Absorption, Distribution, Metabolism, and Excretion of [14C]BS1801, a Selenium-Containing Drug Candidate, in Rats
Abstract
:1. Introduction
2. Results
2.1. Mass Balance
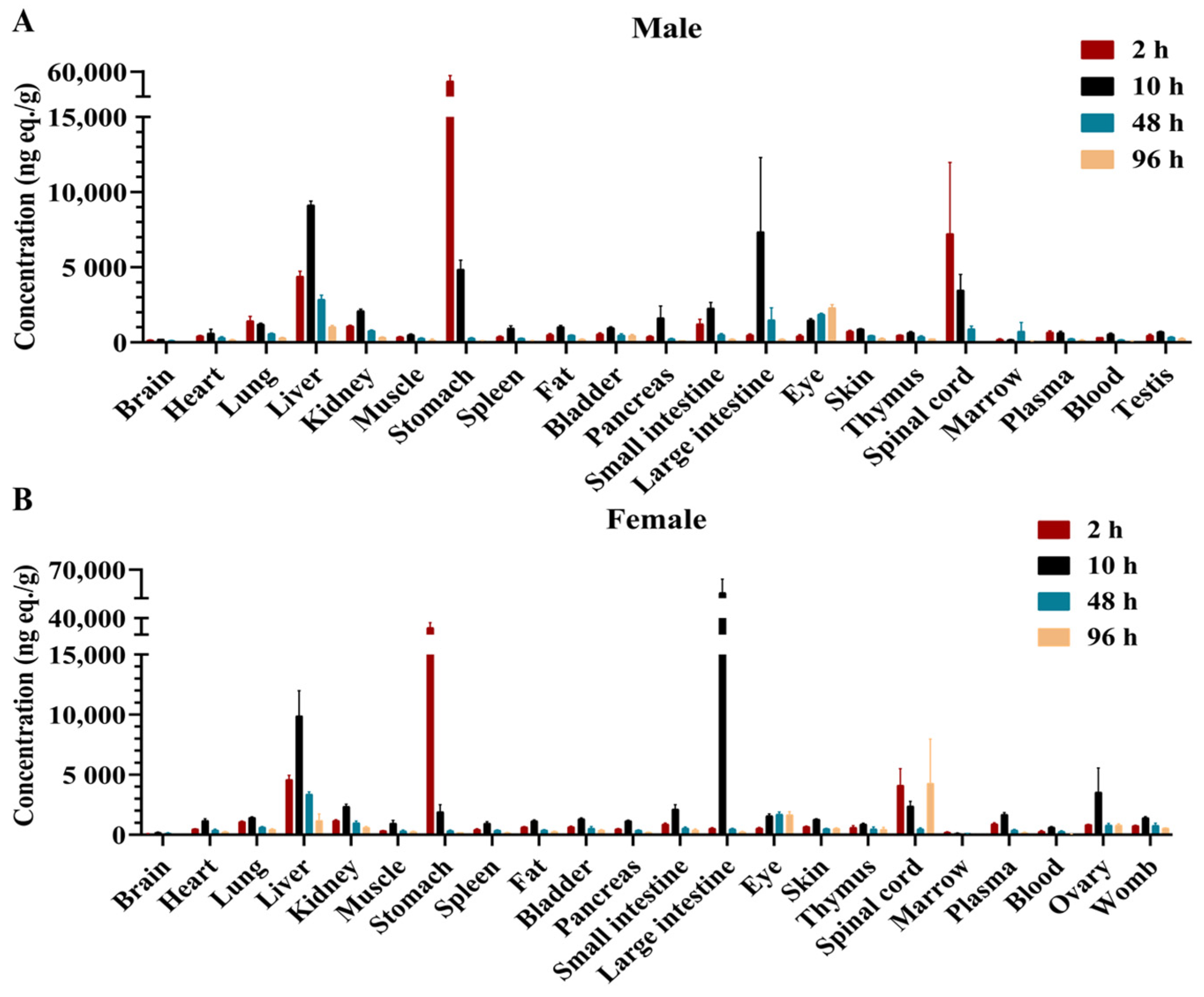
2.2. Tissue Distribution
2.3. Metabolite Identification
2.3.1. HR-MS Analysis of BS1801 and M2 Reference Standards
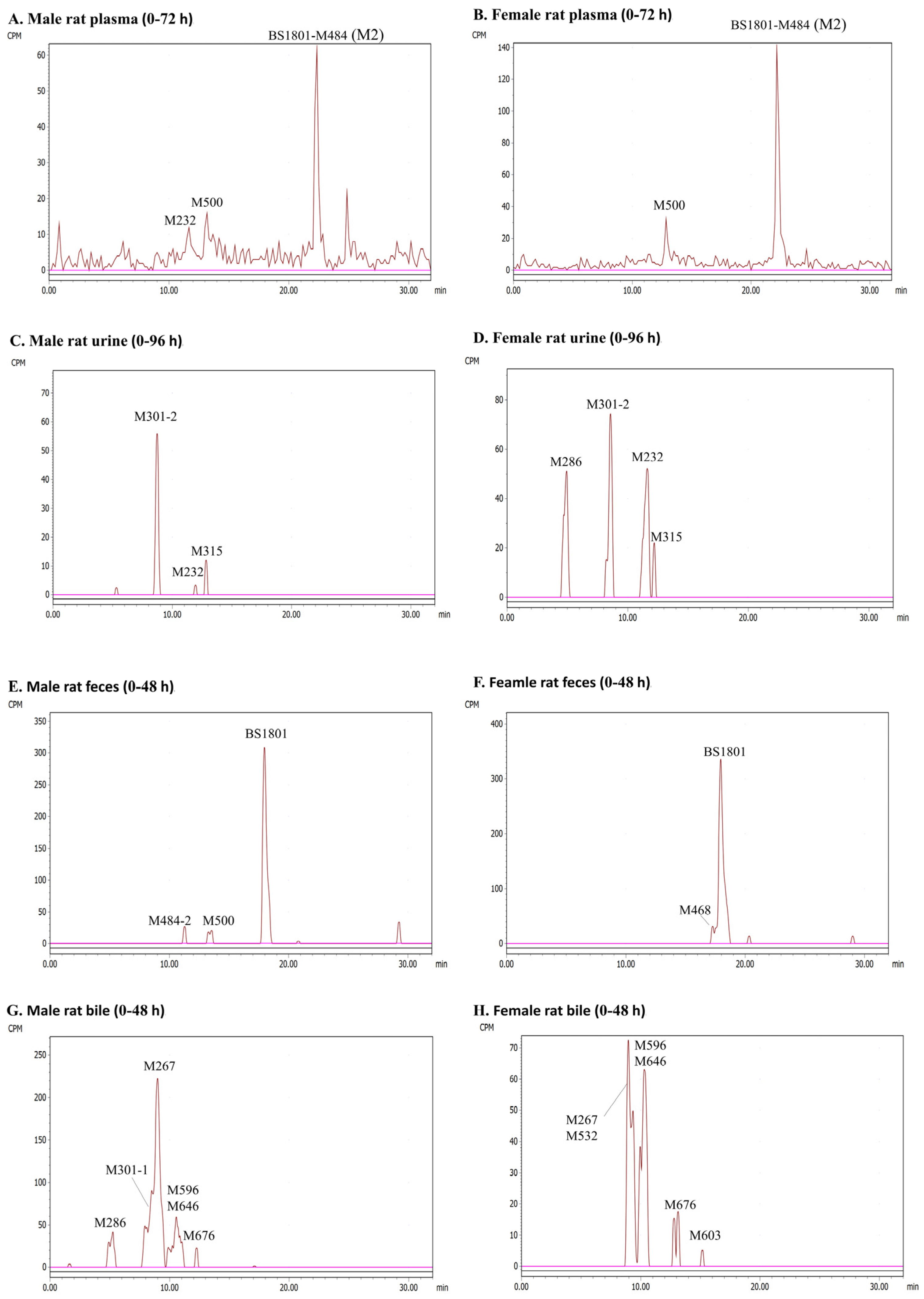
2.3.2. Plasma
2.3.3. Urine
2.3.4. Feces
2.3.5. Bile
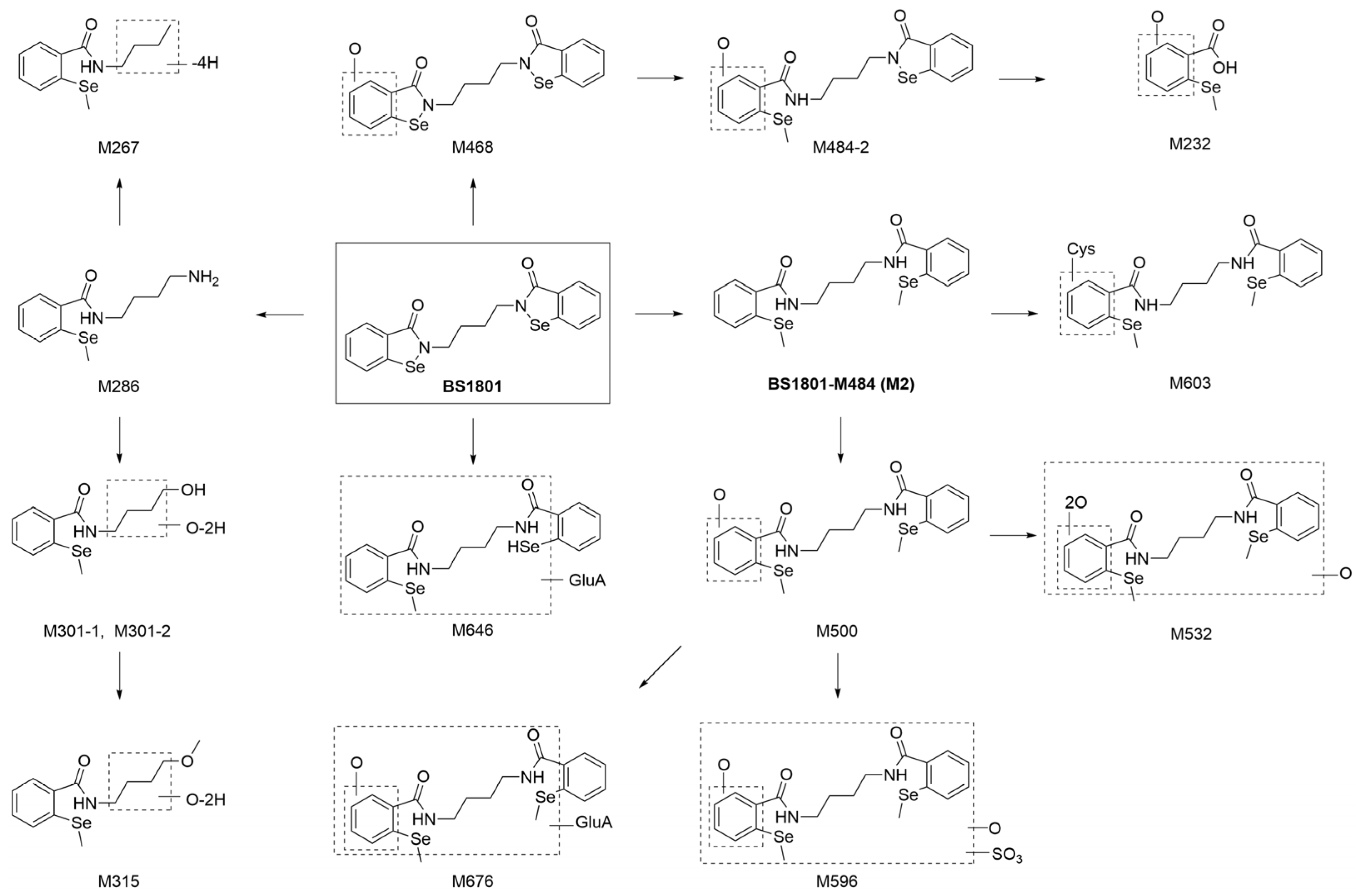
2.3.6. Description of Metabolites
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Dosing Solutions and Oral Administration
4.4. Plasma Samples for Metabolite Identification
4.5. Mass Balance
4.6. Tissue Distribution
4.7. Sample Preparation for Radioactivity Analysis
4.7.1. Urine, Bile, and Plasma Samples
4.7.2. Feces, Blood, and Tissue Samples
4.8. Metabolite Radio-Profiling and Characterization
4.8.1. Sample Preparation
4.8.2. UHPLC-β-RAM/UV/QE MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Ghareeb, H.; Metanis, N. The Thioredoxin System: A Promising Target for Cancer Drug Development. Chem. Eur. J. 2020, 26, 10175–10184. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Peng, S.; Liu, R.; Li, X.; Hou, Y.; Han, X.; Fang, J. Thioredoxin reductase inhibitors: A patent review. Expert Opin. Ther. Pat. 2017, 27, 547–556. [Google Scholar] [CrossRef]
- Chupakhin, E.; Krasavin, M. Thioredoxin reductase inhibitors: Updated patent review (2017-present). Expert Opin. Ther. Pat. 2021, 31, 745–758. [Google Scholar] [CrossRef]
- Chuai, H.; Zhang, S.Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.H.; Huang, J.Q.; Wang, Z.X.; Chen, Y.X.; Dong, Y.L. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 22, 11708. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Jariwalla, R.J.; Gangapurkar, B.; Nakamura, D. Differential sensitivity of various human tumour-derived cell types to apoptosis by organic derivatives of selenium. Br. J. Nutr. 2008, 101, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Dong, Y.; Ganther, H.E. New concepts in selenium chemoprevention. Cancer Metastasis Rev. 2002, 21, 281–289. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Sies, H. The early research and development of ebselen. Biochem. Pharmacol. 2013, 86, 1248–1253. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Dong, C.; Zhao, Y.; Zhou, J.; Yuan, C.; Zou, L. Mechanisms of ebselen as a therapeutic and its pharmacology applications. Future Med. Chem. 2020, 12, 2141–2160. [Google Scholar] [CrossRef] [PubMed]
- Sound Pharmaceuticals lnc. Ebselen. 2022. Available online: https://data.pharmacodia.com/drug#/main/drugInfo (accessed on 12 September 2023).
- Lan, L.; Zhao, F.; Wang, Y.; Zeng, H. The mechanism of apoptosis induced by a novel thioredoxin reductase inhibitor in A549 cells: Possible involvement of nuclear factor-kappaB-dependent pathway. Eur. J Pharmacol. 2007, 555, 83–92. [Google Scholar] [CrossRef]
- Zheng, W.; He, R.; Boada, R.; Subirana, M.A.; Ginman, T.; Ottosson, H.; Valiente, M.; Zhao, Y.; Hassan, M. A general covalent binding model between cytotoxic selenocompounds and albumin revealed by mass spectrometry and X-ray absorption spectroscopy. Sci. Rep. 2020, 10, 1274. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Dou, G.F.; Meng, Z.Y.; Lou, Y.Q.; Zhang, G.L. High performance liquid chromatographic determination of 1,2-[bis(1,2-benzisoselenazolone-3(2H)-ketone)]-ethane (BBSKE), a novel organoselenium compound, in dog plasma using pre-column derivatization and its application in pharmacokinetic study. J. Chromatogr. B 2007, 852, 617–624. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Dou, G.F.; Meng, Z.Y.; Lou, Y.Q.; Zhang, G.L. Development of a rapid and sensitive liquid chromatography/tandem mass spectrometry method for the determination of 1,2-[bis(1,2-benzisoselenazolone-3(2H)-ketone)]-ethane (BBSKE), a novel anti-cancer agent in rat plasma. Biomed. Chromatogr. 2008, 22, 1123–1129. [Google Scholar] [CrossRef]
- Sies, H. Ebselen, a Selenoorganic Compound as Glutathione-Peroxidase Mimic. Free Radic. Biol. Med. 1993, 14, 313–323. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, W.; Sun, R.; Yin, H.; Lin, F.; Liu, Y.; Xu, W.; Zeng, H. Butaselen prevents hepatocarcinogenesis and progression through inhibiting thioredoxin reductase activity. Redox. Biol. 2018, 14, 237–249. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Chen, Y.F.; Bai, M.; Liu, Y.X.; Xu, B.Y.; Sun, R.X.; Zeng, H.H. The antimetastatic effect and underlying mechanisms of thioredoxin reductase inhibitor ethaselen. Free Radic. Biol. Med. 2019, 131, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Jiang, J.; Yin, H.; Ma, J.; Deng, G.; Zhou, J.; Zhong, D. Quantification of the major circulating metabolite of BS1801, an ebselen analog, in human plasma. J. Pharm. Biomed. Anal. 2022, 212, 114638. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, H.; Sun, J.; Zhang, G.; Zhang, Y.; Zeng, H. TrxR/Trx inhibitor butaselen ameliorates pulmonary fibrosis by suppressing NF-κB/TGF-β1/Smads signaling. Biomed. Pharmacother. 2023, 169, 115822. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Jiang, J.; Yin, H.; Zhang, Y.; Li, Y.; Wu, P.; Peng, C.; Wang, Z.; Zhou, J.; Zeng, H.; et al. Investigating the Metabolic Mechanisms of Butaselen, An Ebselen Analog. Curr. Drug Metab. 2022, 23, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Pan, Q.Q.; Zhou, J.Q.; Weng, Y.; Chen, K.L.; Shi, L.; Zhu, G.X.; Chen, C.L.; Li, L.; Geng, M.Y.; et al. Pharmacokinetics, distribution, and excretion of sodium oligomannate, a recently approved anti-Alzheimer’s disease drug in China. J. Pharm. Anal. 2022, 12, 145–155. [Google Scholar] [CrossRef]
- Schulz, J.A.; Stresser, D.M.; Kalvass, J.C. Plasma protein-mediated uptake and contradictions to the free drug hypothesis: A critical review. Drug Metab. Rev. 2023, 55, 205–238. [Google Scholar] [CrossRef]
- Zheng, Y.D.; Zhang, H.; Zhan, Y.; Bian, Y.C.; Ma, S.; Gan, H.X.; Lai, X.J.; Liu, Y.Q.; Gong, Y.C.; Liu, X.F.; et al. Pharmacokinetics, mass balance, and metabolism of [(14)C]vicagrel, a novel irreversible P2Y12 inhibitor in humans. Acta Pharmacol. Sin. 2021, 42, 1535–1546. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Li, J.; Cheng, Z.; Feng, Y.; Ouyang, H.; Du, Z.; Jiang, H. Comprehensive metabolic profiling of Alismatis Rhizoma triterpenes in rats based on characteristic ions and a triterpene database. J. Pharm. Anal. 2021, 11, 96–107. [Google Scholar] [CrossRef]
- Wagner, G.; Schuch, G.; Akerboom, T.P.; Sies, H. Transport of ebselen in plasma and its transfer to binding sites in the hepatocyte. Biochem. Pharmacol. 1994, 48, 1137–1144. [Google Scholar] [CrossRef]
- Tillement, J.P.; Urien, S.; Chaumet-Riffaud, P.; Riant, P.; Bree, F.; Morin, D.; Albengres, E.; Barre, J. Blood binding and tissue uptake of drugs. Recent advances and perspectives. Fundam. Clin. Pharmacol. 1988, 2, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund-Udenaes, M. Active-site concentrations of chemicals—Are they a better predictor of effect than plasma/organ/tissue concentrations? Basic Clin. Pharmacol. Toxicol. 2010, 106, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hop, C.E.C.A.; Wang, Z.; Chen, Q.; Kwei, G. Plasma-Pooling Methods To Increase Throughput for in Vivo Pharmacokinetic Screening. J. Pharm. Sci. 1998, 87, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.A.; Garnett, W.R.; Kline, B.J. Determination of mean valproic acid serum level by assay of a single pooled sample. Clin. Pharmacol. Ther. 1981, 29, 408–413. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xue, M.; He, Y.; Yin, H.; Yang, C.; Zhong, D.; Zeng, H.; Zheng, Y.; Diao, X. Absorption, Distribution, Metabolism, and Excretion of [14C]BS1801, a Selenium-Containing Drug Candidate, in Rats. Molecules 2023, 28, 8102. https://doi.org/10.3390/molecules28248102
Yang C, Xue M, He Y, Yin H, Yang C, Zhong D, Zeng H, Zheng Y, Diao X. Absorption, Distribution, Metabolism, and Excretion of [14C]BS1801, a Selenium-Containing Drug Candidate, in Rats. Molecules. 2023; 28(24):8102. https://doi.org/10.3390/molecules28248102
Chicago/Turabian StyleYang, Cheng, Mingzhen Xue, Yifei He, Hanwei Yin, Chen Yang, Dafang Zhong, Huihui Zeng, Yuandong Zheng, and Xingxing Diao. 2023. "Absorption, Distribution, Metabolism, and Excretion of [14C]BS1801, a Selenium-Containing Drug Candidate, in Rats" Molecules 28, no. 24: 8102. https://doi.org/10.3390/molecules28248102
APA StyleYang, C., Xue, M., He, Y., Yin, H., Yang, C., Zhong, D., Zeng, H., Zheng, Y., & Diao, X. (2023). Absorption, Distribution, Metabolism, and Excretion of [14C]BS1801, a Selenium-Containing Drug Candidate, in Rats. Molecules, 28(24), 8102. https://doi.org/10.3390/molecules28248102






