Effect of Drying Methods on Chemical and Sensory Properties of Cannabis sativa Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Drying Kinetics
2.2. Volatile Compounds
2.2.1. HS-SPME-Arrow and Essential Oils Profile of Fresh Hemp Leaves
| Compound | RI Exp. 1 | RI Adams 2 | RI NIST20 3 | HS-SPME Content % 4 | Essential Oil Content % 4 | Identification 5 |
|---|---|---|---|---|---|---|
| Hexanal | 802 | 801 | 800 | 0.29 ± 0.12 | Nd 6 | MS, RI, AS |
| 3-Hexenal | 804 | 794 | 810 | nd | 1.23 ± 0.09 | MS, RI, AS |
| 2-Hexenal | 851 | 855 | 851 | 1.98 ± 0.27 | 0.74 ± 0.07 | MS, RI, AS |
| 3-Hexen-1-ol | 818 | 859 | 857 | 0.81 ± 0.09 | nd | MS, RI |
| cis-2-Hexen-1-ol | 840 | 867 | 868 | 0.32 ± 0.03 | nd | MS, RI |
| Santolina triene | 893 | 908 | 908 | 0.24 ± 0.05 | nd | MS, RI |
| n-Heptanal | 904 | 895 | 901 | nd | 0.10 ± 0.02 | MS, RI, AS |
| Artemisia triene | 924 | 929 | 921 | nd | 0.34 ± 0.10 | MS, RI |
| α-Pinene | 939 | 939 | 937 | 2.24 ± 0.21 | 2.35 ± 0.32 | MS, RI, AS |
| Camphene | 944 | 954 | 952 | 0.26 ± 0.07 | 0.28 ± 0.09 | MS, RI, AS |
| Benzaldehyde | 946 | 960 | 962 | 0.34 ± 0.04 | 0.53 ± 0.12 | MS, RI, AS |
| Sabinene | 962 | 975 | 974 | 1.08 ± 0.12 | nd | MS, RI |
| 1-Octen-3-ol | 969 | 979 | 980 | 0.28 ± 0.07 | nd | MS, RI, AS |
| β-Pinene | 977 | 980 | 981 | nd | 0.83 ± 0.15 | MS, RI, AS |
| 5-Hepten-2-one, 6-methyl- | 978 | 985 | 986 | 0.34 ± 0.04 | 0.43 ± 0.09 | MS, RI, AS |
| 3-Octanone | 980 | 983 | 985 | nd | 0.16 ± 0.07 | MS, RI, AS |
| β-Myrcene | 982 | 990 | 991 | 8.25 ± 1.45 | nd | MS, RI, AS |
| 2-Pentyl-furan | 990 | 991 | 993 | nd | 1.09 ± 0.05 | MS, RI, AS |
| δ-2-Carene | 1000 | 1001 | 1000 | nd | Tr 7 | MS, RI |
| α-Phellandrene | 1004 | 1002 | 1005 | 0.29 ± 0.09 | 0.30 ± 0.02 | MS, RI, AS |
| trans,trans-2,4-Heptadienal | 1011 | 1007 | 1012 | nd | 0.34 ± 0.08 | MS, RI, AS |
| 3-Carene | 1013 | 1011 | 1013 | 0.25 ± 0.05 | nd | MS, RI |
| Limonene | 1018 | 1029 | 1030 | 5.47 ± 0.88 | 4.67 ± 0.35 | MS, RI, AS |
| p-Cymene | 1028 | 1024 | 1025 | nd | 0.09 ± 0.03 | MS, RI, AS |
| Eucalyptol | 1036 | 1031 | 1032 | nd | 0.11 ± 0.03 | MS, RI, AS |
| β-cis-Ocimene | 1030 | 1037 | 1038 | 0.87 ± 0.16 | nd | MS, RI, AS |
| β-trans-Ocimene | 1042 | 1048 | 1049 | 1.57 ± 0.13 | nd | MS, RI, AS |
| Benzeneacetaldehyde | 1050 | 1051 | 1054 | nd | tr | MS, RI, AS |
| γ-Terpinene | 1052 | 1059 | 1060 | 0.35 ± 0.08 | nd | MS, RI, AS |
| cis-Sabinene hydrate | 1061 | 1070 | 1068 | 0.28 ± 0.05 | nd | MS, RI, AS |
| trans,trans-3,5-Octadien-2-one | 1075 | - | 1073 | nd | 0.25 ± 0.08 | MS, RI |
| Terpinolene | 1082 | 1088 | 1088 | 2.28 ± 0.45 | 0.15 ± 0.05 | MS, RI, AS |
| Linalool | 1095 | 1096 | 1099 | 0.30 ± 0.04 | nd | MS, RI, AS |
| 3,5-Heptadien-2-one, 6-methyl- | 1097 | - | 1102 | nd | 0.16 ± 0.04 | MS, RI |
| n-Nonanal | 1106 | 1100 | 1107 | nd | 0.20 ± 0.09 | MS, RI, AS |
| Fenchol | 1107 | 1116 | 1113 | 0.34 ± 0.07 | 0.16 ± 0.22 | MS, RI |
| trans-Pinene hydrate | 1117 | 1122 | 1121 | 0.31 ± 0.08 | 0.25 ± 0.09 | MS, RI |
| p-Mentha-2,8-dien-1-ol | 1139 | 1137 | 1123 | nd | 0.53 ± 0.12 | MS, RI |
| Pinocarveol | 1143 | 1139 | 1139 | nd | 0.07 ± 0.06 | MS, RI, AS |
| cis-Verbenol | 1144 | 1141 | 1142 | nd | 0.07 ± 0.03 | MS, RI, AS |
| Ipsdienol | 1147 | 1145 | 1147 | 0.22 ± 0.04 | nd | MS, RI |
| Myrcenone | 1150 | 1149 | 1145 | 0.50 ± 0.06 | nd | MS, RI |
| trans,cis-2,6-Nonadienal | 1160 | 1154 | 1155 | 0.44 ± 0.04 | nd | MS, RI |
| trans-β-Terpineol | 1165 | 1161 | 1163 | 0.33 ± 0.08 | nd | MS, RI, AS |
| Borneol | 1169 | 1167 | 1169 | 0.29 ± 0.04 | nd | MS, RI |
| 1-Nonanol | 1180 | 1173 | 1169 | 0.37 ± 0.03 | nd | MS, RI |
| α-Terpineol | 1188 | 1188 | 1189 | nd | 0.33 ± 0.09 | MS, RI, AS |
| Hexyl butanoate | 1191 | 1192 | 1192 | 0.31 ± 0.10 | nd | MS, RI |
| Estragole | 1197 | 1196 | 1196 | 0.96 ± 0.12 | nd | MS, RI, AS |
| Carvone | 1249 | 1243 | 1242 | nd | 0.16 ± 0.04 | MS, RI, AS |
| Geranial | 1275 | 1267 | 1270 | nd | 0.08 ± 0.03 | MS, RI, AS |
| Carvacrol | 1303 | 1299 | 1300 | nd | 0.07 ± 0.02 | MS, RI, AS |
| Guaiacol | 1317 | 1309 | 1309 | nd | 0.23 ± 0.09 | MS, RI, AS |
| Cinnamaldehyde | 1330 | 1331 | 1327 | nd | 0.08 ± 0.02 | MS, RI, AS |
| Eugenol | 1362 | 1359 | 1360 | nd | 0.22 ± 0.09 | MS, RI, AS |
| Ylangene | 1380 | 1375 | 1372 | 0.65 ± 0.06 | nd | MS, RI |
| cis-Jasmone | 1406 | 1392 | 1394 | nd | 0.09 ± 0.02 | MS, RI |
| β-Longipinene | 1410 | 1400 | 1405 | nd | 0.61 ± 0.12 | MS, RI |
| Isocaryophyllene | 1419 | 1408 | 1406 | 3.86 ± 0.99 | nd | MS, RI |
| (E)-β-Caryophyllene | 1435 | 1419 | 1419 | 30.95 ± 2.35 | 33.18 ± 2.76 | MS, RI, AS |
| Humulene | 1452 | 1454 | 1454 | nd | 3.57 ± 0.56 | MS, RI, AS |
| Aristolene | 1455 | - | 1455 | 0.89 ± 0.12 | nd | MS, RI |
| 9-epi-trans-Caryophyllene | 1465 | 1466 | 1464 | nd | 0.44 ± 0.11 | MS, RI |
| α-Humulene | 1470 | 1454 | 1454 | 11.22 ± 0.86 | nd | |
| γ-Selinene | 1478 | 1479 | 1479 | 1.25 ± 0.14 | nd | MS, RI |
| trans-β-Ionone | 1490 | 1488 | 1488 | nd | 0.88 ± 0.21 | MS, RI, AS |
| β-Selinene | 1504 | 1490 | 1489 | 8.95 ± 1.01 | nd | MS, RI |
| α-Selinene | 1513 | 1498 | 1494 | 7.00 ± 0.89 | nd | MS, RI |
| γ-Cadinene | 1515 | 1513 | 1503 | nd | 0.26 ± 0.06 | MS, RI |
| δ-Cadinene | 1524 | 1523 | 1522 | nd | 0.44 ± 0.09 | MS, RI |
| Citronellyl butyrate | 1530 | 1529 | - | nd | 0.11 ± 0.08 | MS, RI |
| epi-Longipinanol | 1558 | 1563 | 1556 | nd | 0.84 ± 0.15 | MS, RI |
| trans-Nerolidol | 1568 | 1563 | 1566 | nd | 0.73 ± 0.13 | MS, RI, AS |
| Caryophyllene oxide | 1589 | 1583 | 1588 | nd | 4.65 ± 0.76 | MS, RI, AS |
| Hexadecane | 1600 | 1600 | 1600 | 2.31 ± 0.32 | nd | |
| Humulene epoxide I | 1603 | 1601 | 1604 | nd | 0.75 ± 0.13 | MS, RI |
| Humulane-1,6-dien-3-ol | 1608 | - | 1619 | nd | 0.12 ± 0.05 | MS, RI |
| Humulene epoxide II | 1609 | 1608 | 1606 | 0.73 ± 0.12 | 8.25 ± 0.45 | MS, RI, AS |
| Javanol isomer II | 1623 | - | 1622 | nd | 0.30 ± 0.07 | MS, RI |
| Caryophylla-4(12),8(13)-dien-5α-ol | 1638 | 1640 | 1640 | nd | 2.02 ± 0.18 | MS, RI |
| 14-hydroxy-cis-Caryophyllene | 1652 | 1657 | 1654 | nd | 2.71 ± 0.15 | MS, RI |
| Allo-Himachalol | 1663 | 1661 | 1662 | nd | 1.45 ± 0.13 | MS, RI |
| 14-hydroxy-9-epi-trans-Caryophyllene | 1677 | 1669 | 1676 | nd | 3.16 ± 0.21 | MS, RI |
| α-Bisabolol | 1688 | 1685 | 1687 | nd | 0.17 ± 0.12 | MS, RI, AS |
| Nootkatone | 1812 | 1806 | 1811 | nd | 0.99 ± 0.06 | MS, RI, AS |
| Phytone | 1848 | - | 1847 | nd | 0.98 ± 0.08 | MS, RI |
| trans,trans-Farnesyl acetone | 1922 | 1913 | 1921 | nd | 0.27 ± 0.05 | MS, RI |
| Phytol | 2115 | 2114 | 2114 | nd | 2.33 ± 0.17 | MS, RI |
| Unknown 1 9 | 2225 | - | - | nd | 0.08 ± 0.04 | - |
| Unknown 2 9 | 2364 | - | - | nd | 0.78 ± 0.11 | - |
| Cannabidivarol | 2401 | - | 2406 | nd | 0.11 ± 0.03 | MS, RI |
| Cannabidiol | 2432 | - | 2430 8 | nd | 12.73 ± 1.21 | MS, RI, AS |
| Cannabichromene | 2438 | - | 2440 8 | nd | 0.23 ± 0.06 | MS, RI |
| Δ9-THC | 2468 | - | 2465 | nd | 0.20 ± 0.04 | MS, RI |
2.2.2. Changes in Aroma Profile during Various Drying Methods
| Compound | Fresh | CD50 | CD60 | CD70 | 240VMD | 360VMD | 480 VMD | CD60/VMD |
|---|---|---|---|---|---|---|---|---|
| Content % 1 | ||||||||
| β-Myrcene | 8.25 a,2 | 11.96 b | 3.95 c | 1.62 f | 1.19 g | 1.30 h | 2.14 e | 2.47 d |
| Limonene | 5.47 a | 5.73 b | 1.95 e | 2.01 e | 0.95 g | 1.40 f | 4.78 c | 3.84 d |
| Terpinolene | 2.28 a | 1.32 c | 6.33 d | 3.34 b | 6.38 d | 9.53 e | 12.29 g | 10.36 f |
| Isocaryophyllene | 3.86 a | 1.95 f | 2.32 d | 2.09 e | 1.13 h | 1.64 g | 2.59 c | 3.08 b |
| (E)-β-Caryophyllene | 30.95 a | 18.03 d | 14.90 e | 13.24 f | 21.43 b | 20.69 c | 9.11 g | 7.10 h |
| α-Humulene | 11.22 a | 5.50 d | 5.26 e | 5.04 f | 7.45 b | 7.16 c | 4.11 g | 3.92 h |
| β-Selinene | 8.95 a | 1.21 h | 1.89 e | 2.05 d | 1.78 f | 1.50 g | 2.35 c | 2.84 b |
| α-Selinene | 7.00 a | 1.51 g | 2.07 e | 2.15 d | 1.19 h | 1.81 f | 2.33 c | 2.80 b |
2.2.3. Changes in Essential Oils Profiles during Various Drying Methods
2.3. Cannabinoids
2.4. Sterols and Triterpenoids
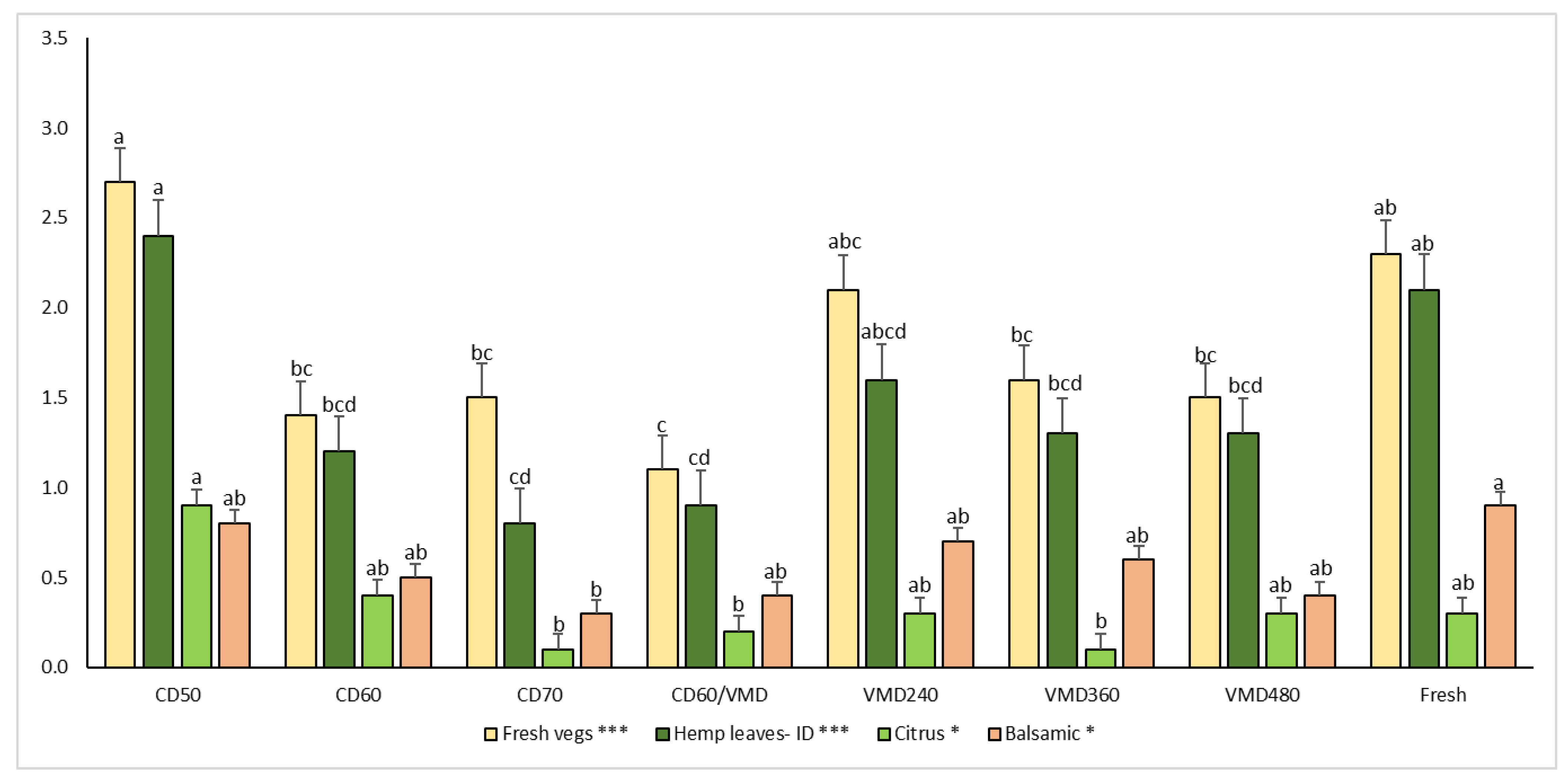
2.5. Sensory Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Drying Methods
3.3. Drying Kinetics and Models
3.4. Sample Preparation and Chromatographical Analysis
3.4.1. Volatile Compounds Analysis
Headspace Solid-Phase Microextraction (HS-SPME)
Essential Oils
3.4.2. Cannabinoids, Sterols and Triterpenoids Analysis
3.4.3. Analysis of Obtained Data
3.5. Sensory Analysis
3.5.1. Ranking Test
3.5.2. Descriptive Test
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial Hemp (Cannabis sativa L.) Agronomy and Utilization: A Review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Noppawan, P.; Bainier, C.; Lanot, A.; McQueen-Mason, S.; Supanchaiyamat, N.; Attard, T.M.; Hunt, A.J. Effect of Harvest Time on the Compositional Changes in Essential Oils, Cannabinoids, and Waxes of Hemp (Cannabis sativa L.). R. Soc. Open Sci. 2022, 9, 211699. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Choong, T.S.Y. Antioxidant Activity, and Volatile and Phytosterol Contents of Strobilanthes Crispus Dehydrated Using Conventional and Vacuum Microwave Drying Methods. Molecules 2019, 24, 1397. [Google Scholar] [CrossRef] [PubMed]
- OrphAnides, A.; GOulAs, V.; GekAs, V. Effect of Drying Method on the Phenolic Content and Antioxidant Capacity of Spearmint. Czech J. Food Sci. 2013, 31, 509–513. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A Review of Drying Methods for Improving the Quality of Dried Herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of Oregano Essential Oil (Origanum vulgare) as Affected by Drying Method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, Á.A. Effects of Drying Methods on the Composition of Thyme (Thymus vulgaris L.) Essential Oil. Dry. Technol. 2013, 31, 224–235. [Google Scholar] [CrossRef]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; García-Garví, J.M.; Carbonell-Barrachina, Á.A.; Szumny, A. Determination of Various Drying Methods’ Impact on Odour Quality of True Lavender (Lavandula angustifolia Mill.) Flowers. Molecules 2019, 24, 2900. [Google Scholar] [CrossRef]
- Meija, J.; McRae, G.; Miles, C.O.; Melanson, J.E. Thermal Stability of Cannabinoids in Dried Cannabis: A Kinetic Study. Anal. Bioanal. Chem. 2022, 414, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Jaidee, W.; Siridechakorn, I.; Nessopa, S.; Wisuitiprot, V.; Chaiwangrach, N.; Ingkaninan, K.; Waranuch, N. Kinetics of CBD, Δ9-THC Degradation and Cannabinol Formation in Cannabis Resin at Various Temperature and PH Conditions. Cannabis Cannabinoid Res. 2022, 7, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Čonić, B.S.; Božin, B.; Torović, L. European Hemp-Based Food Products—Health Concerning Cannabinoids Exposure Assessment. Food Control 2021, 129, 108233. [Google Scholar] [CrossRef]
- Oduola, A.A.; Bruce, R.M.; Shafiekhani, S.; Atungulu, G.G. Impacts of Industrial Microwave and Infrared Drying Approaches on Hemp (Cannabis sativa L.) Quality and Chemical Components. Food Bioprod. Process. 2023, 137, 20–27. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczyński, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Różański, H. Volatile Composition and Sensory Properties as Quality Attributes of Fresh and Dried Hemp Flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Sobolev, A.P.; Giusti, A.M.; Vinci, G.; Cammarone, S.; Tortora, C.; Lamelza, L.; Prencipe, S.A.; et al. Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile. Foods 2022, 11, 3658. [Google Scholar] [CrossRef] [PubMed]
- Chasiotis, V.K.; Tzempelikos, D.A.; Filios, A.E. Assessment of Constant and Time-Varying Temperature Schemes on the Convective Drying Characteristics of Hemp Leaves. Case Stud. Therm. Eng. 2021, 26, 101098. [Google Scholar] [CrossRef]
- Challa, S.K.R.; Misra, N.N.; Martynenko, A. Drying of Cannabis—State of the Practices and Future Needs. Dry. Technol. 2021, 39, 2055–2064. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Altay, K.; Hayaloglu, A.A.; Dirim, S.N. Determination of the Drying Kinetics and Energy Efficiency of Purple Basil (Ocimum basilicum L.) Leaves Using Different Drying Methods. Heat Mass Transf. 2019, 55, 2173–2184. [Google Scholar] [CrossRef]
- Doymaz, İ.; Karasu, S. Effect of Air Temperature on Drying Kinetics, Colour Changes and Total Phenolic Content of Sage Leaves (Salvia officinalis). Qual. Assur. Saf. Crops Foods 2018, 10, 269–276. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of Six Drying Treatments with Respect to Essential Oil Yield, Composition and Color Characteristics of Thymys Daenensis Subsp. Daenensis. Celak Leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Ibrahim, E.; Wang, M.; Radwan, M.; Wanas, A.; Majumdar, C.; Avula, B.; Wang, Y.-H.; Khan, I.; Chandra, S.; Lata, H.; et al. Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, F. Impact of Four Hemp (Cannabis sativa L.) Varieties and Stage of Plant Growth on Yield and Composition of Essential Oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Woźniczka, K.; Konieczyński, P.; Plenis, A.; Bączek, T.; Roszkowska, A. SPME as a Green Sample-Preparation Technique for the Monitoring of Phytocannabinoids and Endocannabinoids in Complex Matrices. J. Pharm. Anal. 2023, 13, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; Finamore, C.; Chini, M.G.; Saviano, G.; De Felice, V.; De Marino, S.; Lauro, G.; Casapullo, A.; Fantasma, F.; Trombetta, F.; et al. Phytochemical Analysis of the Methanolic Extract and Essential Oil from Leaves of Industrial Hemp Futura 75 Cultivar: Isolation of a New Cannabinoid Derivative and Biological Profile Using Computational Approaches. Plants 2022, 11, 1671. [Google Scholar] [CrossRef] [PubMed]
- Nagy, D.U.; Cianfaglione, K.; Maggi, F.; Sut, S.; Dall’Acqua, S. Chemical Characterization of Leaves, Male and Female Flowers from Spontaneous Cannabis (Cannabis sativa L.) Growing in Hungary. Chem. Biodivers. 2019, 16, e1800562. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Hassan, T.; Ali, N.; Lone, A.; Shawl, A.S.; Shah, W.; Qurishi, M.A.; Prakash, P. Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography Mass Spectrometric Analysis of the Volatile Constituents of Cannabis sativa L. from Kashmir. J. Pharm. Res. 2011, 4, 2651–2653. [Google Scholar]
- Cicaloni, V.; Salvini, L.; Vitalini, S.; Garzoli, S. Chemical Profiling and Characterization of Different Cultivars of Cannabis sativa L. Inflorescences by SPME-GC-MS and UPLC-MS. Separations 2022, 9, 90. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Müller, J. Changes of Essential Oil Content and Composition during Convective Drying of Lemon Balm (Melissa officinalis L.). Ind. Crops Prod. 2014, 52, 118–124. [Google Scholar] [CrossRef]
- Szumny, A.; Figiel, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of Rosemary Essential Oil (Rosmarinus Officinalis) as Affected by Drying Method. J. Food Eng. 2010, 97, 253–260. [Google Scholar] [CrossRef]
- Rybka, A.; Krofta, K.; Heřmánek, P.; Honzík, I.; Pokorný, J. Effect of Drying Temperature on the Content and Composition of Hop Oils. Plant Soil. Environ. 2018, 64, 512–516. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Ryu, B.R.; Rana, M.S.; Rahman, M.H.; Lim, J.-D.; Lim, Y.-S. Enhancing the Cannabidiol (CBD) Compound in Formulated Hemp (Cannabis sativa L.) Leaves through the Application of Hot-Melt Extrusion. Processes 2021, 9, 837. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.-S.; Wang, L.-W.; Chang, S.-L.; Wang, P.-D.; Zhao, B. Optimization of Cyclodextrin-Assisted Green Extraction of Cannabidiol from Industrial Hemp Leaves: Release Behavior, Permeability, Bioactivity, and Stability. Ind. Crops Prod. 2022, 188, 115709. [Google Scholar] [CrossRef]
- Das, P.C.; Vista, A.R.; Tabil, L.G.; Baik, O.-D. Postharvest Operations of Cannabis and Their Effect on Cannabinoid Content: A Review. Bioengineering 2022, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Martínez-Tomé, J.; Carbonell-Barrachina, Á.A. Dying Methods Affect the Aroma of Origanum majorana L. Analyzed by GC–MS and Descriptive Sensory Analysis. Ind. Crops Prod. 2015, 74, 218–227. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Kharaghani, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, Á.A.; Tsotsas, E. Drying Kinetics and Microstructural and Sensory Properties of Black Chokeberry (Aronia melanocarpa) as Affected by Drying Method. Food Bioprocess Technol. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. A Review of Thin Layer Drying of Foods: Theory, Modeling, and Experimental Results. Crit. Rev. Food Sci. Nutr. 2010, 50, 441–464. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A New Model for Single-Layer Drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Yaldýz, O.; Ertekýn, C. Thin Layer Solar Drying of Some Vegetables. Dry. Technol. 2001, 19, 583–597. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis: General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
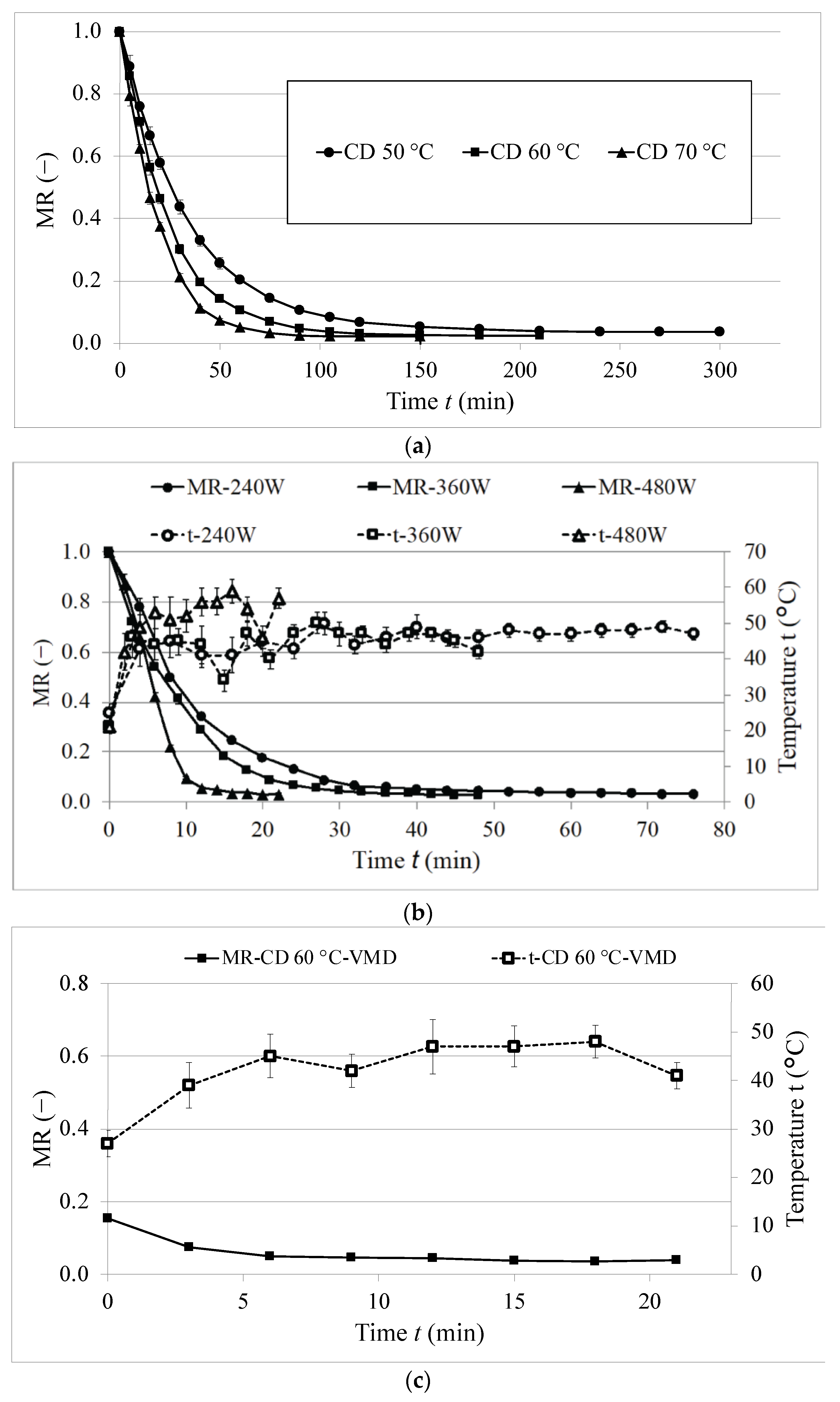
| Model Name | Drying Conditions | Constants | Statistics | ||||
|---|---|---|---|---|---|---|---|
| a | k | b | RMSE | R2 | |||
| Logarithmic | CD50 | 1.000 | 0.0301 | 0.0327 | 0.0102 | 0.9991 | |
| CD60 | 1.000 | 0.0412 | 0.0217 | 0.0125 | 0.9987 | ||
| CD70 | 1.000 | 0.0525 | 0.0129 | 0.0121 | 0.9988 | ||
| VMD240 | 1.000 | 0.0936 | 0.0291 | 0.0181 | 0.9958 | ||
| VMD360 | 1.000 | 0.1111 | 0.0195 | 0.0154 | 0.9972 | ||
| VMD480 | 1.000 | 0.1595 | 0.0055 | 0.0816 | 0.9527 | ||
| CD60-VMD | 0.115 | 0.3897 | 0.0395 | 0.0028 | 0.9966 | ||
| a | k | B | C | RMSE | R2 | ||
| Midilli | CD50 | 1.000 | 0.0288 | 0.9874 | 0.00015 | 0.0082 | 0.9994 |
| CD60 | 1.000 | 0.0307 | 1.0751 | 0.00017 | 0.0104 | 0.9991 | |
| CD70 | 1.000 | 0.0403 | 1.0788 | 0.00018 | 0.0064 | 0.9997 | |
| VMD240 | 1.000 | 0.0638 | 1.1228 | 0.00060 | 0.0143 | 0.9975 | |
| VMD360 | 1.000 | 0.0930 | 1.0120 | 0.00071 | 0.0123 | 0.9984 | |
| VMD480 | 1.000 | 0.0342 | 1.8377 | 0.00178 | 0.0113 | 0.9992 | |
| CD60-VMD | 0.155 | 0.4330 | 0.5643 | 0.01173 | 0.0027 | 0.9974 | |
| a | k | n | RMSE | R2 | |||
| Modified Page | CD50 | 1.000 | 0.0332 | 0.9389 | 0.0218 | 0.9956 | |
| CD60 | 1.000 | 0.0338 | 1.0367 | 0.0185 | 0.9970 | ||
| CD70 | 1.000 | 0.0436 | 1.0465 | 0.0136 | 0.9984 | ||
| VMD240 | 1.000 | 0.0806 | 1.0133 | 0.0277 | 0.9901 | ||
| VMD360 | 1.000 | 0.1087 | 0.9782 | 0.0193 | 0.9957 | ||
| VMD480 | 1.000 | 0.0393 | 1.7355 | 0.0245 | 0.9957 | ||
| CD60-VMD | 0.155 | 0.5665 | 0.3242 | 0.0043 | 0.9959 | ||
| Drying Method | Tmax | tCD | tVMD | Color | ||
|---|---|---|---|---|---|---|
| °C | min | min | L * | a * | b * | |
| CD50 | 50 | 300 | - | 43.27 ± 0.11 a,1 | −4.32 ± 0.89 a | 12.01 ± 0.31 a,c |
| CD60 | 60 | 210 | - | 43.45 ± 0.2 a,d | −3.21 ± 0.29 b | 12.53 ± 0.27 a |
| CD70 | 70 | 150 | - | 44.08 ± 0.24 d | −3.51 ± 0.24 b,c | 14.21 ± 0.22 d |
| VMD240 | 50 | - | 76 | 41.29 ± 0.3 e | −3.31 ± 0.1 b,c | 11.49 ± 0.09 b,c |
| VMD360 | 50 | - | 48 | 42.93 ± 0.18 a,c | −4.02 ± 0.14 a,c | 12.37 ± 0.13 a |
| VMD480 | 59 | - | 22 | 42.09 ± 0.44 b | −3.29 ± 0.26 b,c | 12.4 ± 0.19 a |
| CD60-VMD | 48 | 60 | 21 | 42.49 ± 0.64 b,c | −3.88 ± 0.29 a,b,c | 11.18 ± 0.46 b |
| Compound | Fresh | CD50 | CD60 | CD70 | 240VMD | 360VMD | 480 VMD | CD60/VMD |
|---|---|---|---|---|---|---|---|---|
| Content % 1 | ||||||||
| Limonene | 4.67 a,2 | 0.46 c | 0.45 c | 0.17 d | 1.26 b | 1.06 b | 0.41 c | 0.49 c |
| Caryophyllene | 33.18 a | 20.76 b | 12.50 f | 10.19 g | 13.76 ef | 16.58 d | 14.32 e | 21.12 c |
| Humulene | 3.57 a | 9.96 g | 6.40 c | 4.85 b | 6.79 d | 7.80 e | 7.40 e | 8.28 f |
| Caryophyllene oxide | 4.65 a | 13.44 e | 11.38 cd | 8.92 b | 11.93 d | 10.21 c | 13.82 e | 11.18 cd |
| Humulene epoxide II | 8.25 a | 5.61 b | 4.57 d | 3.46 f | 4.94 c | 4.21 e | 5.72 b | 4.40 ed |
| Caryophylla-4(12),8(13)-dien-5α-ol | 2.02 a | 8.16 b | 12.14 c | 8.24 b | 9.80 b | 9.80 b | 8.75 b | 8.96 b |
| 14-hydroxy-cis-Caryophyllene | 2.71 a | 5.81 b | 7.41 c | 5.87 b | 7.12 c | 6.19 b | 6.00 b | 6.00 b |
| 14-hydroxy-9-epi-trans-Caryophyllene | 3.16 a | 5.36 b | 7.20 e | 5.43 b | 6.66 de | 5.63 bc | 6.23 cd | 5.57 bc |
| TOTAL (mg/100 g) d.w. | 166.13 | 28.13 | 31.15 | 27.51 | 27.12 | 47.75 | 48.02 | 59.95 |
| % recovery of EOs | 100 | 16.93 | 18.75 | 16.55 | 16.32 | 28.56 | 28.91 | 36.08 |
| Compound, TMS | ANOVA | Fresh | CD50 | CD60 | CD70 | 240VMD | 360VMD | 480 VMD | CD60/VMD |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg g−1) 1 | |||||||||
| CBD | NS 2 | 2.19 | 1.98 | 1.87 | 1.88 | 1.89 | 2.01 | 2.08 | 1.95 |
| CBC | NS | 0.05 | 0.06 | 0.06 | 0.08 | 0.07 | 0.07 | 0.08 | 0.05 |
| Δ8-THC-d8 | NS | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 |
| Δ9-THC-d9 | NS | 0.25 | 0.21 | 0.23 | 0.27 | 0.28 | 0.25 | 0.28 | 0.24 |
| CBG | NS | 0.09 | 0.07 | 0.08 | 0.10 | 0.08 | 0.14 | 0.10 | 0.09 |
| CBN | NS | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 |
| CBDA | NS | 6.05 | 5.48 | 5.65 | 5.74 | 5.79 | 5.62 | 5.61 | 5.85 |
| THCA | NS | 0.63 | 0.59 | 0.67 | 0.60 | 0.55 | 0.48 | 0.49 | 0.52 |
| CBGA | NS | 0.19 | 0.20 | 0.23 | 0.20 | 0.17 | 0.19 | 0.16 | 0.14 |
| TOTAL | 9.51 | 8.64 | 8.84 | 8.93 | 8.90 | 8.83 | 8.87 | 8.90 | |
| Compound, TMS | ANOVA | Fresh | CD50 | CD60 | CD70 | 240VMD | 360VMD | 480 VMD | CD60/VMD |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (µg g−1) 1 | |||||||||
| Campesterol | NS 2 | 97.90 | 100.37 | 86.70 | 92.27 | 93.22 | 108.87 | 107.33 | 104.11 |
| Stigmasterol | NS | 44.51 | 36.31 | 35.26 | 49.23 | 33.39 | 43.27 | 51.22 | 50.51 |
| β-Sitosterol | NS | 534.11 | 515.77 | 421.81 | 448.79 | 527.28 | 444.21 | 414.75 | 371.27 |
| β-Amyrin | NS | 65.96 | 40.68 | 60.41 | 64.33 | 49.50 | 60.41 | 65.12 | 56.53 |
| Isofucosterol | NS | 41.38 | 40.56 | 44.94 | 47.94 | 38.27 | 47.31 | 46.65 | 50.64 |
| α-Amyrin | NS | 61.07 | 65.91 | 72.09 | 73.80 | 67.42 | 70.45 | 73.15 | 73.26 |
| Lupeol | NS | 97.45 | 90.30 | 94.21 | 90.78 | 82.49 | 84.80 | 96.59 | 101.87 |
| TOTAL | 855.38 | 889.9 | 815.42 | 867.14 | 891.57 | 869.32 | 874.81 | 808.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśnica, A.; Pachura, N.; Carbonell-Barrachina, Á.A.; Issa-Issa, H.; Szumny, D.; Figiel, A.; Masztalerz, K.; Klemens, M.; Szumny, A. Effect of Drying Methods on Chemical and Sensory Properties of Cannabis sativa Leaves. Molecules 2023, 28, 8089. https://doi.org/10.3390/molecules28248089
Kwaśnica A, Pachura N, Carbonell-Barrachina ÁA, Issa-Issa H, Szumny D, Figiel A, Masztalerz K, Klemens M, Szumny A. Effect of Drying Methods on Chemical and Sensory Properties of Cannabis sativa Leaves. Molecules. 2023; 28(24):8089. https://doi.org/10.3390/molecules28248089
Chicago/Turabian StyleKwaśnica, Andrzej, Natalia Pachura, Ángel A. Carbonell-Barrachina, Hanán Issa-Issa, Dorota Szumny, Adam Figiel, Klaudia Masztalerz, Marta Klemens, and Antoni Szumny. 2023. "Effect of Drying Methods on Chemical and Sensory Properties of Cannabis sativa Leaves" Molecules 28, no. 24: 8089. https://doi.org/10.3390/molecules28248089
APA StyleKwaśnica, A., Pachura, N., Carbonell-Barrachina, Á. A., Issa-Issa, H., Szumny, D., Figiel, A., Masztalerz, K., Klemens, M., & Szumny, A. (2023). Effect of Drying Methods on Chemical and Sensory Properties of Cannabis sativa Leaves. Molecules, 28(24), 8089. https://doi.org/10.3390/molecules28248089











