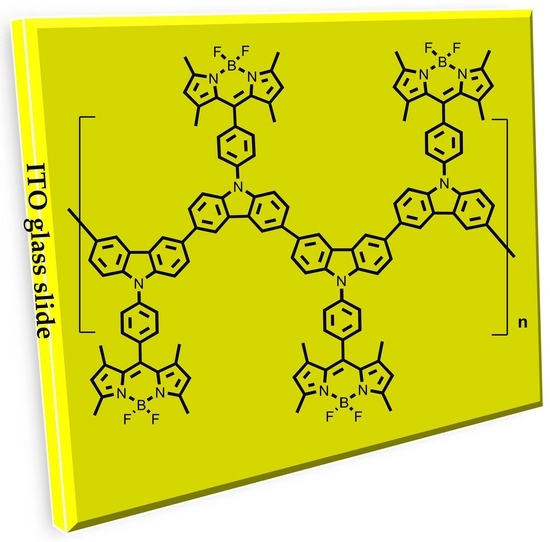
Electropolymerization on ITO-Coated Glass Slides of a Series of π-Extended BODIPY Dyes with Redox-Active Meso-Substituents
Abstract
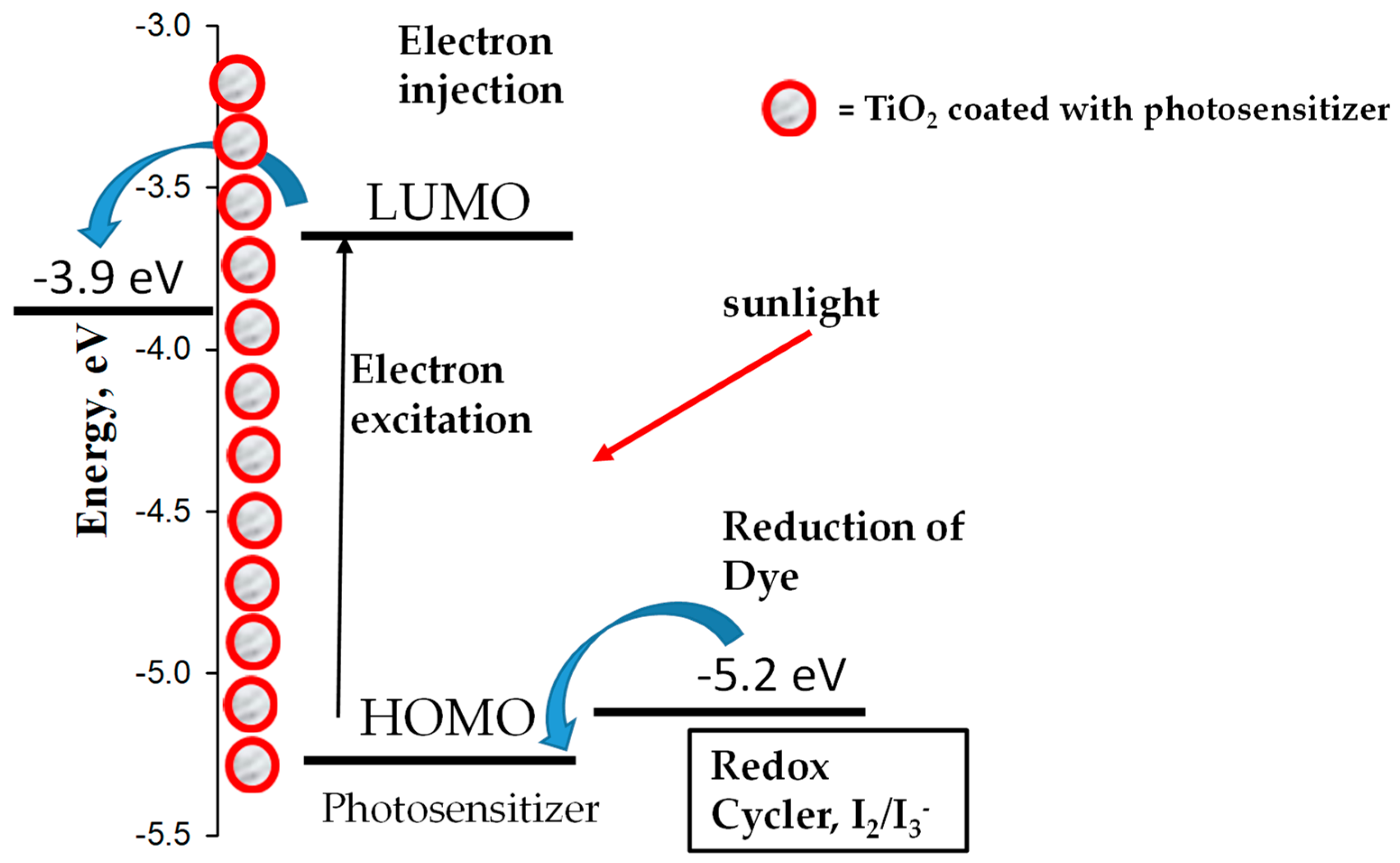
:1. Introduction
2. Results and Discussion
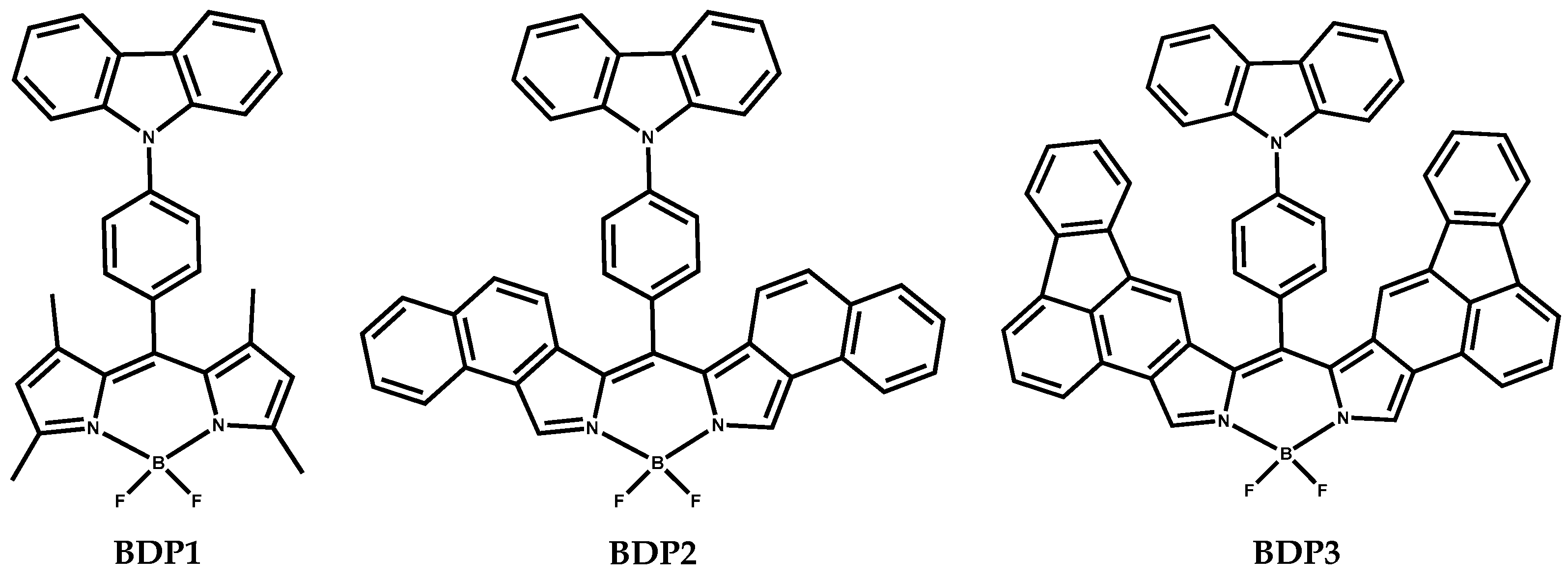
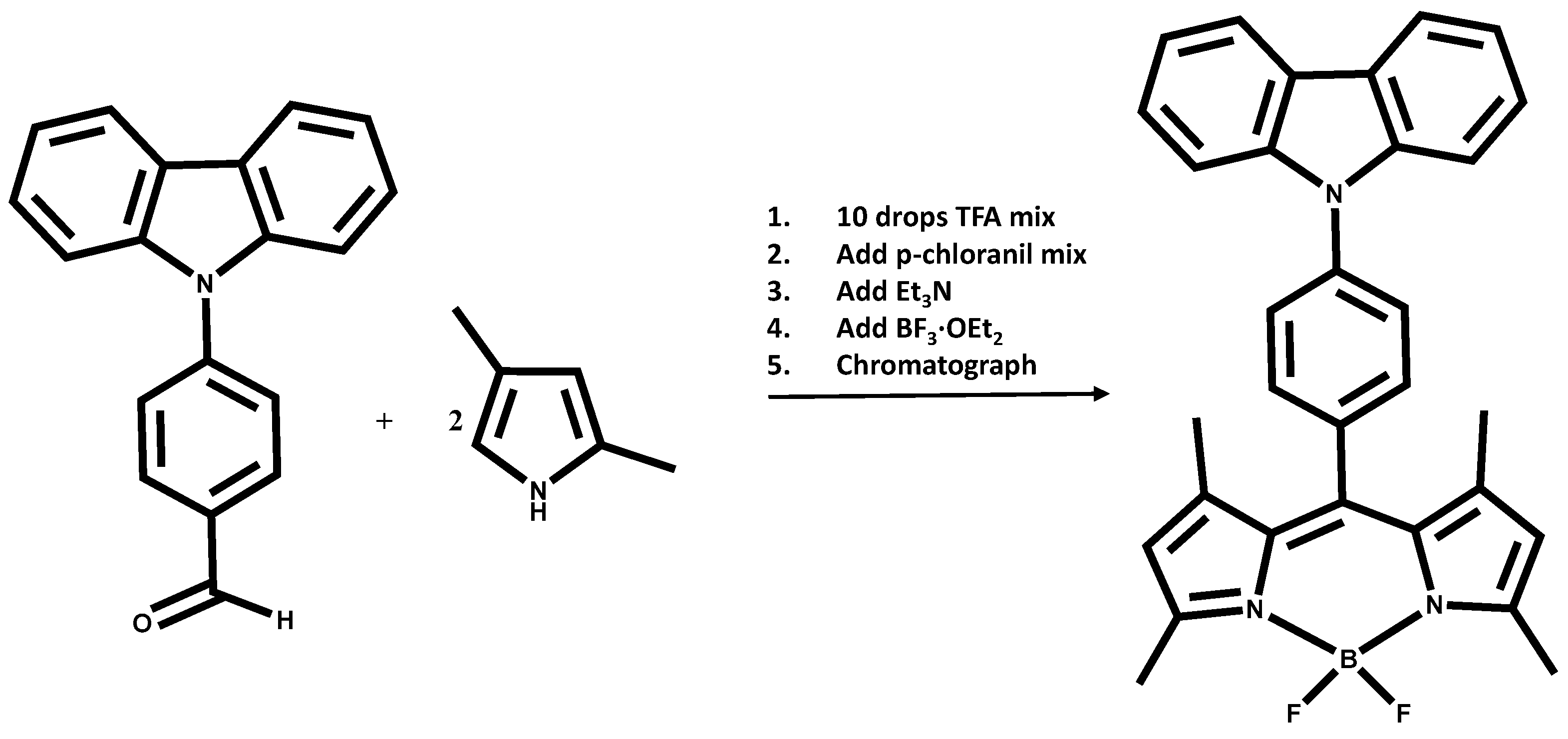
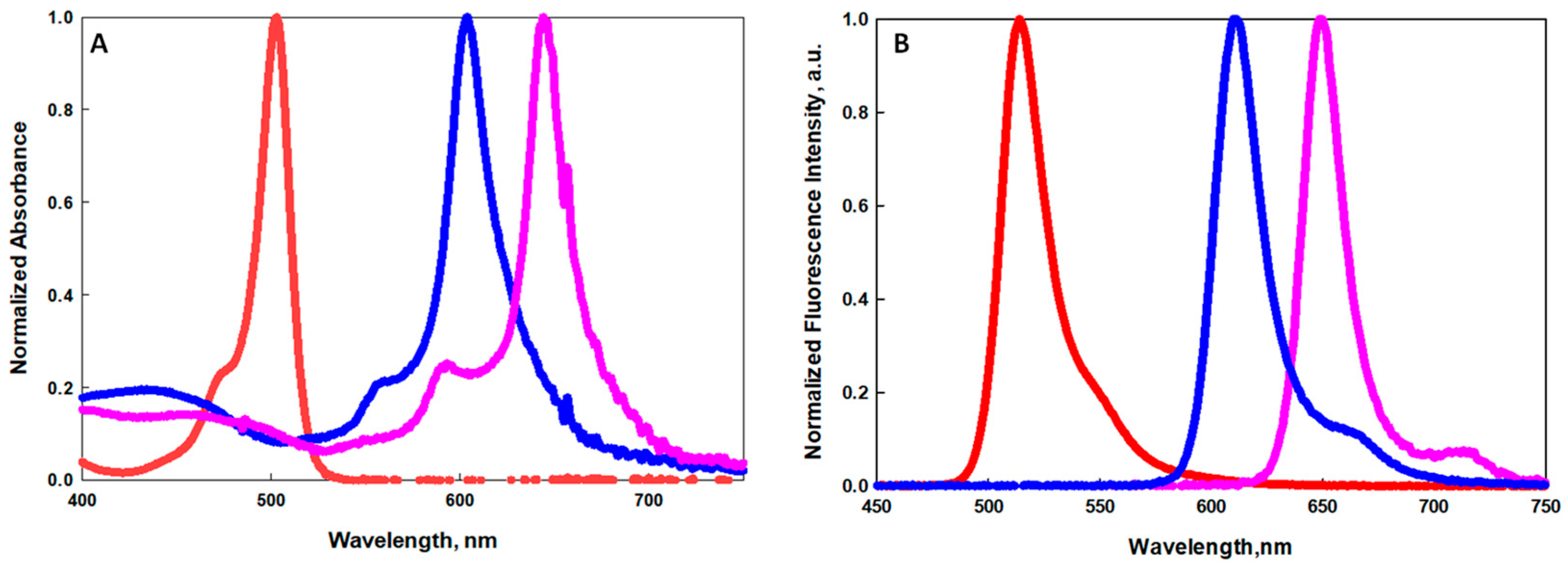
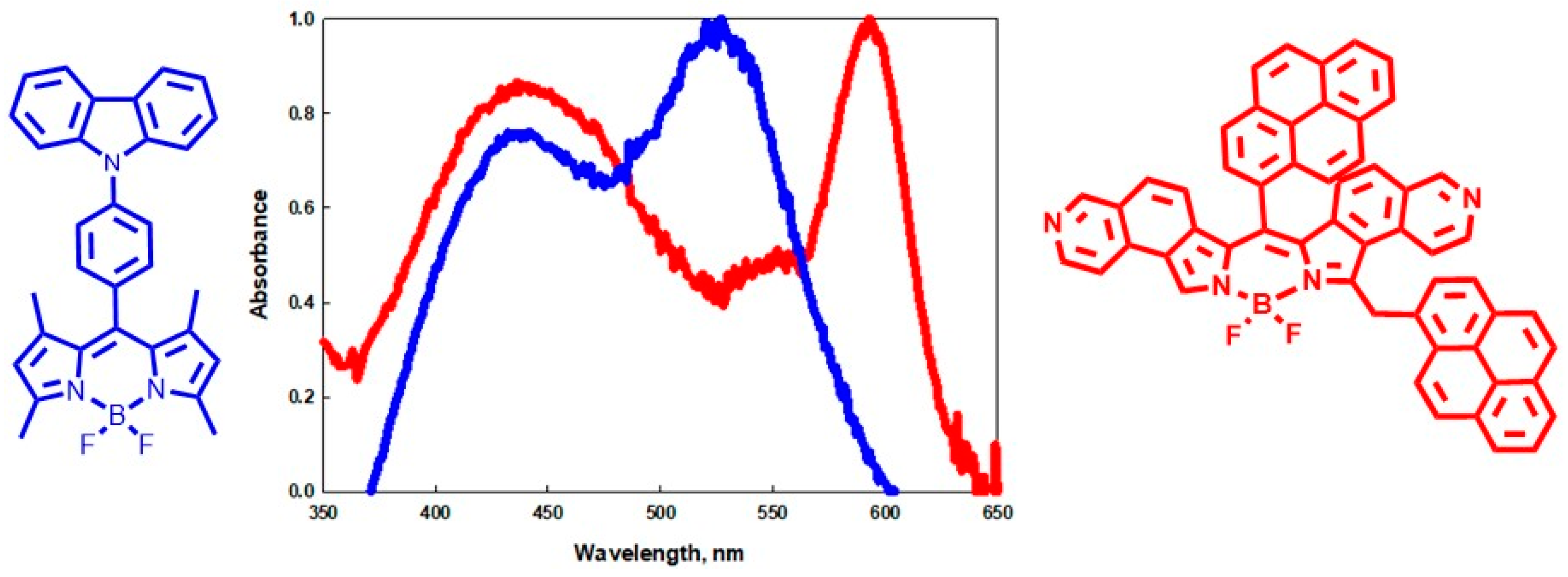
2.1. Synthesis and Spectroscopy
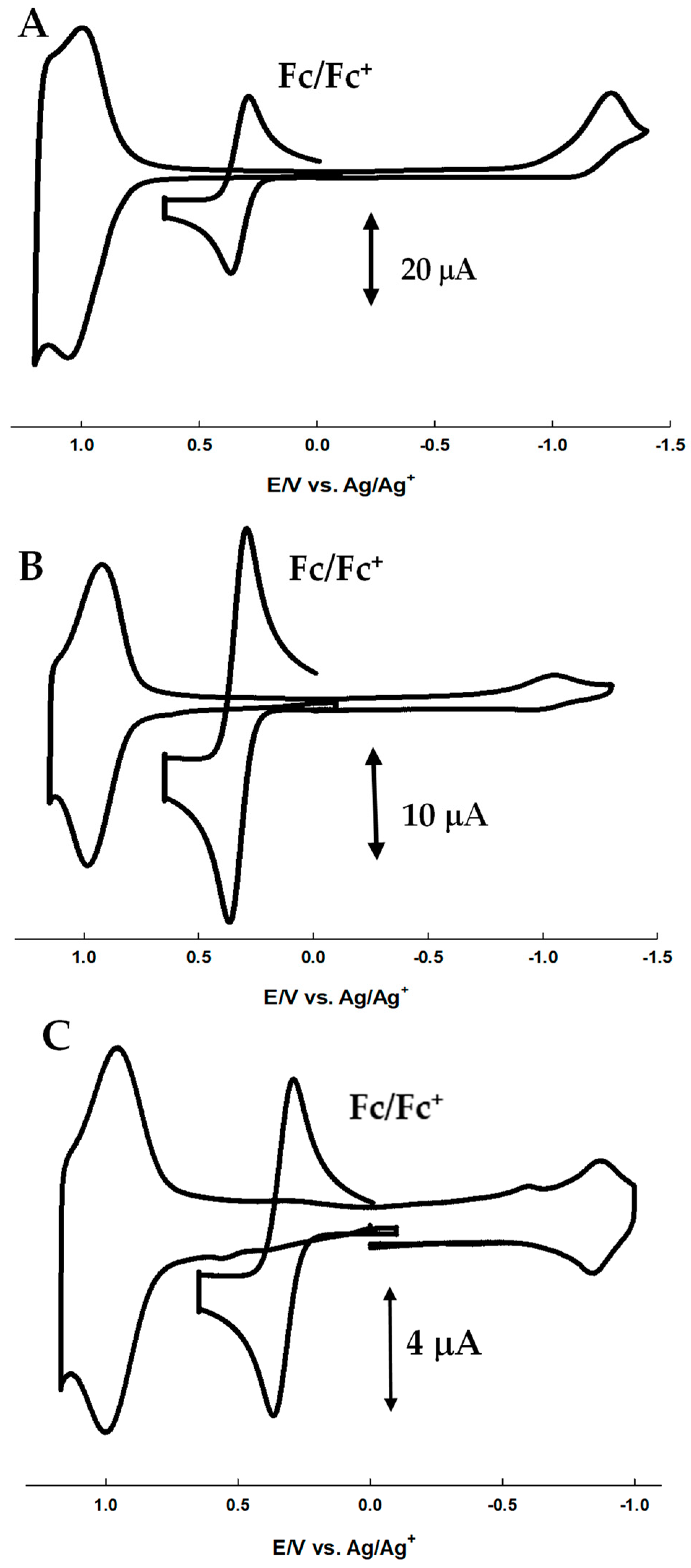
2.2. Solution Cyclic Voltammetry
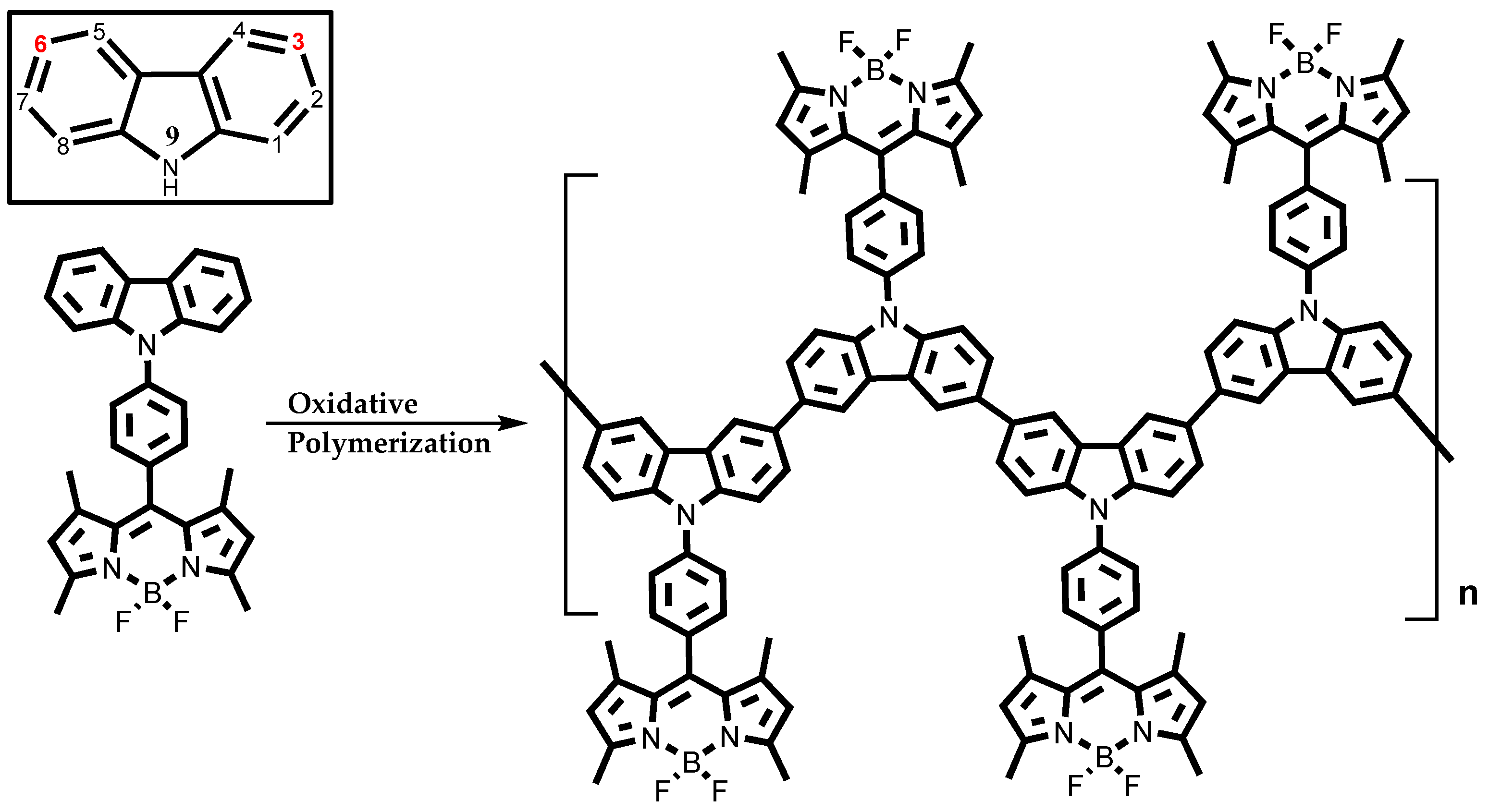
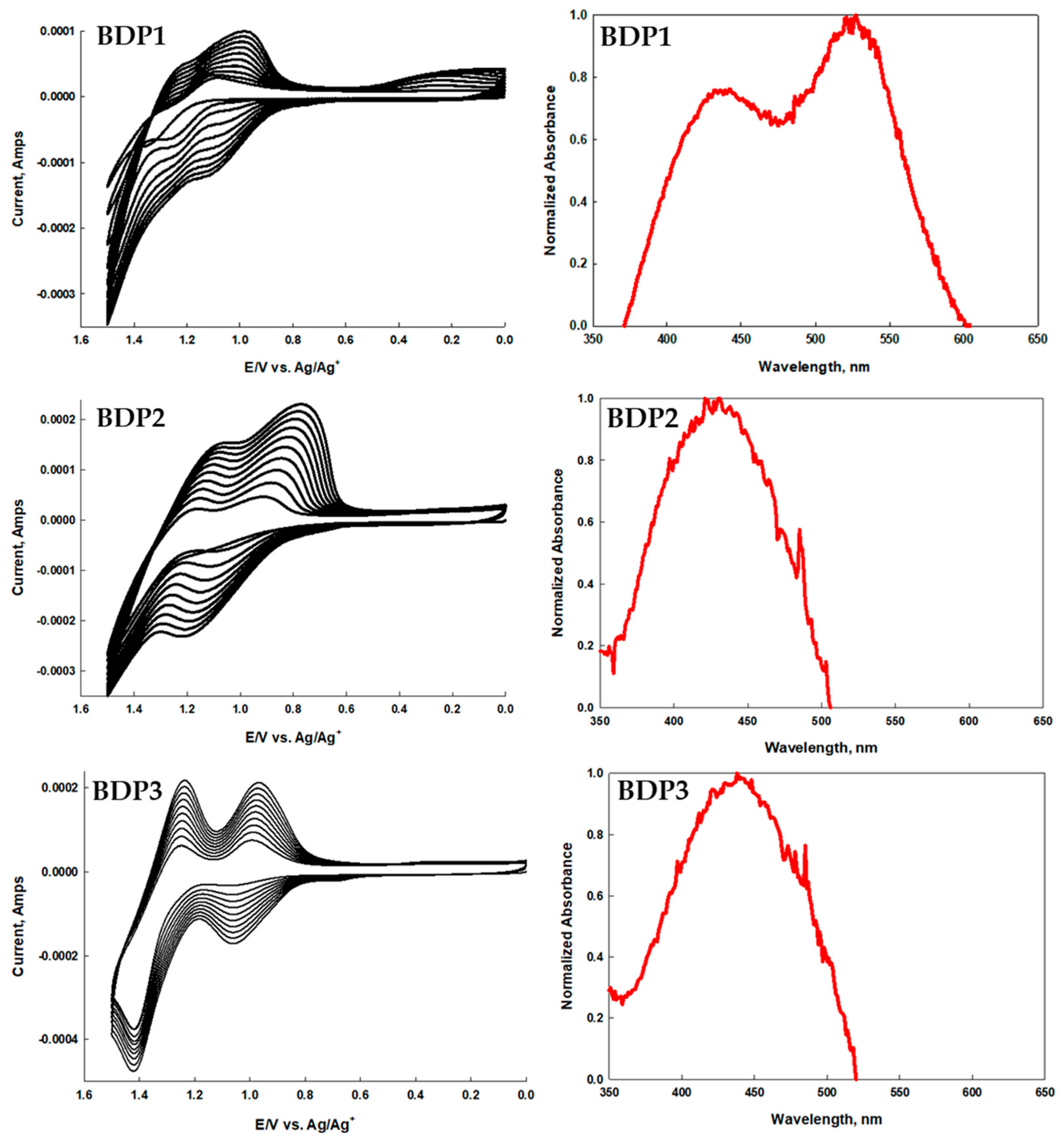
2.3. ITO Film Formation of BDP1–BDP3
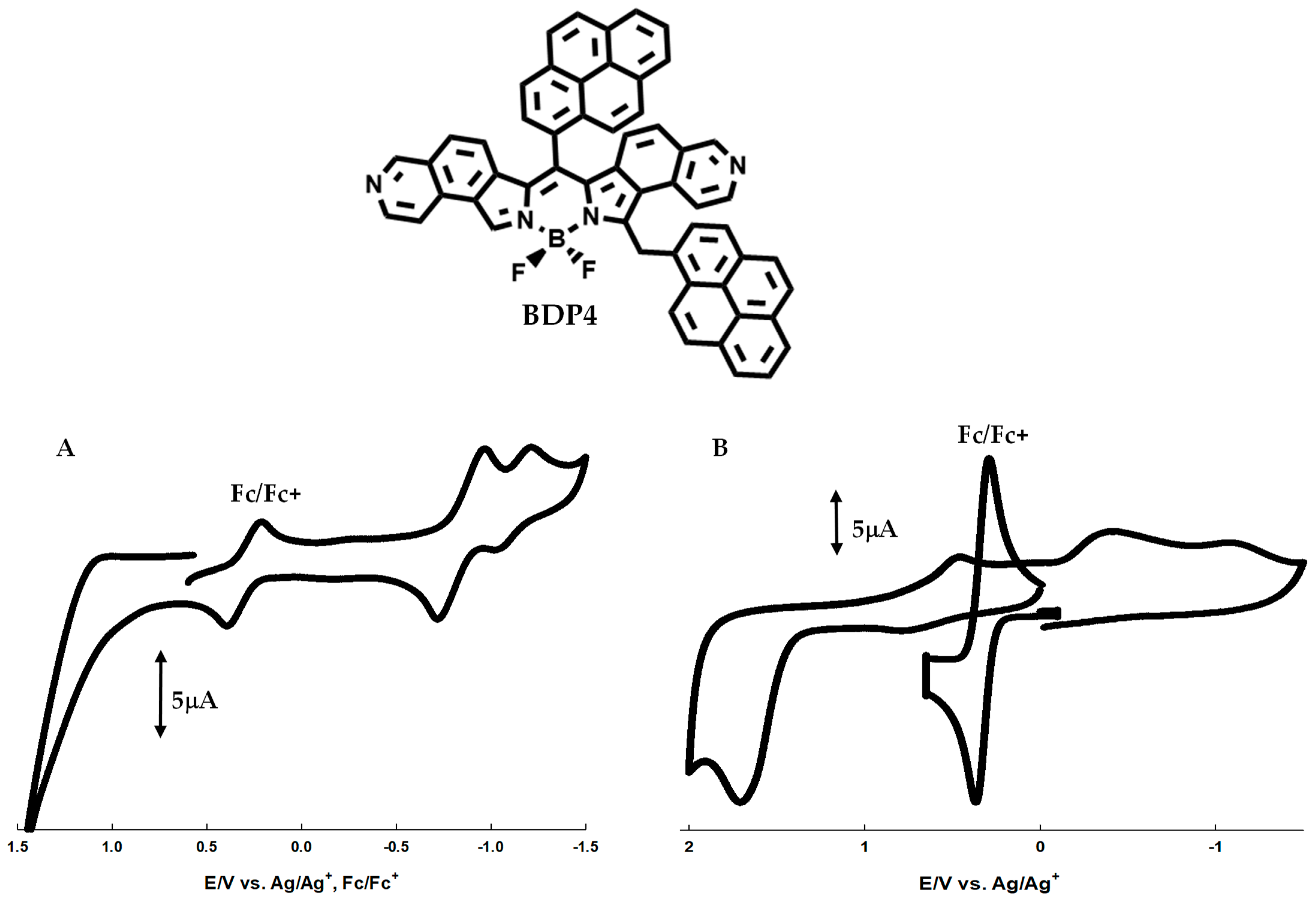
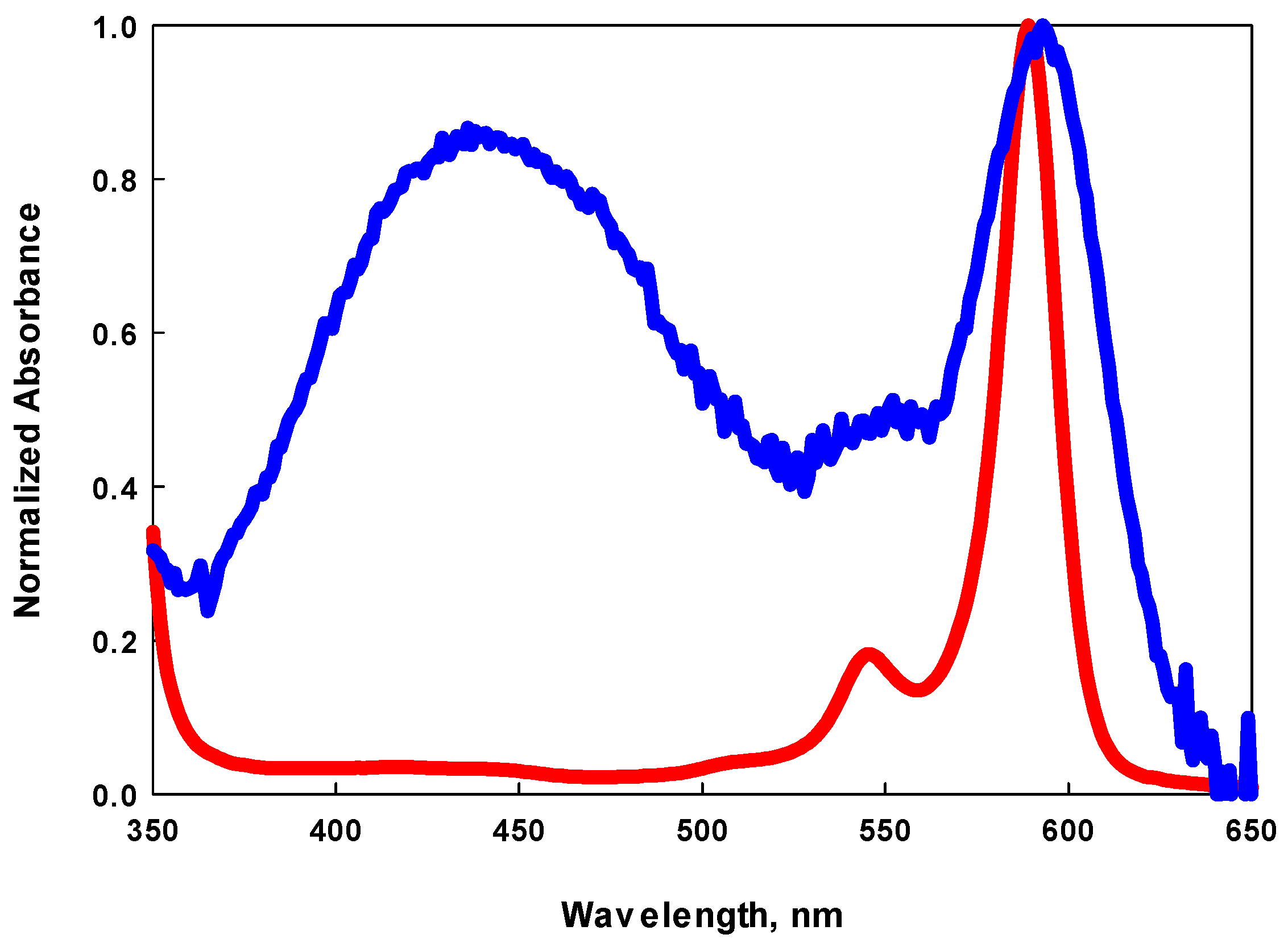
2.4. Investigation of a Pyrene-Substituted Isoquinol BDP Dye
3. Materials and Methods
3.1. General Procedures
3.2. Spectroscopic Measurements
3.3. Electrochemical Measurements
3.4. Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Triebs, A.; Kreuzer, F.H. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 719, 208–223. [Google Scholar] [CrossRef]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396–1455. [Google Scholar] [CrossRef]
- Rana, P.; Singh, N.; Majumdar, P.; Sing, S.P. Evolution of BODIPY/aza-BODIPY dyes for organic photoredox/energy transfer catalysis. Coord. Chem. Rev. 2022, 470, 214698. [Google Scholar] [CrossRef]
- Shukla, V.K.; Chakraborty, G.; Ray, A.K.; Nagaiyan, S. Red and NIR emitting ring-fused BODIPY/aza-BODIPY dyes. Dye. Pigment. 2023, 215, 111245. [Google Scholar] [CrossRef]
- Mao, Z.; Kim, J.H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J.S. Engineering of BODIPY-based theranostics for cancer therapy. Coord. Chem. Rev. 2023, 476, 214908. [Google Scholar] [CrossRef]
- Klfout, H.; Stewart, A.; Elkalifa, M.; He, H. BODIPYs for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2017, 46, 39873–39889. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, B. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Smalley, S.J.; Waterland, M.R.; Telfer, S.G. Heteroleptic Dipyrrin/Bipyridine Complexes of Ruthenium(II). Inorg. Chem. 2009, 48, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.D.; McLean, T.M.; Smalley, S.J.; Waterland, M.R.; Telfer, S.G. Chromophoric dipyrrin complexes capable of binding to TiO2: Synthesis, structure and spectroscopy. Dalton Trans. 2010, 39, 437–445. [Google Scholar] [CrossRef] [PubMed]
- McLean, T.M.; Cleland, D.M.; Lind, S.J.; Gordon, K.C.; Telfer, S.G.; Waterland, M.R. Strongly Absorbing π–π* States in Heteroleptic Dipyrrin/2,2′-Bipyridine Ruthenium Complexes: Excited-State Dynamics from Resonance Raman Spectroscopy. Chem. Asian J. 2010, 5, 2036–2046. [Google Scholar] [CrossRef]
- Weston, M.; Reade, T.J.; Handrup, K.; Champness, N.R.; O’Shea, J.N. Adsorption of Dipyrrin-Based Dye Complexes on a Rutile TiO2(110) Surface. J. Phys. Chem. C 2012, 116, 18184–18192. [Google Scholar] [CrossRef]
- Li, G.; Ray, L.; Glass, E.N.; Kovnir, K.; Khoroshutin, A.; Gorelsky, S.I.; Shatruk, M. Synthesis of Panchromatic Ru(II) Thienyl-Dipyrrin Complexes and Evaluation of Their Light-Harvesting Capacity. Inorg. Chem. 2012, 51, 1614–1624. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Munoz-Garcia, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Erten-Ela, S.; Yilmaz, M.D.; Icli, B.; Dede, Y.; Icli, S.; Akkaya, E.U. A Panchromatic Boradiazaindacene (BODIPY) Sensitizer for Dye-Sensitized Solar Cells. Org. Lett. 2008, 10, 3299–3302. [Google Scholar] [CrossRef]
- Hattori, S.; Ohkubo, K.; Urano, Y.; Sunahara, H.; Nagano, T.; Wada, Y.; Tkachenko, N.V.; Lemmetyinen, H.; Fukuzumi, S. Charge Separation in a Nonfluorescent Donor−Acceptor Dyad Derived from Boron Dipyrromethene Dye, Leading to Photocurrent Generation. J. Phys. Chem. B 2005, 109, 15368–15375. [Google Scholar] [CrossRef]
- Yang, J.; Devillers, C.H.; Fleurat-Lessard, P.; Jiang, H.; Wang, S.; Gros, C.P.; Gupta, G.; Sharma, G.D.; Xu, H. Carbazole-based green and blue-BODIPY dyads and triads as donors for bulk heterojunction organic solar cells. Dalton Trans. 2020, 49, 5606–5617. [Google Scholar] [CrossRef]
- Berkes, B.B.; Bandarenka, A.S.; Inzelt, G. Electropolymerization: Further Insight into the Formation of Conducting Polyindole Thin Films. J. Phys. Chem. C 2015, 119, 1996–2003. [Google Scholar] [CrossRef]
- Rusinek, C.A.; Bange, A.; Warren, M.; Kang, W.; Nahan, K.; Papautsky, I.; Heineman, H.R. Bare and Polymer-Coated Indium Tin Oxide as Working Electrodes for Manganese Cathodic Stripping Voltammetry. Anal. Chem. 2016, 88, 4221–4228. [Google Scholar] [CrossRef]
- Aynaou, A.; Youbi, B.; Ait Himi, M.; Lghazi, Y.; Bahar, J.; El Haimer, C.; Ouedrhiri, A.; Bimaghra, I. Electropolymerization investigation of polyaniline films on ITO substrate. Mater. Today Proc. 2022, 66, 335–340. [Google Scholar] [CrossRef]
- Yan, J.; Sun, C.; Tan, F.; Hu, X.; Chen, P.; Qu, S.; Zhou, S.; Xu, J. Electropolymerized poly(3,4 ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) film on ITO glass and its application in photovoltaic device. Sol. Energy Mater. Sol. Cells 2010, 94, 390–394. [Google Scholar] [CrossRef]
- Jameson, L.P.; Dzyuba, S.V. Expeditious, mechanochemical synthesis of BODIPY dyes. Beilstein J.Org. Chem. 2013, 9, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Swavey, S.; Coladipietro, M.; Burbayea, A.; Krause, J.A. Two-Step synthetic route toward asymmetric and symmetric boron dipyromethenes: Synthesis, optical properties, and electrochemistry. Eur. J. Org. Chem. 2016, 2016, 4429–4435. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; pp. 590–595. [Google Scholar]
- Zeng, W.; Gopalakrishna, T.Y.; Phan, H.; Tanaka, T.; Herng, T.S.; Ding, J.; Osuka, A.; Wu, J. Superoctazethrene: An Open-Shell Graphene-like Molecule Possessing Large Diradical Character but Still with Reasonable Stability. J. Am. Chem. Soc. 2018, 140, 14054–14058. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Polycarbazoles: 25 Years of Progress. Macromol. Rapid Commun. 2005, 26, 761–778. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Nelson, R.F. Anodic oxidation pathways of carbazoles. J. Electrochem. Soc. 1968, 115, 1159–1163. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Balun Kayan, D.; Polat, V. Improvement of electrochemical and structural properties of polycarbazole by simultaneous electrodeposition of chitosan. Turkish J. Chem. 2017, 41, 233–242. [Google Scholar] [CrossRef]
- Li, C.; Xue, J.; Huang, A.; Ma, J.; Qing, F.; Zhou, A.; Wang, Z.; Wang, Y.; Li, J. Poly (N-vinylcarbazole) as an advanced organic cathode for potassium-ion-based dual-ion battery. Electrochim. Acta 2019, 297, 850–855. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Michinobu, T.; Chang, S.-W.; Chiu, Y.-C.; Ke, C.-Y.; Liou, G.-S. Donor–Acceptor Effect of Carbazole-Based Conjugated Polymer Electrets on Photoresponsive Flash Organic Field-Effect Transistor Memories. ACS Appl. Mater. Interfaces 2020, 12, 6144–6150. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.A.; Bodnaryk, W.J.; Mirka, B.; Melville, O.A.; Adronov, A.; Lessard, B.H. Polycarbazole-Sorted Semiconducting Single-Walled Carbon Nanotubes for Incorporation into Organic Thin Film Transistors. Adv. Electron. Mater. 2019, 5, 1800539. [Google Scholar] [CrossRef]
- Soganci, T.; Baygu, Y.; Gök, Y.; Ak, M. Disulfide-linked symmetric N-alkyl carbazole derivative as a new electroactive monomer for electrochromic applications. Synth. Met. 2018, 244, 120–127. [Google Scholar] [CrossRef]
- Ates, M.; Özyilmaz, A.T.; Özyılmaz, A.T. The application of polycarbazole, polycarbazole/nanoclay and polycarbazole/Zn-nanoparticles as a corrosion inhibition for SS304 in saltwater. Prog. Org. Coat. 2015, 84, 50–58. [Google Scholar] [CrossRef]
- Sun, D.; Ren, Z.; Bryce, M.R.; Yan, S. Arylsilanes and siloxanes as optoelectronic materials for organic light-emitting diodes (OLEDs). J. Mater. Chem. C 2015, 3, 9496–9508. [Google Scholar] [CrossRef]
- Srivastava, A.; Chakrabarti, P. Experimental characterization of electrochemically polymerized polycarbazole film and study of its behavior with different metals contacts. Appl. Phys. A 2017, 123, 784. [Google Scholar] [CrossRef]
- Wu, C.-J.J.; Gaharwar, A.K.; Schexnailder, P.J.; Schmidt, G. Development of biomedical polymer-silicate nanocomposites: A materials science perspective. Materials 2010, 3, 2986–3005. [Google Scholar] [CrossRef]
- Pernites, R.; Ponnapati, R.; Felipe, M.J.; Advincula, R. Electropolymerization molecularly imprinted polymer (E-MIP) SPR sensing of drug molecules: Pre-polymerization complexed terthiophene and carbazole electroactive monomers. Biosens. Bioelectron. 2011, 26, 2766–2771. [Google Scholar] [CrossRef]
- Li, J.; Grimsdale, A.C. Carbazole-based polymers for organic photovoltaic devices. Chem. Soc. Rev. 2010, 39, 2399. [Google Scholar] [CrossRef]
- Ci, Z.; Yu, X.; Bao, M.; Wang, C.; Ma, T. Influence of the benzo [d] thiazole-derived π-bridges on the optical and photovoltaic performance of D–π–A dyes. Dye. Pigment. 2013, 96, 619–625. [Google Scholar] [CrossRef]
- El-Emary, T.I.; El-Aal, H.A.K.A.; Mohamed, S.K. Synthesis and Characterization of Assorted Heterocycles Based 3-(9Hcarbazol-9-yl) Propane Hydrazide. Chem. Sin. 2018, 9, 588–598. [Google Scholar]
- Zaia, E.W.; Gordon, M.P.; Yuan, P.; Urban, J.J. Progress and Perspective: Soft Thermoelectric Materials for Wearable and Internet-of-Things Applications. Adv. Electron. Mater. 2019, 5, 1800823. [Google Scholar] [CrossRef]
- Swavey, S.; Heidary, D.; Boyd, E.; Erb, J. Evaluation of Endoplasmic Reticulum Targeting Isoquinol-Based BODIPY Dyes and Their Properties as Molecular Rotors. Eur. J. Org. Chem. 2023, e202300777. [Google Scholar] [CrossRef]
- Lu, G.; Shi, G. Electrochemical polymerization of pyrene in the electrolyte of boron trifluoride diethyl etherate containing trifluoroacetic acid and polyethylene glycol oligomer. J. Electroanal. Chem. 2006, 586, 154–160. [Google Scholar] [CrossRef]
- Bachman, J.C.; Kavian, R.; Graham, D.J.; Kim, D.Y.; Noda, S.; Nocera, D.G.; Shao-Horn, Y.; Lee, S.W. Electrochemical polymerization of pyrene derivatives on functionalized carbon nanotubes for pseudocapacitive electrodes. Nat. Commun. 2015, 6, 7040. [Google Scholar] [CrossRef]
- Xu, L.; Tong, F.; Liu, X.; Lu, K.; Lu, Q. Multifunctional polypyrene/silica hybrid coatings with stable excimer fluorescence and robust superhydrophotobicity derived from electrodeposited polypyrene films. J. Mater. Chem. C 2015, 3, 2086–2092. [Google Scholar] [CrossRef]
- Lash, T.D.; Thompson, M.L.; Werner, T.M.; Spence, J.D. Synthesis of Novel Pyrrolic Compounds from Nitroarenes and Isocyanoacetates Using a Phosphazene Superbase. Synlett 2000, 2, 213–216. [Google Scholar] [CrossRef]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and Synthesis of a Library of BODIPY-Based Environmental Polarity Sensors Utilizing PhotoinducedElectron-Transfer-Controlled Fluorescence ON/OFF Switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swavey, S.; Wright, A. Electropolymerization on ITO-Coated Glass Slides of a Series of π-Extended BODIPY Dyes with Redox-Active Meso-Substituents. Molecules 2023, 28, 8101. https://doi.org/10.3390/molecules28248101
Swavey S, Wright A. Electropolymerization on ITO-Coated Glass Slides of a Series of π-Extended BODIPY Dyes with Redox-Active Meso-Substituents. Molecules. 2023; 28(24):8101. https://doi.org/10.3390/molecules28248101
Chicago/Turabian StyleSwavey, Shawn, and Alexa Wright. 2023. "Electropolymerization on ITO-Coated Glass Slides of a Series of π-Extended BODIPY Dyes with Redox-Active Meso-Substituents" Molecules 28, no. 24: 8101. https://doi.org/10.3390/molecules28248101
APA StyleSwavey, S., & Wright, A. (2023). Electropolymerization on ITO-Coated Glass Slides of a Series of π-Extended BODIPY Dyes with Redox-Active Meso-Substituents. Molecules, 28(24), 8101. https://doi.org/10.3390/molecules28248101