Copper-Catalyzed Azide–Alkyne Cycloaddition-Oriented Multifunctional Bio-Orthogonal Linker BPPA: Design, Synthesis and Evaluation
Abstract
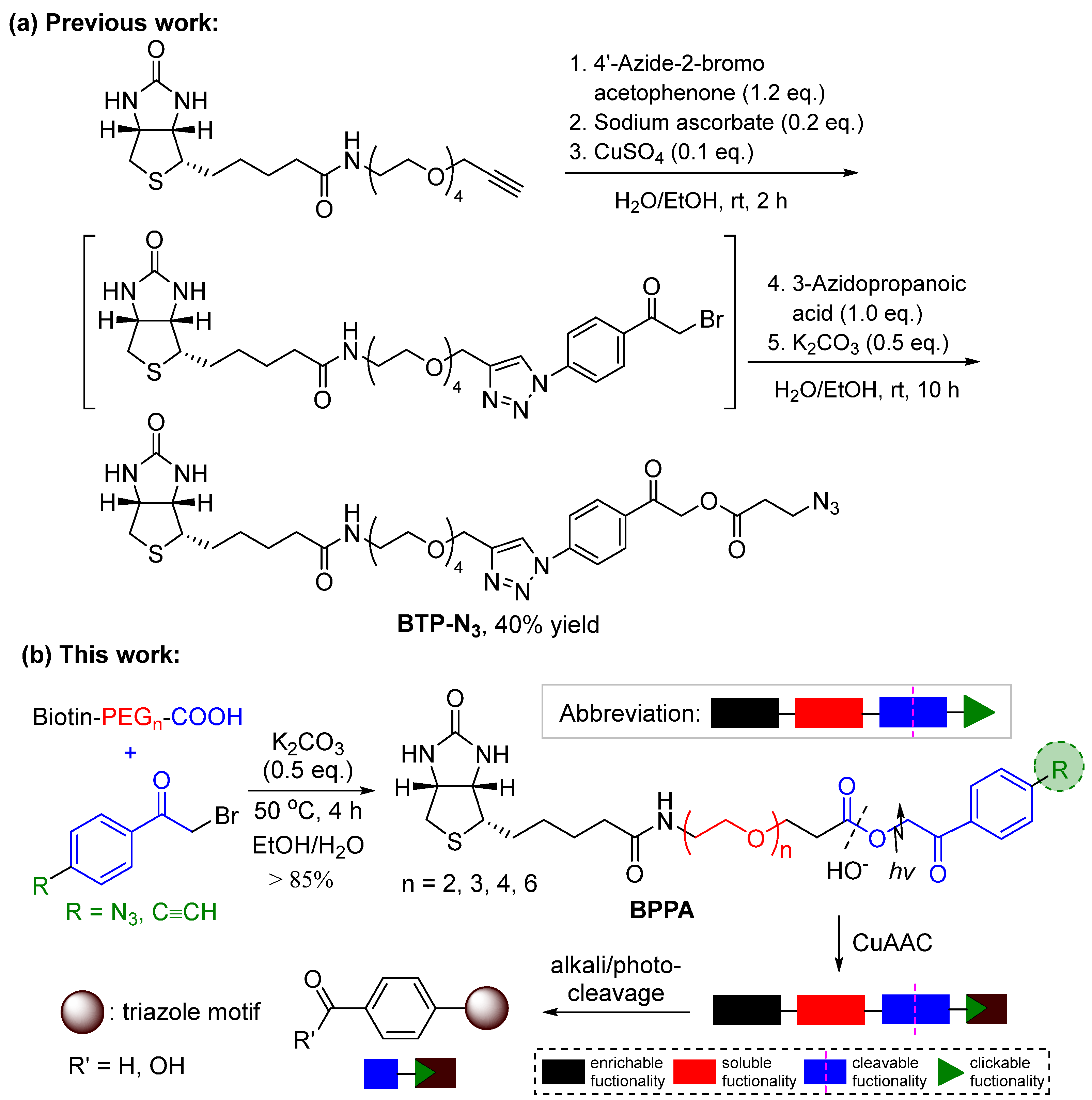
:1. Introduction
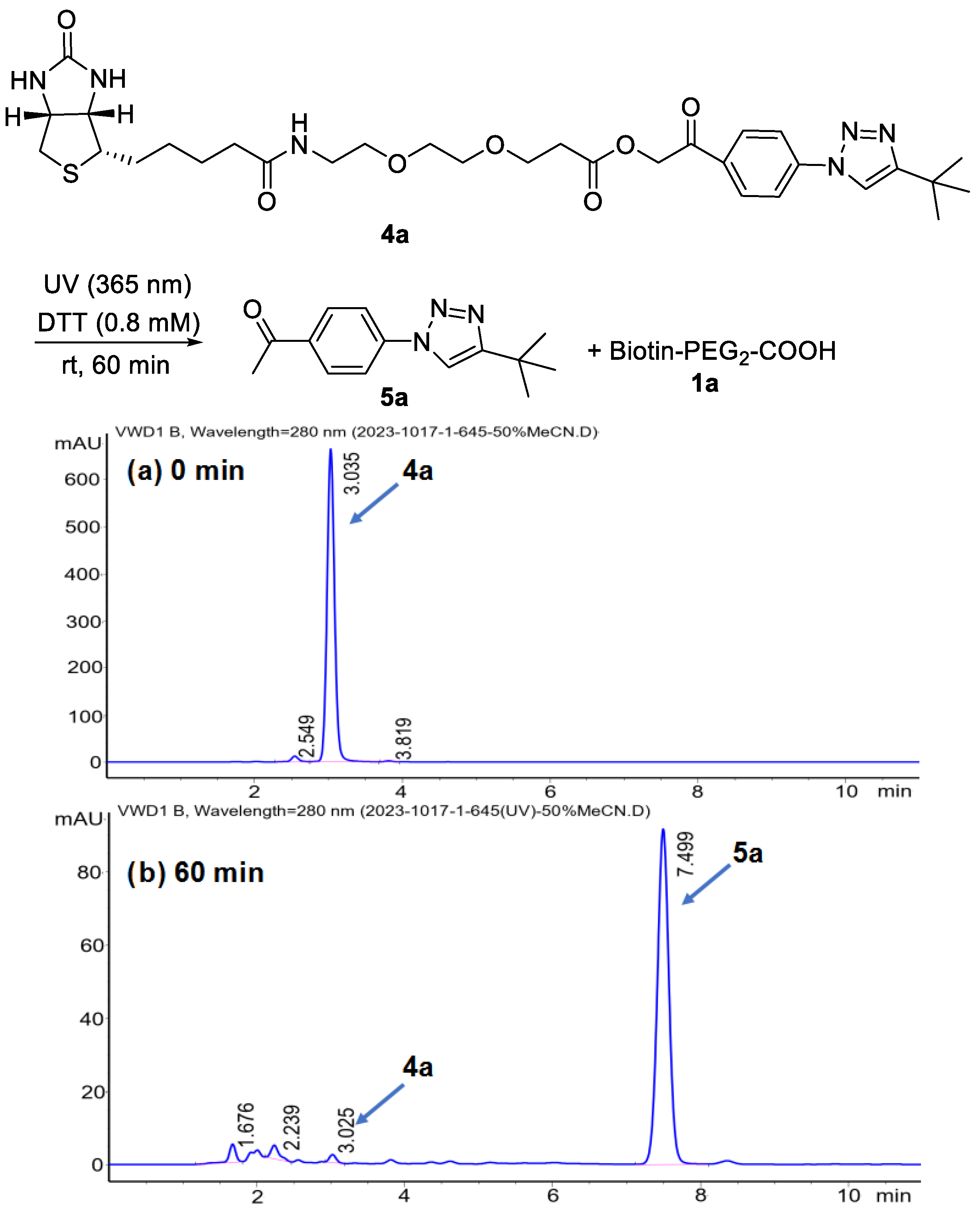
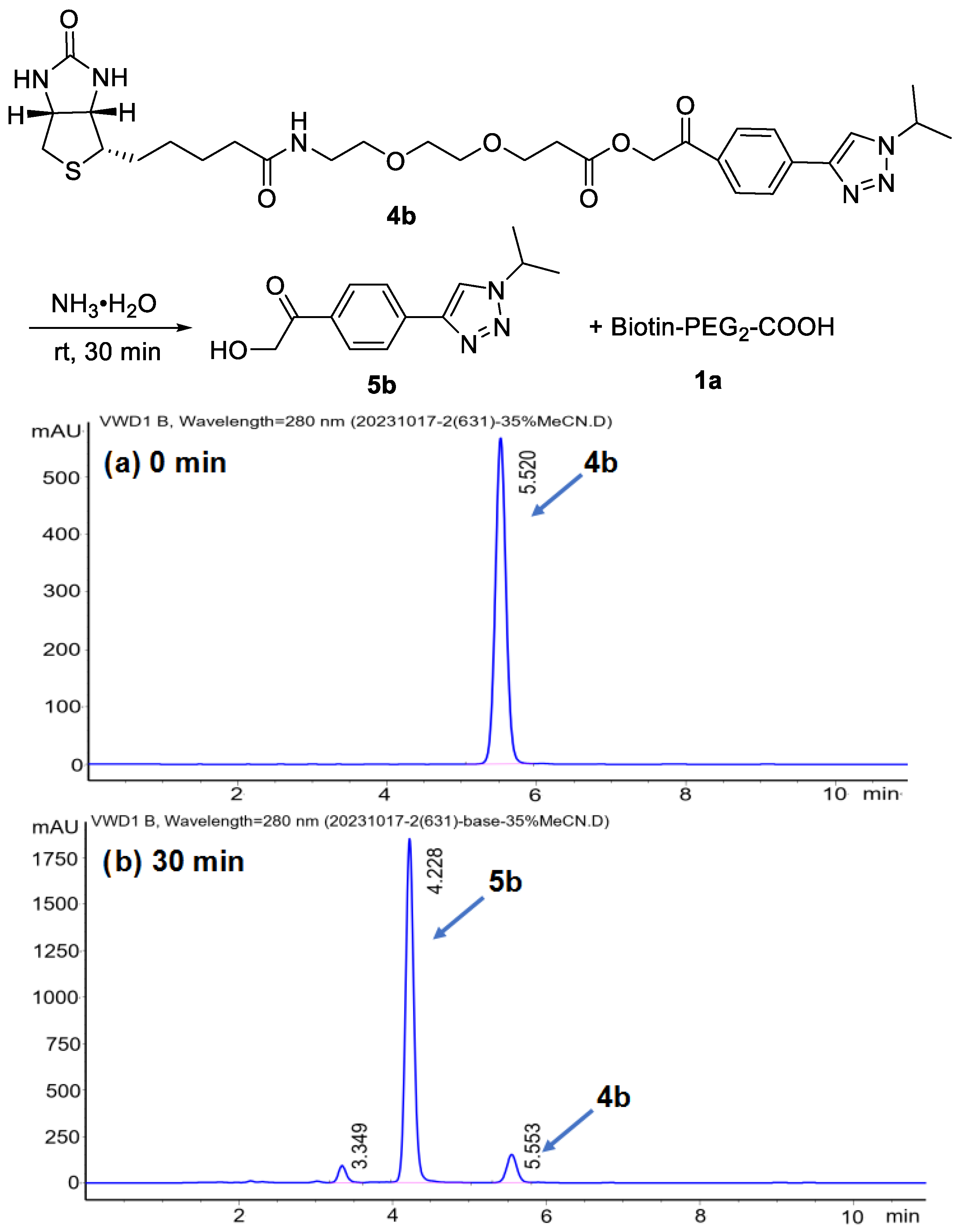
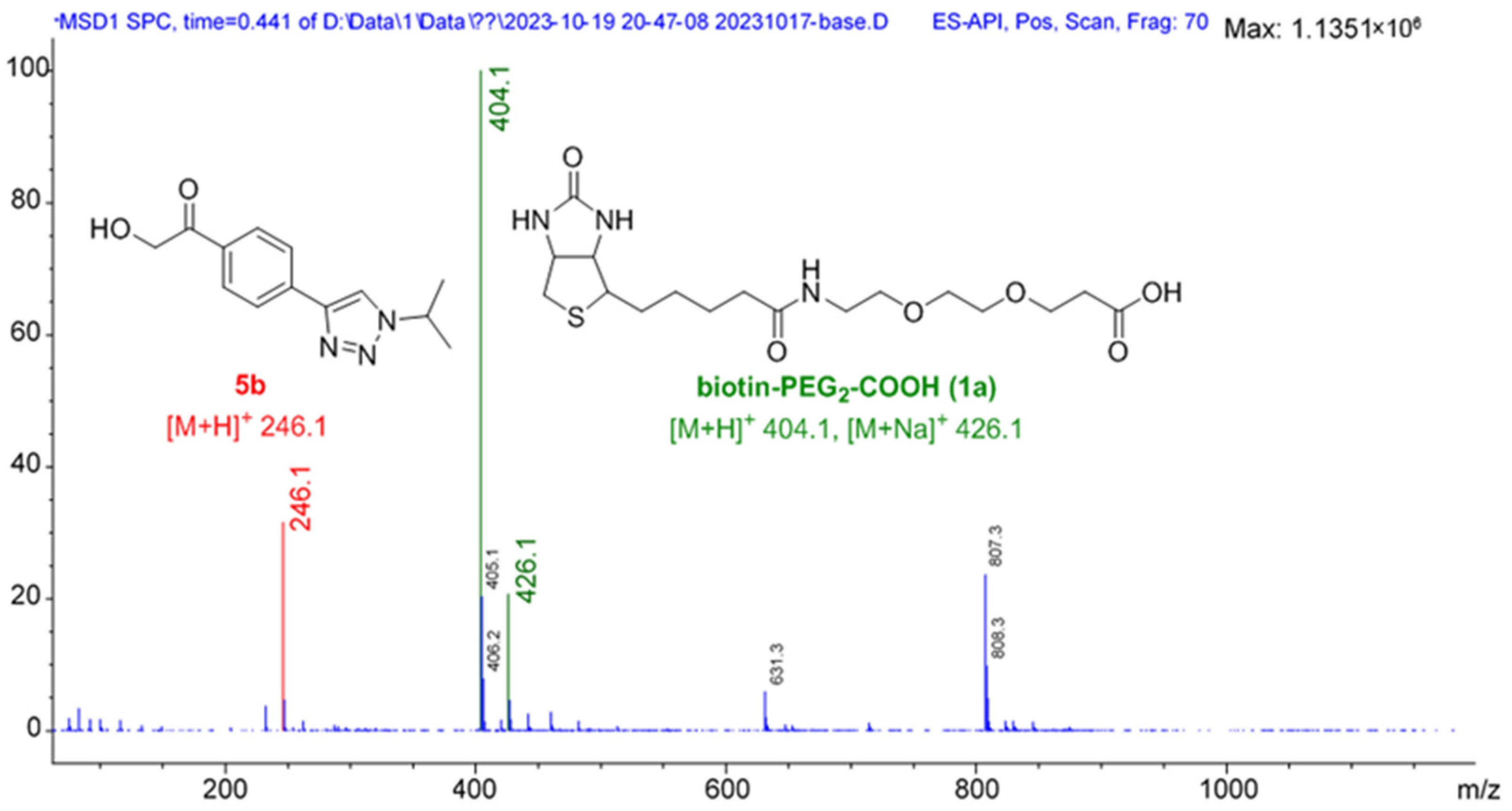
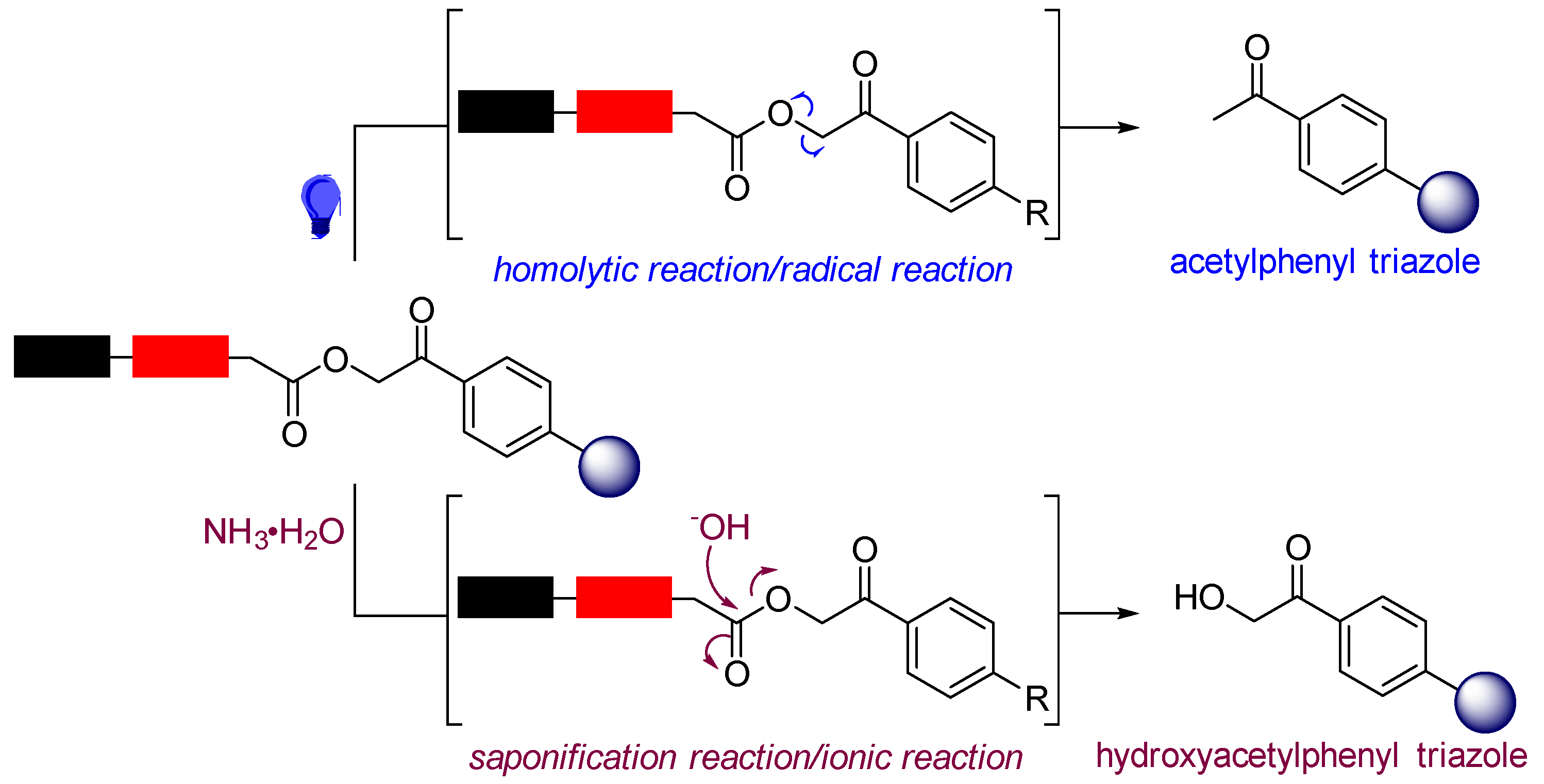
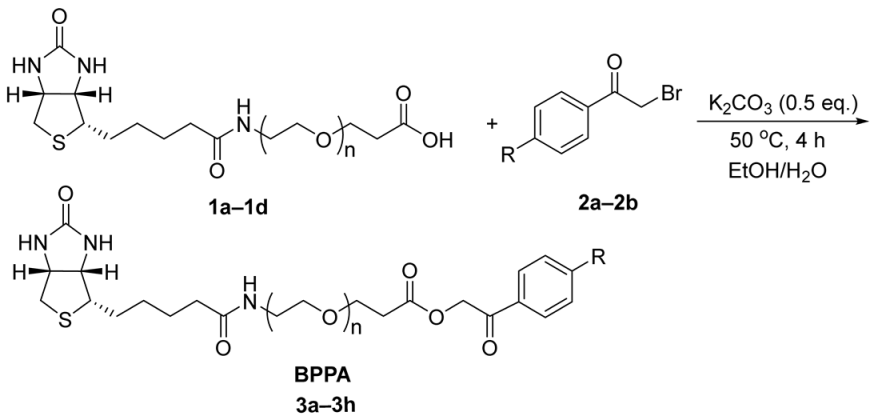
2. Results and Discussion
3. Experimental
- (1)
- General procedure for synthesis of BPPA linker 3
- (2)
- General procedure for synthesis of BPPA-triazole 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schauenburg, D.; Weil, T. Chemical Reactions in Living Systems. Adv. Sci. 2023, 2303396. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.C.; Yu, C.; Kato, D.L.; Bertozzi, C.R. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. USA 2003, 100, 14846–14851. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.R. A special virtual issue celebrating the 2022 Nobel Prize in chemistry for the development of click chemistry and bioorthogonal chemistry. ACS Cent. Sci. 2023, 9, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, H.; Wang, C.; Yao, S.C.L.; Yao, S.Q. Target identification of natural products and bioactive compounds using affinity-based probes. Nat. Prod. Rep. 2016, 33, 612. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Bagatolli, L.A.; Echabe, I.; Arrondo, J.L.R.; Argarana, C.E.; Cantor, C.R.; Fidelio, G.D. Interaction of biotin with streptavidin: Thermostability and conformational changes upon binding. J. Biol. Chem. 1997, 272, 11288–11294. [Google Scholar] [CrossRef] [PubMed]
- Freitag, S.; Le Trong, I.; Chilkoti, A.; Klumb, L.A.; Stayton, P.S.; Stenkamp, R.E. Structural studies of binding site tryptophan mutants in the high-affinity streptavidin-biotin complex. J. Mol. Biol. 1998, 279, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Green, N.M. Avidin. In Advances in Protein Chemistry; Anfinsen, C.B., Edsall, J.T., Richards, F.M., Eds.; Academic Press: New York, NY, USA, 1975; Volume 29, pp. 85–133. [Google Scholar]
- Finn, F.M.; Titus, G.; Montibeller, J.A.; Hofmann, K. Hormone-receptor studies with avidin and biotinylinsulin-avidin complexes. J. Biol. Chem. 1980, 255, 5742–5746. [Google Scholar] [CrossRef] [PubMed]
- Haugland, R.P. Handbook of Fluorescent Probes and Research Products, 9th ed.; Molecular Probes: Eugene, OR, USA, 2002. [Google Scholar]
- Breitinger, H.G.A.; Gee, K.R.; Carpenter, B.K.; Hess, G.P. Toward the development of new photolabile protecting groups that can rapidly release bioactive compounds upon photolysis with visible light. J. Org. Chem. 2003, 68, 8361–8367. [Google Scholar]
- Leriche, G.; Chisholm, L.; Wagner, A. Cleavable linkers in chemical biology. Bioorg. Med. Chem. 2012, 20, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Beard, H.A.; Korovesis, D.; Chen, S.; Verhelst, S.H. Cleavable linkers and their application in MS-based target identification. Mol. Omics. 2021, 17, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z. Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction. Molecules 2016, 21, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Rigolot, V.; Biot, C.; Lion, C. To view your biomolecule, click inside the cell. Angew. Chem. Int. Ed. 2021, 60, 23084–23104. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.; Sieber, S.A. A photolabile linker for the mild and selective cleavage of enriched biomolecules from solid support. J. Org. Chem. 2009, 74, 8476–8479. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Verhelst, S.H.L. Cleavable trifunctional biotin reagents for protein labelling, capture and release. Chem. Commun. 2013, 49, 5366–5368. [Google Scholar] [CrossRef] [PubMed]
- Karaj, E.; Sindi, S.H.; Tillekeratne, L.M.V. Photoaffinity labeling and bioorthogonal ligation: Two critical tools for designing “Fish Hooks” to scout for target proteins. Bioorg. Med. Chem. 2022, 62, 116721–116748. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.K.; Flaxman, H.A.; Wu, H.Y.; Gao, J.X.; Woo, C.M. Discovery of a celecoxib binding site on prostaglandin E synthase (PTGES) with a cleavable chelation-assisted biotin probe. ACS Chem. Biol. 2019, 14, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Anabuki, T.; Tsukahara, M.; Okamoto, M.; Matsuura, H.; Takahashi, K. Novel biotin linker with alkyne and amino groups for chemical labelling of a target protein of a bioactive small molecule. Bioorg. Med. Chem. Lett. 2018, 28, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Wang, S.; Cui, F.; Liu, H.; Gao, Z.; Ying, W.; Shi, E. Comprehensive insight on protein modification by V-type agent: A chemical proteomic approach employing bioorthogonal reaction. Proteomics 2023, e2300039. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Rauch, S.; Eichhorn, K.J.; Stamma, M.; Uhlmann, P. Enzyme immobilization on protein-resistant PNIPAAm brushes: Impact of biotin linker length on enzyme amount and catalytic activity. Colloids Surf. B 2018, 171, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.; Shafi, S.; Zaman, M.S.; Kalia, N.P.; Rajput, V.S.; Mulakayala, C.; Mulakayala, N.; Khan, I.A.; Alam, M.S. Sulfur rich 2-mercaptobenzothiazole and 1,2,3-triazole conjugates as novel antitubercular angent. Eur. J. Med. Chem. 2014, 76, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 4th ed.; Wiley & Sons Inc.: New York, NY, USA, 2007. [Google Scholar]

 | ||||
|---|---|---|---|---|
| Entry | BPPA | R | n | 3 (%) b |
| 1 | 3a | –N3 | 2 | 89 |
| 2 | 3b | –N3 | 3 | 90 |
| 3 | 3c | –N3 | 4 | 87 |
| 4 | 3d | –N3 | 6 | 85 |
| 5 | 3e | –C≡CH | 2 | 90 |
| 6 | 3f | –C≡CH | 3 | 87 |
| 7 | 3g | –C≡CH | 4 | 88 |
| 8 | 3h | –C≡CH | 6 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; He, X.; Li, J.; Shi, E. Copper-Catalyzed Azide–Alkyne Cycloaddition-Oriented Multifunctional Bio-Orthogonal Linker BPPA: Design, Synthesis and Evaluation. Molecules 2023, 28, 8083. https://doi.org/10.3390/molecules28248083
Wang S, He X, Li J, Shi E. Copper-Catalyzed Azide–Alkyne Cycloaddition-Oriented Multifunctional Bio-Orthogonal Linker BPPA: Design, Synthesis and Evaluation. Molecules. 2023; 28(24):8083. https://doi.org/10.3390/molecules28248083
Chicago/Turabian StyleWang, Shuo, Xu He, Junchen Li, and Enxue Shi. 2023. "Copper-Catalyzed Azide–Alkyne Cycloaddition-Oriented Multifunctional Bio-Orthogonal Linker BPPA: Design, Synthesis and Evaluation" Molecules 28, no. 24: 8083. https://doi.org/10.3390/molecules28248083
APA StyleWang, S., He, X., Li, J., & Shi, E. (2023). Copper-Catalyzed Azide–Alkyne Cycloaddition-Oriented Multifunctional Bio-Orthogonal Linker BPPA: Design, Synthesis and Evaluation. Molecules, 28(24), 8083. https://doi.org/10.3390/molecules28248083






