Mechanistic Study on the Possibility of Converting Dissociated Oxygen into Formic Acid on χ-Fe5C2(510) for Resource Recovery in Fischer–Tropsch Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Computational Models and Methods
2.1.1. DFT Calculation Method
2.1.2. DFT Calculation Model
2.1.3. Models and Methods for Calculating kMC
2.2. The Design of Dissociated O Removal Pathways on Hydrogen-Covered χ-Fe5C2(510) Surface
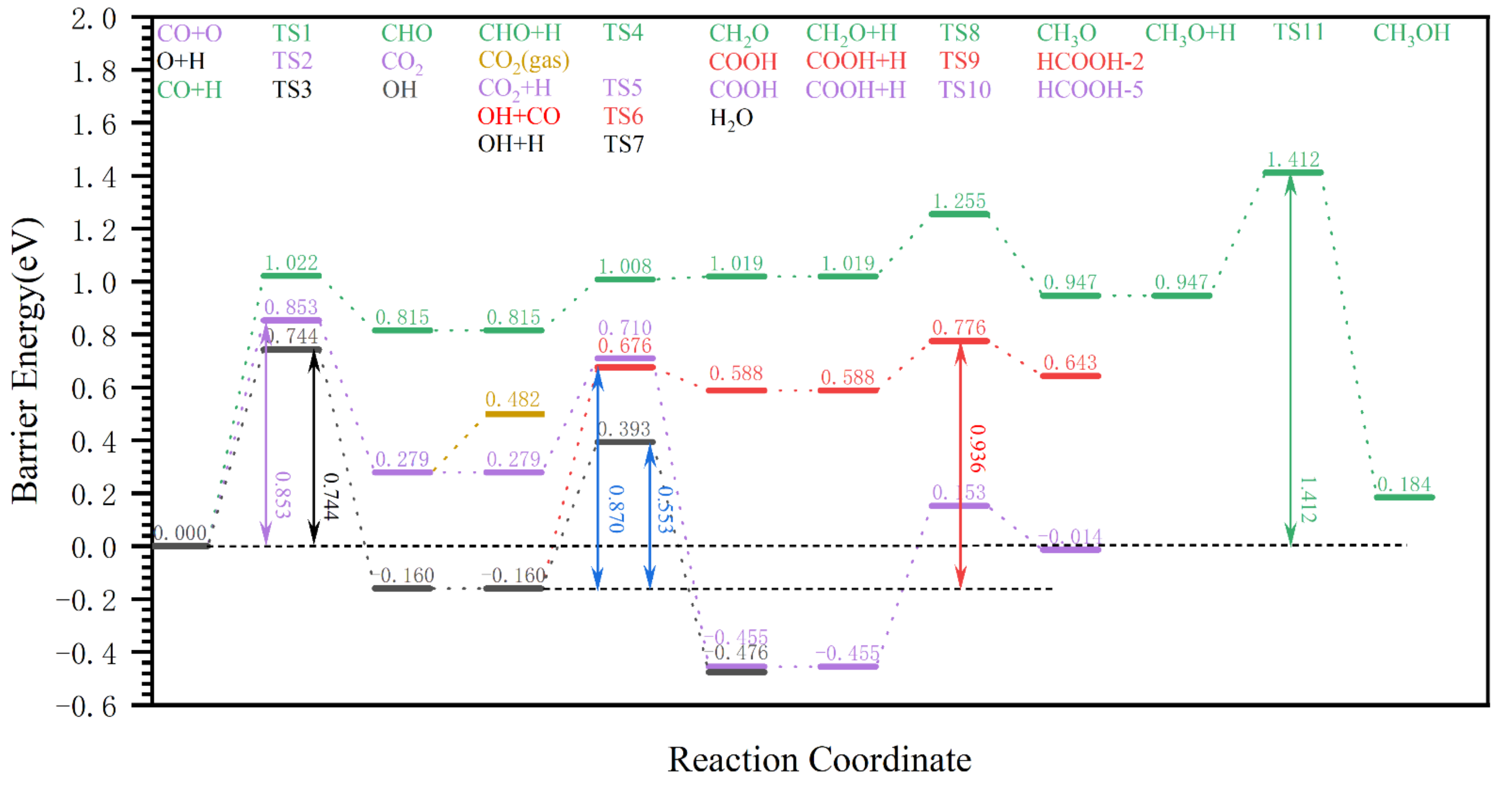
2.3. The Formic Acid Formation Pathway on Hydrogen-Covered χ-Fe5C2(510) Surface
2.3.1. Analysis of Formic Acid Formation Pathway on Hydrogen-Covered χ-Fe5C2 (510) Surface
2.3.2. Charge Analysis of the Rate-Control Step in the Formation of Formic Acid on a Hydrogen-Covered χ-Fe5C2(510) Surface
2.3.3. Summary
2.4. Other Represented Oxygenated Species Formation Pathways on the Hydrogen–Covered χ-Fe5C2(510) Surface and their Competition with Formic Acid
2.4.1. The H2O and CO2 Formation Pathway on the Hydrogen-Covered χ-Fe5C2(510) Surface
2.4.2. The Methanol Formation Pathway on a Hydrogen-Covered χ-Fe5C2(510) Surface
2.4.3. Competitive Analysis of the Formic acid Formation Pathway with the Formation Pathways of other Oxygenated Species on the Reaction of the Hydrogen-Covered χ-Fe5C2(510) Surface
2.4.4. Summary
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulikova, M.V.; Chudakova, M.V.; Kulikov, A.B.; Krylova, A.Y. Fischer–Tropsch Synthesis in the Presence of Catalysts Based on Unmodified Iron Ores. Russ. J. Appl. Chem. 2021, 94, 104–109. [Google Scholar] [CrossRef]
- Mubenesha, S.; Okoye-Chine, C.G.; Ramutsindela, F.K.; Gorimbo, J.; Moyo, M.; Liu, X. The Potential Use of Raw Iron Ore in Fischer-Tropsch Synthesis. Int. J. Pet. Technol. 2021, 8, 99–115. [Google Scholar] [CrossRef]
- Ramutsindela, F.K.; Okoye-Chine, C.G.; Mbuya, C.O.L.; Mubenesha, S.; Gorimbo, J.; Okonye, L.U.; Liu, X.; Hildebrandt, D. The effect of reducing gases on raw iron ore catalyst for Fischer-Tropsch synthesis. J. Taiwan Inst. Chem. Eng. 2022, 131, 104163. [Google Scholar] [CrossRef]
- Ncube, S.; Moyo, M. A Review of the Use of Carbon Nanostructures and Other Reducing Agents During Auto-reduction for Fischer–Tropsch Synthesis and Other Applications. Catal. Lett. 2023, 1–21. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer-Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Mahmoudi, M.; Doustdar, O.; Jahangiri, H.; Tsolakis, A.; Gu, S.; LechWyszynski, M. A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation. Biofuels Eng. 2017, 2, 11–31. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Mubenesha, S. The Use of Iron Ore as a Catalyst in Fischer–Tropsch Synthesis—A Review. Crystals 2022, 12, 1349. [Google Scholar] [CrossRef]
- Van Der Laan, G.P.; Beenackers, A.A.C.M. Kinetics and Selectivity of the Fischer–Tropsch Synthesis: A Literature Review. Catal. Rev. 1999, 41, 255–318. [Google Scholar] [CrossRef]
- Lin, T.; An, Y.; Yu, F.; Gong, K.; Yu, H.; Wang, C.; Sun, Y.; Zhong, L. Advances in Selectivity Control for Fischer–Tropsch Synthesis to Fuels and Chemicals with High Carbon Efficiency. ACS Catal. 2022, 12, 12092–12112. [Google Scholar] [CrossRef]
- Tavares, M.; Westphalen, G.; Araujo Ribeiro de Almeida, J.M.; Romano, P.N.; Sousa-Aguiar, E.F. Modified fischer-tropsch synthesis: A review of highly selective catalysts for yielding olefins and higher hydrocarbons. Front. Nanotechnol. 2022, 4, 978358. [Google Scholar] [CrossRef]
- Filot, I.A.; van Santen, R.A.; Hensen, E.J. The optimally performing Fischer-Tropsch catalyst. Angew. Chem. Int. Ed. 2014, 53, 12746–12750. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Cao, D.-B.; Liu, S.; Yang, Y.; Li, Y.-W.; Wang, J.; Jiao, H. Density functional theory study into H2O dissociative adsorption on the Fe5C2(010) surface. Appl. Catal. A Gen. 2013, 468, 370–383. [Google Scholar] [CrossRef]
- Pham, T.H.; Duan, X.; Qian, G.; Zhou, X.; Chen, D. CO Activation Pathways of Fischer–Tropsch Synthesis on χ-Fe5C2 (510): Direct versus Hydrogen-Assisted CO Dissociation. J. Phys. Chem. C 2014, 118, 10170–10176. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, X.; Yu, Y. Theoretical insights into the removal pathways of adsorbed oxygen on the surface of χ-Fe5C2(5 1 0). Chem. Eng. Sci. 2023, 271, 118576. [Google Scholar] [CrossRef]
- Zhang, M.; Ren, J.; Yu, Y. Insights into the Hydrogen Coverage Effect and the Mechanism of Fischer–Tropsch to Olefins Process on Fe5C2 (510). ACS Catal. 2020, 10, 689–701. [Google Scholar] [CrossRef]
- Andersson, R.; Boutonnet, M.; Järås, S. On-line gas chromatographic analysis of higher alcohol synthesis products from syngas. J. Chromatogr. A 2012, 1247, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, X.; Wang, T.; Wen, X.; Li, Y.-W.; Wang, J.; Jiao, H. Coverage dependent water dissociative adsorption on Fe(110) from DFT computation. Phys. Chem. Chem. Phys. PCCP 2015, 17, 8811–8821. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.; Nabar, R.; Nilekar, A.U.; Ishikawa, A.; Mavrikakis, M.; Iglesia, E. CO activation pathways and the mechanism of Fischer–Tropsch synthesis. J. Catal. 2010, 272, 287–297. [Google Scholar] [CrossRef]
- Seomoon, K. On-line GC and GC–MS analyses of the Fischer–Tropsch products synthesized using ferrihydrite catalyst. J. Ind. Eng. Chem. 2013, 19, 2108–2114. [Google Scholar] [CrossRef]
- Wang, H.Z.; Nie, X.W.; Liu, Y.; Janik, M.J.; Han, X.P.; Deng, Y.D.; Hu, W.B.; Song, C.S.; Guo, X.W. Mechanistic Insight into Hydrocarbon Synthesis via CO2 Hydrogenation on χ-Fe5C2 Catalysts. Acs Appl. Mater. Interfaces 2022, 14, 37637–37651. [Google Scholar] [CrossRef]
- Li, T.; Wen, X.; Li, Y.-W.; Jiao, H. Mechanisms of CO Activation, Surface Oxygen Removal, Surface Carbon Hydrogenation, and C–C Coupling on the Stepped Fe(710) Surface from Computation. J. Phys. Chem. C 2018, 122, 15505–15519. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Ma, H.; Qiao, Y.; Chao, J.; Hou, X.; Wang, Y.; Wang, Y. Combination of pure shift NMR and chemical shift selective filters for analysis of Fischer-Tropsch waste-water. Anal. Chim. Acta 2020, 1110, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, R.; Crous, R.; de Villiers, A.; Sandra, P. Comprehensive two-dimensional gas chromatography for the analysis of Fischer–Tropsch oil products. J. Chromatogr. A 2010, 1217, 8334–8339. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.R.; Pereira, V.B.; Stelzer, K.T.; Gomes, A.O.; Neto, F.R.; Azevedo, D.A. Quantification of trace O-containing compounds in GTL process samples via Fischer-Tropsch reaction by comprehensive two-dimensional gas chromatography/mass spectrometry. Talanta 2015, 144, 627–635. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Liu, Y.; Cao, Y. Formic acid: A versatile renewable reagent for green and sustainable chemical synthesis. Chin. J. Catal. 2015, 36, 1461–1475. [Google Scholar] [CrossRef]
- Gibson, H.W. Chemistry of formic acid and its simple derivatives. Chem. Rev. 1969, 69, 673–692. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source—Recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Ma, H.; Qian, W.; Sun, Q.; Ying, W. Effect of alkalis (Li, Na, and K) on precipitated iron-based catalysts for high-temperature Fischer-Tropsch synthesis. Fuel 2022, 326, 125090. [Google Scholar] [CrossRef]
- Song, L.; Ouyang, S.; Li, P.; Ye, J. Highly selective light olefin production via photothermal Fischer–Tropsch synthesis over α/γ-Fe2O3-derived Fe5C2 under low pressure. J. Mater. Chem. 2022, 10, 16243–16248. [Google Scholar] [CrossRef]
- Cheng, J.; Gong, X.-Q.; Hu, P.; Lok, C.M.; Ellis, P.; French, S. A quantitative determination of reaction mechanisms from density functional theory calculations: Fischer–Tropsch synthesis on flat and stepped cobalt surfaces. J. Catal. 2008, 254, 285–295. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer–Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef] [PubMed]
- Hacene, M.; Anciaux-Sedrakian, A.; Rozanska, X.; Klahr, D.; Guignon, T.; Fleurat-Lessard, P. Accelerating VASP electronic structure calculations using graphic processing units. J. Comput. Chem. 2012, 33, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.; Widom, M. VASP on a GPU: Application to exact-exchange calculations of the stability of elemental boron. Comput. Phys. Commun. 2012, 183, 1422–1426. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W. Comment on “Generalized Gradient Approximation Made Simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Ren, J.; Ai, N.; Ou, D.; Yu, Y. Insights into the Fischer–Tropsch mechanism on χ-Fe5C2(510) based on the hydrogen coverage effect. Mol. Catal. 2023, 538, 112990. [Google Scholar] [CrossRef]
- MacDonald, A.H. Comment on special points for Brillouin-zone integrations. Phys. Rev. B 1978, 18, 5897–5899. [Google Scholar] [CrossRef]
- Methfesel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.; Xiao, P.; Chemelewski, W.; Johnson, D.D.; Henkelman, G. A generalized solid-state nudged elastic band method. J. Chem. Phys. 2012, 136, 074103. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.; Terrell, R.; Henkelman, G. Optimization methods for finding minimum energy paths. J. Chem. Phys. 2008, 128, 134106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, Y.; Deng, D.; Ai, N. Density Functional Theory Study on the Absorption of CO2 by the Ionic Liquid of 1-butyl-3-methylimidazolium Acetate. Adv. Mater. Res. 2013, 807–809, 543–548. [Google Scholar]
- Cheng, J.; Hu, P.; Ellis, P.; French, S.; Kelly, G.; Lok, C.M. An Energy Descriptor to Quantify Methane Selectivity in Fischer−Tropsch Synthesis: A Density Functional Theory Study. J. Phys. Chem. C 2009, 113, 8858–8863. [Google Scholar] [CrossRef]
- Retief, J.J. Powder diffraction data and Rietveld refinement of Hägg-carbide, χ-Fe5C2. Powder Diffr. 1999, 14, 130–132. [Google Scholar] [CrossRef]
- Sun, F.; Huang, W.L.; Li, J. Mesoscale Structures in the Adlayer of A-B 2 Heterogeneous Catalysis. Langmuir 2017, 33, 11582–11589. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO₂ hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef]
- Jansen, A.P.J. An Introduction to Monte Carlo Simulations of Surface Reactions. arXiv 2003, arXiv:cond-mat/0303028. [Google Scholar]
- Turner, C.H.; Ji, J.; Lu, Z.; Lei, Y. Analysis of the propylene epoxidation mechanism on supported gold nanoparticles. Chem. Eng. Sci. 2017, 174, 229–237. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, S.; Nie, X.W.; Ding, F.S.; Song, C.S.; Guo, X.W. Mechanistic Understanding of Hydrocarbon Formation from CO2 Hydrogenation over χ-Fe5C2(111) and the Effect of H2O and Transition Metal Addition. Energy Fuels 2023, 37, 16842–16855. [Google Scholar] [CrossRef]
- Back, S.; Kim, J.H.; Kim, Y.T.; Jung, Y. On the mechanism of high product selectivity for HCOOH using Pb in CO2 electroreduction. Phys. Chem. Chem. Phys. 2016, 18, 9652–9657. [Google Scholar] [CrossRef] [PubMed]
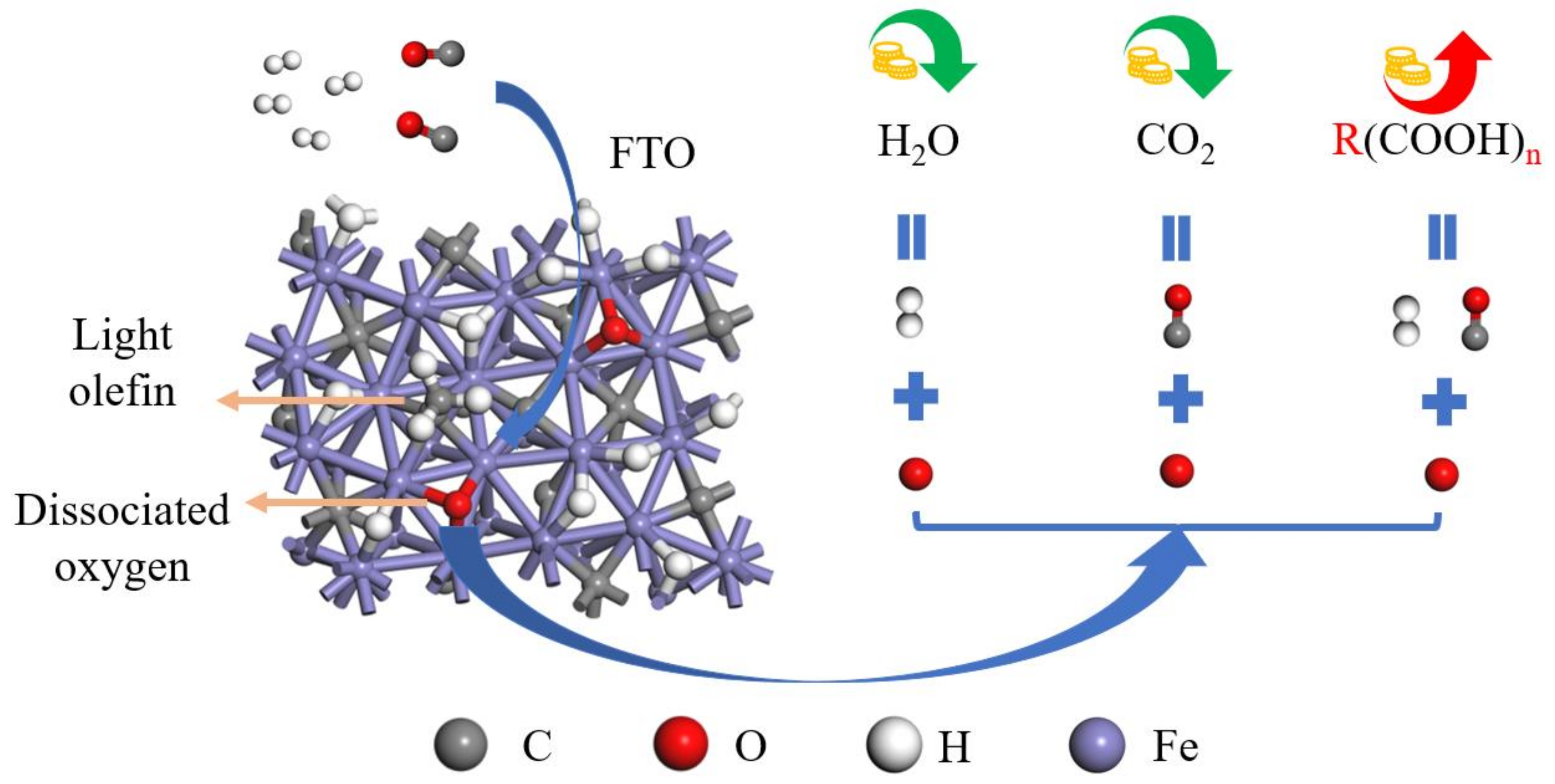





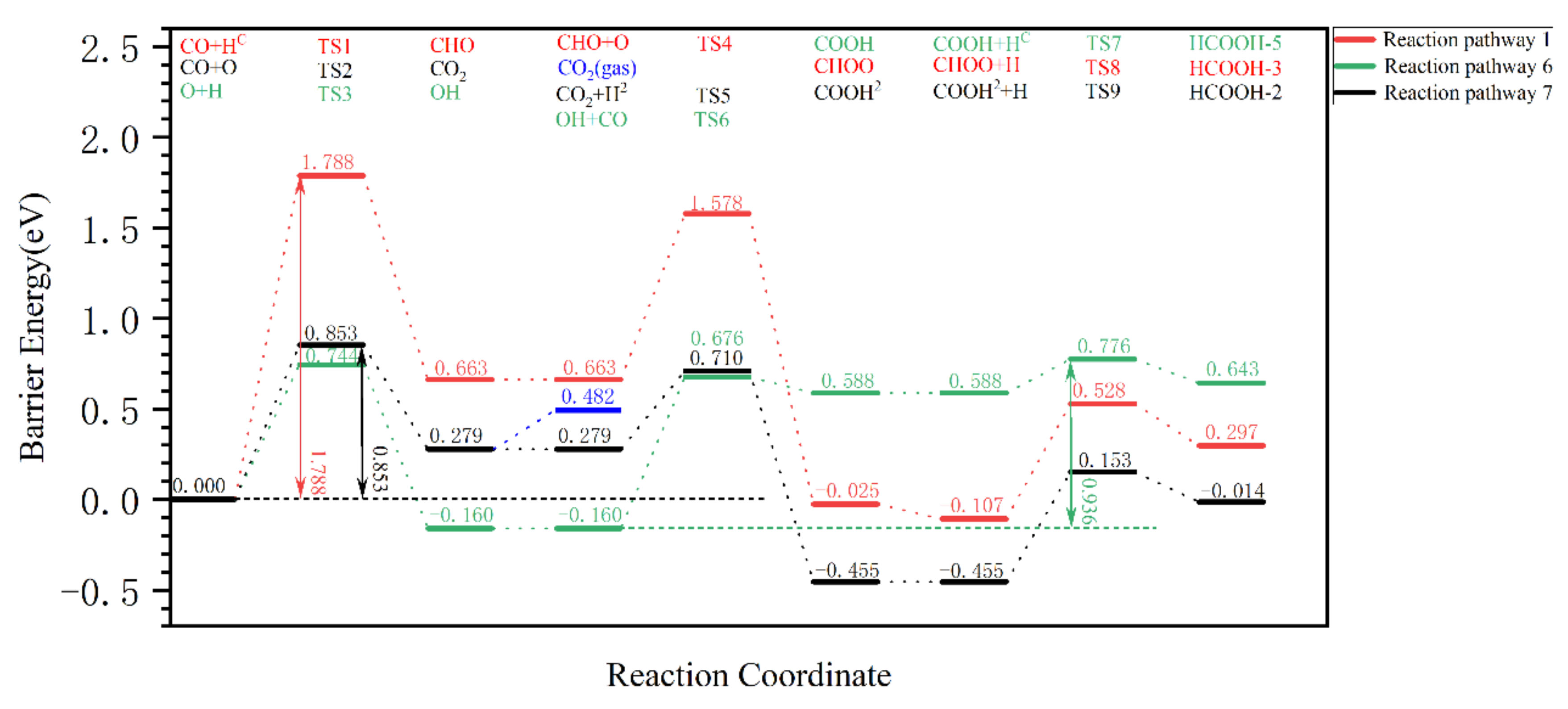
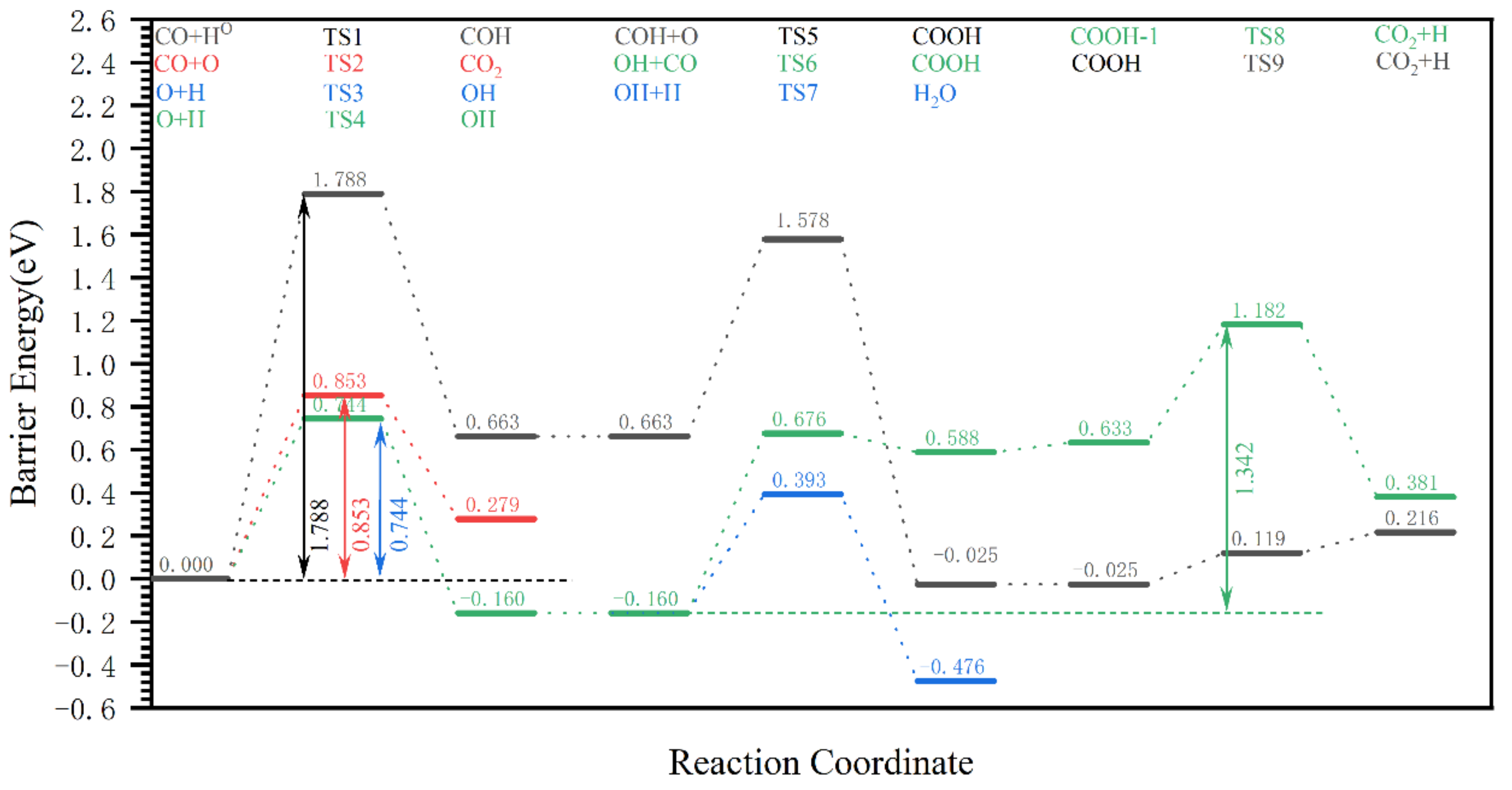
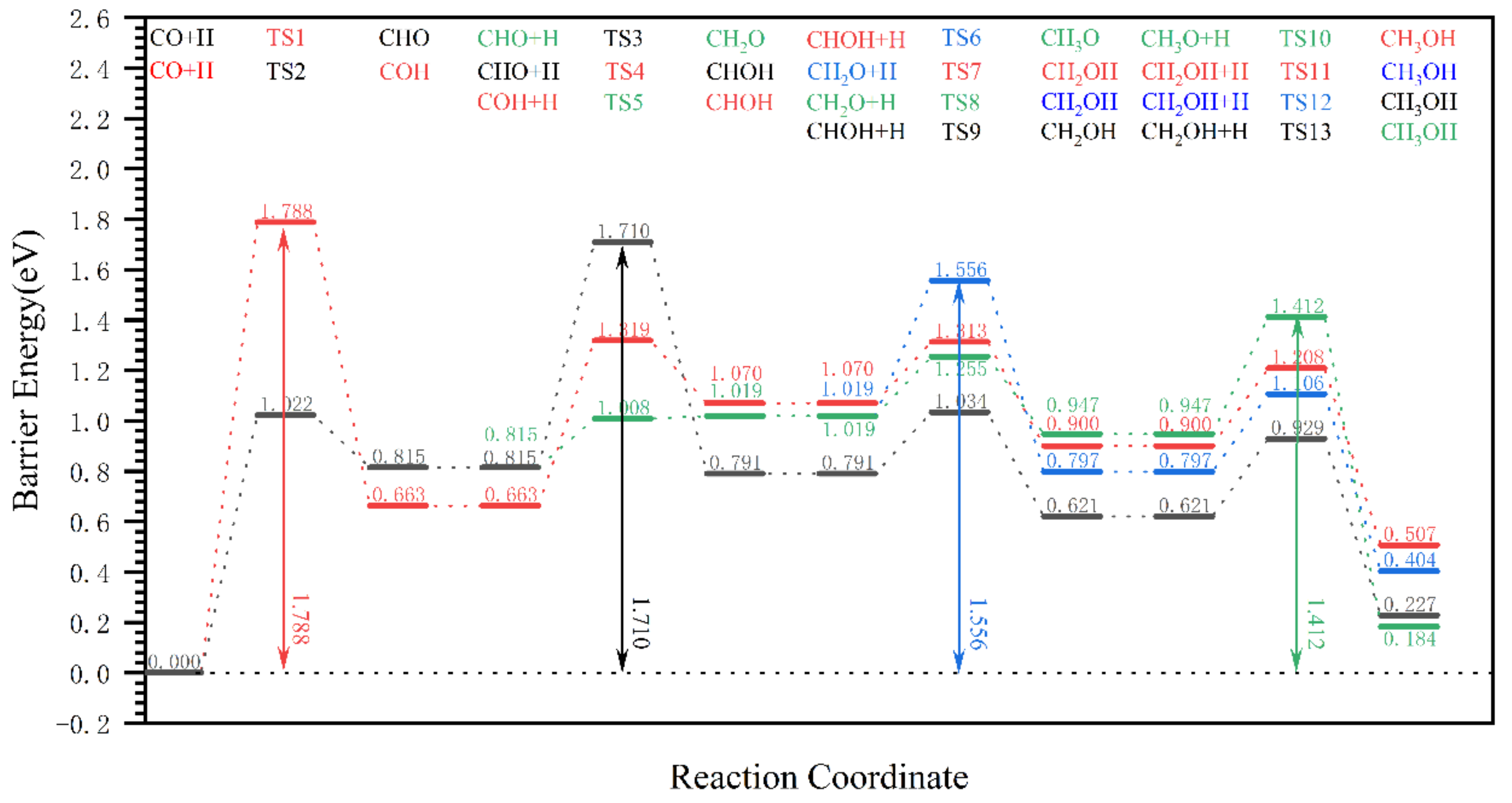
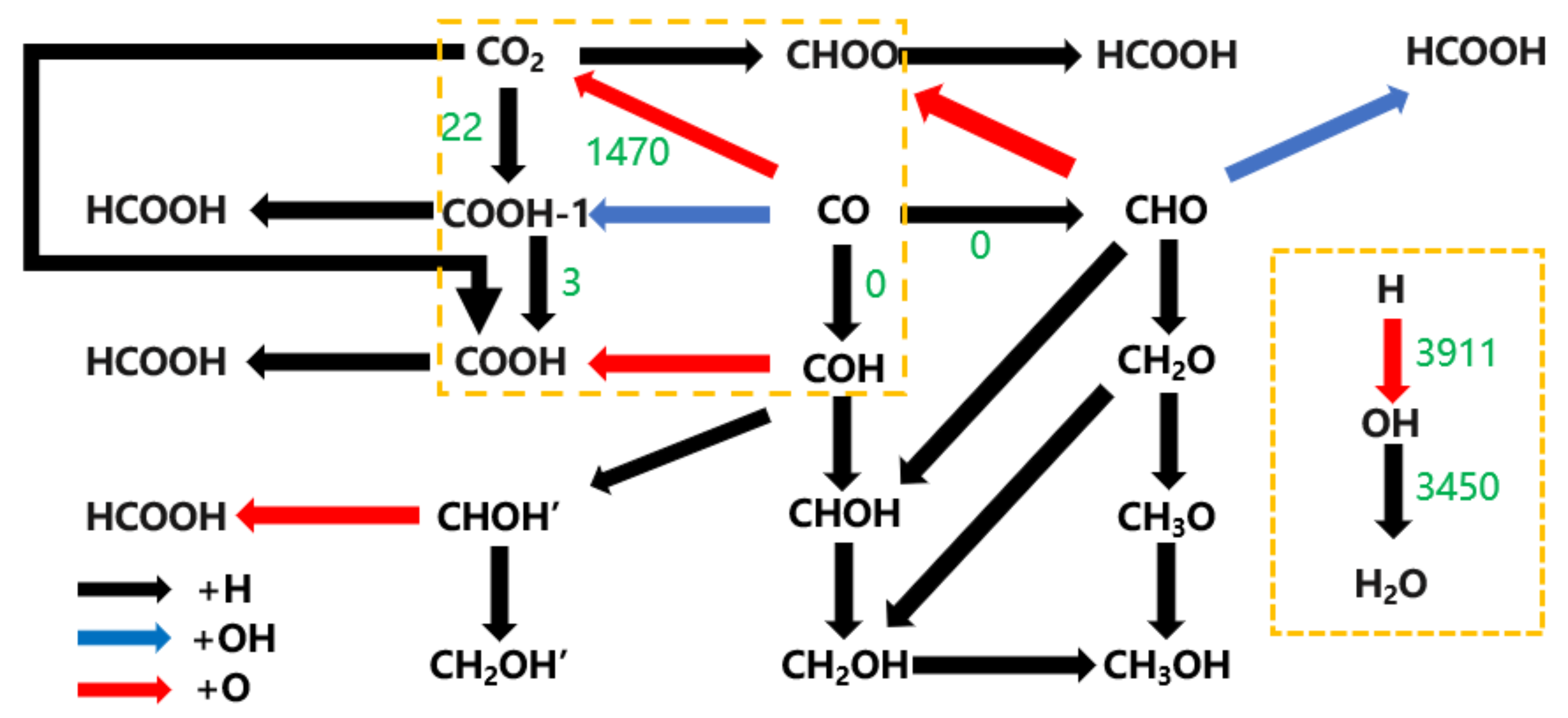
| Reaction Pathway | Step 1: Elementary Reaction | Step 2: Elementary Reaction | Step 3: Elementary Reaction |
|---|---|---|---|
| 1 | CO Oxygen side hydrogenation CO + HO → COH | COH carbon site oxygenated COH + O → COOH | COOH carbon site hydrogenation COOH + HC → HCOOH |
| 2 | COH carbon site hydrogenation COH + HC → CHOH | CHOH carbon site oxygenated CHOH + O → HCOOH | |
| 3 | CO carbon site hydrogenation CO + HC → CHO | CHO carbon site hydrogenation CHO + O → CHOO | CHOO Oxygen side hydrogenation CHOO + HO → HCOOH |
| 4 | CHO Oxygen side hydrogenation CHO + HO → CHOH | CHOH carbon site oxygenated CHOH + O → HCOOH | |
| 5 | CO carbon site hydrogenation CO + HC → CHO | Dissociation O hydrogenation O + H → OH | CHO reacts with OH HCO + OH → HCOOH |
| 6 | Dissociation O hydrogenation O + H → OH | OH attacks the carbon site of CO OH + CO → COOH | COOH carbon site hydrogenation COOH + HC → HCOOH |
| 7 | CO carbon site oxygenated CO + O → CO2 | Hydrogenation of CO2 at the O1 Site CO2 + H → COOH | COOH carbon site hydrogenation COOH + HC → HCOOH |
| 8 | CO carbon site oxygenated CO + O → CO2 | Hydrogenation of CO2 at the O2 CO2 + H → COOH | COOH carbon site hydrogenation COOH + HC → HCOOH |
| Elementary Reaction | Reaction Site | CHARGE | ||
|---|---|---|---|---|
| Initial State | Transition State | End State | ||
| 1. CO + H → CHO | H | 0.795 | 0.840 | 1.170 |
| C | 3.493 | 3.422 | 3.147 | |
| O | 7.013 | 7.002 | 7.070 | |
| 2. CO + H → COH | H | 1.184 | 0.584 | 0.277 |
| C | 3.126 | 3.665 | 3.757 | |
| O | 7.036 | 7.008 | 7.201 | |
| 3. CO + O → CO2 | Dissociated O | 6.820 | 6.774 | 7.012 |
| C | 3.120 | 3.092 | 2.542 | |
| O | 7.031 | 6.976 | 7.015 | |
| 4. CO + OH → COOH | H | 0.206 | 0.407 | 0.296 |
| C | 3.196 | 3.014 | 2.774 | |
| OH | 7.292 | 7.011 | 7.193 | |
| OC | 7.035 | 7.026 | 7.080 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, N.; Lai, C.; Hu, W.; Wang, Q.; Ren, J. Mechanistic Study on the Possibility of Converting Dissociated Oxygen into Formic Acid on χ-Fe5C2(510) for Resource Recovery in Fischer–Tropsch Synthesis. Molecules 2023, 28, 8117. https://doi.org/10.3390/molecules28248117
Ai N, Lai C, Hu W, Wang Q, Ren J. Mechanistic Study on the Possibility of Converting Dissociated Oxygen into Formic Acid on χ-Fe5C2(510) for Resource Recovery in Fischer–Tropsch Synthesis. Molecules. 2023; 28(24):8117. https://doi.org/10.3390/molecules28248117
Chicago/Turabian StyleAi, Ning, Changyi Lai, Wanpeng Hu, Qining Wang, and Jie Ren. 2023. "Mechanistic Study on the Possibility of Converting Dissociated Oxygen into Formic Acid on χ-Fe5C2(510) for Resource Recovery in Fischer–Tropsch Synthesis" Molecules 28, no. 24: 8117. https://doi.org/10.3390/molecules28248117
APA StyleAi, N., Lai, C., Hu, W., Wang, Q., & Ren, J. (2023). Mechanistic Study on the Possibility of Converting Dissociated Oxygen into Formic Acid on χ-Fe5C2(510) for Resource Recovery in Fischer–Tropsch Synthesis. Molecules, 28(24), 8117. https://doi.org/10.3390/molecules28248117






