Insights on Juniperus phoenicea Essential Oil as Potential Anti-Proliferative, Anti-Tyrosinase, and Antioxidant Candidate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield and Composition of the Essential Oils
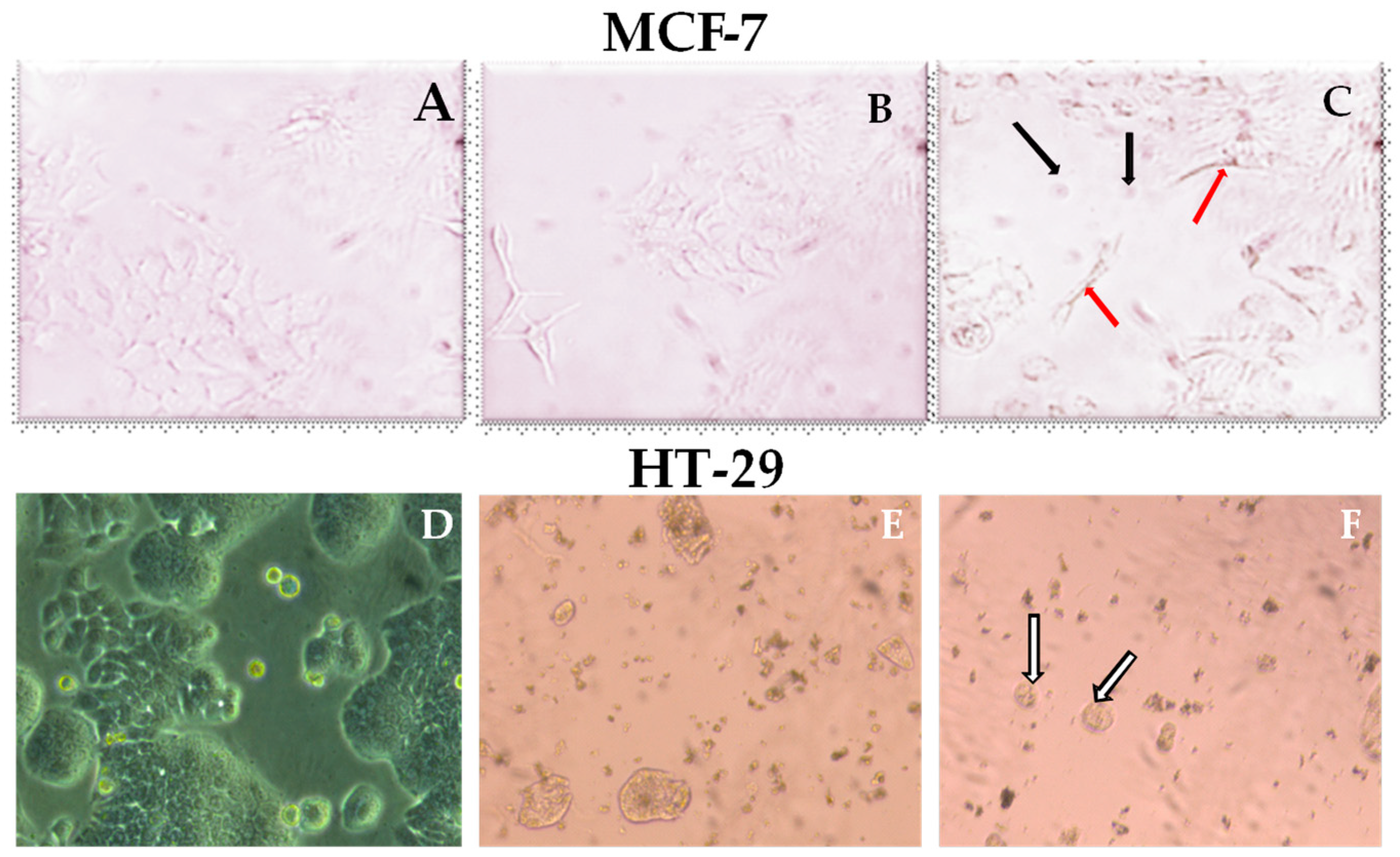
2.2. Cytotoxic Activity of J. phoenicia L. Essential Oils
2.3. Tyrosinase Inhibition Activities of J. phoenicea EOs
2.4. Antioxidant Activity of J. phoenicia L. Essential Oils
3. Materials and Methods
3.1. Plant Material and Essential Oil Extraction
3.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.3. Anti-Proliferative Effect by Resazurin Assay
3.4. Determination of Tyrosinase Inhibition Effects
3.5. Evaluation of Antioxidant Capacities of J. phoenicia EO
3.5.1. DPPH Scavenging Assay
3.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Found. Sci. Technol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical composition and in vitro antibacterial activity of the essential oil of Cedrus atlantica. Int. J. Agric. Biol. 2010, 12, 381–385. [Google Scholar]
- Oladipupo, A.; Lawal, I.; Ogunwande, A. Essential oils from the medicinal plants of Africa. J. Med. Plants Res. 2013, 203–224. [Google Scholar] [CrossRef]
- Ahmad, A.; Viljoen, A. The in vitro antimicrobial activity of Cymbopogon essential oil (lemon grass) and its interaction with silver ions. Phytomedicine 2015, 22, 657–665. [Google Scholar] [CrossRef]
- Piaru, S.P.; Perumal, S.; Cai, L.W.; Mahmud, R.; Majid, A.M.S.; Ismail, S.; Man, C.N. Chemical composition, anti-angiogenic and cytotoxicity activities of the essential oils of Cymbopo gancitratus (lemon grass) against colorectal and breast carcinoma cell lines. J. Essent. Oil Res. 2013, 24, 453–459. [Google Scholar] [CrossRef]
- Obaid, R.; Mughal, J.; Naeem, E.U.; Sadiq, N.; Alsantali, A.; Jassas, R.I.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, K.; Mizerska-Kowalska, M.; Zdzisińska, B.; Cytarska, J.; Baranowska-łączkowska, A.; Jaroch, K.; Łuczykowski, K.; Płaziński, W.; Bojko, B.; Kruszewski, S.; et al. Tropinone-derived alkaloids as potent anticancer agents: Synthesis, tyrosinase inhibition, mechanism of action, DFT calculation, and molecular docking studies. Int. J. Mol. Sci. 2020, 21, 9050. [Google Scholar] [CrossRef] [PubMed]
- Boateng, S.T.; Roya, T.; Torrey, K.; Owunna, U.; Banang-Mbeumia, S.; Basnet, D.; Niedda, E.; Alexander, A.D.; El Hagec, D.; Atchimnaidu, S.; et al. Synthesis, in silico modelling, and in vitro biological evaluation of substituted pyrazole derivatives as potential anti-skin cancer, anti-tyrosinase, and antioxidant agents. J. Enzym. Inhib. Med. Chem. 2023, 38, 2205042. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Chang, W.L.; Chang, C.T.; Hsu, S.L.; Lin, Y.C.; Shih, Y. Cinnamomum cassia essential oil inhibits α-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef]
- Yuanyuan, W.; Baichen, X.; Shuaishuai, X.; Ying, C.; Qinghong, L.; Jun, M.; Yao, C.; Qi, L.; Haopeng, S. Medicinal Prospects of Targeting Tyrosinase: A Feature Review. Curr. Med. Chem. 2023, 30, 2638–2671. [Google Scholar]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S. Afr. J. Bot. 2016, 103, 89–94. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; Van Staden, A.B.; Hosenally, M.; Mahomoodally, M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchia, S.; Bras-Gonc, R.; Bradaiac, A. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004, 215, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Jugreeta, S.B.; Suroowana, S.; Rengasamy, R.R.K.; Mahomoodally, F.M. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Singla, R.K.; De, R.; Efferth, T.; Mezzetti, B.; Uddin, M.S.; Ntie-Kang, F.S.; Wang, D.; Schultz, F.; Kharat, K.R.; Devkota, H.P.; et al. The International Natural Product Sciences Taskforce (INPST) and the power of Twitter networking exemplified through #INPST hashtag analysis. Phytomedicine 2023, 108, 154520. [Google Scholar]
- Wazny, K. Applications of crowdsourcing in health: An overview. J. Glob. Health 2018, 8, 010502. [Google Scholar] [CrossRef]
- Aouadi, M.; Msaada, K.; Sebaia, E.; AidiWannes, W.; Abbassid, M.S.; Akkari, H. Antioxidant, anthelmintic and anti-bacterial activities of red juniper (Juniperus phoenicea L.) essential oil. J. Essent. Oil 2021, 34, 163–172. [Google Scholar]
- Terfaya, B.; Makhloufi, A.; Mekboul, A.; Benlarbi, L.; Abdelouahi, D. In vitro Antifusarial Activity of a Tar Extracted from the Juniperus phoenicea L. Wild in outhwest of Algeria. Phytothérapie 2021, 19, 243–249. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Wasli, H.; Serairi-Beji, R.; Bourgou, S.; Dakhlaoui, S.; Selmi, S.; Khamessi, S.; Hammami, M.; Ksouri, R.; Megdiche-Ksouri, W. In vivo gastroprotective effect and biological potentialities of six Tunisian medicinal plants using multivariate data treatment. Plant Biosyst. 2020, 156, 152–163. [Google Scholar] [CrossRef]
- Bellakhder, J. La Pharmacopée Marocaine Traditionnelle; Ibis Press: Paris, France, 1997; p. 272. [Google Scholar]
- Amer, M.M.A.; Wasif, M.M.; Abo-Aytta, A.M. Chemical and biological evaluation of Juniperus phoenicea as a hypoglycaemic agent. J. Agric. Res. 1994, 21, 1077–1091. [Google Scholar]
- Vourlioti-Arapi, F.; Michaelakis, A.; Evergetis, E.; Koliopoulos, G.; Haroutounian, S.A. Essential oils of indigenous in Greece six Juniperus taxa. Parasitol. Res. 2012, 110, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- El-Sawi, S.A.; Motawae, H.M.; Ali, A.M. Chemical composition, cytotoxic activity and antimicrobial activity of essential oils of leaves and berries of Juniperus phoenicea L. grown in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Keskes, H.; Mnafgui, K.; Hamden, K.; Damak, M.; El Feki, A.; Allouche, N. In vitro antidiabetic, anti-obesity and antioxidant proprieties of Juniperus phoenicea L. leaves from Tunisia. Asian Pac. J. Trop. Biomed. 2014, 4, 649–655. [Google Scholar] [CrossRef]
- Bouyahyaoui, A.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity and chemical analysis of the essential oil of Algerian Juniperus phoenicea. Nat. Prod. Commun. 2016, 11, 519–522. [Google Scholar] [CrossRef]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-Scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. Oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Sci. 2016, 2016, 6392656. [Google Scholar] [PubMed]
- Ennajar, M.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Savagnac, A.; Abderraba, M.; Romdhane, M.J. The influence of organ, season and drying method on chemical composition and antioxidant and antimicrobial activities of Juniperus phoenicea L. essential oils. J. Sci. Food Agric. 2010, 90, 462–470. [Google Scholar] [CrossRef]
- Medini, H.; Elaissi, A.; Khouja, M.L.; Piras, A.; Porcedda, S.; Falconieri, D.; Marongiu, B.; Chemli, R. Chemical composition and antioxidant activity of the essential oil of Juniperus phoenicea L. berries. Nat. Prod. Res. 2011, 25, 1695–1706. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; ÇolAyvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six Algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef]
- Ennajar, M.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Abderraba, M.; Raies, A.; Romdhane, M.J. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea L. (Cupressacees). Food Sci. 2009, 74, 364–371. [Google Scholar] [CrossRef]
- Adams, R.P.; Barrero, A.F.; Lara, A. Comparisons of the Leaf Essential Oils of Juniperus phoenicea, J. phoenicea subsp. Eumediterranea Lebr. & Thiv. and J. phoenicea var. turbinata (Guss.) Parl. J. Essent. Oil Res. 1996, 8, 367–371. [Google Scholar]
- Ramdani, M.; Lograda, T.; Silini, H.; Zeraib, A.; Chalard, P.; Figueredo, G.; Bouchaala, M.; Zerrar, S. Antibacterial activity of essential oils of Juniperus phoenicea from eastern Algeria. J. App. Pharm. Sci. 2013, 3, 022–028. [Google Scholar]
- Rahhal, R.; Hajjouji, H.; Gmouh, S.; Hsaine, M.; Fougrach, H.; Badri, W. Chemical composition, antioxidant and anti-bacterial activities of the essential oils of Juniperus phoenicea, Juniperus thurifera and Juniperus oxycedrus. Mediterr. J. Chem. 2019, 9, 190–198. [Google Scholar]
- Bettaieb, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential oils, phenolics, and antioxidant Activities of different parts of cumin (Cuminumcyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Achak, N.; Romane, A.; Alifriqui, M.; Adams, P.R. Chemical studies of leaf essential oils of three species of Juniperus from Tensift Al Haouz-Marrakech Region (Morocco). J. Essent. Oil Res. 2009, 21, 337–341. [Google Scholar] [CrossRef]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Quilez Del Moral, J.F.; Sanchez-Fernandez, E.; Akssira, M.; Aitigri, M.; Mellouki, F.; Akkad, S. Chemical composition of the essential oil from the leaves of Juniperus phoenicea L. from North Africa. J. Essent. Oil Res. 2006, 18, 168–169. [Google Scholar] [CrossRef]
- Harmouzi, A.; Boughdad, A.; El Ammari, Y.; Chaouch, A. Chemical composition and toxicity of Moroccan Tetracli-nisarticulata and Juniperus phoenicea essential oils against Aphis citricola Goot, 1912 (Homoptera, Aphididae). Res. Chem. Intermed. 2016, 42, 7185–7197. [Google Scholar] [CrossRef]
- Chaftar, N.; Girardot, M.; Quellard, N.; Labanowski, J.; Ghrairi, T.; Hani, K.; Frère, J.; Imbert, C. Activity of six essential oils extracted from Tunisian plants against Legionella pneumophila. Chem. Biodivers. 2015, 12, 1565–1574. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Rezzi, S.; Salgueiro, L.; Bighelli, A.; Casanova, J.; da Cunha, A.P. Infraspecific chemical variability of the leaf essential oil of Juniperus phoenicea var. turbinata from Portugal. Biochem. Syst. Ecol. 2001, 29, 1175–1183. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Eur. Environ. Bur. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Bourgou, S.; Ezzine, Y.; Ben Mansour, R.; Dakhlaoui, S.; Selmi, S.; Bachkouel, S.; Msaada, K.; Aidi-Wannes, W.; Hiroko, I.; Megdiche-Ksouri, W. Preliminary phytochemical analysis, antioxidant, anti-inflammatory and anticancer activities of two Tunisian Ephedra species: Ephedra alata and Ephedra fragilis. S. Afr. J. Bot. 2020, 135, 1–8. [Google Scholar]
- Meringolo, L.; Bonesi, M.; Sicari, V.; Rovito, S.; Passalacqua, N.G.; Rosa Loizzo, M.; Tundis, R. Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities. Plants 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Pistelli, L.; Volatilomic, J.B. Analyses of Tuscan Juniperus oxycedrus L. and in vitro cytotoxic effect of its essential oils on human cell lines. Tenant Energy Optim. Program 2020, 23, 756–771. [Google Scholar] [CrossRef]
- Cole, R.A.; Bansa, A.; Moriarity, D.M.; Haber, W.A.; Setzer, W.N. Chemical composition and cytotoxic activity of the leaf essential oil of Eugenia zuchowskiae from Monteverde, Costa Rica. J. Nat. Med. 2007, 61, 414–417. [Google Scholar] [CrossRef]
- Fraternale, A.; Crinelli, R.; Casabianca, A.; Paoletti, M.F.; Orlandi, C.; Carloni, E.; Smietana, M.; Palamara, A.T.; Magnani, M. Molecules altering the intracellular thiol content modulate NF-kB and STAT-1/IRF-1 signalling pathways and IL-12 p40 and IL27 p28 production in murine macrophages. PLoS ONE 2013, 8, e57866. [Google Scholar] [CrossRef]
- Jin, K.S.; Bak, M.J.; Jun, M.; Lim, H.J.; Jo, W.K.; Jeong, W.S. α-Pinene triggers oxidative stress and related signaling pathways in A549 and HepG2 cells. Food Sci. Biotechnol. 2010, 19, 1325–1332. [Google Scholar] [CrossRef]
- Manuele, M.G.; Ferraro, G.; Anesini, C. Effect of Tilia × viridis flower extract on the proliferation of a lymphoma cell line and on normal murine lymphocytes: Contribution of monoterpenes, especially limonene. Phytother. Res. 2008, 22, 1520–1526. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Y.; Zhu, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. Basic Res. 2019, 25, 6631–6638. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Yang, M. α-Pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 3, 30473536. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Tarik, A.I.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar]
- Habachi, E.; Bettaieb Rebey, I.; Dakhlaoui, S.; Hammami, M.; Sawsen, S.; Msaada, K.; Merah, O.; Bourgou, S. Arbutus unedo: Innovative Source of antioxidant, anti-Inflammatory and anti-tyrosinase phenolics for novel cosmeceuticals. Cosmetics 2022, 9, 143. [Google Scholar] [CrossRef]
- Fiocco, D.; Fiorentino, D.; Frabboni, L.; Benvenuti, S.; Orlandini, G.; Pellatic, F.; Gallone, A. Lavender and peppermint essential oils as effective mushroom tyrosinase inhibitors: A basic study. Flavour Fragr. J. 2011, 26, 441–446. [Google Scholar] [CrossRef]
- Ho, J.C. Chemical composition and bioactivity of essential oil of seed and leaf from Alpinia speciosa grown in Taiwan. J. Chin. Chem. Soc. 2010, 57, 758–763. [Google Scholar] [CrossRef]
- Masturra, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J Agric. Food Chem. 2006, 22, 2309–2913. [Google Scholar]
- Yang, C.H.; Huang, Y.C.; Tsai, M.L.; Cheng, C.Y.; Liu, L.L.; Yen, Y.W.; Chen, W.L. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 2015, 37, 550–554. [Google Scholar] [CrossRef]
- Albrecht, U.W.; Madisch, A. Therapeutic potentials associated with biological properties of Juniper berry oil (Juniperus communis L.) and its therapeutic use in several diseases—A Review. Bioact. Compd. Health Dis. 2022, 5, 174–185. [Google Scholar] [CrossRef]
- Chang, T.S. An Updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Bouzouita, N.; Kachouri, F.; Ben Halima, M.; Chaabouni, M.M. Composition chimique et activités antioxydante, antimicrobienne et insecticide de l’huile essentielle de Juniperus phœnicea. J. Société Chim. Tunis. 2008, 10, 119–125. [Google Scholar]
- Wang, C.Y.; Chen, Y.W.; Hou, C.Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Inter. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef] [PubMed]
- In-Young, C.; Lim, H.; Hwang, S.; Lee, J.C.; Cho, G.S.; Kim, W.K. Anti-ischemic and anti-inflammatory activity of (S)-cis-verbenol. Free Radic. Res. 2010, 44, 541–551. [Google Scholar]
- Ben Mansour, R.; Beji, R.S.; Wasli, H.; Zekri, S.; Ksouri, R.; Megdiche-Ksouri, W.; Cardoso, S.M. Gastroprotective Effect of Microencapsulated Myrtus communis Essential Oil against Ethanol/HCl-Induced Acute Gastric Lesions. Molecules 2022, 27, 1566. [Google Scholar] [CrossRef]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxy loninerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Wasli, H.; Jelali, N.; Silva, A.M.S.; Ksouri, R.; Cardoso, S.M. Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (Anethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Ind. Crops Prod. 2018, 126, 466–467. [Google Scholar] [CrossRef]



| Relative Percentage | ||||
|---|---|---|---|---|
| Compounds | KI a | KI b | Leaves | Berries |
| α-Pinene | 940 | 939 | 44.2 | 83.6 |
| Verbenene | 975 | 967 | - | 0.8 |
| β-Pinene | 981 | 980 | 1.1 | 1.4 |
| β-Myrcene | 988 | 991 | 3.1 | 0.9 |
| α-Phellandrene | 1005 | 1005 | 2.1 | - |
| δ-3-Carene | 1011 | 1011 | 2.4 | 1.7 |
| o-Cymene | 1028 | 1026 | 0.9 | - |
| Limonene | 1032 | 1031 | - | 1.7 |
| β-Phellandrene | 1031 | 1031 | 18.0 | - |
| α-Terpinolene | 1089 | 1088 | 1.5 | - |
| Campholene aldehyde | 1025 | 1025 | - | 1.3 |
| Trans-verbenol | 1144 | 1144 | - | 2.1 |
| Camphor | 1145 | 1143 | - | 1.0 |
| Trans-p-Menth-2-Ene-1,8-Diol | 1340 | 1344 | - | 2.5 |
| Camphene | 954 | 953 | 15.0 | 1.5 |
| Trans-caryophyllene | 1420 | 1418 | 1.5 | - |
| β-Selinene | 1419 | 1419 | 0.9 | - |
| Germacrene-D | 1480 | 1481 | 1.5 | - |
| (+)-epi-Bicyclosesquiphellandrene | 1482 | 1482 | 0.8 | - |
| δ-cadinene | 1525 | 1524 | 2.2 | - |
| Elemol | 1547 | 1549 | 0.7 | - |
| γ-Elemene | 1429 | 1429 | 1.5 | - |
| α-Longipinene | 1345 | 1347 | 1.2 | - |
| β-Bisabolene | 1507 | 1509 | - | 1.4 |
| α-Cadinol | 1653 | 1653 | 1.3 | - |
| Monoterpenes hydrocarbons | 88.3 | 91.6 | ||
| Oxygenated monoterpenes | - | 6.9 | ||
| Sesquiterpenes hydrocarbons | 9.6 | 1.4 | ||
| Oxygenated sesquiterpenes | 2.1 | - | ||
| Yield | 1.69 | 0.45 | ||
| Cell Type | Leaves | Berries |
|---|---|---|
| HT-29 | 38 ± 0.98 a | 15 ± 0.43 b |
| MCF-7 | 40 ± 1.22 b | 60 ± 2.14 a |
| H9C2 | 12 ± 0.77 a | 12 ± 0.93 a |
| Samples | Monophenolase (μg/mL) | Diphenolase (μg/mL) |
|---|---|---|
| Leaves | 944 ± 0.22 a | 371 ± 0.42 a |
| Berries | 455 ± 1.19 b | 109 ± 0.74 b |
| Kojic-acid | 7.06 ± 0.24 | 52.01 ± 1.98 |
| Variables | Monophenolase | Diphenolase | α-Pinene | β-Phellandrene | Camphene |
|---|---|---|---|---|---|
| Monophenolase | 1 | 1 | −0.91 | −0.94 | −0.99 |
| Diphenolase | 1 | 1 | −0.87 | −1 | −0.96 |
| α-Pinene | −0.91 | −0.87 | 1 | 0.74 | 0.99 |
| β-Phellandrene | −0.94 | −1 | 0.74 | 1 | 0.81 |
| Camphene | −0.99 | −0.96 | 0.99 | 0.81 | 1 |
| Samples | DPPH Test IC50 | FRAP Test EC50 |
|---|---|---|
| Leaf (mg/mL) | 11.5 ± 0.04 b | 14.5 ± 1.67 b |
| Berry (mg/mL) | 14.0 ± 3.25 a | 17.0 ± 0.96 a |
| BHT (µg/mL) * | 19.5 ± 1.33 * | ND |
| Vitamin C (µg/mL) * | 14.5 ± 0.52 * | 28.3 ± 0.98 * |
| α-Tocopherol (µg/mL) * | 17.8 ± 1.78 * | 22.5 ± 3.77 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, R.B.; Wasli, H.; Bourgou, S.; Khamessi, S.; Ksouri, R.; Megdiche-Ksouri, W.; Cardoso, S.M. Insights on Juniperus phoenicea Essential Oil as Potential Anti-Proliferative, Anti-Tyrosinase, and Antioxidant Candidate. Molecules 2023, 28, 7547. https://doi.org/10.3390/molecules28227547
Mansour RB, Wasli H, Bourgou S, Khamessi S, Ksouri R, Megdiche-Ksouri W, Cardoso SM. Insights on Juniperus phoenicea Essential Oil as Potential Anti-Proliferative, Anti-Tyrosinase, and Antioxidant Candidate. Molecules. 2023; 28(22):7547. https://doi.org/10.3390/molecules28227547
Chicago/Turabian StyleMansour, Rim Ben, Hanen Wasli, Soumaya Bourgou, Saber Khamessi, Riadh Ksouri, Wided Megdiche-Ksouri, and Susana M. Cardoso. 2023. "Insights on Juniperus phoenicea Essential Oil as Potential Anti-Proliferative, Anti-Tyrosinase, and Antioxidant Candidate" Molecules 28, no. 22: 7547. https://doi.org/10.3390/molecules28227547
APA StyleMansour, R. B., Wasli, H., Bourgou, S., Khamessi, S., Ksouri, R., Megdiche-Ksouri, W., & Cardoso, S. M. (2023). Insights on Juniperus phoenicea Essential Oil as Potential Anti-Proliferative, Anti-Tyrosinase, and Antioxidant Candidate. Molecules, 28(22), 7547. https://doi.org/10.3390/molecules28227547








