Engineering a New IFN-ApoA-I Fusion Protein with Low Toxicity and Prolonged Action
Abstract
1. Introduction
2. Results
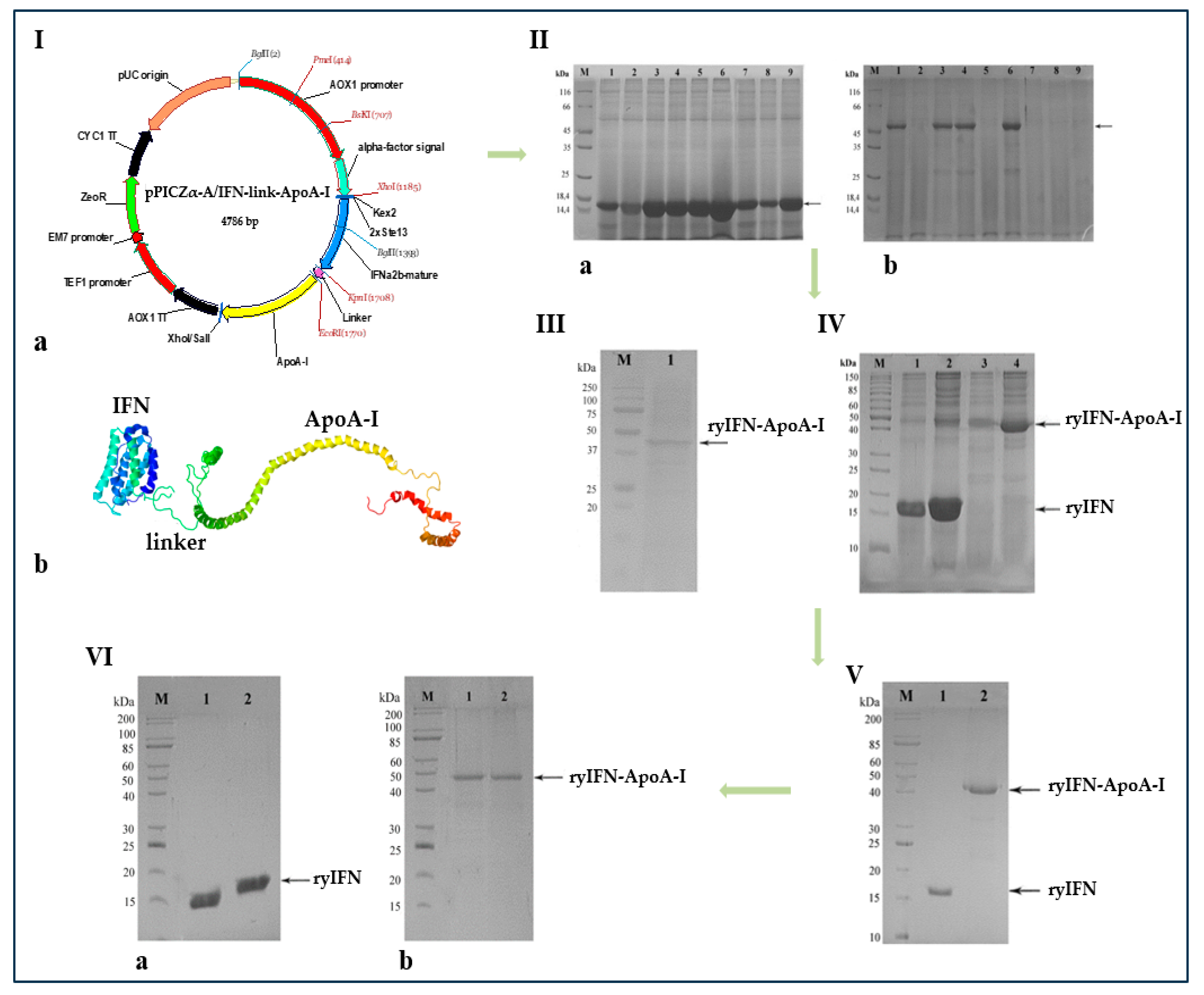
2.1. Creation of Recombinant Plasmids Encoding an Authentic and Chimeric Form of Mature Human IFN
2.2. Transformation of P. pastoris Cells with Recombinant Plasmids and Screening of Transformants
2.3. Fermentation and Purification of ryIFN and ryIFN-ApoA-I
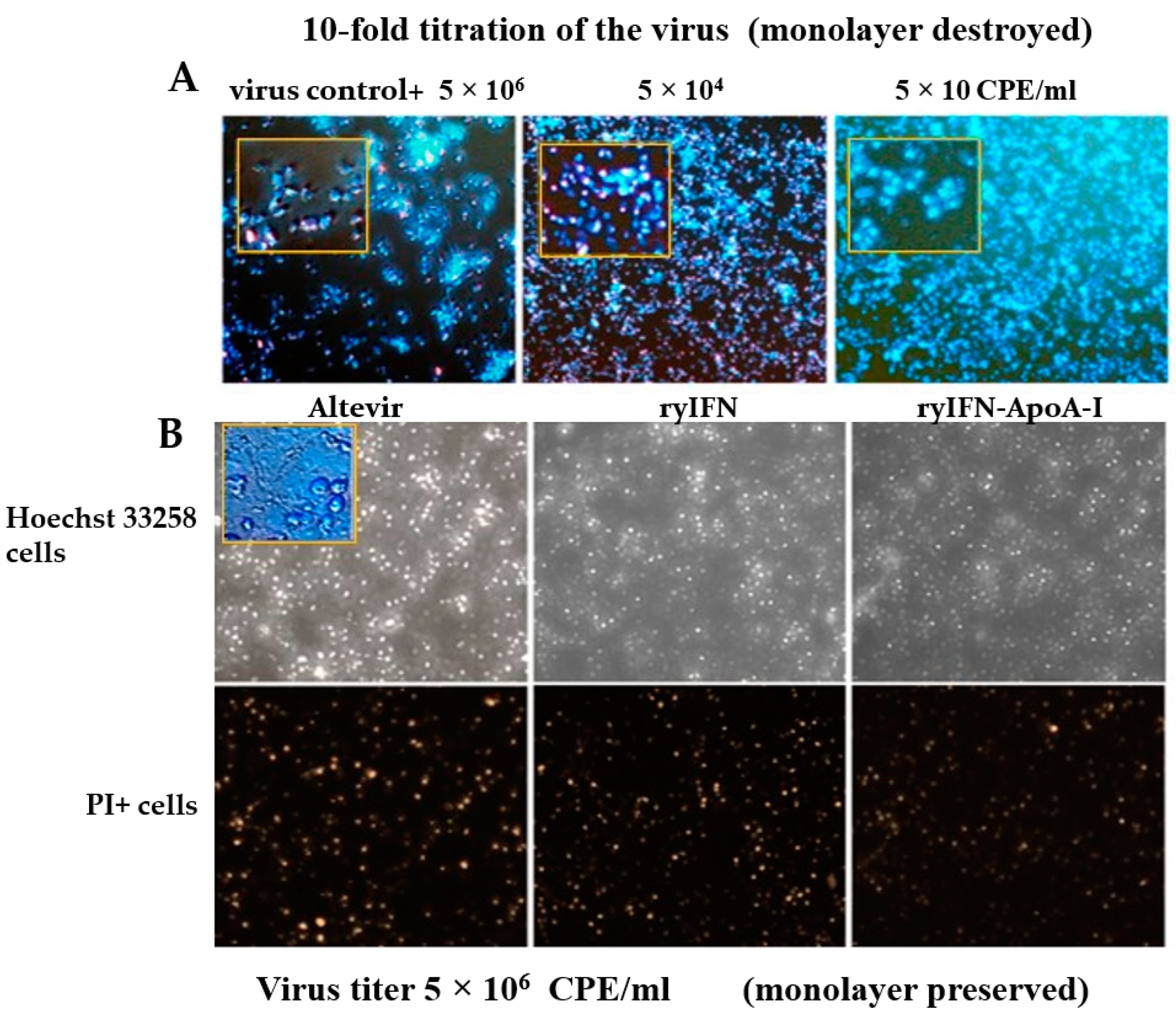
2.4. Antiviral Activity of ryIFN and ryIFN-ApoA-I
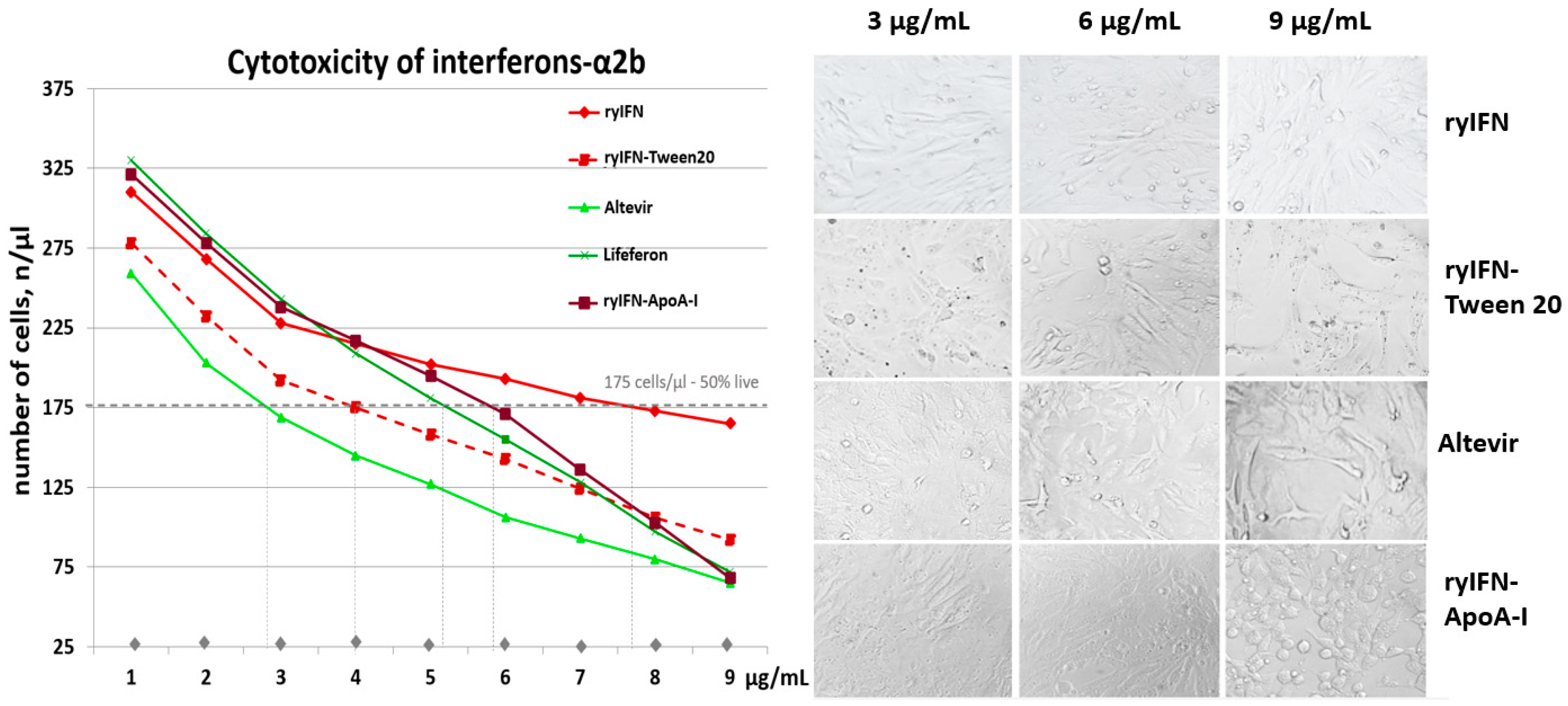
2.5. Cytotoxic Effect of Recombinant Interferons
2.6. Pharmacokinetics of ryIFN and ryIFN-ApoA-I
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial and Yeast Strains and Plasmid Vectors
4.3. Construction of the Recombinant Plasmids pPICZα-A/IFN and pPICZα-A/IFN-link-ApoA-I
4.4. Transformation of Electrocompetent E. coli Cells and Screening of Transformants
4.5. Transformation of Electrocompetent P. pastoris str. X33 Cells and Transformant Screening
4.6. Cultivation of P. pastoris Clones Producing Recombinant ryIFN and ryIFN-ApoA-I
4.7. Isolation and Purification of Recombinant Proteins
- (a)
- RyIFN purification
- (b)
- RyIFN-ApoA-I purification
4.8. Analysis of ryIFN and ryIFN-ApoA-I by SDS-PAGE under Reducing and Non-Reducing Conditions
4.9. Determination of the Antiviral Activity of ryIFN and ryIFN-ApoA-I
- (a)
- Antiviral activity against the equine vesicular stomatitis virus
- (b)
- Antiviral activity against SARS-CoV-2
4.10. Determination of the Cytotoxicity of ryIFN and ryIFN-ApoA-I
4.11. Pharmacokinetics
4.12. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scagnolari, C.; Antonelli, G. Antiviral activity of the interferon α family: Biological and pharmacological aspects of the treatment of chronic hepatitis C. Expert Opin. Biol. Ther. 2013, 13, 693–711. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Gogas, H.; Kirkwood, J.M. IFN-α in the Treatment of Melanoma. J. Immunol. 2012, 189, 3789–3793. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, T.Y. Recurrent Classical Type of Kaposi’s Sarcoma Treated by Interferon-alpha. Ann. Dermatol. 2008, 20, 162–165. [Google Scholar] [CrossRef]
- Kujawski, L.A.; Talpaz, M. The role of interferon-alpha in the treatment of chronic myeloid leukemia. Cytokine Growth Factor Rev. 2007, 18, 459–471. [Google Scholar] [CrossRef]
- Ozer, H.; Anderson, J.R.; Peterson, B.A.; Budman, D.R.; Cooper, M.R.; Kennedy, B.J.; Silver, R.T.; Henderson, E.S.; Duggan, D.B.; Barcos, M.; et al. Combination trial of subcutaneous recombinant alpha 2 b interferon and oral cyclophosphamide in follicular low-grade non-Hodgkin’s lymphoma. Med. Pediatr. Oncol. 1994, 22, 228–235. [Google Scholar] [CrossRef]
- Wills, R.J. Clinical pharmacokinetics of interferons. Clin. Pharmacokinet. 1990, 19, 390–399. [Google Scholar] [CrossRef]
- Kaser, A.; Enrich, B.; Ludwiczek, O.; Vogel, W.; Tilg, H. Interferon-alpha (IFN-alpha) enhances cytotoxicity in healthy volunteers and chronic hepatitis C infection mainly by the perforin pathway. Clin. Exp. Immunol. 1999, 118, 71–77. [Google Scholar] [CrossRef]
- Sleijfer, S.; Bannink, M.; Van Gool, A.R.; Kruit, W.H.; Stoter, G. Side effects of interferon-alpha therapy. Pharm. World Sci. 2005, 27, 423–431. [Google Scholar] [CrossRef]
- Schmid, M.; Kreil, A.; Jessner, W.; Homoncik, M.; Datz, C.; Gangl, A.; Ferenci, P.; Peck-Radosavljevic, M. Suppression of haematopoiesis during therapy of chronic hepatitis C with different interferon alpha mono and combination therapy regimens. Gut 2005, 54, 1014–1020. [Google Scholar] [CrossRef]
- Fritz-French, C.; Tyor, W. Interferon-α (IFNα) neurotoxicity. Cytokine Growth Factor Rev. 2012, 23, 7–14. [Google Scholar] [CrossRef]
- Gilbert, G.J. Recombinant interferon-alpha-induced chorea and frontal subcortical dementia. Neurology 2002, 59, 1821. [Google Scholar] [CrossRef]
- Hui, B.S.M.; Zhi, L.R.; Retinasamy, T.; Arulsamy, A.; Law, C.S.W.; Shaikh, M.F.; Yeong, K.Y. The Role of Interferon-α in Neuro-degenerative Diseases: A Systematic Review. J. Alzheimer’s Dis. 2023, 94, S45–S66. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.R.; Wang, B.; Wan, Y.W.; Chiu, G.; Cole, A.; Yin, Z.; Propson, N.E.; Xu, Y.; Jankowsky, J.L.; Liu, Z.; et al. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J. Clin. Investig. 2020, 130, 1912–1930. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G. The role of type I interferons in the pathogenesis and treatment of COVID-19. Front. Immunol. 2020, 11, 595739. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Fang, J.W.; Rouzier-Panis, R.; Raffanel, C.; Sabo, R.; Gupta, S.K.; Salfi, M.; Jacobs, S. Pegylated interferon-alpha2b: Pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin. Pharmacol. Ther. 2000, 68, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.M.; Jarvis, B. Peginterferon-alpha-2a (40 kD): A review of its use in the management of chronic hepatitis C. Drugs 2001, 61, 2263–2288. [Google Scholar] [CrossRef]
- Grace, M.J.; Lee, S.; Bradshaw, S.; Chapman, J.; Spond, J.; Cox, S.; DeLorenzo, M.; Brassard, D.; Wylie, D.; Carlson, S.C.; et al. Site of pegylation and polyethylene glycol molecule size attenuate interferon-antiviral and antiproliferative activities through the JAK/STAT signaling pathway. J. Biol. Chem. 2005, 280, 6327–6336. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Duttaroy, A.; Kanakaraj, P.; Osborn, B.L.; Schneider, H.; Pickeral, O.K.; Chen, C.; Zhang, G.; Kaithamana, S.; Singh, M.; Schulingkamp, R.; et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes 2005, 54, 251–258. [Google Scholar] [CrossRef]
- Osborn, B.L.; Sekut, L.; Corcoran, M.; Poortman, C.; Sturm, B.; Chen, G.; Mather, D.; Lin, H.L.; Parry, T.J. Albutropin: A growth hormone–albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur. J. Pharmacol. 2002, 456, 149–158. [Google Scholar] [CrossRef]
- Nolte, M.W.; Nichols, T.C.; Mueller-Cohrs, J.; Merricks, E.P.; Pragst, I.; Zollner, S.; Dickneite, G. Improved kinetics of rIX-FP, a recombinant fusion protein linking factor IX with albumin in cynomolgus monkeys and hemophilia B dogs. J. Thromb. Haemost. 2008, 10, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, S.; Chuang, V.T.; Ishima, Y.; Nakajou, K.; Furukawa, M.; Watanabe, H.; Maruyama, T.; Otagiri, M. Albumin fusion of thioredoxin—The production and evaluation of its biological activity for potential therapeutic applications. J. Control Release 2010, 147, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Halpern, W.; Riccobene, T.A.; Agostini, H.; Baker, K.; Stolow, D.; Gu, M.L.; Hirsch, J.; Mahoney, A.; Carrell, J.; Boyd, E.; et al. Albugranin, a recombinant human granulocyte colony stimulating factor (G-CSF) genetically fused to recombinant human albumin induces prolonged myelopoietic effects in mice and monkeys. Pharm. Res. 2002, 19, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chen, Z.; Yang, Z.Y.; Wang, T.Y.; Zhou, L.; Wu, J.B.; Zhou, L.F. Preparation and characterization of a potent, long-lasting recombinant human serum albumin-interferon-alpha2b fusion protein expressed in Pichia pastoris. Eur. J. Pharm. Biopharm. 2007, 67, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.U.; Ahmed, N.; Khan, M.A.; Tahir, S.; Zafar, A.U. Production of potent long-lasting consensus interferon using albumin fusion technology in Pichia pastoris expression system. Protein Expr. Purif. 2020, 166, 105509. [Google Scholar] [CrossRef]
- Osborn, B.L.; Olsen, H.S.; Nardelli, B.; Murray, J.H.; Zhou, J.X.; Garcia, A.; Moody, G.; Zaritskaya, L.S.; Sung, C. Pharmacokinetic and pharmacodynamic studies of a human serum albumininterferon- alpha fusion protein in Cynomolgus monkeys. J. Pharmacol. Exp. Ther. 2002, 303, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Bain, V.G.; Kaita, K.D.; Yoshida, E.M.; Swain, M.G.; Heathcote, E.J.; Neumann, A.U.; Fiscella, M.; Osborn, B.L.; Cronin, P.W.; Freimuth, W.W.; et al. A phase 2 study to evaluate the antiviral activity, safety, and pharmacokinetics of recombinant human albumin-interferon alfa fusion protein in genotype 1 chronic hepatitis C patients. J. Hepatol. 2006, 44, 671–678. [Google Scholar] [CrossRef]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins (HDL)—Nature’s Multi-Functional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef]
- Kim, S.I.; Shin, D.; Choi, T.H.; Lee, J.C.; Cheon, G.J.; Kim, K.Y.; Park, M.; Kim, M. Systemic and Specific Delivery of Small Interfering RNAs to the Liver Mediated by Apolipoprotein A–I. Mol. Ther. 2007, 15, 1145–1152. [Google Scholar] [CrossRef]
- Li, J.; Han, M.; Li, J.; Ge, Z.; Wang, Q.; Zhou, K.; Yina, X. Sterically stabilized recombined HDL composed of modified apolipoprotein A–I for efficient targeting toward glioma cells. Drug Deliv. 2020, 27, 530–541. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, Q.; Zhao, Q.; Zheng, J.; Li, Y.; Wang, S.; Yuan, Y. Development of synthetic high-density lipoprotein-based ApoA-I mimetic peptide-loaded docetaxel as a drug delivery nanocarrier for breast cancer chemotherapy. Drug Deliv. 2019, 26, 708–716. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Stein, Y. Atheroprotective mechanisms of HDL. Atherosclerosis 1999, 144, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.; Waldeck, A.R.; Witting, P.K.; Rye, K.A.; Stocker, R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 1998, 273, 6088–6095. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H. Importance of Apolipoprotein A-I and A-II Composition in HDL and Its Potential for Studying COVID-19 and SARS-CoV-2. Medicines 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Castaing-Berthou, A.; Malet, N.; Radojkovic, C.; Cabou, C.; Gayral, S.; Martinez, L.O.; Laffargue, M. PI3Kβ Plays a Key Role in Apolipoprotein A-I-Induced Endothelial Cell Proliferation through Activation of the Ecto-F 1-ATPase/P2Y 1 Receptors. Cell. Physiol. Biochem. 2017, 42, 579–593. [Google Scholar] [CrossRef]
- Trakaki, A.; Marsche, G. Current Understanding of the Immunomodulatory Activities of High-Density Lipoproteins. Biomedicines 2021, 9, 587. [Google Scholar] [CrossRef]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef]
- Duman-Özdamar, Z.E.; Binay, B. Production of Industrial Enzymes via Pichia pastoris as a Cell Factory in Bioreactor: Current Status and Future Aspects. Protein J. 2021, 40, 367–376. [Google Scholar] [CrossRef]
- Weinacker, D.; Rabert, C.; Zepeda, A.B.; Figueroa, C.A.; Pessoa, A.; Farías, J.G. Applications of recombinant Pichia pastoris in the healthcare industry. Braz. J. Microbiol. 2014, 44, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- de Sá Magalhães, S.; Keshavarz-Moore, E. Pichia pastoris (Komagataella phaffii) as a Cost-Effective Tool for Vaccine Production for Low- and Middle-Income Countries (LMICs). Bioengineering 2021, 8, 119. [Google Scholar] [CrossRef]
- Mossel, E.C.; Huang, C.; Narayanan, K.; Makino, S.; Tesh, R.B.; Peters, C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J. Virol. 2005, 79, 3846–3850. [Google Scholar] [CrossRef] [PubMed]
- Emeny, J.M.; Morgan, M.J. Regulation of the interferon system: Evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Huang, B.; Zhao, L.; Deng, Y.; Ren, J.; Tan, W. Novaferon Effectively Inhibits Ancestral SARS-CoV-2 and Omicron Variant in Vitro, 2022. China CDC Wkly. 2022, 4, 509–512. [Google Scholar]
- Mamaev, A.L.; Beklemishev, A.B. Cloning and analysis of expression of synthetic human apolipoprotein a1 genes in Escherichia coli and methylotrophic yeasts Pichia pastoris. SB RAMS Bull. 2014, 34, 37–49. [Google Scholar]
- Pykhtina, M.; Miroshnichenko, S.; Romanov, V.; Grazhdantseva, A.; Kochneva, G.; Beklemishev, A. Construction of Recombinant Human GM-CSF and GM-CSF-ApoA-I Fusion Protein and Evaluation of Their Biological Activity. Pharmaceuticals 2021, 14, 459. [Google Scholar] [CrossRef]
- Pykhtina, M.B.; Romanov, V.P.; Miroshnichenko, S.M.; Beklemishev, A.B. Production and analysis of the biological properties of recombinant human granulocyte colony stimulating factor chimeric form. Sib. Sci. Med. J. 2019, 39, 37–45. [Google Scholar]
- Pykhtina, M.B.; Romanov, V.P.; Miroshnichenko, S.M.; Beklemishev, A.B. Construction of a Pichia pastoris strain efficiently producing recombinant human granulocyte-colony stimulating factor (rhG-CSF) and study of its biological activity on bone marrow cells. Mol. Biol. Rep. 2020, 47, 607–620. [Google Scholar] [CrossRef]
- Bahrami, A.; Shojaosadati, S.A.; Khalilzadeh, R.; Mohammadian, J.; Farahani, E.V.; Masoumian, M.R. Prevention of human granulocyte colony-stimulating factor protein aggregation in recombinant Pichia pastoris fed-batch fermentation using additives. Biotechnol. Appl. Biochem. 2009, 52, 141–148. [Google Scholar] [CrossRef]
- Sadaf, S.; Arshad, H.; Waheed Akhtar, M. A non-ionic surfactant reduces the induction time and enhances expression levels of bubaline somatotropin in Pichia pastoris. Mol. Biol. Rep. 2014, 41, 855–863. [Google Scholar] [CrossRef]
- Kerwin, B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Gong, X.; Yang, Z.Y.; Wang, T.Y.; Ma, G.C.; Ma, Q.J.; Wu, J. Expression in Pichia pastoris and properties of human serum albumin-interferon alpha2b chimera. Sheng Wu Gong Cheng Xue Bao 2006, 22, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, S.; Usynin, I.; Dudarev, A.; Nimaev, V.; Solovieva, A. Apolipoprotein A-I Supports MSCs Survival under Stress Conditions. Int. J. Mol. Sci. 2020, 21, 4062. [Google Scholar] [CrossRef] [PubMed]
- Castañeda Ruiz, A.J.; Shetab Boushehri, M.A.; Phan, T.; Carle, S.; Garidel, P.; Buske, J.; Lamprech, A. Alternative Excipients for Protein Stabilization in Protein Therapeutics: Overcoming the Limitations of Polysorbates. Pharmaceutics 2022, 14, 2575. [Google Scholar] [CrossRef]
- Li, D.; Wu, X.; Yu, X.; Huang, Q.; Tao, L. Synergistic effect of non-ionic surfactants tween 80 and PEG6000 on cytotoxicity of insecticides. Environ. Toxicol. Pharmacol. 2015, 39, 677–682. [Google Scholar] [CrossRef]
- Weiszhar, Z.; Czucz, J.; Revesz, C.; Rosivall, L.; Szebeni, J.; Rozsnyay, Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm Sci. 2012, 45, 492–498. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, X.; Tang, B.; Chang, C.; Liu, G.; Feng, L.; Yu, Y.; Zhang, D.; Hou, J. Tween-20 transiently changes the surface morphology of PK-15 cells and improves PCV2 infection. BMC Vet. Res. 2018, 14, 138. [Google Scholar] [CrossRef]
- Ochoa, M.C.; Fioravanti, J.; Duitman, E.H.; Medina-Echeverz, J.; Palazon, A.; Arina, A.; Dubrot, J.; Alfaro, C.; Morales-Kastresana, A.; Murillo, O.; et al. Liver gene transfer of interkeukin-15 constructs that become part of circulating high density lipoproteins for immunotherapy. PLoS ONE 2012, 7, e52370. [Google Scholar] [CrossRef]
- Medina-Echeverz, J.; Fioravanti, J.; Díaz-Valdés, N.; Frank, K.; Aranda, F.; Gomar, C.; Ardaiz, N.; Dotor, J.; Umansky, V.; Prieto, J.; et al. Harnessing high density lipoproteins to block transforming growth factor beta and to inhibit the growth of liver tumor metastases. PLoS ONE 2014, 9, e96799. [Google Scholar] [CrossRef]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Fernandez-Barrena, M.G.; Urtasun, R.; Elizalde, M.; Barcena-Varela, M.; Jiménez, M.; Chang, H.C.; Barbero, R.; et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: Development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut 2017, 66, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, J.; González, I.; Medina-Echeverz, J.; Larrea, E.; Ardaiz, N.; González-Aseguinolaza, G.; Prieto, J.; Berraondo, P. Anchoring interferon alpha to apolipoprotein A-I reduces hematological toxicity while enhancing immunostimulatory properties. Hepatology 2011, 53, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Viengkhou, B.; Hofer, M.J. Breaking down the cellular responses to type I interferon neurotoxicity in the brain. Front. Immunol. 2023, 14, 1110593. [Google Scholar] [CrossRef] [PubMed]
- Hyka, N.; Dayer, J.M.; Modoux, C.; Kohno, T.; Edwards, C.K.; Roux-Lombard, P.; Burger, D. Apolipoprotein A-I inhibits the production of interleukin-1β and tumor necrosis factor-α by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001, 97, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.H.; Duch, M.; Pedersen, F.S. Increased cloning efficiency by temperature-cycle ligation. Nucleic Acids Res. 1996, 24, 800–801. [Google Scholar] [CrossRef][Green Version]
- Chepurnov, A.A.; Sharshov, K.A.; Kazachinskaya, E.I.; Kononova, Y.V.; Kazachkova, E.A.; Khripkova, O.P.; Yurchenko, K.S.; Alekseev, A.Y.; Voevoda, M.I.; Shestopalov, A.M. Antigenic properties of the SARS-CoV-2/human/RUS/Nsk-FRCFTM-1/2020 coronavirus isolate, recovered of a patient in Novosibirsk. J. Infectol. 2020, 12, 42–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]

| Protein | Cmax (pg/mL) | SE of Cmax | AUC0-inf_obs (pg/mL × h) | MRT (H) | T1/2 (h) | Tmax (h) |
|---|---|---|---|---|---|---|
| ryIFN | 37.897 | 6.0 | 4.715 | 118.140 | 6.701 | 1.000 |
| ryIFN-ApoA-I | 39.771 | 16.296 | 6.645 | 445.881 | 12.205 | 4.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroshnichenko, S.; Pykhtina, M.; Kotliarova, A.; Chepurnov, A.; Beklemishev, A. Engineering a New IFN-ApoA-I Fusion Protein with Low Toxicity and Prolonged Action. Molecules 2023, 28, 8014. https://doi.org/10.3390/molecules28248014
Miroshnichenko S, Pykhtina M, Kotliarova A, Chepurnov A, Beklemishev A. Engineering a New IFN-ApoA-I Fusion Protein with Low Toxicity and Prolonged Action. Molecules. 2023; 28(24):8014. https://doi.org/10.3390/molecules28248014
Chicago/Turabian StyleMiroshnichenko, Svetlana, Mariya Pykhtina, Anastasiia Kotliarova, Alexander Chepurnov, and Anatoly Beklemishev. 2023. "Engineering a New IFN-ApoA-I Fusion Protein with Low Toxicity and Prolonged Action" Molecules 28, no. 24: 8014. https://doi.org/10.3390/molecules28248014
APA StyleMiroshnichenko, S., Pykhtina, M., Kotliarova, A., Chepurnov, A., & Beklemishev, A. (2023). Engineering a New IFN-ApoA-I Fusion Protein with Low Toxicity and Prolonged Action. Molecules, 28(24), 8014. https://doi.org/10.3390/molecules28248014






