Human Health Risk Assessment of the Photocatalytic Oxidation of BTEX over TiO2/Volcanic Glass
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst and Catalyst Support
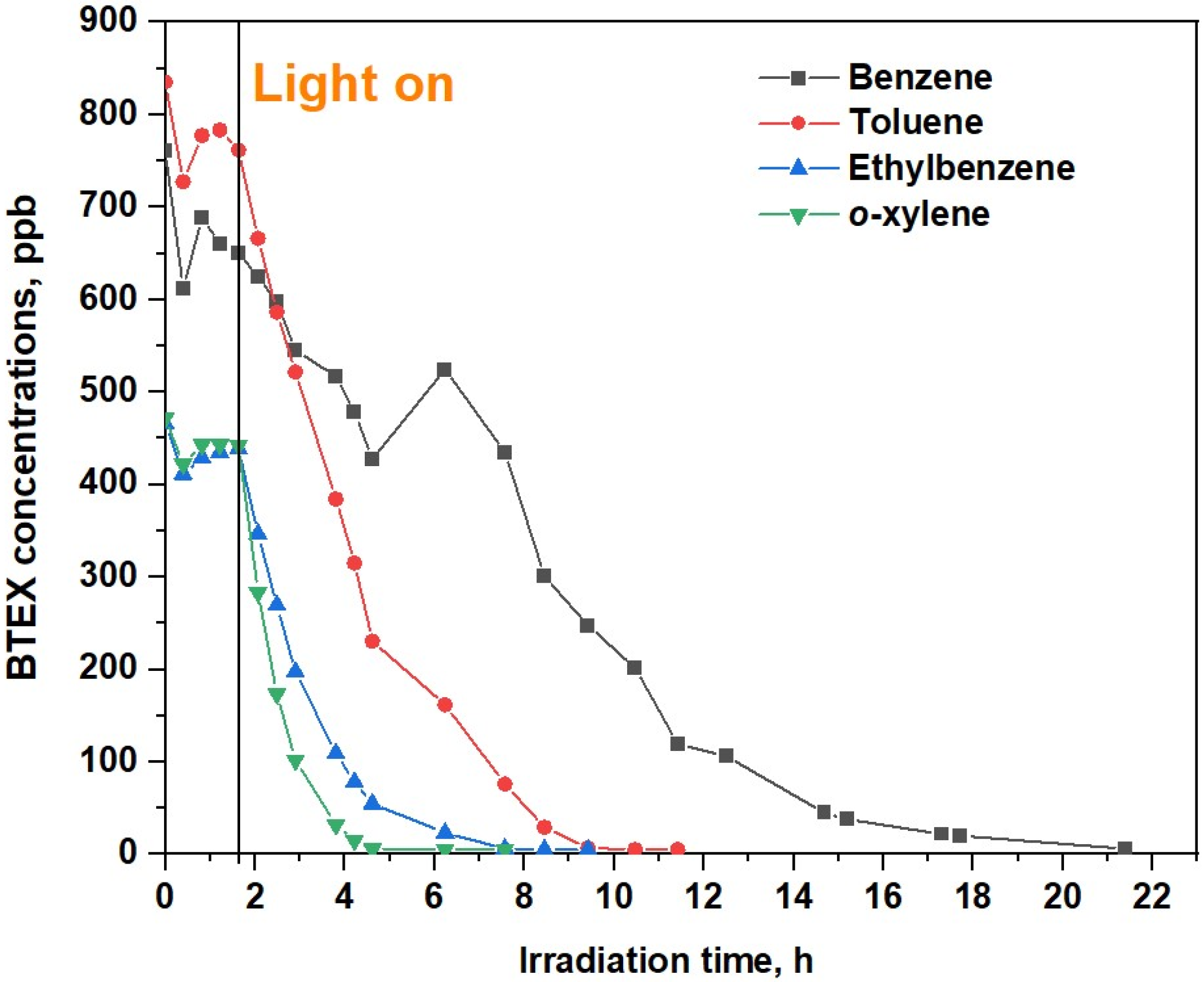
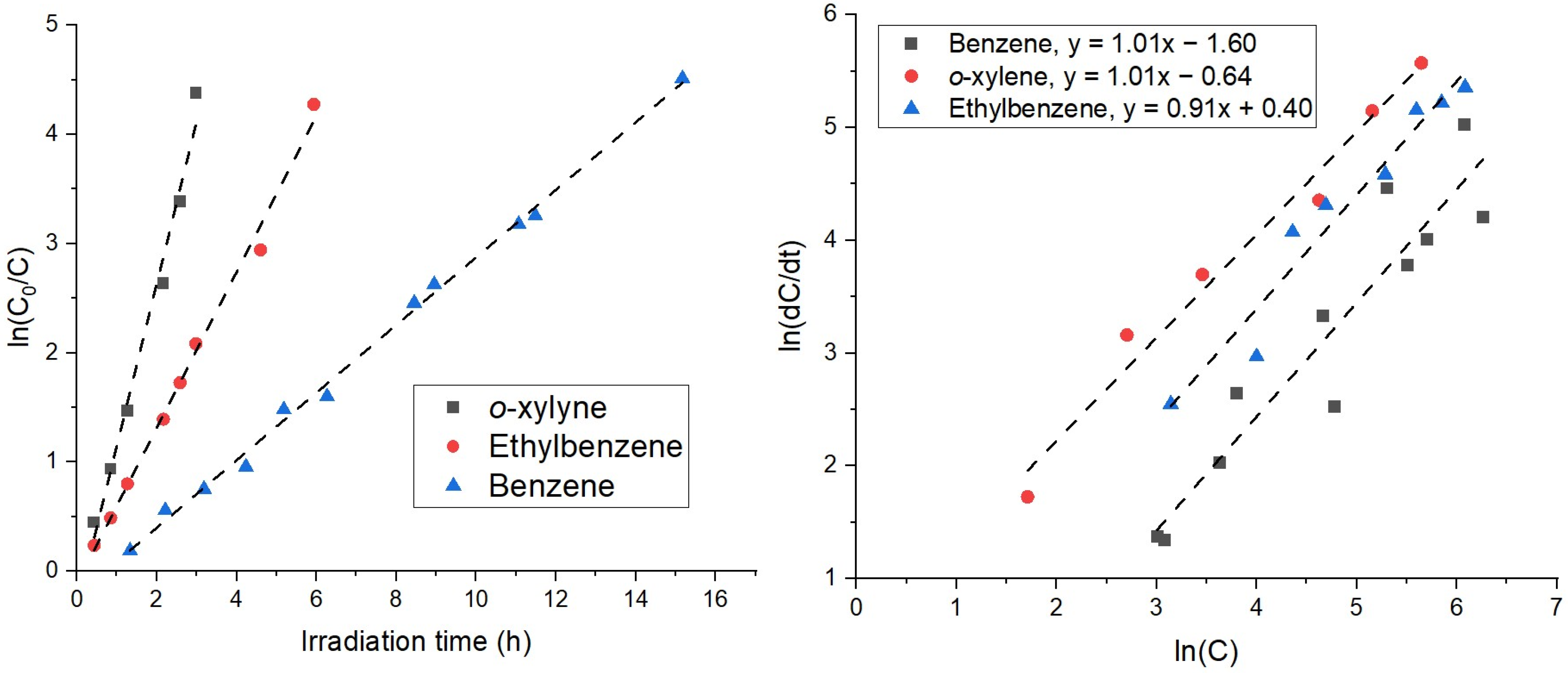
2.2. Recirculation Test in the Chamber
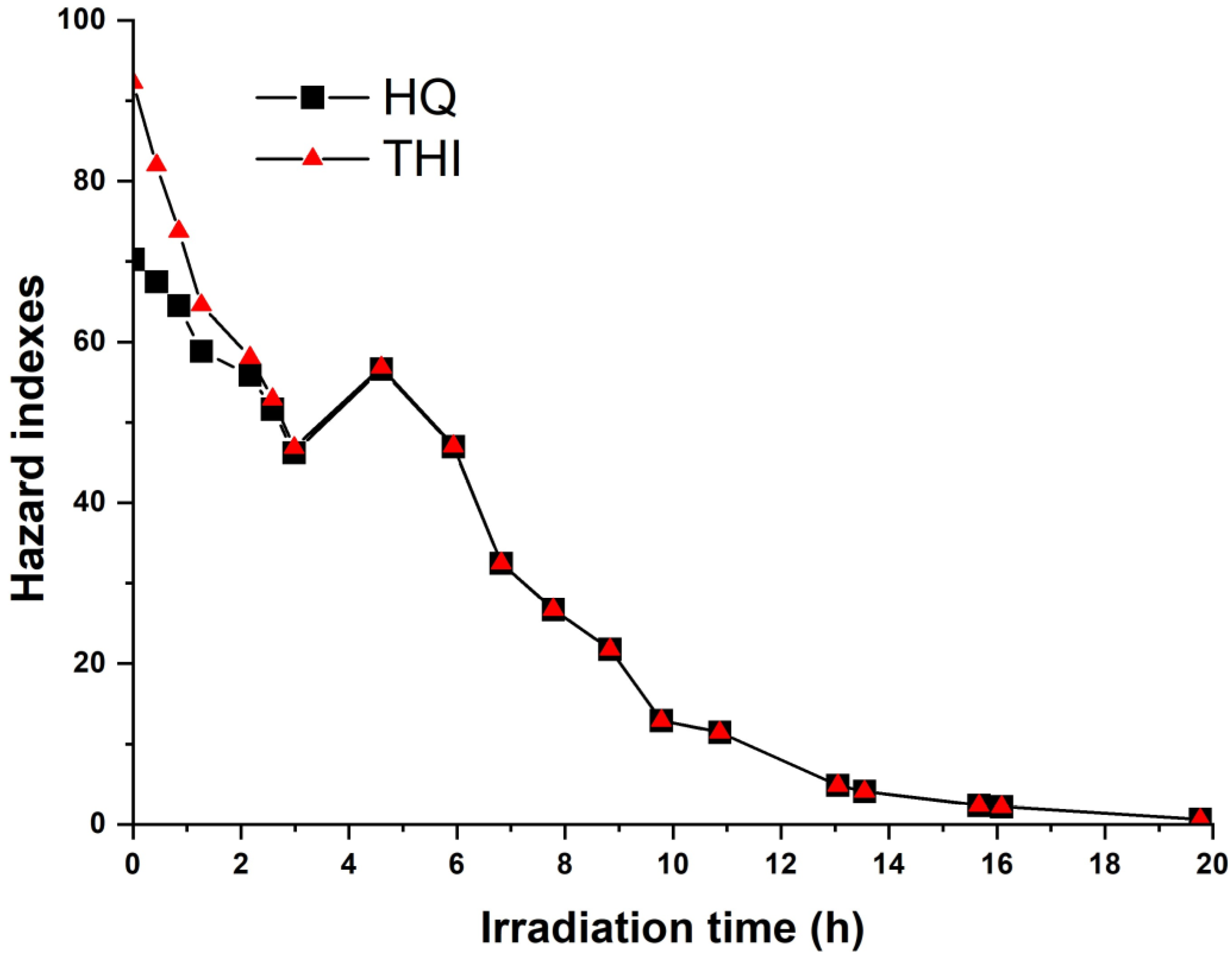
2.3. Hazard Quotients and Cancer Risk Estimates
| Pollutant | Benzene CAS 71-43-2 | Toluene CAS 108-88-3 | Ethylbenzene CAS 100-41-4 | Xylenes CAS 1330-20-7 CAS 106-42-3 CAS 108-38-3 CAS 95-47-6 | Ref. |
|---|---|---|---|---|---|
| DIRECTIVE 2008/50/EC | 5 μg/m3 Calendar year | - | - | - | [27] |
| EPA-Derived Reference Concentration for Inhalation Exposure (RfC) | 3 × 10−2 mg/m3 | 5 mg/m3 | 1 mg/m3 | XYLENES 1 × 10−1 mg/m3 | [28] |
| ACGIH, TLV | TWA 0.5 ppm STEL 2.5 ppm | TWA 20 ppm | TWA 20 ppm | [29] | |
| NIOSH, REL | Ca TWA 0.1 ppm ST 1 ppm | TWA 100 ppm (375 mg/m3) ST 150 ppm (560 mg/m3) | TWA 100 ppm (435 mg/m3) ST 125 ppm (545 mg/m3) | For each xylenes separately TWA 100 ppm (435 mg/m3) ST 150 ppm (655 mg/m3) | [30] |
| OSHA, PEL | TWA 1 ppm ST 5 ppm |
TWA 200 ppm C 300 ppm 500 ppm (10 min maximum peak) | TWA 100 ppm (435 mg/m3) | For each xylenes separately TWA 100 ppm (435 mg/m3) | [31] |
| ATSDR-Developed Minimal Risk Levels |
0.009 ppm (acute) 0.006 ppm (int) 0.003 ppm (Chr.) |
2 ppm (acute) 1 ppm (Chr.) |
5 ppm (acute) 2 ppm (int) 0.06 ppm (Chr.) |
XYLENES, MIXED 2 ppm (acute) 0.6 ppm (int) 0.05 ppm (Chr.) | [23] |
| Maximum permissible concentration—KZ |
0.1
mg/m3 (24 h) 0.3 mg/m3 single max | 0.6 mg/m3 single max | 0.02 mg/m3 single max | XYLENES, MIXED 0.2 mg/m3 | [25] |
| Inhalation Unit Risk | 7.8 × 10−6 per µg/m3 | [28] |
3. Materials and Methods
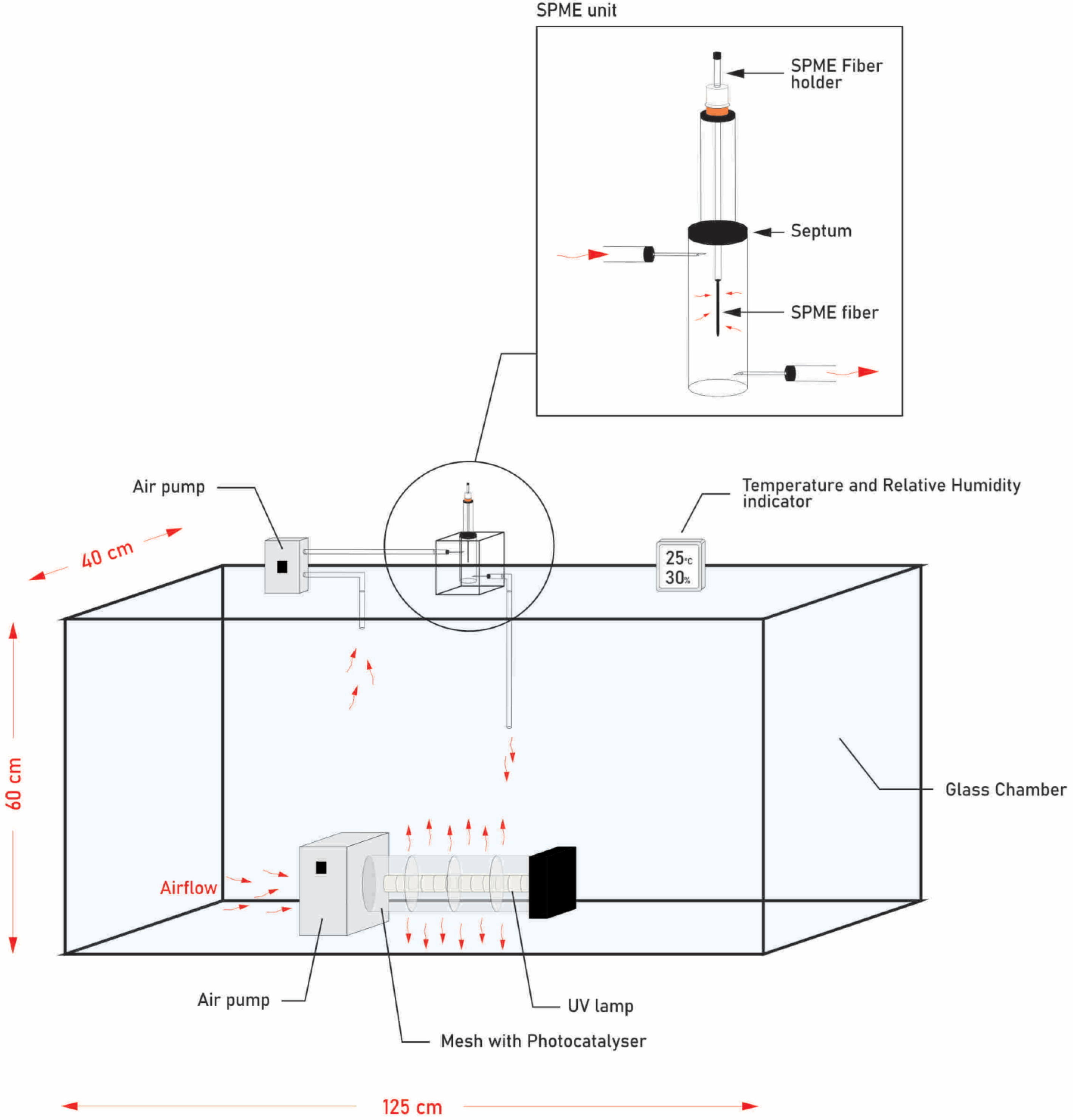
3.1. Experimental Set-Up
3.2. BTEX Concentration Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, X.; Chen, H.; Oliver, B.G. The Health Effects of Traffic-Related Air Pollution: A Review Focused the Health Effects of Going Green. Chemosphere 2022, 289, 133082. [Google Scholar] [CrossRef]
- Chen, H.; Oliver, B.G.; Pant, A.; Olivera, A.; Poronnik, P.; Pollock, C.A.; Saad, S. Effects of Air Pollution on Human Health—Mechanistic Evidence Suggested by in Vitro and in Vivo Modelling. Environ. Res. 2022, 212, 113378. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine Emissions with Air Pollutants and Greenhouse Gases and Their Control Technologies. J. Clean. Prod. 2022, 376, 134260. [Google Scholar] [CrossRef]
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New Look at BTEX: Are Ambient Levels a Problem? Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase—A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Chen, R.; Li, J.; Wang, H.; Chen, P.; Dong, X.; Sun, Y.; Zhou, Y.; Dong, F. Photocatalytic Reaction Mechanisms at a Gas–Solid Interface for Typical Air Pollutant Decomposition. J. Mater. Chem. A 2021, 9, 20184–20210. [Google Scholar] [CrossRef]
- Tulebekov, Y.; Orazov, Z.; Satybaldiyev, B.; Snow, D.D.; Schneider, R.; Uralbekov, B. Reaction Steps in Heterogeneous Photocatalytic Oxidation of Toluene in Gas Phase—A Review. Molecules 2023, 28, 6451. [Google Scholar] [CrossRef] [PubMed]
- Debono, O.; Hequet, V.; Le Coq, L.; Locoge, N.; Thevenet, F. VOC Ternary Mixture Effect on Ppb Level Photocatalytic Oxidation: Removal Kinetic, Reaction Intermediates and Mineralization. Appl. Catal. B Environ. 2017, 218, 359–369. [Google Scholar] [CrossRef]
- Dhada, I.; Sharma, M.; Nagar, P.K. Quantification and Human Health Risk Assessment of By-Products of Photo Catalytic Oxidation of Ethylbenzene, Xylene and Toluene in Indoor Air of Analytical Laboratories. J. Hazard. Mater. 2016, 316, 1–10. [Google Scholar] [CrossRef]
- Lyulyukin, M.N.; Kolinko, P.A.; Selishchev, D.S.; Kozlov, D.V. Hygienic Aspects of TiO2-Mediated Photocatalytic Oxidation of Volatile Organic Compounds: Air Purification Analysis Using a Total Hazard Index. Appl. Catal. B Environ. 2018, 220, 386–396. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Zhu, Y.; Lamson, J.J.; Zhao, R. Determination and Risk Assessment of By-Products Resulting from Photocatalytic Oxidation of Toluene. Appl. Catal. B Environ. 2009, 89, 570–576. [Google Scholar] [CrossRef]
- Hay, S.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.; Suib, S. The Viability of Photocatalysis for Air Purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Yao, C.; Ma, Y.; Zhao, J. Recent Advances of Photocatalytic Degradation for BTEX: Materials, Operation, and Mechanism. Chem. Eng. J. 2023, 455, 140461. [Google Scholar] [CrossRef]
- Orazov, Z.K.; Tulebekov, Y.; Bakhadur, A.M.; Uralbekov, B.M. Kinetic Model of Photocatalytic Oxidation of Dye (Orange II) by Superoxide Radicals. Chem. Bull. Kazakh Natl. Univ. 2023, 110, 4. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic Oxidation Technology for Indoor Environment Air Purification: The State-of-the-Art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Devine, J.D.; Gardner, J.E.; Brack, H.P.; Layne, G.D.; Rutherford, M.J. Comparison of Microanalytical Methods for Estimating H2O Contents of Silicic Volcanic Glasses. Am. Mineral. 1995, 80, 319–328. [Google Scholar] [CrossRef]
- Roy, B.N. Infrared Spectroscopy of Lead and Alkaline-Earth Aluminosilicate Glasses. J. Am. Ceram. Soc. 1990, 73, 846–855. [Google Scholar] [CrossRef]
- Wysoczanski, R.; Tani, K. Spectroscopic FTIR Imaging of Water Species in Silicic Volcanic Glasses and Melt Inclusions: An Example from the Izu-Bonin Arc. J. Volcanol. Geotherm. Res. 2006, 156, 302–314. [Google Scholar] [CrossRef]
- Obee, T.N.; Hay, S.O. The Estimation of Photocatalytic Rate Constants Based on Molecular Structure: Extending to Multi-Component Systems. J. Adv. Oxid. Technol. 1999, 4, 147–152. [Google Scholar]
- Dos Reis Vargas, M.; De Castro, E.A.S.; Politi, J.R.D.S.; Gargano, R.; Martins, J.B.L. BTEX Adsorption on TiO2 Anatase and Rutile Surfaces: DFT Functionals. J. Mol. Model. 2019, 25, 137. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Available online: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality (accessed on 3 November 2023).
- Agency for Toxic Substances and Disease Registry. ATSDR Website. Available online: https://wwwn.cdc.gov/tsp/mrls/mrlslisting.aspx (accessed on 3 November 2023).
- U.S. EPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); Office of Superfund Remediation and Technology Innovation Environmental Protection Agency: Washington, DC, USA, 2009; Volume I, pp. 1–68. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/partf_200901_final.pdf (accessed on 14 February 2022).
- Hygienic Standards for Atmospheric Air in Urban and Rural Settlements, in the Territories of Industrial Organizations. Order of the Minister of Health of the Republic of Kazakhstan Dated August 2, 2022 No. KR DSM-70. Available online: https://adilet.zan.kz/rus/docs/v2200029011#z10 (accessed on 3 November 2023).
- Ibragimova, O.P.; Omarova, A.; Bukenov, B.; Zhakupbekova, A.; Baimatova, N. Seasonal and Spatial Variation of Volatile Organic Compounds in Ambient Air of Almaty City, Kazakhstan. Atmosphere 2021, 12, 1592. [Google Scholar] [CrossRef]
- EU Parlament. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. Off. J. Eur. Union 2008, 29, 169–212. [Google Scholar]
- EPA Website. Available online: https://www.epa.gov/iris/basic-information-about-integrated-risk-information-system (accessed on 3 November 2023).
- American Conference of Governmental Industrial Hygienists. ACGIH Website. Available online: https://www.acgih.org/science/tlv-bei-guidelines/ (accessed on 3 November 2023).
- U.S. National Institute for Occupational Safety and Health. NIOSH Web Site. Available online: https://www.cdc.gov/niosh/npg/pgintrod.html (accessed on 3 November 2023).
- U.S. Occupational Safety and Health Administration. OSHA Web Site. Available online: https://www.osha.gov/annotated-pels (accessed on 3 November 2023).
- Pachoulis, M.; Maggos, T.; Panagopoulos, P.; Dasopoulou, M.; Balla, D.; Stamatelopoulou, A.; Manousakas, M.I.; Eleftheriadis, K.; Saraga, D. Population Health Risks Assessment from Air Pollution Exposure in an Industrialized Residential Area in Greece. Atmosphere 2022, 13, 615. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.; Shi, C.; Chang, Y.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.; et al. Identifying a Universal Activity Descriptor and a Unifying Mechanism Concept on Perovskite Oxides for Green Hydrogen Production. Adv. Mater. 2023, 35, 2305074. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Ibragimova, O.P.; Baimatova, N.; Kenessov, B. Low-Cost Quantitation of Multiple Volatile Organic Compounds in Air Using Solid-Phase Microextraction. Separations 2019, 6, 51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaiyl, M.; Tulebekov, Y.; Nurpeisov, N.; Satybaldiyev, B.; Snow, D.D.; Uralbekov, B. Human Health Risk Assessment of the Photocatalytic Oxidation of BTEX over TiO2/Volcanic Glass. Molecules 2023, 28, 8119. https://doi.org/10.3390/molecules28248119
Smaiyl M, Tulebekov Y, Nurpeisov N, Satybaldiyev B, Snow DD, Uralbekov B. Human Health Risk Assessment of the Photocatalytic Oxidation of BTEX over TiO2/Volcanic Glass. Molecules. 2023; 28(24):8119. https://doi.org/10.3390/molecules28248119
Chicago/Turabian StyleSmaiyl, Madi, Yerzhigit Tulebekov, Nurbek Nurpeisov, Bagdat Satybaldiyev, Daniel D. Snow, and Bolat Uralbekov. 2023. "Human Health Risk Assessment of the Photocatalytic Oxidation of BTEX over TiO2/Volcanic Glass" Molecules 28, no. 24: 8119. https://doi.org/10.3390/molecules28248119
APA StyleSmaiyl, M., Tulebekov, Y., Nurpeisov, N., Satybaldiyev, B., Snow, D. D., & Uralbekov, B. (2023). Human Health Risk Assessment of the Photocatalytic Oxidation of BTEX over TiO2/Volcanic Glass. Molecules, 28(24), 8119. https://doi.org/10.3390/molecules28248119







