Abstract
A two-step, one-pot synthesis of 3-substituted 1H-dibenzo[e,g]indazoles in good to high yields via a LiOtBu-promoted intramolecular 1,3-dipolar cyclization of 2′-alkynyl-biaryl-2-aldehyde N-tosylhydrazones was developed. The N-Ts-hydrazones used were prepared in situ via the reactions of 2′-alkynyl-biaryl-2-aldehydes and TsNHNH2 (p-methylbenzenesulfonohydrazide). Two types of signals related to the hydrogen bonds, forming in several products, were observed in the 1H NMR spectra recorded in DMSO-d6, assigned to N-H bonds in their dimeric species of product and tautomer.
1. Introduction
1,3-dipolar cycloadditions of azides to alkynes, as the most important representative reactions in click chemistry and bio-orthogonal chemistry, have attracted enormous attention in the past decades [1,2,3,4,5]. Besides azides, diazo compounds’ 1,3-dipoles could also be used in 1,3-dipolar cycloadditions to react with alkynes, providing diverse pyrazole-based skeletons [6,7]. Recently, numerous elegant works, involving cycloadditions between diazo compounds (or their N-tosylhydrazone precursors) and alkynes, were reported [8,9,10,11,12,13,14,15,16,17,18,19,20]. However, the design of N-tosylhydrazones for intramolecular 1,3-dipolar cycloadditions to construct π-extended pyrazole-based skeletons is rarely reported.
Nowadays, indazole derivatives comprising a pyrazole ring represent one of the most important heterocyclic scaffolds in the pharmaceutical industry [21,22,23], possessing a variety of biological activities, such as antimicrobial [24], anti-inflammatory [25], and anti-HIV [26] properties. 1H-indazoles, as one of the tautomeric forms of indazole, have more thermodynamic stability than 2H-indazoles [27].
Since the synthesis of 2H-dibenzo[e,g]indazoles has already been reported [28], in the present work, we opted for a synthetic method towards 1H-dibenzo[e,g]indazoles, providing more possibilities of indazole-based derivatives in a further exploration of pharmaceutical molecules or larger polycyclic aromatic compounds (PACs).
In 1975, Jones’s group reported a pyrolytic method towards 1H-dibenzo[e,g]indazole from 2′-ethynyl-biaryl-2-aldehyde N-tosylhydrazone sodium-salt in a quantitative yield [29] (Scheme 1a). In 2013, Zhan’s group synthesized 3-phenyl-substituted 1H-dibenzo[e,g]indazole (2a) in a 60% yield from a ring-expansion strategy of 9-(phenylethynyl)-9H-fluoren-9-ol [30] (Scheme 1b). We note that only one example was given in each of the cited references, and either high temperatures or complicated starting materials were required.
Scheme 1.
The syntheses of some 1H-dibenzo[e,g]indazoles from different starting materials [29,30].
Based on our previous studies on the use of N-tosylhydrazones in cyclizations [31,32,33], herein we report a one-pot synthetic method towards 3-substituted 1H-dibenzo[e,g]indazoles (2) from 2′-alkynyl-biaryl-2-aldehyde N-tosylhydrazones, which was then optimized to a one-pot two-steps manner starting from 2′-alkynyl-biaryl-2-aldehydes (1) (Scheme 1c). In addition, in order to explain the observed two types of N-H signals in the 1H NMR spectra in DMSO-d6, the formation of dimeric species is proposed, which is supported by the X-ray structure of one product with the studies of DFT (density functional theory) calculations using the Gaussian 09 program [34] with an SMD solvation model [35].
2. Results and Discussion
2.1. Synthesis
2.1.1. Optimization of Model Reaction Conditions
Our investigations started from hydrazone 1a′, easily available from biarylaldehyde 1a and TsNHNH2 (p-methylbenzenesulfonohydrazide) in methanol at room temperature. When the reaction of hydrazone 1a′ (1.0 equiv.) and LiOtBu (1.5 equiv.) in tetrahydrofuran (THF) was heated at 100 °C for 2 h, 3-phenyl-1H-dibenzo[e,g]indazole (2a) could be isolated in an 89% yield (Table 1, entry 1). When the temperature was progressively diminished at 50 °C, 45 °C, 35 °C, or 25 °C, the yields of 2a did not significantly decrease except for 25 °C (Table 1, entries 2–5). Repeating the reaction in THF at 45 °C for 1 h, the yield of 2a could be maintained in an 88% yield (Table 1, entry 6). Since hydrazone 1a′ was prepared in methanol, we then examined the reaction of hydrazone 1a′ in this solvent, rather than in THF, but the yield of 2a decreased to 68% (Table 1, entry 7).

Table 1.
Optimization of the model’s reaction conditions in the case of compound 2a.
Therefore, the condensation between biarylaldehyde 1a and TsNHNH2 in THF at 45 °C was further examined to explore the possibility of developing a two-step, one-pot procedure 1a → 2a. It was found that 1a could be totally converted into hydrazone 1a′ after 1 h (TLC monitoring). Moreover, when LiOtBu (1.5 equiv.) and 2.5 mL of THF were added to the reaction mixture of entry 6, 2a could be obtained in an 88% yield after additional heating for 1 h. We also tried a one-step process at 45 °C: by adding TsNHNH2 and LiOtBu at the same time, 79% of 2a could be acquired. Thus, the one-pot/two-step process in entry 6 was considered as the optimized condition.
We also examined the formation of 2a from 1a using other inorganic alkali, such as NaOtBu, KOtBu, Li2CO3, Na2CO3, K2CO3, and Cs2CO3. As shown in Table 2, the use of NaOtBu and KOtBu resulted in the formation of 2a in 81% and 85% yields, respectively (entries 2 and 3), similar to the yield of LiOtBu (entry 1). However, in the presence of Li2CO3, Na2CO3, K2CO3, and Cs2CO3, 2a only formed in 9–14% yields (entries 4–7). These results support the proposed mechanism depicted in Scheme 2 (vide infra), in which a tert-butoxide anion (tBuO−) made a main contribution to the intramolecular cyclization by promoting the formation of the diazo zwitterion A.

Table 2.
Optimal alkali bases used in the synthesis of compound 2a.

Scheme 2.
Proposed mechanism of 2a formation.
2.1.2. Substrates’ Expansion under Optimized Conditions
Thus, the extension of the above optimized methodology as a one-pot/two-step synthesis of 3-substituted-1H-dibenzo[e,g]indazoles 2b–p starting from 2′-alkynyl-biaryl-2-aldehydes 1b–p via an intramolecular 1,3-dipolar cycloaddition is depicted in Chart 1. The corresponding p-Ts-hydrazones of 1b–p, as crucial transformations, exhibited their quantitative feasibility by being modulated with the influence of alkyne-substituents R1 and Ar-substituents R2 and R3.

Chart 1.
Extension of the optimized methodology as one-pot/two-step synthesis of 3-substituted-1H-dibenzo[e,g]indazoles 2b–p a. a Reaction conditions: 1 (1.0 mmol), TsNHNH2 (1.1 equiv.) in 5.0 mL of THF at 45 °C for 1 h, then LiOtBu (1.5 equiv.) and additional 2.5 mL of THF at 45 °C for 1 h. In each case, the yield refers to the effective amount of isolated compound.
Aromatic alkynyl substrates bearing either electron-donating groups (R1−X = p-methoxy (1b), R1−X = p-methyl (1c)) or electron-withdrawing groups (R1−X = p-fluoro (1d), R1−X = p-chloro (1e)) underwent the condensation reactions smoothly to give 2b–e in 80–88% yields. Moreover, pyridyl-(1i), thienyl-(1j), and silyl-(1k) substituted substrates showed a good tolerance, providing 2i–k in 78–93% yields. However, the substrate having an alkyl alkynyl group (1l) showed a slightly lower reactivity, giving 2l in a 61% yield.
In addition, the introduction of methyl (1f), chloro (1g), and trifluoromethyl (1h) groups at the position of R2 showed a similar reactivity to 1a in producing 2f–h in 83–86% yields. In the case of the substrate having chloro and silyl groups (1m), the corresponding product 2m could be also obtained in an 82% yield. More interestingly, three pyridyl-fused analogues of 2a, 2n–p were also successfully synthesized in 88%, 90%, and 75% yields, respectively.
2.2. Proposed Mechanism
The proposed mechanism of 3-phenyl-1H-dibenzo[e,g]indazole (2a) formation is depicted in Scheme 2. In the presence of tBuO−, diazo zwitterion A forms from hydrazone 1a′; then, an intramolecular nucleophilic cycloaddition occurs in the 1,3-dipole C to afford D, which, then, allows aromatization to take place to give the final product 2a.
2.3. Structural Analysis
2.3.1. X-ray Data of Compound 2a
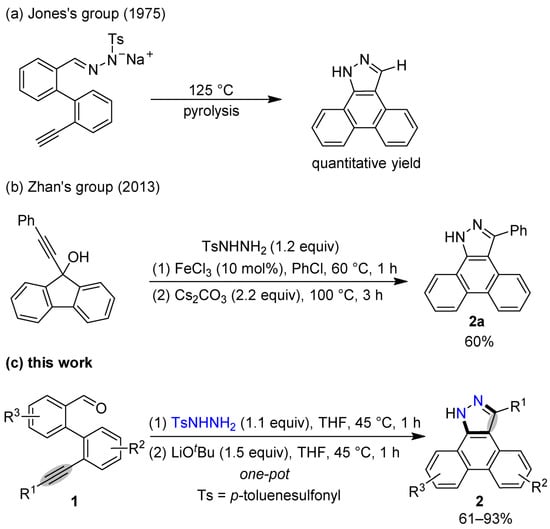
A suitable single crystal of compound 2a was obtained through a slow evaporation from its petroleum ether/dichloromethane (5:1 v/v) solution [36]. The X-ray crystal data (Figure 1) indicate that the existence of intermolecular NH-N hydrogen bonds promotes the formation of a 2a dimer. The length of the NH–N hydrogen bond in the 2a dimer is 2.103 Å, and the N-N distance of the NH–N hydrogen bond is 2.845 Å.
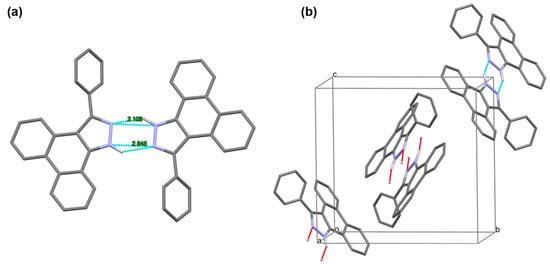
Figure 1.
X-ray crystal structure of compound 2a. Carbon atoms are shown in gray, nitrogen atoms in purple, and N-H hydrogen atoms in white. The hydrogen atoms on the benzene rings are omitted for clarity. (a) View face-on to the aromatic rings showing the hydrogen bonds in the 2a dimer. Annotated with the NH–N hydrogen bonds’ length and N-N distance. (b) View of a unit cell. The blue lines show the NH–N hydrogen bonds in the 2a dimer. The red lines show the NH–N hydrogen bonds between 2a and another omitted molecule.
2.3.2. DFT Calculation of Dimeric Species of 2a and Its Tautomer
In a DMSO-d6 solvent, two types of proton peaks assigned to the N-H bond were observed in the 1H NMR spectra of 2a, 2d, 2f, 2h, 2i, 2j, 2l, 2n, 2o, and 2p. On the basis of a DFT calculation, it is favorable to form the dimeric species with a definitely lower energy than that of the sum of the two isolated monomers resulted from the hydrogen bond in the solution; thus, there are expected to be three types of dimers, as shown in Chart 2, including a 2a dimer-anti, a 2a/2a tautomer dimer-syn, and a tautomer dimer-anti, taking two phenyl groups as reference, centered on a six-membered H-bonding chelate. We selected 2a as the representative sample for calculating the binding energies of these three dimers with DFT using the Gaussian 09 program at a B3LYP-D3(BJ)/ma-TZVP [37,38,39] level. An SMD solvation model was employed with the default settings. The basis set superposition error (BSSE) was corrected using the counterpoise (CP) method of Boys and Bernardi [40]. The calculation results disclose that the binding energies for the formation of the 2a dimer-anti, 2a/2a tautomer dimer-syn, and tautomer dimer-anti are −8.29 kcal/mol, −8.50 kcal/mol, and −8.79 kcal/mol, respectively (Figure 2). Considering the slight differences of binding energies among these three types of dimers, the observed two types of N-H signals in the 1H NMR spectra in DMSO-d6 are assigned to N-H hydrogen atoms in 2a (14.27 ppm) and 2a tautomer (13.98 ppm) in dimeric species, respectively
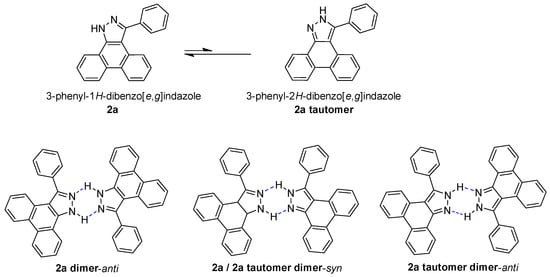
Chart 2.
Structures of the expected three types of dimeric species in solution. The blue dashed lines show the NH–N hydrogen bonds.
Figure 2.
The calculation results of the binding energies of two types of dimeric species of 2a in DMSO-d6. The calculations were performed using the Gaussian 09 program at a B3LYP-D3(BJ)/ma-TZVP level. The blue dashed lines show the NH–N hydrogen bonds. The red circles show N-H hydrogen atoms and peak of 2a, when green circles show that of 2a tautomer.
2.3.3. Temperature Gradient Experiment of Compound 2a in DMSO-d6
A temperature gradient experiment of compound 2a in DMSO-d6 was performed from 25 °C to 125 °C; the corresponding 1H NMR spectra are recorded in Figure 3. With the temperature increasing, it is shown that two 1H sharp signals gradually coalesced into a unique, broad signal at 14.03 ppm at 125 °C, which results from the fast chemical exchange of 2a and 2a tautomer at relatively higher temperature.
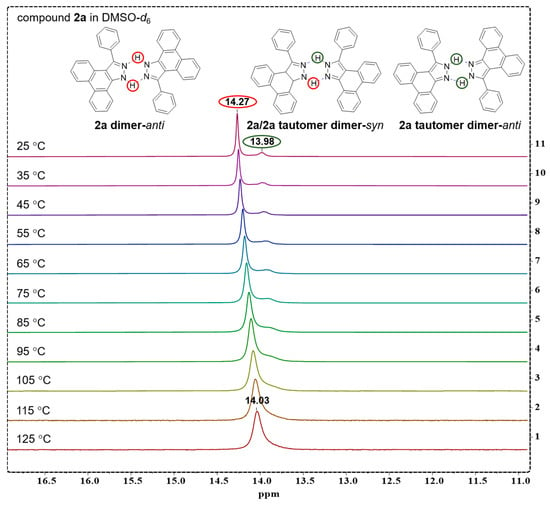
Figure 3.
1H NMR spectra of compound 2a recorded in DMSO-d6 with increasing temperature from 25 °C to 125 °C. The red circles show N-H hydrogen atoms and peak of 2a, when green circles show that of 2a tautomer.
3. Materials and Methods
3.1. Materials
All commercially available reagents, solvents, and metal salts are analytically pure and were used without further purification. 1-bromo-2-iodobenzene (CAS 583-55-1), 2-bromo-1-iodo-4-methylbenzene (CAS 71838-16-9), 2-bromo-4-chloro-1-iodobenzene (CAS 31928-44-6), 2-bromo-1-iodo-4-(trifluoromethyl)benzene (CAS 481075-58-5), 3-bromo-2-iodopyridine (CAS 408502-43-2), 4-bromo-3-iodopyridine (CAS 917969-51-8), 3-bromo-4-iodopyridine (CAS 89167-19-1), ethynylbenzene (CAS 536-74-3), 1-ethynyl-4-methoxybenzene (CAS 768-60-5), 1-ethynyl-4-methylbenzene (CAS 766-47-2), 1-ethynyl-4-fluorobenzene (CAS 766-98-3), 1-chloro-4-ethynylbenzene (CAS 873-73-4), 2-ethynylpyridine (CAS 1945-84-2), 2-ethynylthiophene (CAS 4298-52-6), ethynyltriisopropylsilane (CAS 89343-06-6), (2-formylphenyl)boronic acid (CAS 40138-16-7), (4-chloro-2-formylphenyl)boronic acid (CAS 913835-76-4), 4-methylbenzenesulfonohydrazide (CAS 1576-35-8), and dichloroditriphenylphosphor palladium (CAS 13965-03-2) were purchased from Bidepharm; tetrahydrofuran (CAS 109-99-9), triethylamine (CAS 121-44-8), and methyl alcohol (CAS 67-56-1) were purchased from Aladdin; hept-1-yne (CAS 628-71-7), potassium fluoride (CAS 7789-23-3), and 1,4-dioxane (CAS 123-91-1) were purchased from Meryer (Shanghai, China); cuprous iodide (CAS 7681-65-4) and lithium t-butoxide (CAS 1907-33-1) were purchased from Macklin (Shanghai, China); the H2O used was ultrapure water.
3.2. General Methods
Column chromatography was performed on silica gel (300–400 mesh). Thin-layer chromatography (TLC) was performed on 0.2 mm silica gel-coated glass sheets. The NMR spectra were recorded on a JEOL ECS-400 instrument operating at 400 and 100 MHz for the 1H and 13C nuclei, respectively. All chemical shifts (δH, δC, δF) are given in parts per million (ppm); all homocoupling patterns (nJH,H) are given in hertz (Hz). No TMS was added; the chemical shifts were measured against the solvent peak taken as a reference signal; CDCl3, δH = 7.26 ppm, and δC = 77.16 ppm; DMSO-d6, δH = 2.50 ppm, and δC = 39.52 ppm. The high-resolution mass spectroscopy (HRMS) spectra were obtained using high-resolution mass spectrometers with an electrospray ionization (ESI) source. The single-crystal X-ray diffraction data were obtained using a SuperNova (Agilent Technologies, Oxfordshire, UK) diffractometer with a Cu Kα radiation at a low temperature (173.15 K). All the NMR charts for the prepared starting materials and the products are reported in the Supplementary Materials.
3.3. General Procedure for the Preparation of 2′-Alkynyl-biaryl-2-aldehydes 1a–p
(1) A THF (5.0 mL) and Et3N (5.0 mL) solution containing 1-bromo-2-iodobenzenes (2.0 mmol), CuI (5.0 mol%, 19.0 mg, 0.1 mmol), and PdCl2(PPh3)2 (5.0 mol%, 70.2 mg, 0.1 mmol) in a 25 mL screw-capped thick-walled Pyrex tube with stirring under N2 was dropwise added to terminal alkynes (2.4 mmol) at room temperature over 5 min. The obtained mixture was then stirred at room temperature under N2 for 12 h. After the reaction was completed (TLC monitoring, eluent pure petroleum ether), the reaction mixture was filtrated through a short pad of celite. The solution was then concentrated under reduced pressure to remove the volatiles, and the crude residue was purified using column chromatography on silica gel (eluent pure petroleum ether) to obtain the desired compounds Sa–Sp (checked with GC-MS) in 75–95% yields.
(2) A 1,4-dioxane (10.0 mL) and H2O (1.0 mL) solution containing S (1.5 mmol), phenylboronic acids (1.65 mmol), PdCl2(PPh3)2 (5.0 mol%, 52.7 mg, 0.075 mmol), and KF (261.0 mg, 4.5 mmol) in a 25 mL screw-capped thick-walled Pyrex tube was stirred under N2 at 100 °C in an oil bath for 12 h. After the reaction was completed, the reaction mixture was cooled to room temperature (TLC monitoring, eluent petroleum ether/ethyl acetate, 15/1 v/v) and filtrated through a short pad of celite. The solution was then concentrated under reduced pressure to remove the volatiles, and the crude residue was purified using column chromatography on silica gel (eluent petroleum ether/ethyl acetate, gradient mixture ratio from 30/1 to 15/1 v/v) to afford product 1a–p in 24–94% yields.
Compounds 1a, 1c, 1e, 1f, 1g are known compounds, which were confirmed by their 1H NMR and 13C NMR spectroscopic data [41].
3.4. Analytical Data of Compound 1a–p
- 2′-(Phenylethynyl)-(1,1′-biphenyl)-2-carbaldehyde (1a). Pale yellow oil (355 mg, 1.26 mmol, 84% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.94 (s, 1H), 8.09 (dd, 3JH,H = 7.8 Hz, 4JH,H = 1.5 Hz, 1H), 7.68–7.64 (m, 2H), 7.54 (t, 3JH,H = 7.6 Hz, 1H), 7.46–7.38 (m, 4H), 7.25–7.22 (m, 4H), 7.17–7.15 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δC 192.0, 144.4, 140.4, 134.3, 133.6, 132.1, 131.4, 130.4, 128.6, 128.4, 128.3, 127.0, 123.8, 122.8, 93.9, 88.3 ppm.
- 2′-[(4-Methoxyphenyl)ethynyl]-(1,1′-biphenyl)-2-carbaldehyde (1b). Yellow oil (332 mg, 1.07 mmol, 71% yield). Rf = 0.55 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.93 (s, 1H), 8.08 (dd, 3JH,H = 7.9 Hz, 4JH,H = 1.5 Hz, 1H), 7.66–7.59 (m, 2H), 7.52 (dd app. t, 3JH,H = 7.5 Hz, 1H), 7.43–7.36 (m, 4H), 7.12–7.08 (m, 2H), 6.77–6.74 (m, 2H), 3.74 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.9, 159.8, 144.5, 140.1, 134.3, 133.5, 132.8, 131.8, 131.4, 130.3, 128.3, 128.2, 128.2, 126.8, 124.1, 114.9, 114.0, 94.0, 87.1, 55.3 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C22H17O2 313.1223, found 313.1223.
- 2′-(p-Tolylethynyl)-(1,1′-biphenyl)-2-carbaldehyde (1c). Pale yellow oil (417 mg, 1.41 mmol, 94% yield). Rf = 0.50 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.93 (s, 1H), 8.08 (dd, 3JH,H = 7.7 Hz, 4JH,H = 1.5 Hz, 1H), 7.66–7.61 (m, 2H), 7.52 (t, 3JH,H = 7.6 Hz, 1H), 7.44–7.36 (m, 4H), 7.07–7.02 (m, 4H), 2.29 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.9, 144.4, 140.3, 138.7, 134.3, 133.5, 132.0, 131.4, 131.3, 130.3, 129.1, 128.3, 128.3, 126.9, 124.0, 119.7, 94.1, 87.7, 21.6 ppm.
- 2′-[(4-Fluorophenyl)ethynyl]-(1,1′-biphenyl)-2-carbaldehyde (1d). Pale yellow oil (333 mg, 1.11 mmol, 74% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.93 (s, 1H), 8.08 (dd, 3JH,H = 7.8 Hz, 4JH,H = 1.5 Hz, 1H), 7.67–7.61 (m, 2H), 7.53 (t, 3JH,H = 7.6 Hz, 1H), 7.46–7.38 (m, 4H), 7.16–7.11 (m, 2H), 6.95–6.90 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.9, 162.7 (d, J = 249.6 Hz), 144.3, 140.3, 134.3, 133.6, 133.3 (d, J = 8.3 Hz), 132.0, 131.4, 130.3, 128.6, 128.3, 126.9, 123.6, 118.8 (d, J = 3.6 Hz), 115.7 (d, J = 22.2 Hz), 92.8, 88.0. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C21H14FO 301.1023, found 301.1023.
- 2′-[(4-Chlorophenyl)ethynyl]-(1,1′-biphenyl)-2-carbaldehyde (1e). Pale yellow oil (436 mg, 1.38 mmol, 92% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.92 (s, 1H), 8.08 (dd, 3JH,H = 7.8 Hz, 4JH,H = 1.5 Hz, 1H), 7.69–7.62 (m, 2H), 7.55 (t, 3JH,H = 7.5 Hz, 1H), 7.49–7.40 (m, 4H), 7.22–7.19 (m, 2H), 7.09–7.07 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.9, 144.3, 140.5, 134.6, 134.3, 133.6, 132.6, 132.1, 131.4, 130.4, 128.8, 128.8, 128.4, 128.4, 127.0, 123.5, 121.3, 92.7, 89.3 ppm.
- 5′-Methyl-2′-(phenylethynyl)-(1,1′-biphenyl)-2-carbaldehyde (1f). Pale yellow oil (404 mg, 1.36 mmol, 91% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.94 (s, 1H), 8.08 (dd, 3JH,H = 7.9 Hz, 4JH,H = 1.6 Hz, 1H), 7.66–7.61 (m, 1H), 7.53–7.49 (m, 2H), 7.42 (d, 3JH,H = 7.5 Hz, 1H), 7.23–7.20 (m, 5H), 7.16–7.14 (m, 2H), 2.41 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3) δC 192.0, 144.5, 140.3, 138.8, 134.3, 133.5, 131.9, 131.3, 131.3, 131.1, 129.1, 128.3, 128.2, 126.9, 123.0, 120.8, 93.1, 88.5, 21.6 ppm.
- 5′-Chloro-2′-(phenylethynyl)-(1,1′-biphenyl)-2-carbaldehyde (1g). Pale yellow solid (374 mg, 1.18 mmol, 79% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). m.p. 83.7–84.2 °C. 1H NMR (400 MHz, CDCl3) δH 9.93 (s, 1H), 8.09 (dd, 3JH,H = 7.8 Hz, 4JH,H = 1.5 Hz, 1H), 7.67 (td, 3JH,H = 7.4 Hz, 4JH,H = 1.5 Hz, 1H), 7.58–7.54 (m, 2H), 7.42–7.39 (m, 3H), 7.26–7.21(m, 4H), 7.16–7.13 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.4, 142.9, 142.1, 134.5, 134.3, 133.8, 133.1, 131.4, 131.2, 130.3, 128.8, 128.6, 128.4, 127.3, 122.5, 94.8, 87.3 ppm.
- 2′-(Phenylethynyl)-5′-(trifluoromethyl)-(1,1′-biphenyl)-2-carbaldehyde (1h). Pale yellow solid (483 mg, 1.38 mmol, 92% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). m.p. 79.5–80.1 °C. 1H NMR (400 MHz, CDCl3) δH 9.92 (s, 1H), 8.11 (dd, 3JH,H = 7.8 Hz, 4JH,H = 1.5 Hz, 1H), 7.75–7.67 (m, 4H), 7.58 (t, 3JH,H = 7.6 Hz, 1H), 7.41 (d, 3JH,H = 7.8 Hz, 1H), 7.28–7.26 (m, 3H), 7.18–7.15 (m, 2H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.1, 142.7, 141.2, 134.3, 133.9, 132.4, 131.6, 131.3, 130.3 (q, J = 32.7 Hz), 129.1, 129.0, 128.5, 127.6, 127.5, 126.9 (q, J = 3.8 Hz), 125.1 (q, J = 3.5 Hz), 123.9 (q, J = 273.7 Hz), 122.1, 96.4, 87.1 ppm. 19F NMR (376 MHz, Chloroform-d) δ −62.53. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C22H14F3O 351.0991, found 351.0991.
- 2′-(Pyridin-2-ylethynyl)-(1,1′-biphenyl)-2-carbaldehyde (1i). Pale yellow solid (378 mg, 1.34 mmol, 89% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). m.p. 104.6–104.9 °C. 1H NMR (400 MHz, CDCl3) δH 9.95 (s, 1H), 8.50 (d, 3JH,H = 3.3 Hz, 1H), 8.09 (d, 3JH,H = 7.7 Hz, 1H), 7.74 (dd, 3JH,H = 7.3 Hz, 4JH,H = 1.7 Hz, 1H), 7.66 (td, 3JH,H = 7.5 Hz, 4JH,H = 1.4 Hz, 1H), 7.55–7.39 (m, 6H), 7.15–7.11 (m, 1H), 7.00 (d, 3JH,H = 7.8 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.6, 149.9, 143.9, 142.8, 140.6, 136.0, 134.1, 133.5, 132.6, 131.3, 130.3, 129.1, 128.3, 128.3, 127.1, 126.8, 122.8, 122.6, 92.7, 87.8 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14NO 284.1070, found 284.1069.
- 2′-(Thiophen-2-ylethynyl)-(1,1′-biphenyl)-2-carbaldehyde (1j). Pale yellow oil (363 mg, 1.26 mmol, 84% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.91 (s, 1H), 8.08 (dd, 3JH,H = 7.7 Hz, 4JH,H = 1.5 Hz, 1H), 7.67–7.59 (m, 2H), 7.52 (t, 3JH,H = 7.6 Hz, 1H), 7.45–7.36 (m, 4H), 7.18 (dd, 3JH,H = 5.1 Hz, 4JH,H = 1.2 Hz, 1H), 6.98 (dd, 3JH,H = 3.7 Hz, 4JH,H = 1.2 Hz, 1H), 6.88 (dd, 3JH,H = 5.2 Hz, 3JH,H = 3.6 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.7, 144.1, 140.1, 134.2, 133.5, 132.0, 131.6, 131.3, 130.3, 128.5, 128.3, 128.2, 127.7, 127.1, 127.0, 123.4, 122.6, 92.0, 87.3 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C19H13OS 289.0682, found 289.0682.
- 2′-[(Triisopropylsilyl)ethynyl]-(1,1′-biphenyl)-2-carbaldehyde (1k). White solid (391 mg, 1.08 mmol, 72% yield). Rf = 0.50 (petroleum ether/ethyl acetate, 10/1 v/v). m.p. 68.0–68.3 °C. 1H NMR (400 MHz, CDCl3) δH 9.85 (s, 1H), 8.01 (d, 3JH,H = 7.7 Hz, 1H), 7.61–7.58 (m, 2H), 7.46 (t, 3JH,H = 7.6 Hz, 1H), 7.41–7.34 (m, 3H), 7.31–7.28 (m, 1H), 0.91 (s, 21H) ppm. 13C NMR (100 MHz, CDCl3) δC 191.6, 144.6, 140.7, 134.1, 133.4, 132.7, 131.0, 130.1, 128.3, 128.1, 128.0, 127.0, 123.9, 105.2, 95.7, 18.5, 11.1. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C24H31OSi 363.2139, found 363.2138.
- 2′-(Hept-1-yn-1-yl)-(1,1′-biphenyl)-2-carbaldehyde (1l). Pale yellow oil (99 mg, 0.36 mmol, 24% yield). Rf = 0.50 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.85 (s, 1H), 8.03 (d, 3JH,H = 7.7 Hz, 1H), 7.64–7.60 (m, 1H), 7.50–7.49 (m, 2H), 7.35–7.32 (m, 4H), 7.25 (s, 1H), 2.17–2.13 (s, 2H), 1.33–1.26 (m, 2H), 1.21–1.16 (m, 2H), 1.11–1.05 (m, 2H), 0.83–0.79 (m, 3H) ppm. 13C NMR (100 MHz, CDCl3) δC 192.0, 144.7, 140.2, 134.1, 133.4, 132.0, 131.2, 130.1, 128.1, 128.0, 127.7, 126.7, 124.5, 95.5, 79.5, 30.8, 27.8, 22.2, 19.3, 14.0 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H21O 277.1587, found 277.1587.
- 4-Chloro-2′-[(triisopropylsilyl)ethynyl]-(1,1′-biphenyl)-2-carbaldehyde (1m). Pale yellow oil (422 mg, 1.07 mmol, 71% yield). Rf = 0.50 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.77 (s, 1H), 7.98 (d, 3JH,H = 2.6 Hz, 1H), 7.62–7.56 (m, 2H), 7.44–7.37 (m, 2H), 7.34 (d, 3JH,H = 8.2 Hz, 1H), 7.30–7.28 (m, 1H), 0.92 (s, 21H) ppm. 13C NMR (100 MHz, CDCl3) δC 190.4, 142.8, 139.5, 135.3, 134.7, 133.3, 132.9, 132.6, 130.0, 128.5, 128.4, 126.9, 124.0, 104.9, 96.4, 18.5, 11.1 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C21H24ClOSi 355.1279, found 355.1278.
- 2-[2-(Phenylethynyl)pyridin-3-yl]benzaldehyde (1n). Pale yellow oil (365 mg, 1.29 mmol, 86% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.96 (s, 1H), 8.69 (dd, 3JH,H = 4.7, 4JH,H = 1.8 Hz, 1H), 8.11 (dd, 3JH,H = 7.8 Hz, 4JH,H = 1.4 Hz, 1H), 7.73–7.67 (m, 2H), 7.59 (t, 3JH,H = 7.5 Hz, 1H), 7.43–7.36 (m, 2H), 7.30–7.19 (m, 5H) ppm. 13C NMR (100 MHz, CDCl3) δC 190.8, 149.7, 142.8, 141.4, 137.5, 136.6, 134.2, 133.7, 131.7, 131.3, 129.1, 128.9, 128.3, 127.7, 122.6, 121.6, 93.7, 87.7 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14NO 284.1070, found 284.1069.
- 2-[3-(Phenylethynyl)pyridin-4-yl]benzaldehyde (1o). Pale yellow oil (386 mg, 1.36 mmol, 91% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.93 (s, 1H), 8.86 (s, 1H), 8.65 (d, 3JH,H = 5.1 Hz, 1H), 8.11 (dd, 3JH,H = 7.9, 1.5 Hz, 1H), 7.71 (td, 3JH,H = 7.5 Hz, 4JH,H = 1.5 Hz, 1H), 7.61 (t, 3JH,H = 7.7 Hz, 1H), 7.43–7.40 (m, 1H), 7.34 (d, 3JH,H = 5.1 Hz, 1H), 7.31–7.20 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3) δC 190.6, 152.5, 148.7, 147.8, 141.1, 133.8, 131.4, 130.7, 129.3, 129.0, 128.4, 127.8, 124.2, 122.0, 120.6, 96.7, 84.9 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14NO 284.1070, found 284.1069.
- 2-[4-(Phenylethynyl)pyridin-3-yl]benzaldehyde (1p). Pale yellow oil (378 mg, 1.33 mmol, 89% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 10/1 v/v). 1H NMR (400 MHz, CDCl3) δH 9.95 (s, 1H), 8.67–8.66 (m, 2H), 8.12 (d, 3JH,H = 7.8 Hz, 1H), 7.71 (td, 3JH,H = 7.4, 4JH,H = 1.4 Hz, 1H), 7.60 (t, 3JH,H = 7.6 Hz, 1H), 7.49 (d, 3JH,H = 5.1 Hz, 1H), 7.44 (d, 3JH,H = 7.6 Hz, 1H), 7.32–7.19 (m, 5H) ppm. 13C NMR (100 MHz, CDCl3) δC 190.8, 150.2, 149.3, 140.1, 135.0, 134.4, 133.8, 131.6, 131.6, 131.4, 129.4, 129.0, 128.4, 127.7, 125.1, 121.5, 98.2, 85.7 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14NO 284.1070, found 284.1069.
3.5. General Procedure for the Preparation of 1H-Dibenzo[e,g]indazoles 2a–p
A THF (5.0 mL) solution containing 2′-alkynyl-biaryl-2-aldehydes (1, 282.0 mg, 1.0 mmol) and p-toluenesulfonhydrazide (204.9 mg, 1.1 mmol) in a 25 mL screw-capped thick-walled Pyrex tube was stirred at 45 °C for 1 h. After the reaction was completed (TLC monitoring, eluent petroleum ether/ethyl acetate, 1/1 v/v), LiOtBu (120.0 mg, 1.5 mmol) and additional THF (2.5 mL) were added, and then the mixture was stirred at 45 °C for 1 h. After the reaction was completed (TLC monitoring, eluent petroleum ether/ethyl acetate, 1/1 v/v), the crude residue was directly purified using column chromatography on silica gel (eluent petroleum ether/ethyl acetate, gradient mixture ratio from 5/1 to 2/1 v/v) to afford products 2a–p in 61–93% yields.
3.6. Analytical Data of Compound 2a–p
- 3-Phenyl-1H-dibenzo[e,g]indazole (2a) [30]. White solid (259 mg, 0.88 mmol, 88% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 260.4–260.8 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.24–13.95 (s, 1H), 8.78–8.71 (m, 2H), 8.55 (d, 3JH,H = 7.8 Hz, 1H), 8.02 (d, 3JH,H = 8.1 Hz, 1H), 7.74–7.69 (m, 4H), 7.59–7.54 (m, 3H), 7.50–7.40 (m, 2H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 147.3, 137.2, 135.4, 129.6, 128.6, 128.3, 127.5, 127.4, 127.1, 124.9, 124.1, 124.0, 122.6, 122.3, 121.0, 112.5 ppm.
- 3-(4-Methoxyphenyl)-1H-dibenzo[e,g]indazole (2b). White solid (285 mg, 0.88 mmol, 88% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 204.2–204.7 °C. 1H NMR (400 MHz, DMSO-d6) δH 13.97 (s, 1H), 8.71 (dd, 2JH,H = 16.9 Hz, 3JH,H = 8.1 Hz, 2H), 8.56 (d, 3JH,H = 7.7 Hz, 1H), 8.05 (d, 3JH,H = 7.3 Hz, 1H), 7.74–7.63 (m, 4H), 7.49–7.40 (m, 2H), 7.14 (d, 3JH,H = 8.4 Hz, 2H), 3.84 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 159.3, 147.2, 137.2, 130.9, 129.6, 127.4, 127.3, 127.1, 124.8, 124.0, 123.9, 122.6, 122.4, 121.1, 114.0, 112.6, 55.1 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C22H17N2O 325.1335, found 325.1334.
- 3-(p-Tolyl)-1H-dibenzo[e,g]indazole (2c). White solid (262 mg, 0.85 mmol, 85% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 199.6–200.0 °C. 1H NMR (400 MHz, DMSO-d6) δH 11.79 (s, 1H), 8.71 (dd, 2JH,H = 17.1 Hz, 3JH,H = 8.1 Hz, 2H), 8.57 (d, 3JH,H = 7.7 Hz, 1H), 8.06 (d, 3JH,H = 7.7 Hz, 1H), 7.73 (t, 3JH,H = 7.5 Hz, 1H), 7.66 (t, 3JH,H = 7.5 Hz, 1H), 7.61 (d, 3JH,H = 7.8 Hz, 2H), 7.48–7.36 (m, 4H), 2.40 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 145.1, 139.0, 137.8, 131.4, 129.7, 129.4, 129.2, 127.5, 127.4, 127.3, 127.1, 125.6, 124.9, 124.1, 123.9, 122.6, 122.3, 112.3, 20.9 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C22H17N2 309.1386, found 309.1385.
- 3-(4-Fluorophenyl)-1H-dibenzo[e,g]indazole (2d). White solid (262 mg, 0.84 mmol, 84% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 235.7–236.2 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.31–14.03 (s, 1H), 8.70–8.58 (m, 3H), 8.00 (s, 1H), 7.80–7.62 (m, 4H), 7.43–7.39 (m, 4H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 162.2 (d, J = 245.1 Hz), 146.4, 137.3, 131.8, 131.7, 131.6, 129.6, 127.4, 127.3, 127.1, 124.9, 124.1, 124.0, 122.6, 122.4, 121.0, 115.5 (d, J = 21.5 Hz), 112.6 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C21H14FN2 313.1136, found 313.1134.
- 3-(4-Chlorophenyl)-1H-dibenzo[e,g]indazole (2e). White solid (262 mg, 0.80 mmol, 80% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 265.3–265.9 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.18 (s, 1H), 8.76 (dd, 2JH,H = 17.0 Hz, 3JH,H = 8.0 Hz, 2H), 8.53 (dd, 3JH,H = 7.7 Hz, 4JH,H = 1.7 Hz, 1H), 7.96 (d, 3JH,H = 7.5 Hz, 1H), 7.76–7.68 (m, 4H), 7.66–7.64 (m, 2H), 7.53–7.44 (m, 2H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 144.8, 138.5, 133.6, 133.3, 131.3, 129.6, 128.7, 127.6, 127.4, 127.4, 127.1, 127.1, 125.0, 124.1, 123.9, 122.6, 122.4, 121.7, 112.5 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C21H14ClN2 329.0840, found 329.0838.
- 6-Methyl-3-phenyl-1H-dibenzo[e,g]indazole (2f). White solid (265 mg, 0.86 mmol, 86% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 235.6–235.9 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.22–13.93 (s, 1H), 8.76–8.52 (m, 3H), 7.94 (d, 3JH,H = 8.3 Hz, 1H), 7.76–7.52 (m, 7H), 7.19 (d, 3JH,H = 8.4 Hz, 1H), 2.44 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 147.1, 137.0, 135.6, 134.0, 129.6, 129.5, 128.5, 128.4, 128.2, 127.5, 127.3, 127.1, 124.8, 123.9, 122.5, 122.3, 121.1, 112.6, 21.2 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C22H17N2 309.1386, found 309.1385.
- 6-Chloro-3-phenyl-1H-dibenzo[e,g]indazole (2g). White solid (276 mg, 0.84 mmol, 84% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 286.9–287.3 °C. 1H NMR (400 MHz, DMSO-d6) δH 13.77 (s, 1H), 8.73–8.69 (m, 2H), 8.51 (d, 3JH,H = 7.6 Hz, 1H), 7.92 (d, 3JH,H = 8.6 Hz, 1H), 7.75–7.64 (m, 4H), 7.60–7.52 (m, 3H), 7.40 (dd, 3JH,H = 8.6 Hz, 4JH,H = 2.1 Hz, 1H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 129.8, 129.5, 129.2, 128.7, 128.5, 128.1, 127.4, 127.0, 125.8, 124.3, 124.2, 123.6, 122.3, 111.7. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C21H14ClN2 329.0840, found 329.0838.
- 3-Phenyl-6-(trifluoromethyl)-1H-dibenzo[e,g]indazole (2h). White solid (300 mg, 0.83 mmol, 83% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 276.9–277.3 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.29–14.10 (s, 1H), 8.88–8.84 (m, 1H), 8.72–8.65 (m, 1H), 8.55–8.47 (m, 1H), 8.07–8.02 (m, 1H), 7.71–7.51 (m, 8H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 147.7, 138.0, 135.0, 129.8, 129.5, 128.8, 128.6, 128.4, 128.1, 127.6, 127.0, 126.0, 125.0 (q, J = 31.7 Hz), 124.1, 123.3 (d, J = 6.7 Hz), 122.8, 122.3, 121.1 (d, J = 15.7 Hz), 111.7 ppm. 19F NMR (376 MHz, DMSO-d6) δ −60.08. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C22H14F3N2 363.1104, found 363.1102.
- 3-(Pyridin-2-yl)-1H-dibenzo[e,g]indazole (2i). White solid (230 mg, 0.78 mmol, 78% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 209.3–209.7 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.44–14.28 (s, 1H), 9.04–9.02 (m, 1H), 8.86 (d, 3JH,H = 4.9 Hz, 1H), 8.79–8.58 (m, 3H), 8.06–7.97 (m, 2H), 7.78–7.67 (m, 2H), 7.52–7.49 (m, 3H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 154.3, 148.8, 147.1, 137.8, 137.0, 129.7, 127.5, 127.5, 127.4, 127.3, 127.0, 125.6, 125.2, 124.3, 124.0, 123.7, 123.1, 122.3, 120.9, 113.5. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14N3 296.1182, found 296.1181.
- 3-(Thiophen-2-yl)-1H-dibenzo[e,g]indazole (2j). White solid (273 mg, 0.91 mmol, 91% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 255.4–255.9 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.40–14.17 (s, 1H), 8.71–8.56 (m, 3H), 8.34–8.31 (m, 1H), 7.76–7.73 (m, 2H), 7.66 (t, 3JH,H = 7.7 Hz, 1H), 7.56 (d, 3JH,H = 3.6 Hz, 1H), 7.52–7.46 (m, 2H), 7.32–7.30 (m, 1H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 140.4, 137.4, 136.1, 129.6, 128.0, 127.7, 127.5, 127.3, 127.1, 126.9, 125.1, 124.1, 124.0, 122.6, 122.3, 120.8, 113.2. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C19H13N2S 301.0794, found 301.0793.
- 3-(Triisopropylsilyl)-1H-dibenzo[e,g]indazole (2k). White solid (348 mg, 0.93 mmol, 93% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 87.8–88.3 °C. 1H NMR (400 MHz, CDCl3) δH 12.12 (s, 1H), 8.75 (d, 3JH,H = 7.6 Hz, 1H), 8.58–8.53 (m, 2H), 8.27 (d, 3JH,H = 7.8 Hz, 1H), 7.64–7.46 (m, 4H), 1.78 (hept, 3JH,H = 7.5 Hz, 3H), 1.12 (d, 3JH,H = 7.7 Hz, 18H) ppm. 13C NMR (100 MHz, CDCl3) δC 130.5, 129.1, 128.7, 127.3, 127.2, 126.5, 126.0, 125.3, 124.0, 123.6, 123.4, 123.2, 18.8, 12.7 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C24H31N2Si 375.2251, found 375.2251.
- 3-Pentyl-1H-dibenzo[e,g]indazole (2l). White solid (176 mg, 0.61 mmol, 61% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 188.5–188.9 °C. 1H NMR (400 MHz, DMSO-d6) δH 13.69–13.49 (s, 1H), 8.78–8.67 (m, 2H), 8.44 (d, 3JH,H = 7.5 Hz, 1H), 8.24–8.15 (m, 1H), 7.69–7.52 (m, 4H), 3.19 (t, 3JH,H = 7.6 Hz, 2H), 1.82–1.80 (m, 2H), 1.42–1.32 (m, 4H), 0.87 (t, 3JH,H = 7.1 Hz, 3H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 147.3, 137.1, 129.4, 127.6, 127.3, 127.1, 124.4, 124.0, 123.1, 122.2, 121.1, 112.4, 31.2, 28.9, 27.7, 21.9, 13.9 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H21N2 289.1699, found 289.1698.
- 10-Chloro-3-(triisopropylsilyl)-1H-dibenzo[e,g]indazole (2m). White solid (335 mg, 0.82 mmol, 82% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 191.1–191.7 °C. 1H NMR (400 MHz, CDCl3) δH 11.96 (s, 1H), 8.68 (s, 1H), 8.52 (d, 3JH,H = 7.9 Hz, 1H), 8.47 (d, 3JH,H = 9.0 Hz, 1H), 8.23 (d, 3JH,H = 7.7 Hz, 1H), 7.58–7.50 (m, 3H), 1.76 (hept, 3JH,H = 7.5 Hz, 3H), 1.15 (d, 3JH,H = 7.6 Hz, 18H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 133.3, 129.0, 128.7, 128.5, 127.6, 126.9, 125.7, 125.5, 125.1, 124.0, 123.9, 122.8, 18.9, 12.7 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C24H30ClN2Si 409.1861, found 409.1860.
- 3-Phenyl-1H-benzo[f]pyrazolo[3,4-h]quinoline (2n). White solid (260 mg, 0.88 mmol, 88% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 265.7–266.2 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.39–14.16 (s, 1H), 9.02–8.92 (m, 1H), 8.78–8.56 (m, 3H), 8.29–8.14 (m, 2H), 7.79–7.62 (m, 2H), 7.55–7.42 (m, 4H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 148.6, 148.2, 147.4, 145.9, 144.6, 139.9, 139.7, 134.6, 131.7, 130.1, 129.9, 129.6, 128.9, 128.5, 128.0, 127.9, 127.6, 127.4, 126.0, 124.1, 123.8, 123.4, 122.4, 122.3, 120.8, 120.5, 120.1, 113.4, 112.5 ppm. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14N3 296.1182, found 296.1181.
- 3-Phenyl-1H-benzo[f]pyrazolo[3,4-h]isoquinoline (2o). White solid (266 mg, 0.90 mmol, 90% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 277.9–278.4 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.37–14.12 (s, 1H), 9.22 (s, 1H), 8.82–8.72 (m, 1H), 8.56–8.52 (m, 3H), 7.85–7.59 (m, 7H) ppm. 13C NMR (100 MHz, DMSO-d6) δC 146.9, 145.1, 144.1, 137.7, 135.1, 132.5, 129.6, 129.4, 128.7, 128.5, 127.7, 127.5, 124.7, 122.4, 117.5, 110.6. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd. for C20H14N3 296.1182, found 296.1181.
- 3-Phenyl-1H-benzo[h]pyrazolo[4,3-f]isoquinoline (2p). White solid (221 mg, 0.75 mmol, 75% yield). Rf = 0.40 (petroleum ether/ethyl acetate, 1/1 v/v). m.p. 324.3–324.8 °C. 1H NMR (400 MHz, DMSO-d6) δH 14.46–14.20 (s, 1H), 10.06–9.95 (m, 1H), 9.04–8.87 (m, 1H), 8.56–8.48 (m, 2H), 7.86–7.55 (m, 8H). HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14N3 296.1182, found 296.1181. The 13C NMR spectroscopic data could not be recorded due to the poor solubility in deuterated solvents, such as DMSO-d6, CDCl3.
4. Conclusions
In conclusion, we have developed a one-pot two-steps synthetic method towards 3-substituted 1H-dibenzo[e,g]indazoles in good to high yields via a LiOtBu-promoted intramolecular cyclization of 2′-alkynyl-biaryl-2-aldehyde N-tosylhydrazones under mild conditions. The starting 2′-alkynyl-biaryl-2-aldehyde N-tosylhydrazones were prepared in situ through the reactions of 2′-alkynyl-biaryl-2-aldehydes with TsNHNH2 (p-methylbenzenesulfonohydrazide). In addition, two types of N-H signals were observed in the 1H-NMR spectra in DMSO-d6, which are assigned to hydrogen atoms of N-H in 2a and 2a tautomer in their dimeric species, respectively.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28248061/s1: the general procedure for the synthesis of starting materials, the copies of NMR charts of new starting materials, and all products, as well as X-ray structural details of 2a. References [42,43,44] are cited in the supplementary materials.
Author Contributions
Investigation, writing—original draft preparation, J.L.; supervision, writing—review and editing, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, with grants number 21673124 and 21972072.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
We thank the Tsinghua Xuetang Talents Program for the computational hardware and software support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W. Recent developments in the chemistry of pyrazoles. Adv. Heterocycl. Chem. 2018, 126, 55–107. [Google Scholar] [CrossRef]
- Mix, K.A.; Aronoff, M.R.; Raines, R.T. Diazo compounds: Versatile tools for chemical biology. ACS Chem. Biol. 2016, 11, 3233–3244. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Vicente, J.d.; Bonnert, R.V. A novel one-pot method for the preparation of pyrazoles by 1,3-dipolar cycloadditions of diazo compounds generated in situ. J. Org. Chem. 2003, 68, 5381–5383. [Google Scholar] [CrossRef]
- Jiang, N.; Li, C.-J. Novel 1,3-dipolar cycloaddition of diazocarbonyl compounds to alkynes catalyzed by InCl3 in water. Chem. Commun. 2004, 394–395. [Google Scholar] [CrossRef]
- Moran, J.; McKay, C.S.; Pezacki, J.P. Strain-promoted 1,3-dipolar cycloadditions of diazo compounds with cyclooctynes. Can. J. Chem. 2011, 89, 148–151. [Google Scholar] [CrossRef]
- McGrath, N.A.; Raines, R.T. Diazo compounds as highly tunable reactants in 1,3-dipolar cycloaddition reactions with cycloalkynes. Chem. Sci. 2012, 3, 3237–3240. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-L.; Ge, Y.-C.; He, T.; Zhang, L.; Fu, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. An efficient one-pot synthesis of 3,5-disubstituted 1H-pyrazoles. Synthesis 2012, 44, 1577–1583. [Google Scholar] [CrossRef]
- Friscourt, F.; Fahrni, C.J.; Boons, G.-J. Fluorogenic strain-promoted alkyne-diazo cycloadditions. Chem. Eur. J. 2015, 21, 13996–14001. [Google Scholar] [CrossRef] [PubMed]
- Aronoff, M.R.; Gold, B.; Raines, R.T. 1,3-dipolar cycloadditions of diazo compounds in the presence of azides. Org. Lett. 2016, 18, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.; Aronoff, M.R.; Raines, R.T. 1,3-dipolar cycloaddition with diazo groups: Noncovalent interactions overwhelm strain. Org. Lett. 2016, 18, 4466–4469. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qiu, S.; Chen, Y.; Wu, L. K2CO3-promoted pyrazoles synthesis from 1,3-dipolar cycloaddition of N-tosylhydrazones with acetylene gas. ChemistrySelect 2020, 5, 12034–12037. [Google Scholar] [CrossRef]
- Bubyrev, A.; Dar’in, D.; Kantin, G.; Krasavin, M. Synthetic studies towards CH-diazomethane sulfonamides: A novel type of diazo reagents. Eur. J. Org. Chem. 2020, 27, 4112–4115. [Google Scholar] [CrossRef]
- Ruffell, K.; Smith, F.R.; Lewis, W.; Green, M.T.; Hayes, C.J.; Nicolle, S.M.; Moody, C.J. Diazophosphonates: Effective surrogates for diazoalkanes in pyrazole synthesis. Chem. Eur. J. 2021, 27, 13703–13708. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, L.; Wu, H.; He, Y.; Wu, G. Divergent trideuteromethylthiolation and aminotrideuteromethylthiolation of alkenes with N-fluorobenzenesulfonimide and CD3SSO3Na. Org. Lett. 2023, 25, 7078–7082. [Google Scholar] [CrossRef]
- Ajvazi, N.; Stavber, S. Alcohols in direct carbon-carbon and carbon-heteroatom bond-forming reactions: Recent advances. Arkivoc 2018, 2018, 288–329. [Google Scholar] [CrossRef]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012, 21, 1509–1523. [Google Scholar] [CrossRef]
- Denya, I.; Malan, S.; Joubert, J. Indazole derivatives and their therapeutic applications: A patent review (2013–2017). Expert Opin. Ther. Pat. 2018, 28, 441–453. [Google Scholar] [CrossRef]
- Zhang, S.-G.; Liang, C.-G.; Zhang, W.-H. Recent advances in indazole-containing derivatives: Synthesis and biological perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef] [PubMed]
- Minu, M.; Thangadurai, A.; Wakode, S.R.; Agrawal, S.S.; Narasimhan, B. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorg. Med. Chem. Lett. 2009, 19, 2960–2964. [Google Scholar] [CrossRef] [PubMed]
- Cheekavolu, C.; Muniappan, M. In vivo and in vitro anti-inflammatory activity of indazole and its derivatives. J. Clin. Diagn. Res. 2016, 10, FF01–FF06. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Rodgers, J.D.; McHugh, R.J., Jr.; Johnson, B.L.; Cordova, B.C.; Klabe, R.M.; Bacheler, L.T.; Erickson-Viitanen, S.; Ko, S.S. Unsymmetrical cyclic ureas as HIV-1 protease inhibitors: Novel biaryl indazoles as P2/P2′ substituents. Bioorg. Med. Chem. Lett. 1999, 9, 3217–3220. [Google Scholar] [CrossRef] [PubMed]
- Öğretir, C.; Kaypak, N.F. Quantum chemical calculations on the annular tautomerism of some indazole derivatives. 1. A gas phase study. J. Mol. Struct. THEOCHEM 2002, 583, 137–144. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Du, Z.; Hua, H.; Chen, S. One-pot synthesis of 2H-phenanthro [9, 10-c] pyrazoles from isoflavones by two dehydration processes. Green Chem. 2013, 15, 1048–1054. [Google Scholar] [CrossRef]
- Mykytka, J.P.; Jones, W.M. Formation of a bicyclo [4.1,0]heptatriene by intramolecular addition of a carbene to a carbon-carbon triple bond. J. Am. Chem. Soc. 1975, 97, 5933–5935. [Google Scholar] [CrossRef]
- Hao, L.; Hong, J.-J.; Zhu, J.; Zhan, Z.-P. One-pot synthesis of pyrazoles through a four-step cascade sequence. Chem. Eur. J. 2013, 19, 5715–5720. [Google Scholar] [CrossRef]
- Liu, H.; Lu, L.; Hua, R. [Cu(maloNHC)]-catalyzed synthesis of 2-aryl pyrazolo[5,1-a]isoquinolines by annulation of N′-(2-((trimethylsilyl)ethynyl)benzylidene)hydrazides with terminal aromatic alkynes. Tetrahedron 2017, 73, 6428–6435. [Google Scholar] [CrossRef]
- Lv, J.; Liu, H.; Lu, L.; Hua, R. Copper(I)-catalyzed syntheses of benzo[b]fluorenes by the cascade reactions of 2-alkynylbenzaldehyde N-tosylhydrazones and aromatic terminal alkynes. J. Org. Chem. 2022, 87, 16011–16018. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Hua, R. Copper(I)-catalyzed reactions of 2′-cyano-biaryl-2-aldehyde N-tosylhydrazones with terminal alkynes: An approach towards 9-amino-10-alkynylphenanthrene modules. Adv. Synth. Catal. 2023, 365, 3360–3366. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- The Structural Data for 2a Are Available Free of Charge from the Cambridge Crystallographic Data Center with Reference Number CCDC 2270088. Available online: https://www.ccdc.cam.ac.uk (accessed on 15 June 2023).
- Goerigk, L.; Grimme, S. Efficient and accurate double-hybrid-meta-GGA density functionals-evaluation with the extended GMTKN30 database for general main group thermochemistry, kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2010, 7, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, X.; Truhlar, D.G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 2011, 128, 295–305. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F.D. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Bera, K.; Sarkar, S.; Jalal, S.; Jana, U. Synthesis of Substituted Phenanthrene by Iron (III)-Catalyzed Intramolecular Alkyne–Carbonyl Metathesis. J. Org. Chem. 2012, 77, 8780–8786. [Google Scholar] [CrossRef]
- Bruker. SHELXTL. In Structure Determination Programs; Version 5.10; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Milburn, G. International Tables for X-ray Crystallography; Tables 4.2.6.8 and 6.1.1.4; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; Volume C. [Google Scholar]
- Oxford. CrysAlisPro, Agilent Technologies, Version 1.171.36.32; Oxford Diffraction Ltd.: Oxfordshire, UK, 2013.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
