Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications
Abstract
:1. Introduction
2. Results and Discussion
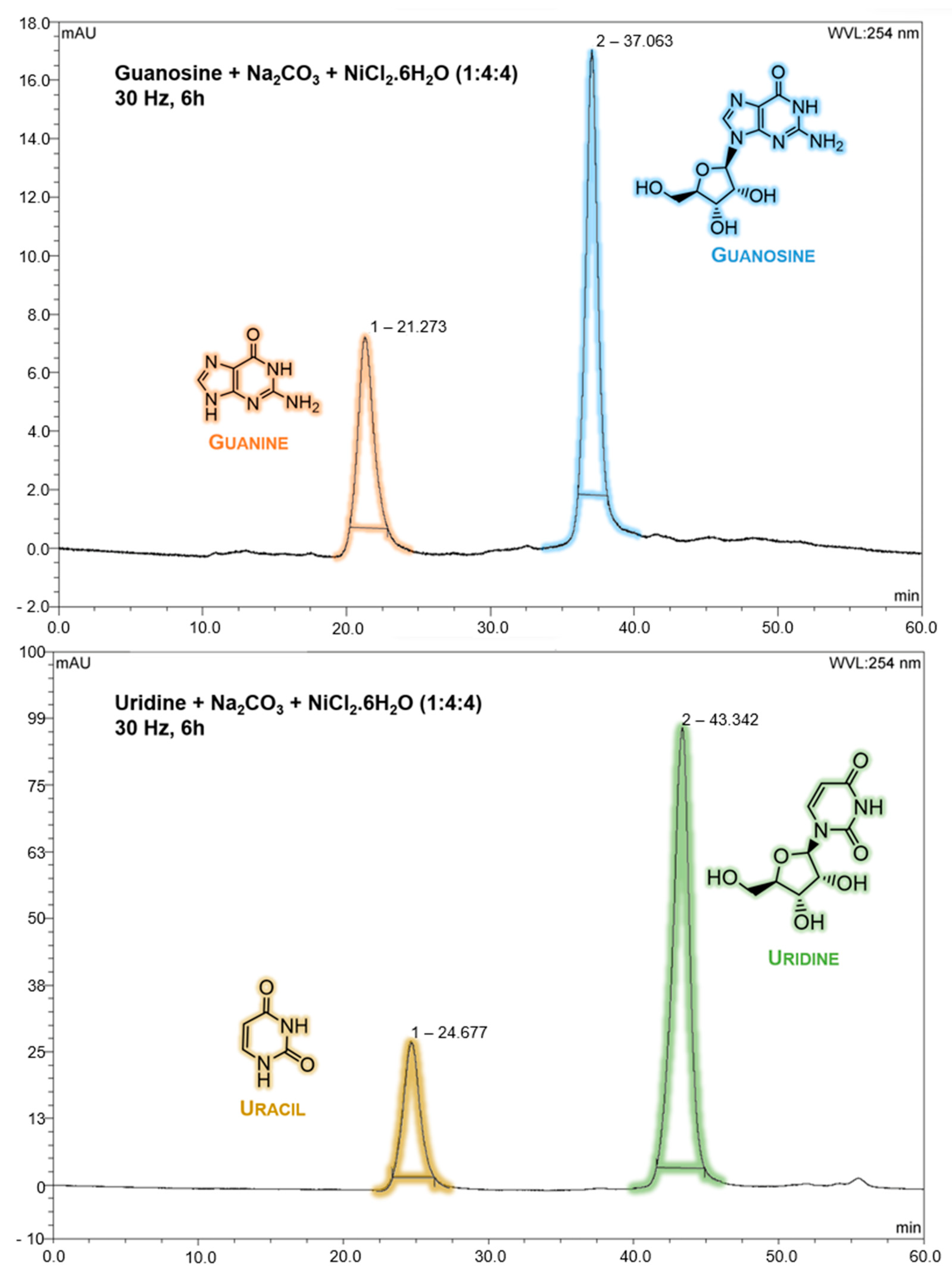
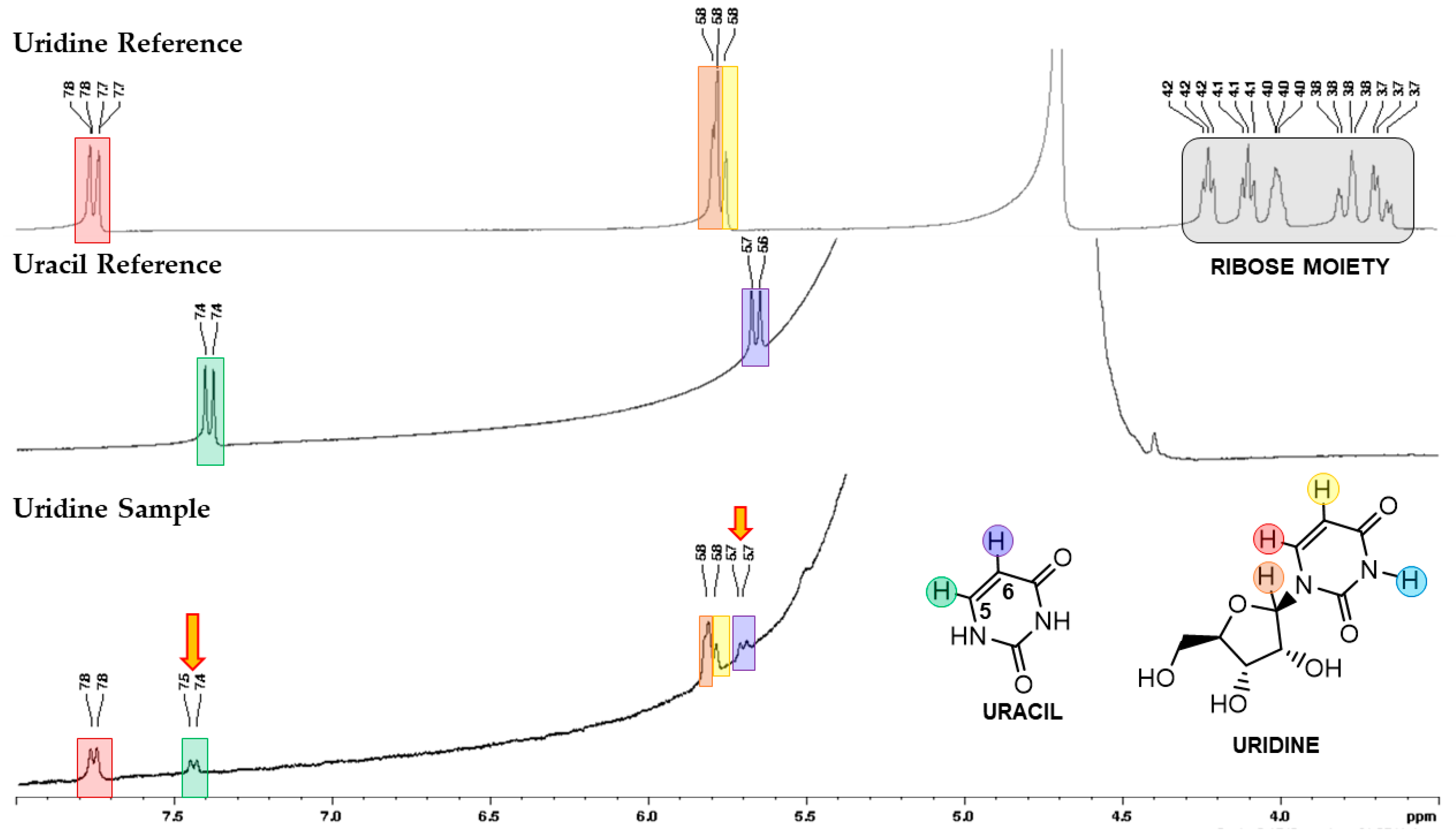
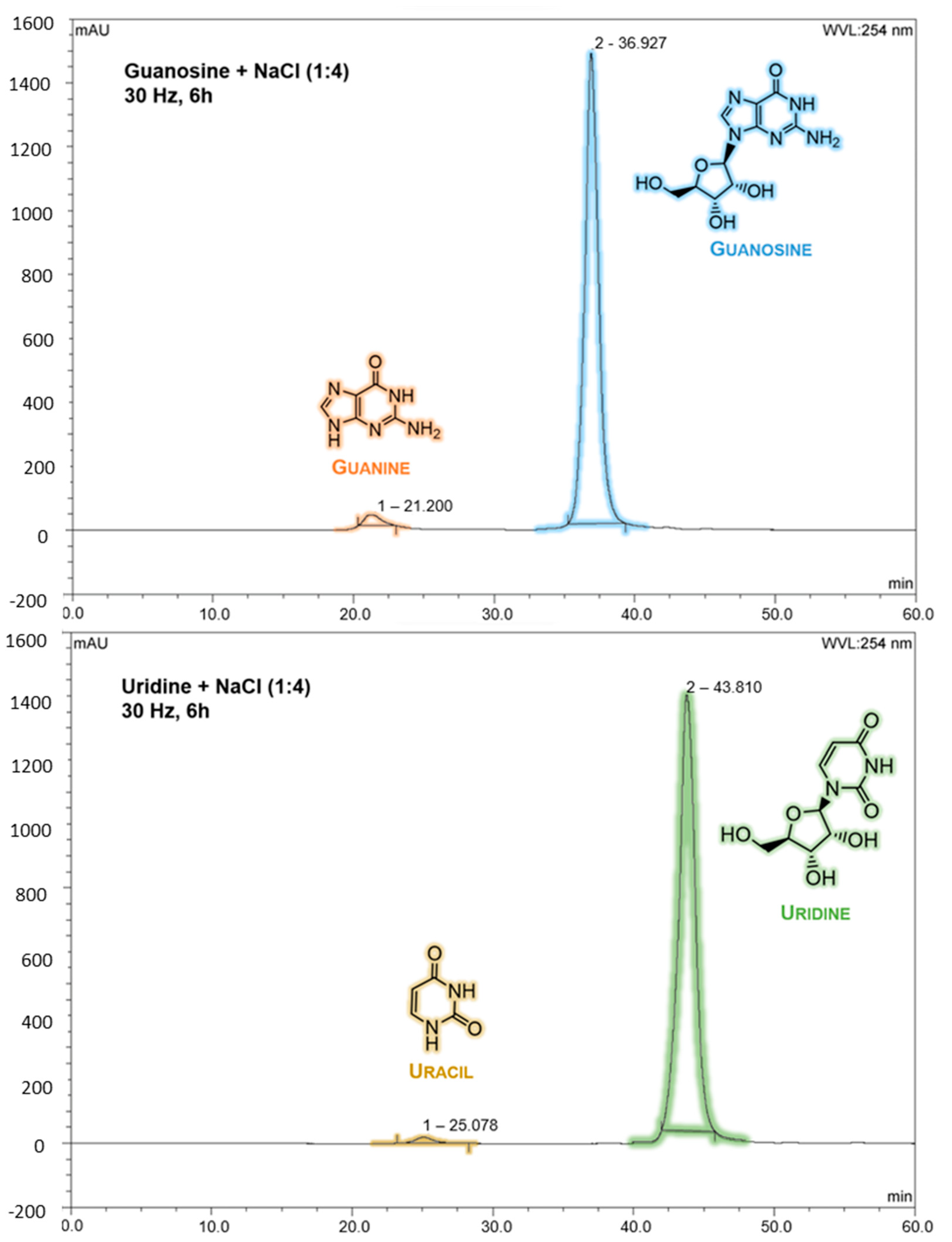
2.1. Mechanochemical Degradation Studies of Guanosine and Uridine
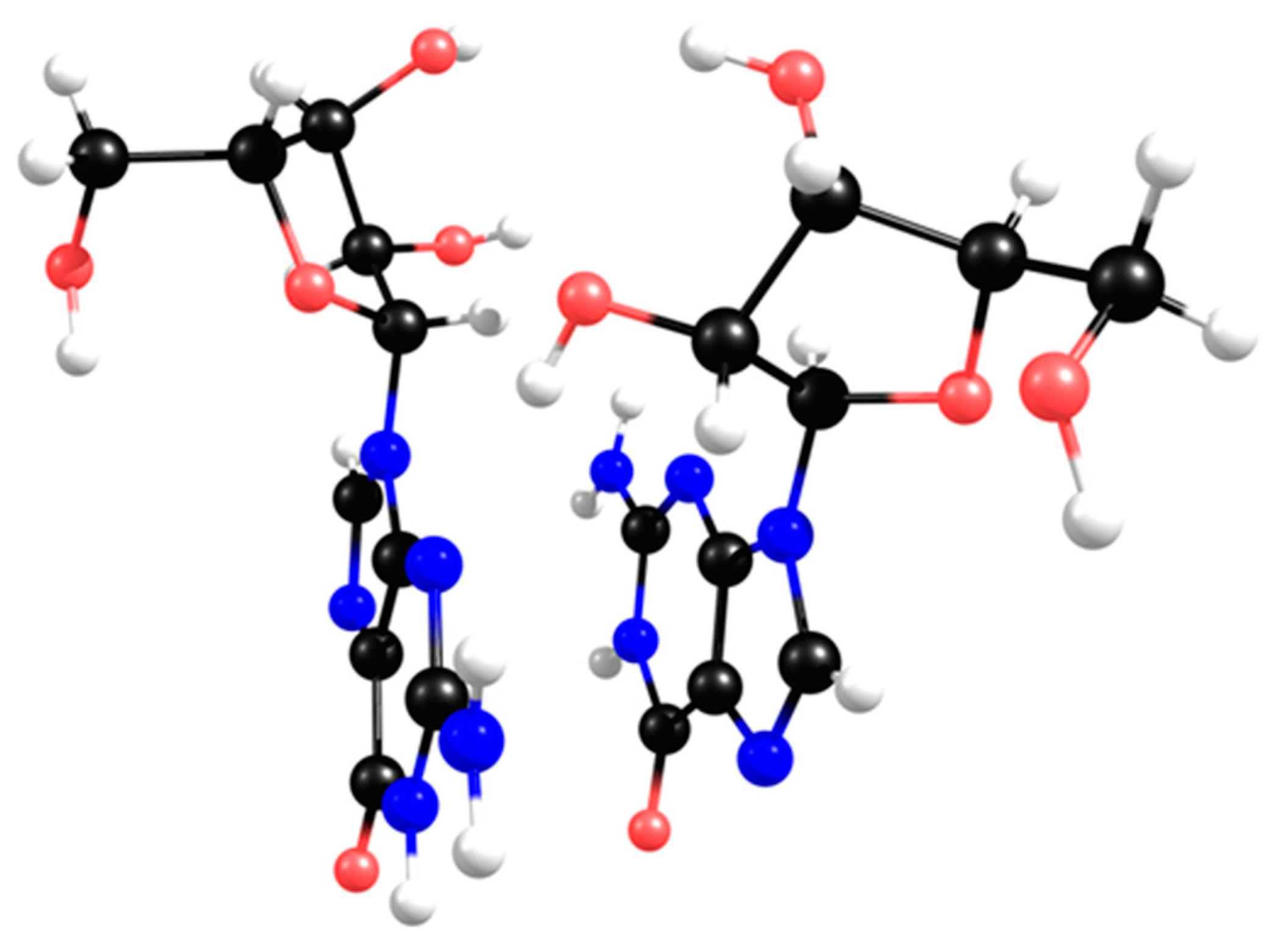
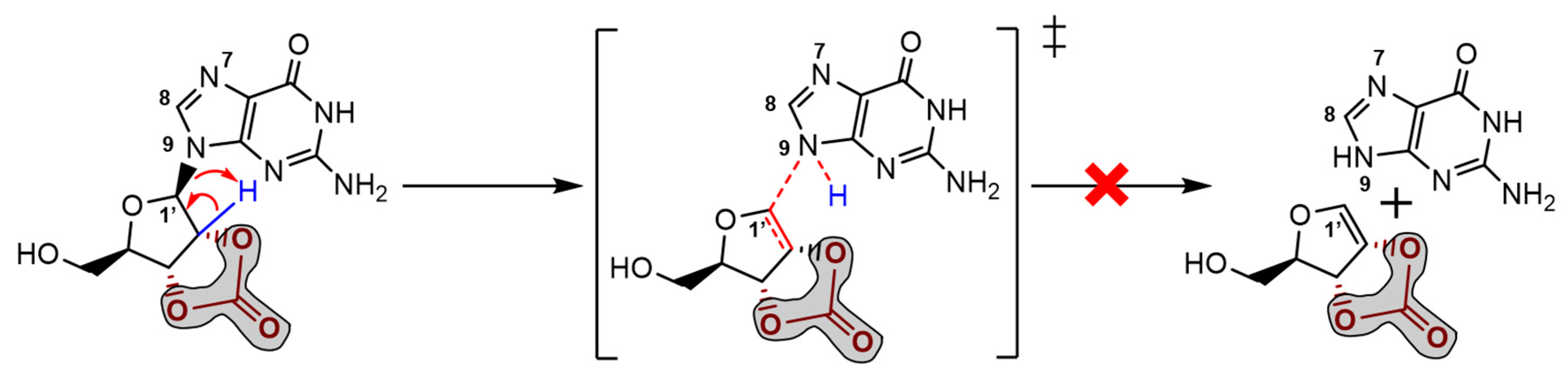
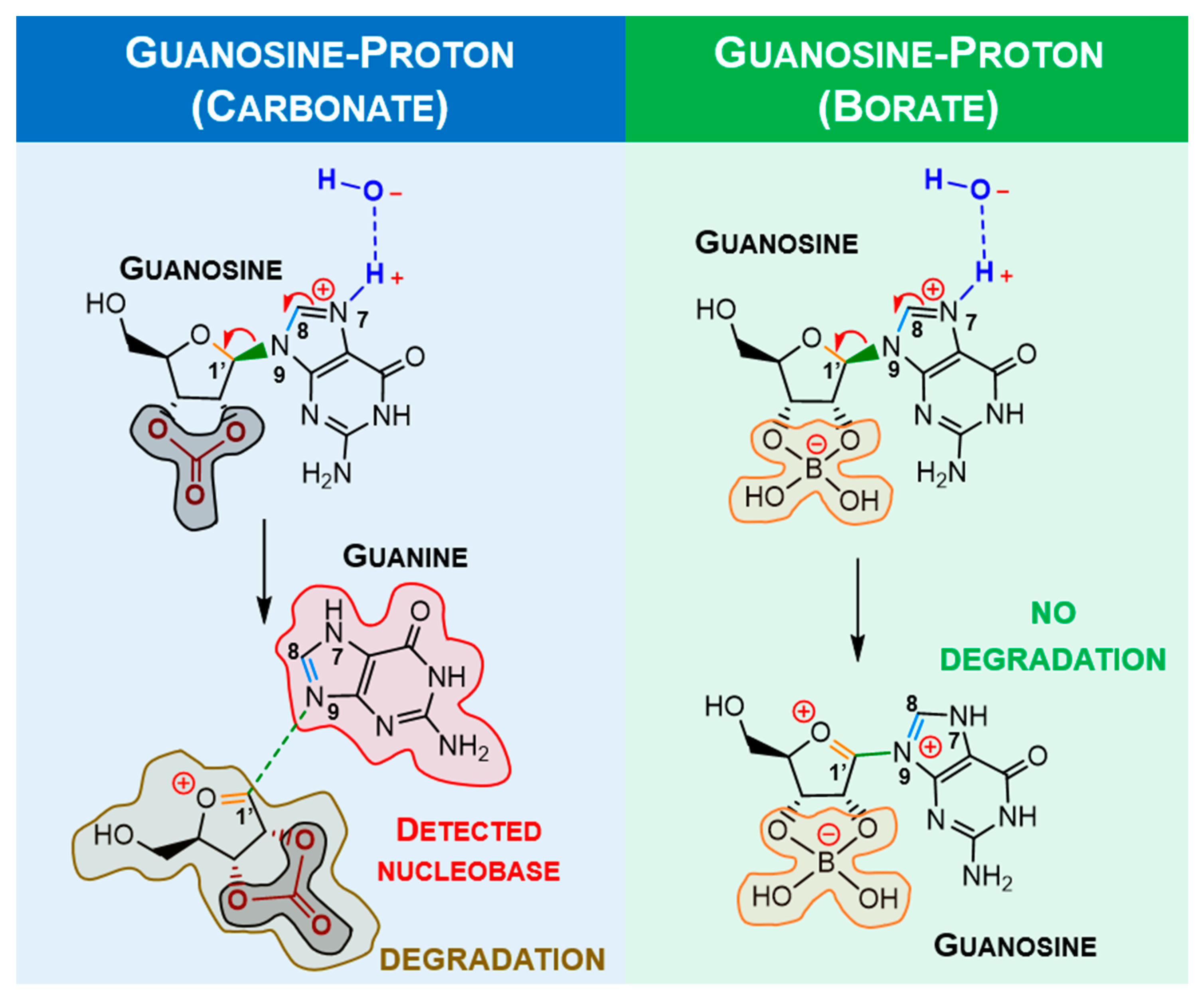
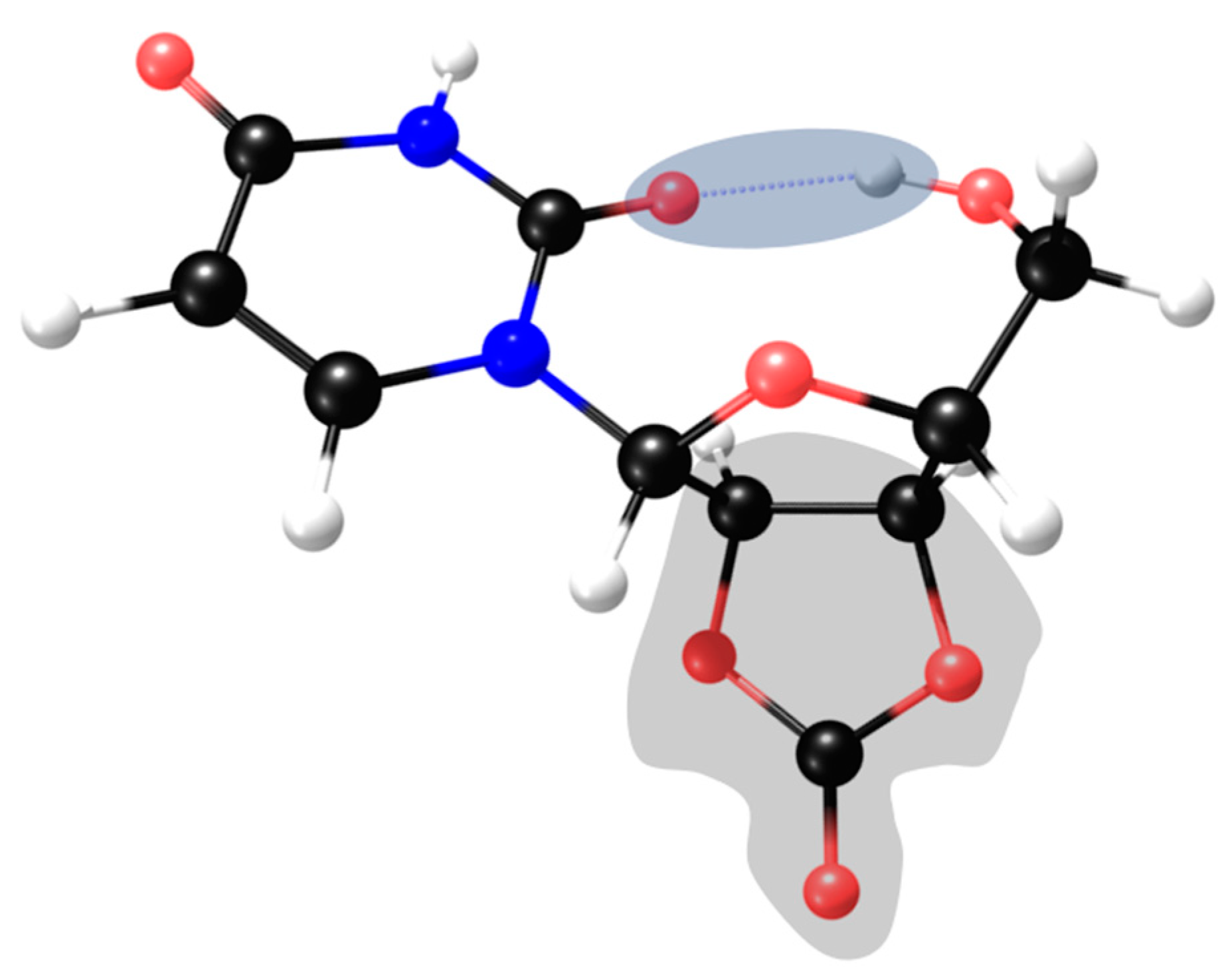
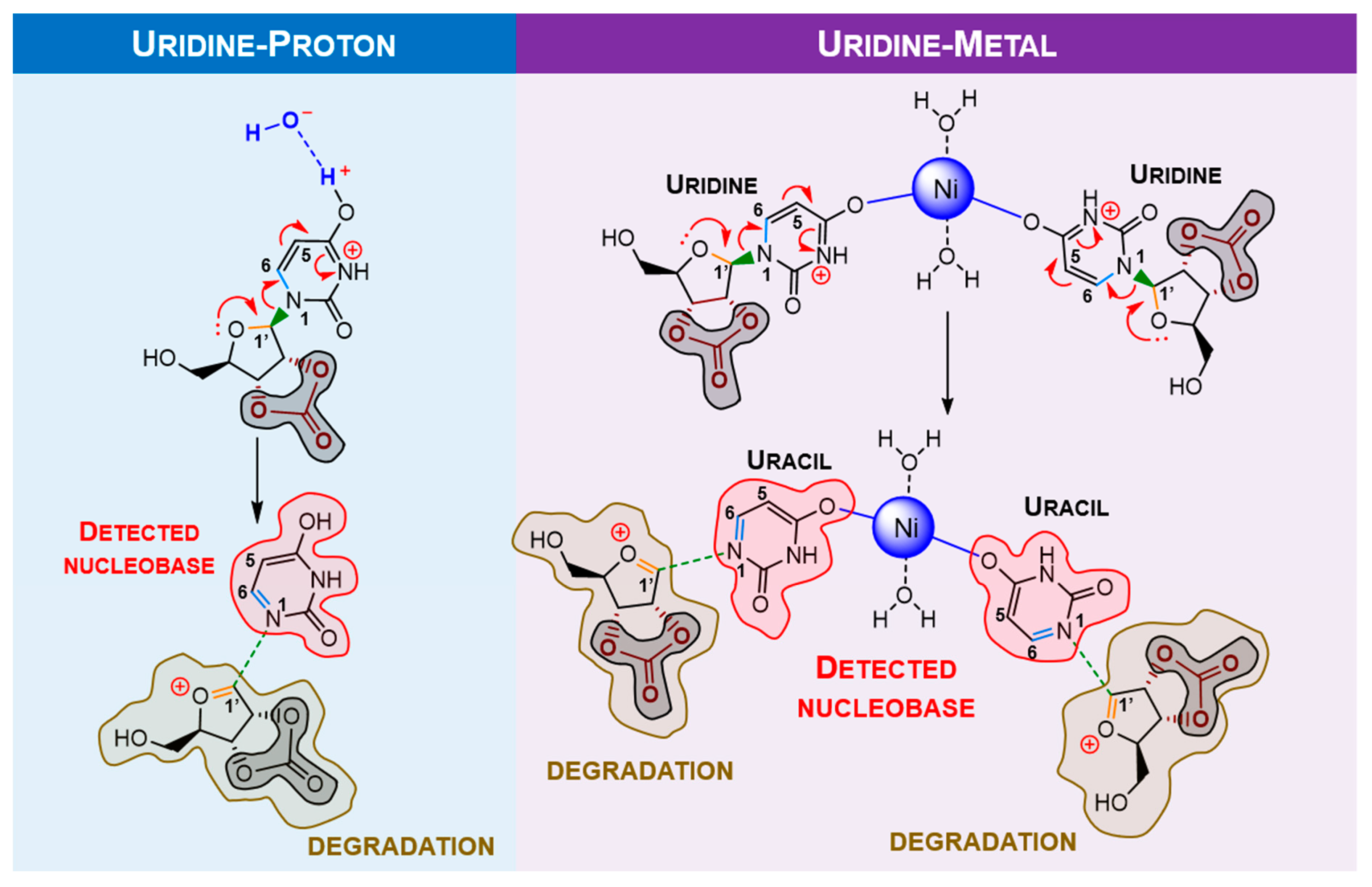
2.2. Ribonucleoside Reactivity and Degradation Mechanism by DFT Calculations
3. Materials and Methods
3.1. Milling Process
3.2. HPLC Analysis
3.3. NMR Spectroscopy Analysis
3.4. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- André, V.; Duarte, M.T.; Gomes, C.S.B.; Sarraguça, M.C. Mechanochemistry in Portugal-A Step towards Sustainable Chemical Synthesis. Molecules 2021, 27, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Ap-proaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef] [PubMed]
- Pagola, S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals 2023, 13, 124. [Google Scholar] [CrossRef]
- Zhou, J.; Hsu, T.-G.; Wang, J. Mechanochemical Degradation and Recycling of Synthetic Polymers. Angew. Chem. Int. Ed. 2023, 62, e202300768. [Google Scholar] [CrossRef]
- Kaupp, G.J.C. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Kaiser, R.P.; Krake, E.F.; Backer, L.; Urlaub, J.; Baumann, W.; Handler, N.; Buschmann, H.; Beweries, T.; Holzgrabe, U.; Bolm, C. Ball milling—A new concept for predicting degradation profiles in active pharmaceutical ingredients. Chem. Commun. 2021, 57, 11956–11959. [Google Scholar] [CrossRef]
- Krake, E.F.; Backer, L.; Andres, B.; Baumann, W.; Handler, N.; Buschmann, H.; Holzgrabe, U.; Bolm, T.; Beweries, C. Mechanochemical Oxidative Degradation of Thienopyridine Containing Drugs: Toward a Simple Tool for the Prediction of Drug Stability. ACS Cent. Sci. 2023, 9, 1150–1159. [Google Scholar] [CrossRef]
- Li, J.; Nagamani, C.; Moore, J.S. Polymer Mechanochemistry: From Destructive to Productive. Acc. Chem. Res. 2015, 48, 2181–2190. [Google Scholar] [CrossRef]
- Rennekamp, B.; Karfusehr, C.; Kurth, M.; Ünal, A.; Monego, D.; Riedmiller, K.; Gryn’ova, G.; Hudson, D.M.; Gräter, F. Collagen breaks at weak sacrificial bonds taming its mechanoradicals. Nat. Commun. 2023, 14, 2075. [Google Scholar] [CrossRef]
- Wang, X.-J.; You, J.-Z. Study on the molecular structure and thermal stability of pyrimidine nucleoside analogs. J. Therm. Anal. Calorim. 2015, 120, 1009–1025. [Google Scholar] [CrossRef]
- Wang, X.-J.; You, J.-Z. Study on the molecular structure and thermal stability of purine nucleoside analogs. J. Anal. Appl. Pyrolysis 2015, 111, 1–14. [Google Scholar] [CrossRef]
- Cataldo, F. Radiolysis and radioracemization of RNA ribonucleosides: Implications for the origins of life. J. Radioanal. Nucl. Chem. 2018, 318, 1649–1661. [Google Scholar] [CrossRef]
- Rios, A.C.; Yu, H.T.; Tor, Y. Hydrolytic Fitness of N-glycosyl Bonds: Comparing the Deglycosylation Kinetics of Modified, Alternative and Native Nucleosides. J. Phys. Org. Chem. 2015, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Marques, M.M.; da Silva, J.A.L.; da Silva, J. Interactions of D-ribose with polyatomic anions, and alkaline and alkaline-earth cations: Possible clues to environmental synthesis conditions in the pre-RNA world. New J. Chem. 2008, 32, 2043–2049. [Google Scholar] [CrossRef]
- Franco, A.; da Silva, J.A.L. Boron in Prebiological Evolution. Angew. Chem. Int. Ed. 2021, 60, 10458. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate Minerals Stabilize Ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Zhou, L.X.; Zhang, Y.F. Theoretical Study on the Interaction between β-D-ribose(RI) and Bivalent and Monova-lent Cations. Acta Phys.-Chim. Sin. 2002, 18, 253–259. [Google Scholar] [CrossRef]
- Bandwar, R.P.; Rao, C.P. Transition metal–saccharide chemistry and biology: An emerging field of multidisciplinary in-terest. Curr. Sci. 1997, 72, 788–796. [Google Scholar]
- Thewalt, U.; Bugg, C.E.; Marsh, R.E. The crystal structure of guanosine dihydrate and inosine dihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 1089–1101. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Cagnetta, G.; Robertson, J.; Huang, J.; Zhang, K.; Yu, G. Mechanochemical destruction of halogenated organic pollutants: A critical review. J. Hazard. Mater. 2016, 313, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Stolar, T.; Grubešić, S.; Cindro, N.; Meštrović, E.; Užarević, K.; Hernández, J.G. Mechanochemical Prebiotic Peptide Bond Formation. Angew. Chem. Int. Ed. 2021, 60, 12727. [Google Scholar] [CrossRef] [PubMed]
- Martins, Z.; Price, M.C.; Goldman, N.; Sephton, M.A.; Burchell, M.J. Shock synthesis of amino acids from impacting cometary and icy planet surface analogues. Nat. Geosci. 2013, 6, 1045–1049. [Google Scholar] [CrossRef]
- Bischoff, A.; Scott, E.R.D.; Metzler, K.; Goodrich, C.A. Nature and Origins of Meteoritic Breccias. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, H.Y., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; p. 679. [Google Scholar]
- Silber, E.A.; Boslough, M.; Hocking, W.K.; Gritsevich, M.; Whitaker, R.W. Physics of meteor generated shock waves in the Earth’s atmosphere—A review. Adv. Space Res. 2018, 62, 489–532. [Google Scholar] [CrossRef]
- Ryan, E.V. Asteroid Fragmentation and Evolution of Asteroids. Annu. Rev. Earth Planet. Sci. 2000, 28, 367–389. [Google Scholar] [CrossRef]
- Rubin, A.E. Mineralogy of meteorite groups. Meteorit. Planet. Sci. 1997, 32, 231–247. [Google Scholar] [CrossRef]
- Golden, D.C.; Ming, D.W.; Zolensky, M.E. Chemistry and mineralogy of oxidation products on the surface of the Hoba nickel-iron meteorite. Meteoritics 1995, 30, 418–422. [Google Scholar] [CrossRef]
- Goryunov, M.V.; Maksimova, A.A.; Oshtrakh, M.I.J.M. Advances in Analysis of the Fe-Ni-Co Alloy and Iron-Bearing Minerals in Meteorites by Mössbauer Spectroscopy with a High Velocity Resolution. Minerals 2023, 13, 1126. [Google Scholar] [CrossRef]
- Rubin, A.E.; Ma, C. Meteoritic minerals and their origins. Geochemistry 2017, 77, 325–385. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of Abiotic Nucleotide Synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.M.; Teichert, J.S.; Trapp, O. Prebiotic Nucleoside Synthesis: The Selectivity of Simplicity. Chem. Eur. J. 2020, 26, 14776. [Google Scholar] [CrossRef]
- Cleaves, H. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331–345. [Google Scholar] [CrossRef]




| Bond | Carbonate (CO32−) | Borate (BO33−) | ||
|---|---|---|---|---|
| Neutral | Protonate | Neutral | Protonate | |
| C8-N9 | 138.1 | 135.0 | 138.1 | 134.9 |
| N9-C1′ | 147.2 | 148.4 | 147.2 | 146.5 |
| C1′-O | 141.5 | 139.0 | 141.5 | 139.7 |
| Bond | Neutral | Proton (H+) | Metal (Ni2+) |
|---|---|---|---|
| C8-N9 | 138.1 | 135.0 | 136.1 |
| N9-C1′ | 147.2 | 148.4 | 148.1 |
| C1′-O | 141.5 | 139.0 | 139.2 |
| Bond | Neutral | Proton (H+) | Metal (Ni2+) |
|---|---|---|---|
| C6-N1 | 138.6 | 135.1 | 135.3 |
| N1-C1′ | 146.4 | 149.9 | 149.8 |
| C1′-O | 140.6 | 138.5 | 138.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, G.P.; da Silva, J.A.L.; André, V.; Galvão, A.M. Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications. Molecules 2023, 28, 8006. https://doi.org/10.3390/molecules28248006
Maia GP, da Silva JAL, André V, Galvão AM. Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications. Molecules. 2023; 28(24):8006. https://doi.org/10.3390/molecules28248006
Chicago/Turabian StyleMaia, Gustavo P., José Armando Luísa da Silva, Vânia André, and Adelino M. Galvão. 2023. "Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications" Molecules 28, no. 24: 8006. https://doi.org/10.3390/molecules28248006
APA StyleMaia, G. P., da Silva, J. A. L., André, V., & Galvão, A. M. (2023). Shock-Induced Degradation of Guanosine and Uridine Promoted by Nickel and Carbonate: Potential Applications. Molecules, 28(24), 8006. https://doi.org/10.3390/molecules28248006







