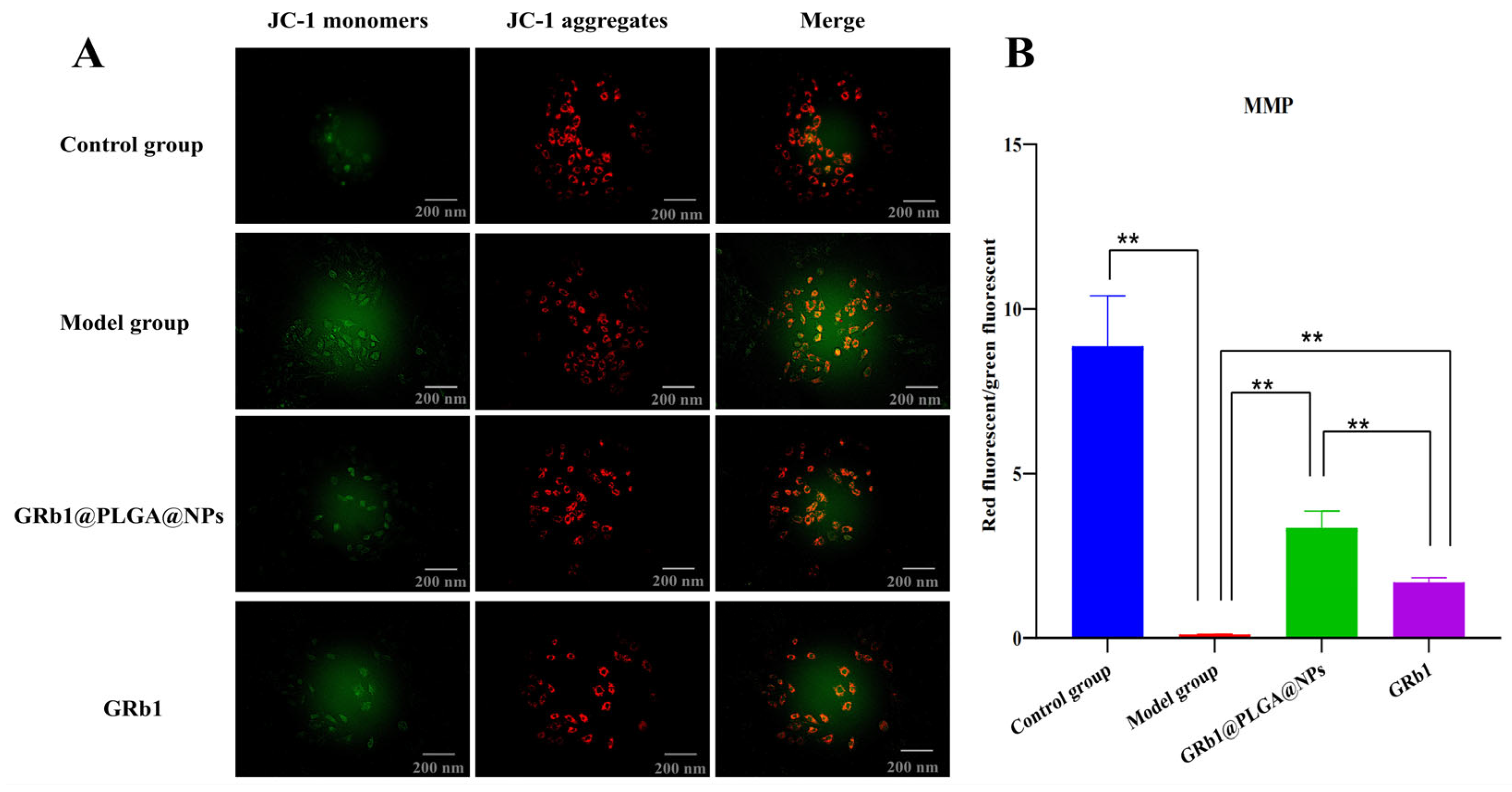
2.6. GRb1@PLGA@NPs Mitigated the Decrease in MMP and Suppressed Apoptosis in H9c2 Cells
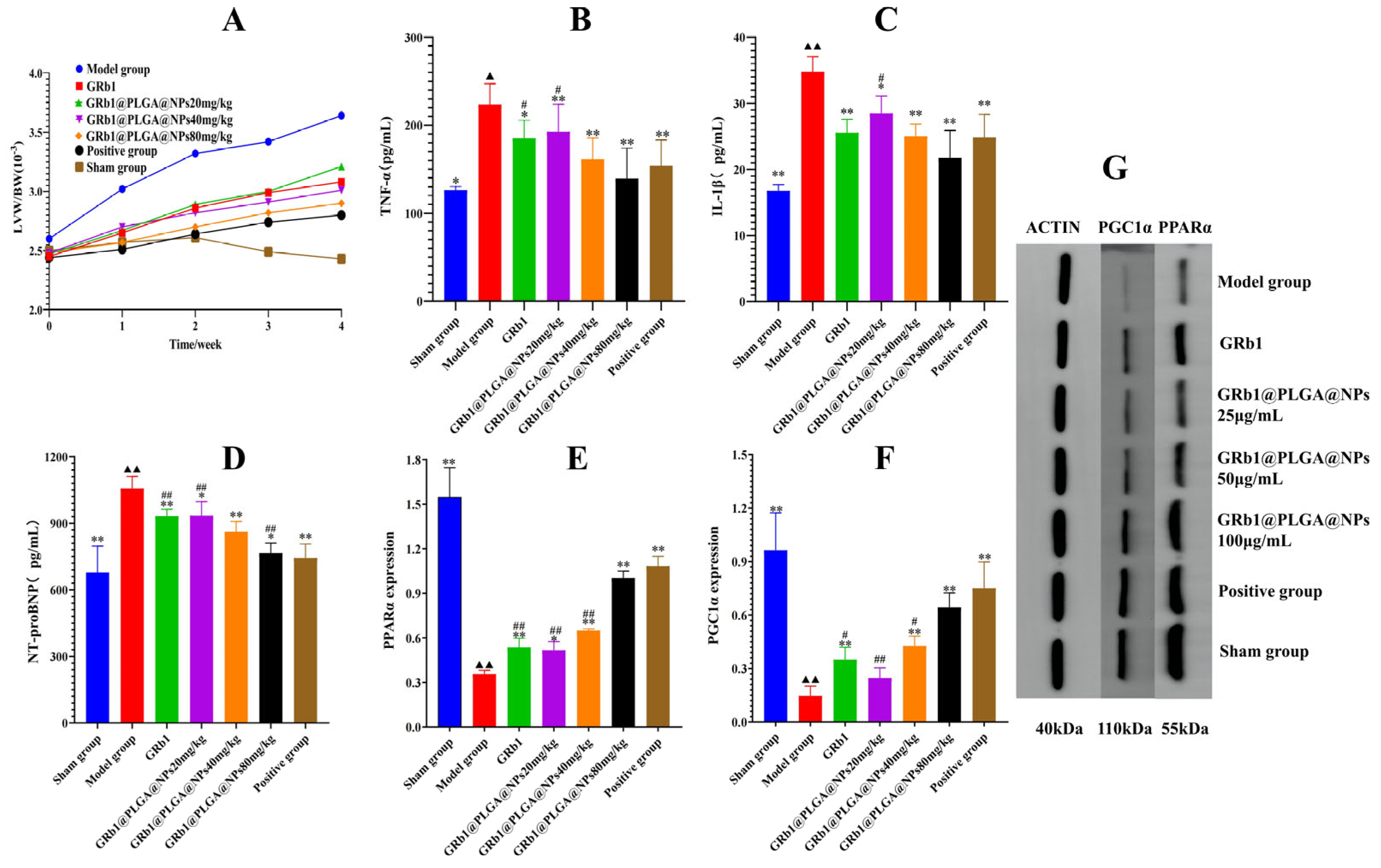
2.7. Effects of GRb1@PLGA@NPs on the General Condition of HF Rats
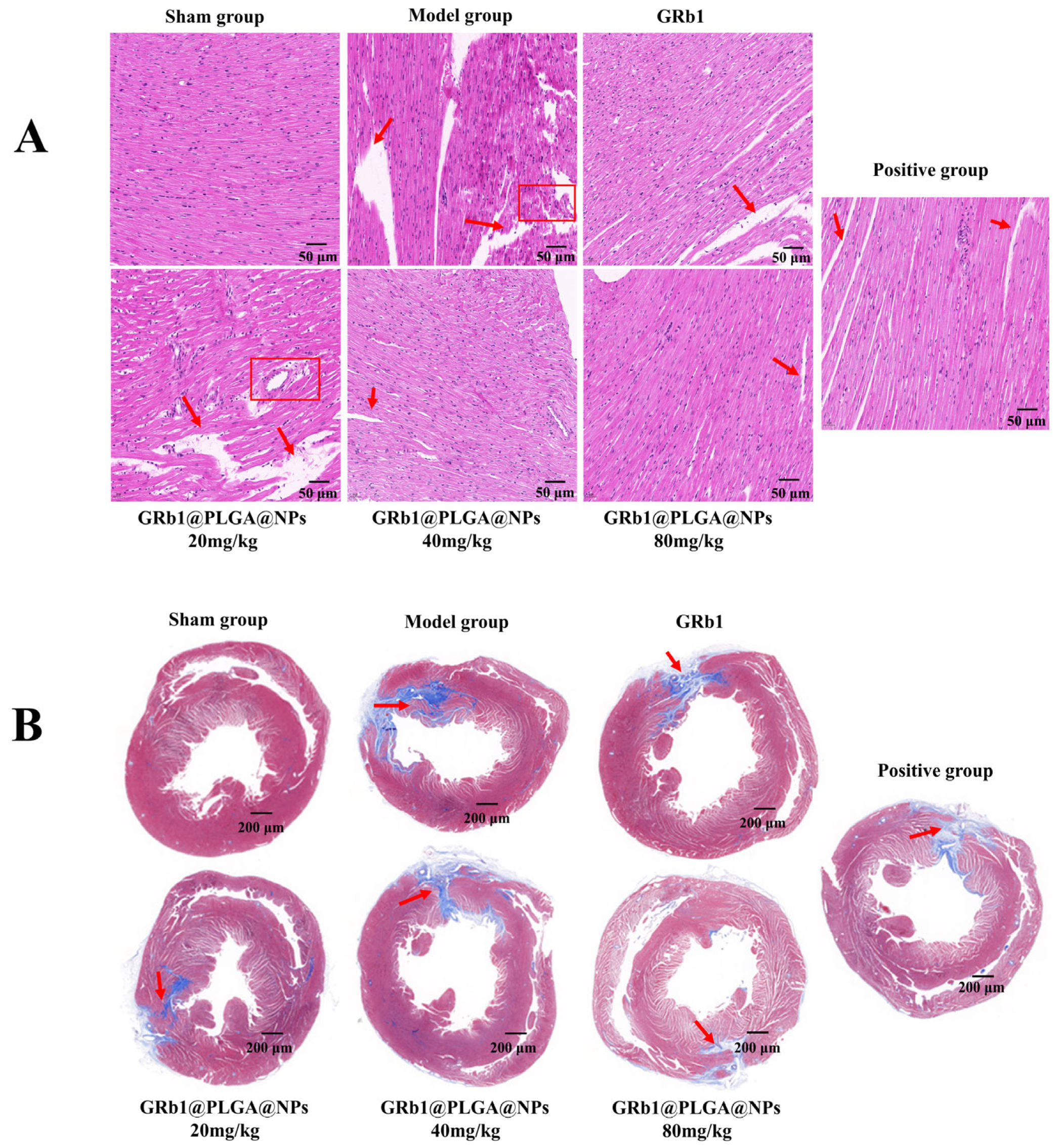
2.8. GRb1@PLGA@NPs Improves Histopathological Characteristics in HF Rats
2.9. GRb1@PLGA@NPs Reduce Levels of NT-proBNP, TNF-α, and IL-1β in HF Rats
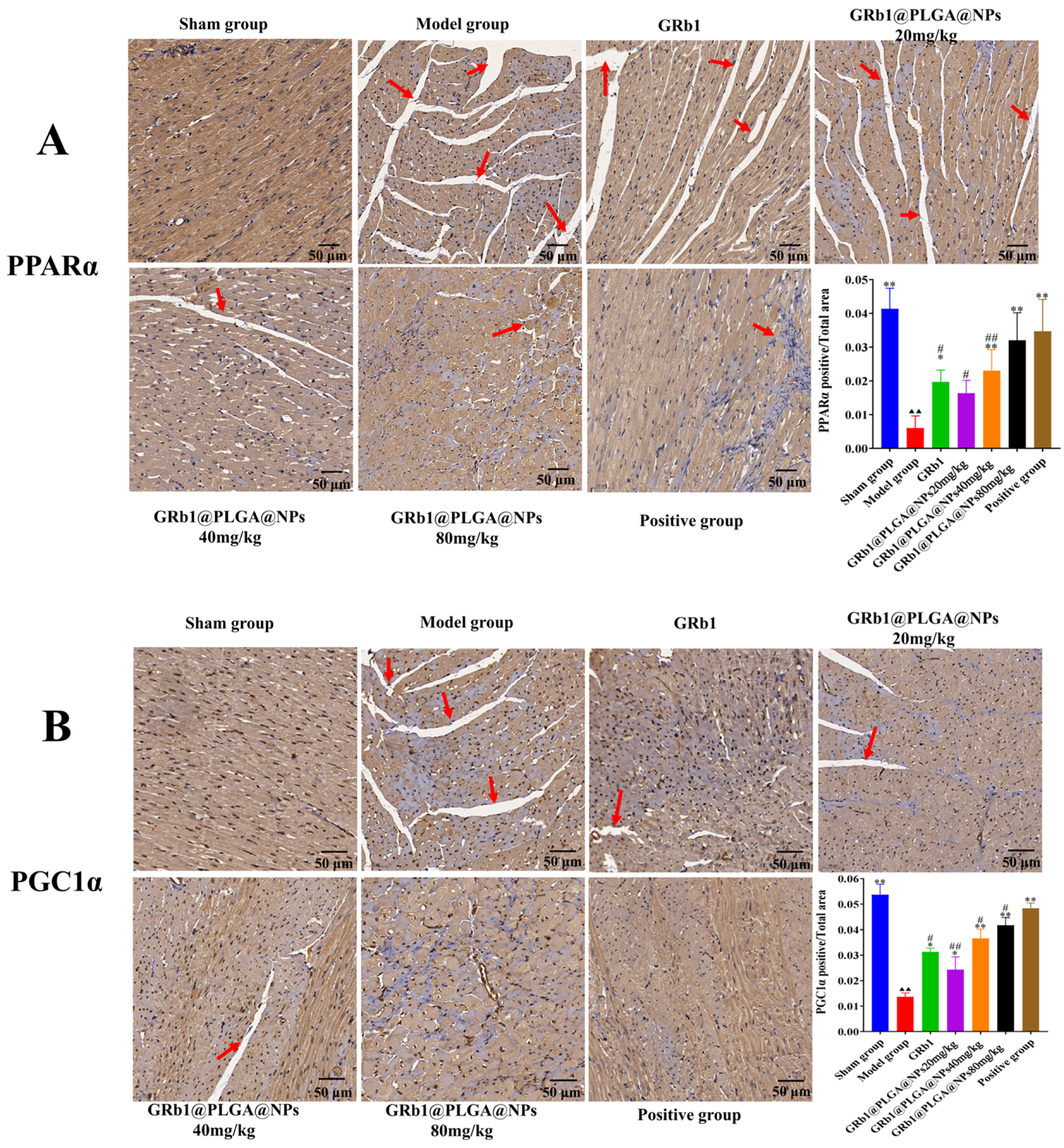
2.10. GRb1@PLGA@NPs Upregulate the Protein Expression of PPARα and PGC1α, Promoting Energy Metabolism in HF Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of GRb1@PLGA@NPs
4.3. Animal Experiment
4.4. Cell Culture and Processing
4.5. Cytotoxicity Assay
4.6. The Protective Effects of GRb1@PLGA@NPs on Hypoxia–Reoxygenation Injured Cardiomyocytes
4.7. Determination of LDH, SOD and MDA Content
4.8. Determination of ROS Content
4.9. Changes in Mitochondrial Membrane Potential (MMP)
4.10. Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of HF Biomarkers and Associated Inflammatory Factors
4.11. Histopathological Examination
4.12. Immunohistochemical Analysis
4.13. Western Blot Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansur, A.d.P.; Pereira-Barretto, A.C.; del Carlo, C.H.; Avakian, S.D.; Nakagawa, N.K.; Cesar, L.A.M.; Bocchi, E.A. Sex Differences in Prognosis of Heart Failure Due to Ischemic and Nonischemic Cardiomyopathy. J. Clin. Med. 2023, 12, 5323. [Google Scholar] [CrossRef]
- Achlaug, L.; Awwad, L.; Goncalves, I.L.; Goldenberg, T.; Aronheim, A. Tumor Growth Ameliorates Cardiac Dysfunction and Suppresses Fibrosis in a Mouse Model for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2023, 24, 12595. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.; Mazroua, M.S.; ElSaban, M.; Najam, N.; Kothari, A.S.; Mansoor, T.; Amal, T.; Lee, J.; Kashyap, R. Effect of Dapagliflozin in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Glob. Heart 2023, 18, 45. [Google Scholar] [CrossRef]
- Schirone, L.; Vecchio, D.; Valenti, V.; Forte, M.; Relucenti, M.; Angelini, A.; Zaglia, T.; Schiavon, S.; D’ambrosio, L.; Sarto, G.; et al. MST1 mediates doxorubicin-induced cardiomyopathy by SIRT3 downregulation. Cell. Mol. Life Sci. 2023, 80, 245. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, T.; Zhang, S.; Li, W.; Wang, K.; Dong, N.; Wu, Q. Corin deficiency impairs cardiac function in mouse models of heart failure. Front. Cardiovasc. Med. 2023, 10, 1164524. [Google Scholar] [CrossRef]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Dasgupta, S.; Niewold, E.L.; Li, C.; Li, Q.; Luo, X.; Tan, L.; Ferdous, A.; Lorenzi, P.L.; et al. ATF4 Protects the Heart from Failure by Antagonizing Oxidative Stress. Circ. Res. 2022, 131, 91–105. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Maekawa, S.; Furihata, T.; Kakutani, N.; Setoyama, D.; Ueda, K.; Nambu, H.; Hagiwara, H.; Handa, H.; Fumoto, Y.; et al. Succinyl-CoA-based energy metabolism dysfunction in chronic heart failure. Proc. Natl. Acad. Sci. USA 2022, 119, e2203628119. [Google Scholar] [CrossRef]
- Liu, P.; Pan, Q. Butein Inhibits Oxidative Stress Injury in Rats with Chronic Heart Failure via ERK/Nrf2 Signaling. Cardiovasc. Ther. 2022, 2022, 8684014. [Google Scholar] [CrossRef]
- Ng, M.L.; Ang, X.; Yap, K.Y.; Ng, J.J.; Goh, E.C.H.; Khoo, B.B.J.; Richards, A.M.; Drum, C.L. Novel Oxidative Stress Biomarkers with Risk Prognosis Values in Heart Failure. Biomedicines 2023, 11, 917. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Yu, S.; Zhang, L.; Jiang, J.; Zhou, Q. Herceptin induces ferroptosis and mitochondrial dysfunction in H9c2 cells. Int. J. Mol. Med. 2022, 49, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wen, P.; Zhang, D.; Li, D.; Gao, Q.; Liu, H.; Di, Y. PGAM5 expression levels in heart failure and protection ROS-induced oxidative stress and ferroptosis by Keap1/Nrf2. Clin. Exp. Hypertens. 2023, 45, 2162537. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Z.; Yan, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-α pathway-dependent fatty acid oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a Pivotal Factor in Lipid and Metabolic Regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Hou, N.; Huang, Y.; Cai, S.-A.; Yuan, W.-C.; Li, L.-R.; Liu, X.-W.; Zhao, G.-J.; Qiu, X.-X.; Li, A.-Q.; Cheng, C.-F.; et al. Puerarin ameliorated pressure overload-induced cardiac hypertrophy in ovariectomized rats through activation of the PPARα/PGC-1 pathway. Acta Pharmacol. Sin. 2021, 42, 55–67. [Google Scholar] [CrossRef]
- Miao, W.; Chen, M.; Chen, M.; Cui, C.; Zhu, Y.; Luo, X.; Wu, B. Nr2f2 Overexpression Aggravates Ferroptosis and Mitochondrial Dysfunction by Regulating the PGC-1α Signaling in Diabetes-Induced Heart Failure Mice. Mediat. Inflamm. 2022, 2022, 8373389. [Google Scholar] [CrossRef]
- Chen, L.; Wei, N.; Jiang, Y.; Yuan, C.; Xu, L.; Li, J.; Kong, M.; Chen, Y.; Wang, Q. Comparative pharmacokinetics of seven bioactive components after oral administration of crude and processed Qixue Shuangbu Prescription in chronic heart failure rats by microdialysis combined with UPLC-MS/MS. J. Ethnopharmacol. 2023, 303, 116035. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lin, Y.; Haider, N.; Moly, P.; Wang, L.; Zhou, W. Ginsenoside Rb1 protects human vascular smooth muscle cells against resistin-induced oxidative stress and dysfunction. Front. Cardiovasc. Med. 2023, 10, 1164547. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Geng, N.; Chen, T.; Xiao, Q.; Zhang, H.; Huo, H.; Jiang, L.; Shao, Q.; He, B. Ginsenoside Rb1 Improves Post-Cardiac Arrest Myocardial Stunning and Cerebral Outcomes by Regulating the Keap1/Nrf2 Pathway. Int. J. Mol. Sci. 2023, 24, 5059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Niu, K.-M.; Wu, Y.-J.; Du, K.-R.; Qi, L.-W.; Zhou, Y.-B.; Sun, H.-J. A dual Keap1 and p47phox inhibitor Ginsenoside Rb1 ameliorates high glucose/ox-LDL-induced endothelial cell injury and atherosclerosis. Cell Death Dis. 2022, 13, 824. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lu, H.; Xiao, Y.; Guo, Z.; Li, Y. Preparation, characterization and in vivo pharmacokinetic study of ginsenoside Rb1-PLGA nanoparticles. Sci. Rep. 2023, 13, 18472. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Li, J.; Liang, L.; Zeng, J.; Wen, M.; Pan, L.; Lv, D.; Liu, M.; Cheng, Y.; et al. Ginsenoside Rb1 promotes the activation of PPARα pathway via inhibiting FADD to ameliorate heart failure. Eur. J. Pharmacol. 2023, 947, 175676. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Y.; Xiong, M.; Li, M.; Wang, P.; Cui, J.; Du, X.; Chen, Y.; Zhang, T. Dual Activity of Ginsenoside Rb1 in Hypertrophic Cardiomyocytes and Activated Macrophages: Implications for the Therapeutic Intervention of Cardiac Hypertrophy. J. Inflamm. Res. 2021, 14, 1789–1806. [Google Scholar] [CrossRef]
- Yang, T.; Miao, Y.; Zhang, T.; Mu, N.; Ruan, L.; Duan, J.; Zhu, Y.; Zhang, R. Ginsenoside Rb1 inhibits autophagy through regulation of Rho/ROCK and PI3K/mTOR pathways in a pressure-overload heart failure rat model. J. Pharm. Pharmacol. 2018, 70, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S. Nano-based drug delivery system for therapeutics: A comprehensive review. Biomed. Phys. Eng. Express 2023, 9, 052002. [Google Scholar] [CrossRef]
- Li, Y.; Che, J.; Chang, L.; Guo, M.; Bao, X.; Mu, D.; Sun, X.; Zhang, X.; Lu, W.; Xie, J. CD47- and Integrin α4/β1-Comodified-Macrophage-Membrane-Coated Nanoparticles Enable Delivery of Colchicine to Atherosclerotic Plaque. Adv. Healthc. Mater. 2022, 11, e2101788. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Huang, L.-H.; Huang, H. Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery. Int. J. Mol. Sci. 2020, 21, 739. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef]
- Ren, S.; Wang, Y.; Zhang, Y.; Yan, P.; Xiao, D.; Zhao, Y.; Jia, W.; Ding, L.; Dong, H.; Wei, C.; et al. Paeoniflorin alleviates AngII-induced cardiac hypertrophy in H9c2 cells by regulating oxidative stress and Nrf2 signaling pathway. Biomed. Pharmacother. 2023, 165, 115253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, S.; Wei, G.; Sun, Y.; Li, C.; Si, X.; Chen, Y.; Tang, Z.; Li, X.; Chen, Y.; et al. CircRNA Samd4 induces cardiac repair after myocardial infarction by blocking mitochondria-derived ROS output. Mol. Ther. 2022, 30, 3477–3498. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Guo, J.; Yan, J.; Zhang, X.; Qu, H.; Yang, T.; Liu, Q.; Xu, H.; Zhou, H. Luhong Formula and Hydroxysafflor yellow A protect cardiomyocytes by inhibiting autophagy. Phytomedicine 2023, 110, 154636. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, Y.; Wang, Y.; Liu, F.; Deng, S.; Xue, W.; Wang, Y. Ursolic Acid Ameliorated Neuronal Damage by Restoring Microglia-Activated MMP/TIMP Imbalance In Vitro. Drug Des. Dev. Ther. 2023, 17, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, H.; Qian, H.; Li, D.; Xu, H.; Chen, J.; Zhong, J.; Wu, W.; Yang, H.; Chen, X.; et al. Linggui Zhugan decoction delays ventricular remodeling in rats with chronic heart failure after myocardial infarction through the Wnt/β-catenin signaling pathway. Phytomedicine 2023, 120, 155026. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.R.; Kaur, S.; Gera, R. N-Terminal Pro-B-Type Natriuretic Peptide as a Marker of Severity of Heart Failure in Children with Congenital Heart Diseases. Pediatr. Cardiol. 2023, 44, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Yao, L.; Lu, B.; Gui, M.; Zhou, X.; Fu, D. Yixin Granules Reduce Myocardial Inflammation and Fibrosis in Rats with Heart Failure by Inhibiting the Expression of ADAMTS8. Int. Heart J. 2023, 64, 741–749. [Google Scholar] [CrossRef]
- Zhu, Z.-D.; Zhang, M.; Wang, Z.; Jiang, C.-R.; Huang, C.-J.; Cheng, H.-J.; Guan, Q.-Y.; Su, T.-T.; Wang, M.-M.; Gao, Y.; et al. Chronic β-adrenergic stress contributes to cardiomyopathy in rodents with collagen-induced arthritis. Acta Pharmacol. Sin. 2023, 44, 1989–2003. [Google Scholar] [CrossRef]
- Pu, Y.; Cheng, C.K.; Zhang, H.; Luo, J.; Wang, L.; Tomlinson, B.; Huang, Y. Molecular mechanisms and therapeutic perspectives of peroxisome proliferator-activated receptor α agonists in cardiovascular health and disease. Med. Res. Rev. 2023, 43, 2086–2114. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Luo, W.; Yu, T.; Liang, S.; Sun, J.; Zhang, Y.; Han, X.; Long, X.; Liang, G.; Li, G. Corynoline protects ang II-induced hypertensive heart failure by increasing PPARα and Inhibiting NF-κB pathway. Biomed. Pharmacother. 2022, 150, 113075. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, Y.; Zhang, Y.; Xu, H.; Wang, L.; Tian, J.; Zhang, F.; Yang, H. Xinshubao tablet ameliorates myocardial injury against heart failure via the DCN/PPARα/PGC-1α/P300 pathway. Biomed. Pharmacother. 2023, 166, 115285. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Lu, H.; Wang, Z.; Liu, C.; Xiao, Y.; Guo, Z.; Li, Y. Therapeutic Potential of Ginsenoside Rb1-PLGA Nanoparticles for Heart Failure Treatment via the ROS/PPARα/PGC1α Pathway. Molecules 2023, 28, 8118. https://doi.org/10.3390/molecules28248118
Du L, Lu H, Wang Z, Liu C, Xiao Y, Guo Z, Li Y. Therapeutic Potential of Ginsenoside Rb1-PLGA Nanoparticles for Heart Failure Treatment via the ROS/PPARα/PGC1α Pathway. Molecules. 2023; 28(24):8118. https://doi.org/10.3390/molecules28248118
Chicago/Turabian StyleDu, Lixin, Huiling Lu, Ziyan Wang, Chengxin Liu, Yifei Xiao, Zhihua Guo, and Ya Li. 2023. "Therapeutic Potential of Ginsenoside Rb1-PLGA Nanoparticles for Heart Failure Treatment via the ROS/PPARα/PGC1α Pathway" Molecules 28, no. 24: 8118. https://doi.org/10.3390/molecules28248118
APA StyleDu, L., Lu, H., Wang, Z., Liu, C., Xiao, Y., Guo, Z., & Li, Y. (2023). Therapeutic Potential of Ginsenoside Rb1-PLGA Nanoparticles for Heart Failure Treatment via the ROS/PPARα/PGC1α Pathway. Molecules, 28(24), 8118. https://doi.org/10.3390/molecules28248118




