Modified Diatomaceous Earth in Heparin Recovery from Porcine Intestinal Mucosa
Abstract
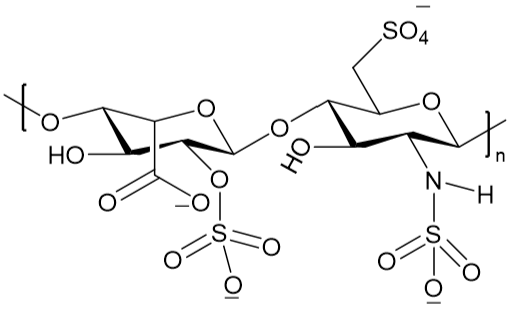
:1. Introduction
2. Results and Discussion
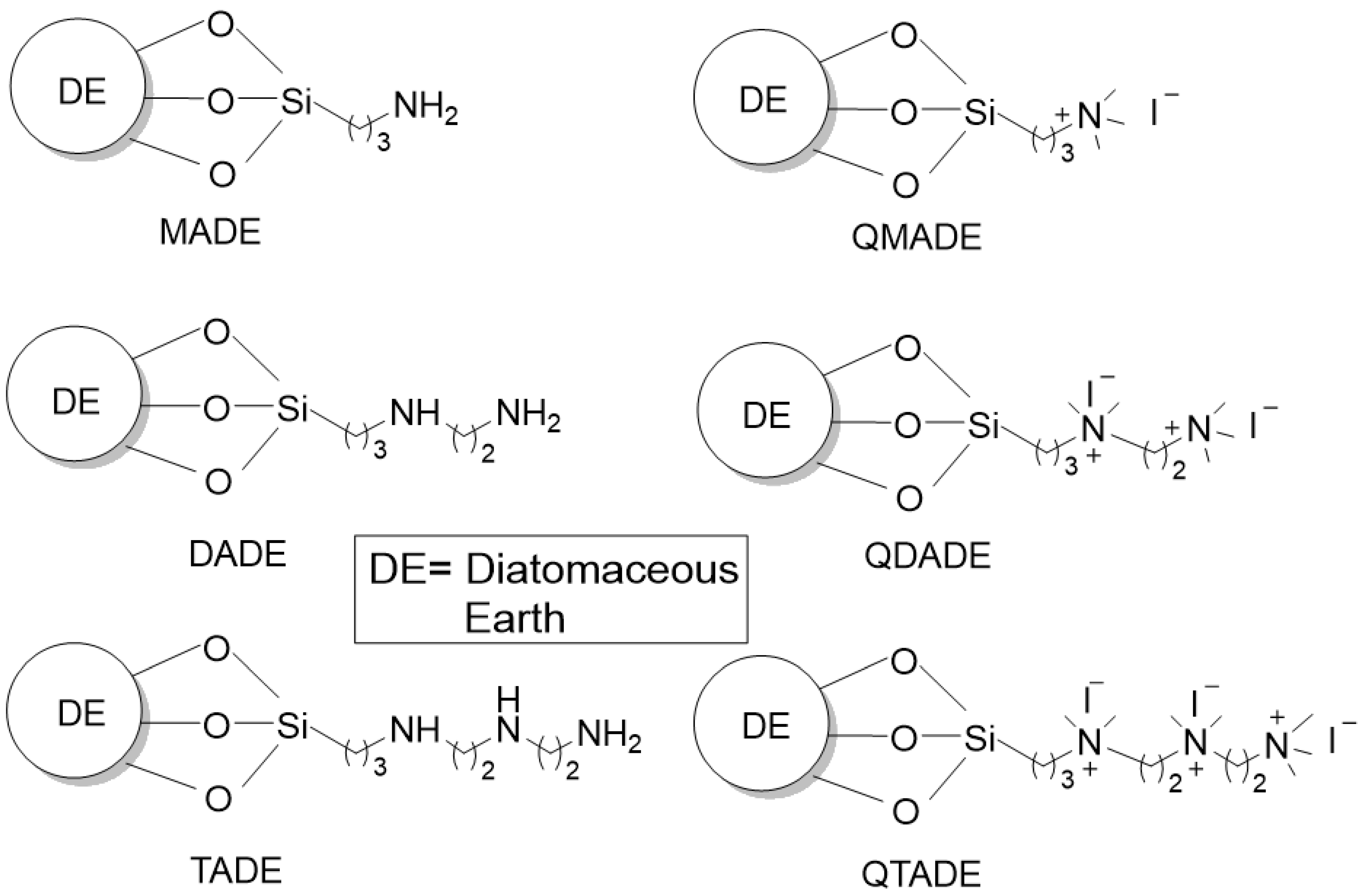
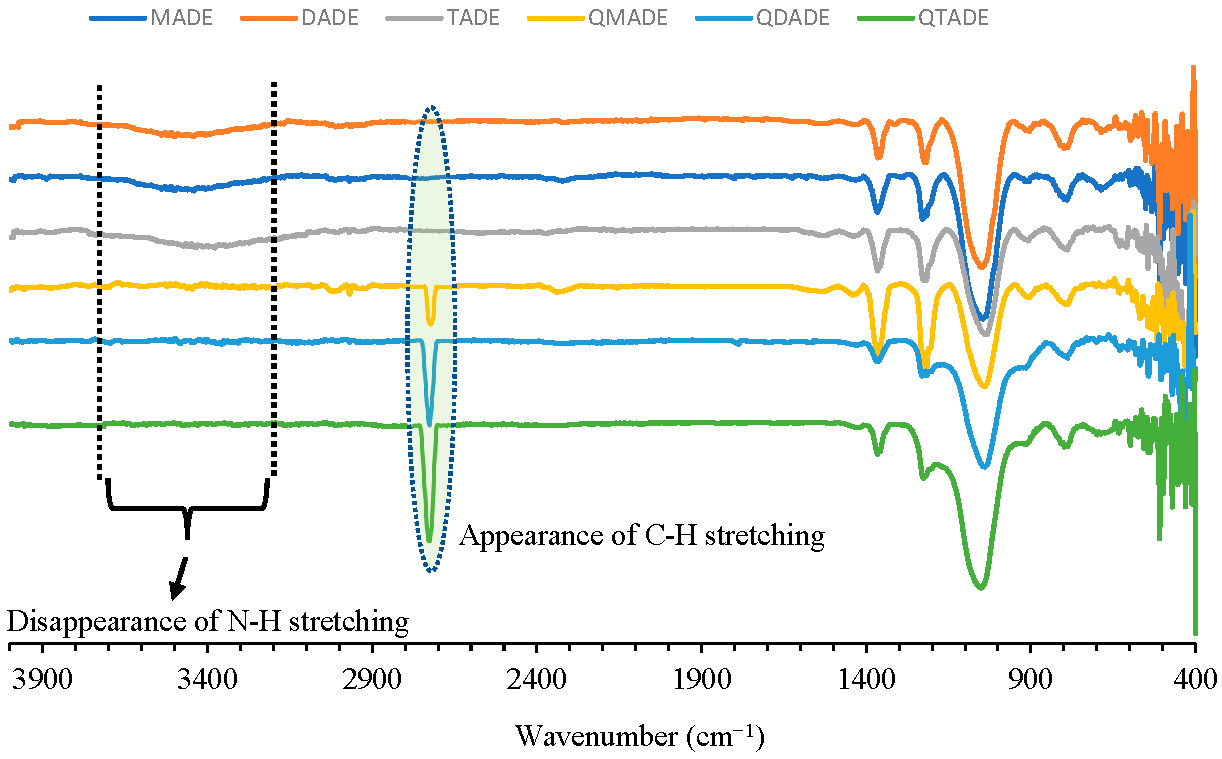
2.1. Preparation and IR Identification of Quaternary Ammonium-Functionalized Diatomaceous Earth
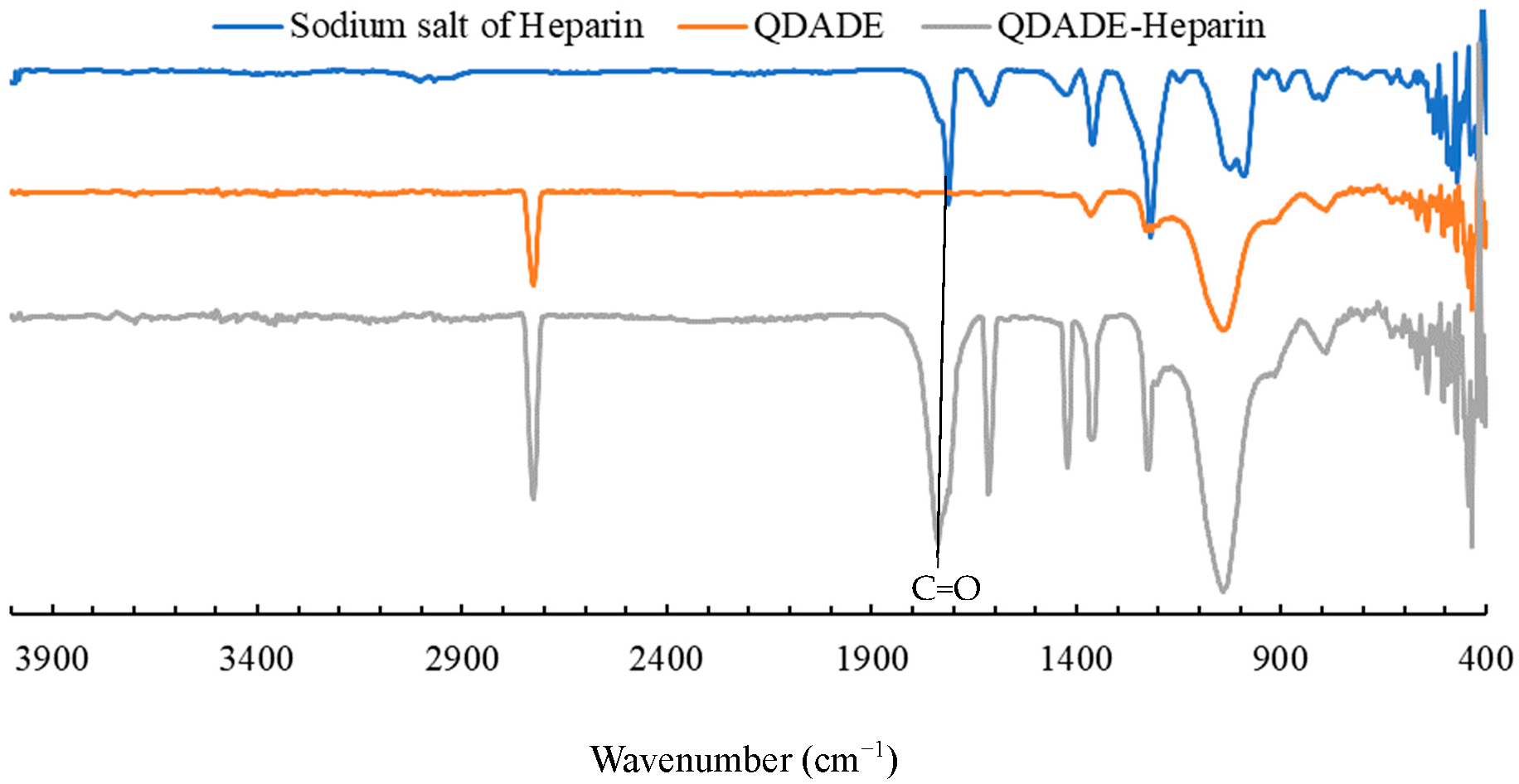
2.2. Heparin Adsorption Studies
2.2.1. Molecular Weight Calculations Using Viscosity
2.2.2. pH Optimization
2.2.3. Optimization of the Adsorption Dosage
2.2.4. Temperature and Duration of Adsorption Effect on Heparin Uptake in Real Sample
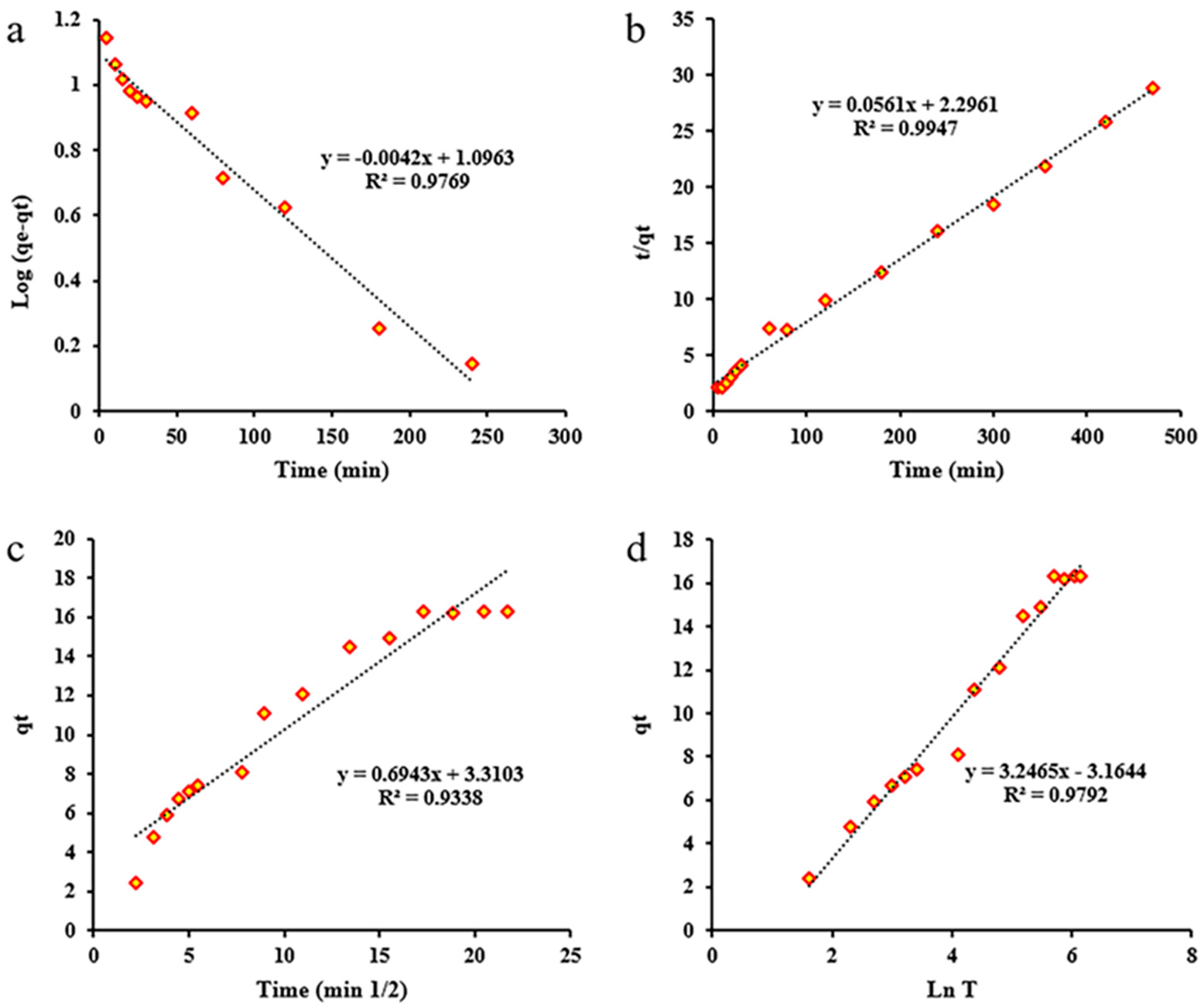
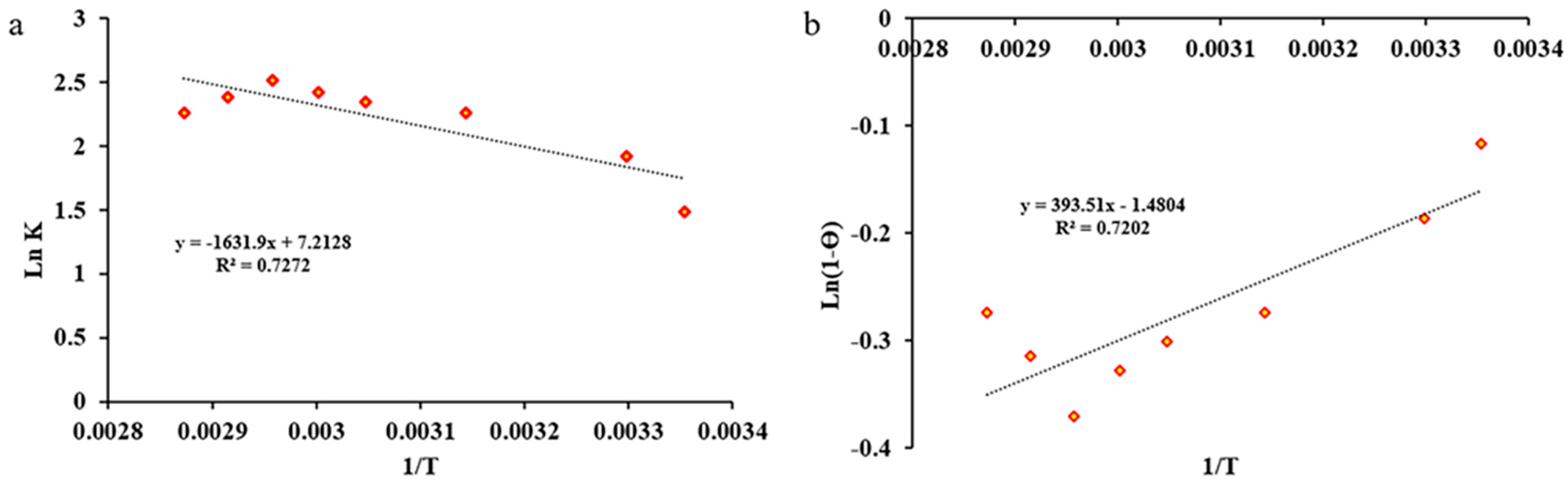
2.2.5. Kinetic and Thermodynamic Studies
Kinetic Study
| Model | Linear Equation | Parameter | Value |
|---|---|---|---|
| Pseudo-first order | Ln(qe − qt) = Lnqe − k1 × t | K1 | 0.0096726 |
| qe (cal) | 12.482 | ||
| R2 | 0.9769 | ||
| Pseudo-second order | t/qe = 1/(k2 × qe2) + (1/qe) × t | K2 | 0.00137 |
| qe (cal) | 17.825 | ||
| R2 | 0.9947 | ||
| Intraparticle Diffusion | qt = ki × √t + xi | Kdiff | 0.6943 |
| C | 3.3103 | ||
| R2 | 0.9338 | ||
| Elovich | Log(C0/(Co-qt × m)) = Log(k0/2.303 × V) + αLogt | β | 0.30802 |
| α | 1.224 | ||
| R2 | 0.9792 | ||
| qe (exp) | 16.3 |
Thermodynamic Study
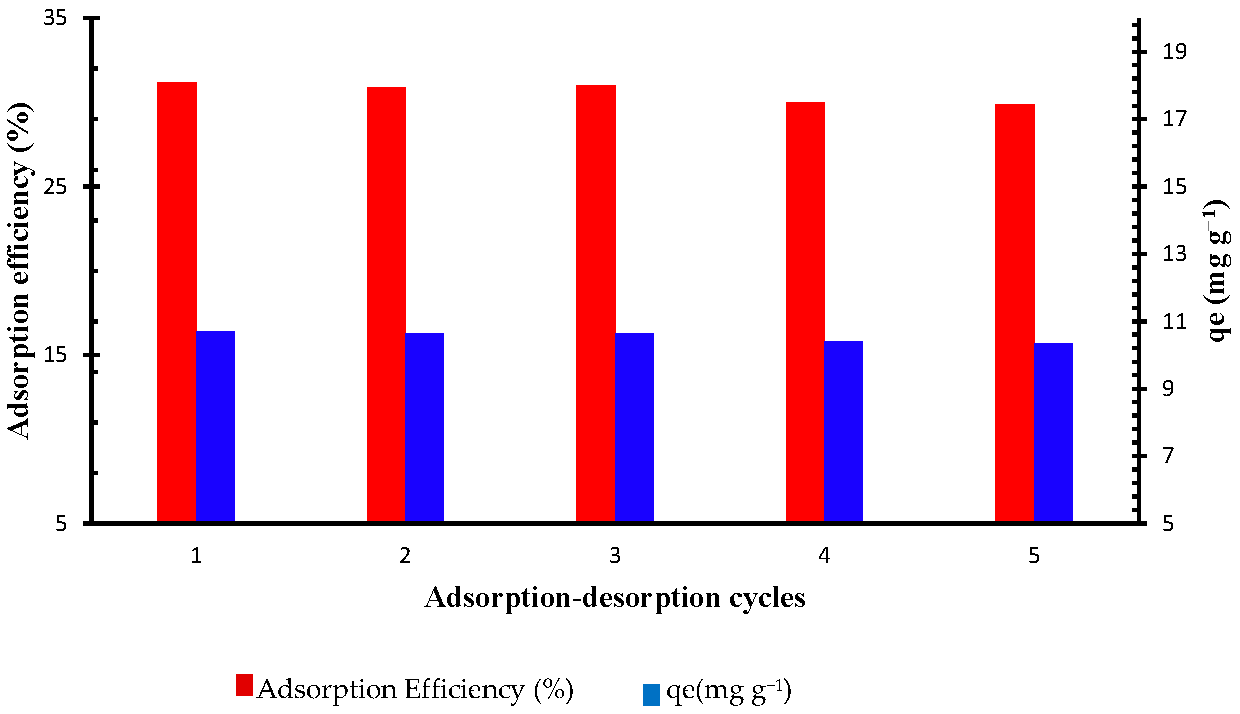
2.2.6. Sorbet Reusability
2.2.7. Sheep Plasma Clotting Assay
3. Experimental
3.1. Synthesis
3.1.1. Synthesis of Amine-Functionalized DE [29,64]
3.1.2. Methyl Iodide Treatment of Amine-Functionalized DEs [11,37]
3.1.3. Sample Preparation for IR Measurements
3.1.4. Viscosity Measurement
3.2. Solutions and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DE | Diatomaceous earth |
| MADE | 3-Aminopropyl-functionalized DE |
| DADE | 3-(Ethylenediamino)propyl-functionalized DE |
| TADE | 3-(Diethylenetriamino)propyl-functionalized DE |
| QMADE | Quaternarized MADE |
| QDADE | Quaternarized DADE |
| QTADE | Quaternarized TADE |
References
- Lee, M.S.; Kong, J. Heparin: Physiology, Pharmacology, and Clinical Application. Rev. Cardiovasc. Med. 2015, 16, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A. Heparin-based nanoparticles: An overview of their applications. J. Nanomater. 2018, 2018, 9780489. [Google Scholar] [CrossRef]
- Scott, L.J.; Perry, C.M. Tramadol. Drugs 2000, 60, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Turpie, A.; Hirsh, J. Heparin. Nova Scotia Med. Bull. 1979, 58, 25–29. [Google Scholar]
- Urbinati, C.; Milanesi, M.; Lauro, N.; Bertelli, C.; David, G.; D’Ursi, P.; Rusnati, M.; Chiodelli, P. HIV-1 tat and heparan sulfate proteoglycans orchestrate the setup of in cis and in trans cell-surface interactions functional to lymphocyte trans-endothelial migration. Molecules 2021, 26, 7488. [Google Scholar] [CrossRef] [PubMed]
- Barrowcliffe, T. History of heparin. In Heparin-A Century of Progress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. [Google Scholar]
- Anderson, J.; Saenko, E. Editorial I: Heparin Resistance; Oxford University Press: Oxford, UK, 2002; Volume 88, pp. 467–469. [Google Scholar]
- Flengsrud, R.; Larsen, M.L.; Ødegaard, O.R. Purification, characterization and in vivo studies of salmon heparin. Thromb. Res. 2010, 126, e409–e417. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Rudd, T.; Yates, E. New Applications of Heparin and Other Glycosaminoglycans. Molecules 2017, 22, 749. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, J.; Baauw, A. Method for Preparation of Heparin from Mucosa. International Patent Application No. WO2010110654A12010, 30 September 2010. [Google Scholar]
- Karimi Abdolmaleki, M.; Das, A.; Khambhati, D.P.; Shafiee, A.; Dimas, K.; Velazquez, C.A.; Davachi, S.M.; Choubtarash Abardeh, S. Efficient and Economic Heparin Recovery from Porcine Intestinal Mucosa Using Quaternary Ammonium-Functionalized Silica Gel. Bioengineering 2022, 9, 606. [Google Scholar] [CrossRef]
- Karimi Abdolmaleki, M.; Ganta, D.; Shafiee, A.; Velazquez, C.A.; Khambhati, D.P. Efficient Heparin Recovery from Porcine Intestinal Mucosa Using Zeolite Imidazolate Framework-8. Molecules 2022, 27, 1670. [Google Scholar] [CrossRef]
- Van der Meer, J.-Y.; Kellenbach, E.; Van den Bos, L.J. From Farm to Pharma: An Overview of Industrial Heparin Manufacturing Methods. Molecules 2017, 22, 1025. [Google Scholar] [CrossRef]
- Hoke, D.E.; Carson, D.D.; Höök, M. A heparin binding synthetic peptide from human HIP/RPL29 fails to specifically differentiate between anticoagulantly active and inactive species of heparin. J. Negat. Results Biomed. 2003, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Linhardt, R.J.; Ampofo, S.A.; Fareed, J.; Hoppensteadt, D.; Folkman, J.; Mulliken, J.B. Isolation and characterization of human heparin. Biochemistry 1992, 31, 12441–12445. [Google Scholar] [CrossRef] [PubMed]
- Haley, R.A.; Ringo, J.M.; Hopgood, H.; Denlinger, K.L.; Das, A.; Waddell, D.C. Graduate student designed and delivered: An upper-level online course for undergraduates in green chemistry and sustainability. J. Chem. Educ. 2018, 95, 560–569. [Google Scholar] [CrossRef]
- Vrieling, E.G.; Beelen, T.P.M.; van Santen, R.A.; Gieskes, W.W.C. Diatom silicon biomineralization as an inspirational source of new approaches to silica production. In Progress in Industrial Microbiology; Osinga, R., Tramper, J., Burgess, J.G., Wijffels, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 35, pp. 39–51. [Google Scholar]
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tatè, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite silica nanoparticles for drug delivery. Nanoscale Res. Lett. 2014, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Polizos, G.; Winter, K.; Lance, M.J.; Meyer, H.M.; Armstrong, B.L.; Schaeffer, D.A.; Simpson, J.T.; Hunter, S.R.; Datskos, P.G. Scalable superhydrophobic coatings based on fluorinated diatomaceous earth: Abrasion resistance versus particle geometry. Appl. Surf. Sci. 2014, 292, 563–569. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; De Stefano, L. Synthetic vs natural: Diatoms bioderived porous materials for the next generation of healthcare Nanodevices. Adv. Healthc. Mater. 2017, 6, 1601125. [Google Scholar] [CrossRef] [PubMed]
- Van Garderen, N.; Clemens, F.J.; Kaufmann, J.; Urbanek, M.; Binkowski, M.; Graule, T.; Aneziris, C.G. Pore analyses of highly porous diatomite and clay based materials for fluidized bed reactors. Microporous Mesoporous Mater. 2012, 151, 255–263. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Oyewole, R.O. Insights into the adsorption of heavy metals from wastewater using diatomaceous earth. Sep. Sci. Technol. 2014, 49, 1787–1806. [Google Scholar] [CrossRef]
- Salih, S.S.; Mahdi, A.; Kadhom, M.; Ghosh, T.K. Competitive adsorption of As (III) and As (V) onto chitosan/diatomaceous earth adsorbent. J. Environ. Chem. Eng. 2019, 7, 103407. [Google Scholar] [CrossRef]
- Salih, S.S.; Ghosh, T.K. Highly efficient competitive removal of Pb (II) and Ni (II) by chitosan/diatomaceous earth composite. J. Environ. Chem. Eng. 2018, 6, 435–443. [Google Scholar] [CrossRef]
- Izuagie, A.A.; Gitari, W.M.; Gumbo, J.R. Defluoridation of groundwater using diatomaceous earth: Optimization of adsorption conditions, kinetics and leached metals risk assessment. Desalination Water Treat. 2016, 57, 16745–16757. [Google Scholar] [CrossRef]
- Kodali, J.; Pavuluri, S.; Arunraj, B.; Kumar, A.S.K.; Rajesh, N. Tapping the potential of a glucosamine polysaccharide-diatomaceous earth hybrid adsorbent in the solid phase extraction of a persistent organic pollutant and toxic pesticide 4, 4′-DDT from water. RSC Adv. 2022, 12, 5489–5500. [Google Scholar] [CrossRef] [PubMed]
- Cicco, S.R.; Giangregorio, M.M.; Rocchetti, M.T.; di Bari, I.; Mastropaolo, C.; Labarile, R.; Ragni, R.; Gesualdo, L.; Farinola, G.M.; Vona, D. Improving the In Vitro Removal of Indoxyl Sulfate and p-Cresyl Sulfate by Coating Diatomaceous Earth (DE) and Poly-vinyl-pyrrolidone-co-styrene (PVP-co-S) with Polydopamine. Toxins 2022, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Perera, H.J.; Khatiwada, B.K.; Paul, A.; Mortazavian, H.; Blum, F.D. Superhydrophobic surfaces with silane-treated diatomaceous earth/resin systems. J. Appl. Polym. Sci. 2016, 133, 44072. [Google Scholar] [CrossRef]
- Perera, H.J.; Mortazavian, H.; Blum, F.D. Surface properties of silane-treated diatomaceous earth coatings: Effect of alkyl chain length. Langmuir 2017, 33, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Perera, H.J.; Goyal, A.; Banu, H.; Alhassan, S.M. Enhanced oil-spill removal and recovery from water bodies using diatomaceous earth and C18-silane-grafted polyurethane. Emergent Mater. 2022, 6, 499–509. [Google Scholar] [CrossRef]
- Uthappa, U.; Sriram, G.; Brahmkhatri, V.; Kigga, M.; Jung, H.-Y.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Xerogel modified diatomaceous earth microparticles for controlled drug release studies. New J. Chem. 2018, 42, 11964–11971. [Google Scholar] [CrossRef]
- Delasoie, J.; Zobi, F. Natural diatom biosilica as microshuttles in drug delivery systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef]
- López-Cebral, R.; Peng, G.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Chen, J.; Silva, T.H.; Reis, R.L. Dual delivery of hydrophilic and hydrophobic drugs from chitosan/diatomaceous earth composite membranes. J. Mater. Sci. Mater. Med. 2018, 29, 21. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Men, J.; Guo, J.; Zhou, W.; Dong, N.; Pang, X.; Gao, B. Preparation of Cationic Functional Polymer Poly(Acryloxyethyltrimethyl Ammonium Chloride)/SiO2 and Its Adsorption Characteristics for Heparin. Korean J. Chem. Eng. 2017, 34, 1889–1895. [Google Scholar] [CrossRef]
- Orihara, K.; Hikichi, A.; Arita, T.; Muguruma, H.; Yoshimi, Y. Heparin molecularly imprinted polymer thin flm on gold electrode by plasma-induced graft polymerization for label-free biosensor. J. Pharm. Biomed. Anal. 2018, 151, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, H.; Godec, M.; Arshadi, M.; Padilla-Zakour, O.I.; Abbaspourrad, A. Multi-porous quaternized chitosan/polystyrene microbeads for scalable, efficient heparin recovery. Chem. Eng. J. 2018, 348, 399–408. [Google Scholar] [CrossRef]
- Valimaki, S.; Khakalo, A.; Ora, A.; Johansson, L.-S.; Rojas, O.J.; Kostiainen, M.A. Effect of PEG–PDMAEMA block copolymer architecture on polyelectrolyte complex formation with heparin. Biomacromolecules 2016, 17, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Clements, D.J.; Pophristic, V.; Ivanov, I.; Vemparala, S.; Bennett, J.S.; Klein, M.L.; Winkler, J.D.; DeGrado, W.F. The design and evaluation of heparin-binding foldamers. Angew. Chem. Int. Ed. 2005, 44, 6685–6689. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, H.; Arshadi, M.; Enayati, M.; Abbaspourrad, A. Highly Efficient Recovery of Heparin Using a Green and Low-Cost Quaternary Ammonium Functionalized Halloysite Nanotube. ACS Sustain. Chem. Eng. 2018, 6, 15349–15360. [Google Scholar] [CrossRef]
- Arshadi, M.; Eskandarloo, H.; Enayati, M.; Godec, M.; Abbaspourrad, A. Highly Water-Dispersible and Antibacterial Magnetic Clay Nanotubes Functionalized with Polyelectrolyte Brushes: High Adsorption Capacity and Selectivity toward Heparin in Batch and Continuous System. Green Chem. 2018, 20, 5491–5508. [Google Scholar] [CrossRef]
- Shenoi, R.A.; Kalathottukaren, M.T.; Travers, R.J.; Lai, B.F.L.; Creagh, A.L.; Lange, D.; Yu, K.; Weinhart, M.; Chew, B.H.; Du, C.; et al. Affinity-Based Design of a Synthetic Universal Reversal Agent for Heparin Anticoagulants. Sci. Transl. Med. 2014, 6, ra150–ra260. [Google Scholar] [CrossRef]
- Dinda, A.K.; Tripathy, D.R.; Das, A.; Dasgupta, S. Comparison of the ribonucleolytic activity of the dityrosine cross-linked Ribonuclease A dimer with its monomer in the presence of inhibitors. Int. J. Biol. Macromol. 2014, 63, 107–113. [Google Scholar] [CrossRef]
- Mecca, T.; Cunsolo, F. Polycationic Calix[8]Arene Receptors Grafted onto Polymeric Matrix: Smart Material for Heparin Neutralization. Polym. Adv. Technol. 2009, 21, 752–757. [Google Scholar] [CrossRef]
- Kamiński, K.; Zazakowny, K.; Szczubiałka, K.; Nowakowska, M. PH-Sensitive Genipin-Cross-Linked Chitosan Microspheres for Heparin Removal. Biomacromolecules 2008, 9, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J. IR Spectroscopic Method for Determination of Silicone Cross-Linking; Pressure Sensitive Tape Council: Chicago, IL, USA, 2016. [Google Scholar]
- Ullah, H.; Azizli, K.; Man, Z.B.; Ismail, M.B.C. Synthesis and Characterization of Urea-Formaldehyde Microcapsules Containing Functionalized Polydimethylsiloxanes. Procedia Eng. 2016, 148, 168–175. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Gatlin, D.M.; Das, A.; Loftin, B.; Krause, J.A.; Abe, M.; Gudmundsdottir, A.D. Laser Flash Photolysis of Nanocrystalline α-Azido-p-Methoxy-Acetophenone. Org. Biomol. Chem. 2017, 15, 7380–7386. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; InTech: Rijeka, Croatia, 2016; Chapter 1. [Google Scholar]
- Enayati, M.; Karimi Abdolmaleki, M.; Abbaspourrad, A. Synthesis of Cross-Linked Spherical Polycationic Adsorbents for Enhanced Heparin Recovery. ACS Biomater. Sci. Eng. 2020, 6, 2822–2831. [Google Scholar] [CrossRef] [PubMed]
- Pandian, V.; Thangavel, B.; Thirugnanasambandan, S. Low-Molecular Weight Molluscan Glycosaminoglycan from Bivalve Katelysia Opima (Gmelin). Methods Find. Exp. Clin. Pharmacol. 2008, 30, 175–180. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Das, A.; Thomas, S.S.; Garofoli, A.A.; Chavez, K.A.; Krause, J.A.; Bohne, C.; Gudmundsdottir, A.D. Steric Demand and Rate-Determining Step for Photoenolization of Di-Ortho-Substituted Acetophenone Derivatives. Photochem. Photobiol. 2019, 95, 154–162. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green synthesis of Zinc oxide nanoparticles for enhanced adsorption of lead ions from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Hossainian, H.; Kokhdan, S.N. Comparison of activated carbon and multiwalled carbon nanotubes for efficient removal of eriochrome cyanine R (ECR): Kinetic, isotherm, and thermodynamic study of the removal process. J. Chem. Eng. Data 2011, 56, 3227–3235. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Alizadeh, A.; Malekhosseini, Z.; Ranjbar, M. Removal of bromocresol green from aqueous solution via adsorption on Ziziphus nummularia as a new, natural, and low-cost adsorbent: Kinetic and thermodynamic study of removal process. J. Chem. Eng. Data 2011, 56, 3738–3746. [Google Scholar] [CrossRef]
- Das, A.; Lao, E.A.; Gudmundsdottir, A.D. Photoenolization of O-Methylvalerophenone Ester Derivative. Photochem. Photobiol. 2016, 92, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S. Adsorption properties of tetracycline onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. PLoS ONE 2013, 8, e79254. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, H.; Arshadi, M.; Abbaspourrad, A. Magnetic Dendritic Halloysite Nanotube for Highly Selective Re-covery of Heparin Digested from Porcine Intestinal Mucosa. ACS Sustain. Chem. Eng. 2018, 6, 14561–14573. [Google Scholar] [CrossRef]
- García, N.; Benito, E.; Guzmán, J.; Tiemblo, P. Use of p-Toluenesulfonic Acid for the Controlled Grafting of Alkoxysilanes onto Silanol Containing Surfaces: Preparation of Tunable Hydrophilic, Hydrophobic, and Super-Hydrophobic Silica. J. Am. Chem. Soc. 2007, 129, 5052–5060. [Google Scholar] [CrossRef]
- Shafiee, A.; Karimi Abdolmaleki, M.; Laurencin, Y.; Vela, G.G.; Velazquez, C.A.; Irwin, D.J.G.; Davachi, S.M.; Forbes, C.B.; MacQuarrie, S. Amine-Functionalized Biochar: Highly Re-Useable and Green Alternative for Heparin Recovery from Porcine Intestinal Mucosa. Sustain. Chem. Pharm. 2023, 33, 101040. [Google Scholar] [CrossRef]
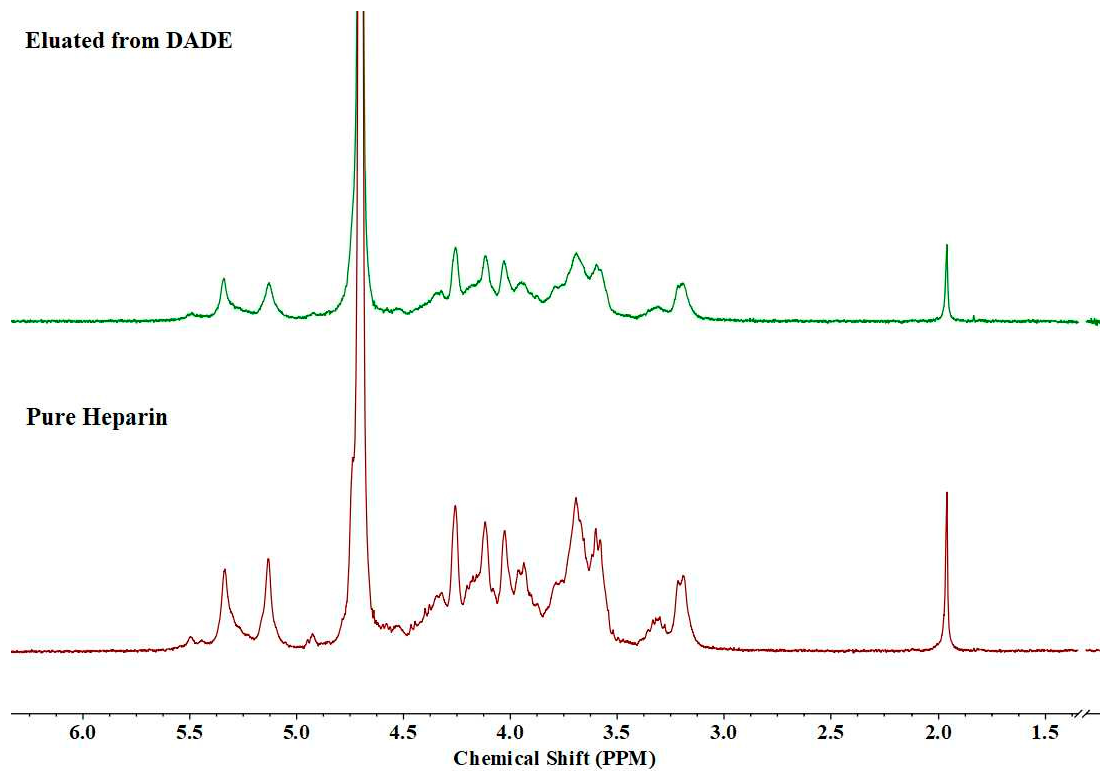

| Compound | Adsorption Efficiency (%) | Adsorption Capacity (mg·g−1) |
|---|---|---|
| DE | below 5 | below 3 |
| MADE | below 5 | below 3 |
| DADE | below 5 | below 3 |
| TADE | below 5 | below 3 |
| QMADE | 23 | 12.1 |
| QDADE | 62 | 32.6 |
| QTADE | 36 | 19 |
| ∆H° (kJ/mol.K) | ∆S° (kJ/mol) | S* | Ea (kJ/mol) | Temperature (K) | |||||||
| 298.15 | 303.15 | 318.15 | 328.15 | 333.15 | 338.15 | 343.15 | 348.15 | ||||
| 13.56 | 59.96 | 0.2275 | 3.271 | ∆G (KJ/mol) | |||||||
| −17.86 | −18.16 | −19.06 | −19.66 | −19.96 | −20.26 | −20.56 | −20.86 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Khambhati, D.P.; Longoria, N.D.; Tabibi, A.; Davachi, S.M.; Dimas, K.; Laurencin, Y.; Carmona, L.; Avalos, P.Z.; Karimi Abdolmaleki, M. Modified Diatomaceous Earth in Heparin Recovery from Porcine Intestinal Mucosa. Molecules 2023, 28, 7982. https://doi.org/10.3390/molecules28247982
Das A, Khambhati DP, Longoria ND, Tabibi A, Davachi SM, Dimas K, Laurencin Y, Carmona L, Avalos PZ, Karimi Abdolmaleki M. Modified Diatomaceous Earth in Heparin Recovery from Porcine Intestinal Mucosa. Molecules. 2023; 28(24):7982. https://doi.org/10.3390/molecules28247982
Chicago/Turabian StyleDas, Anushree, Devang P. Khambhati, Niko D. Longoria, Alireza Tabibi, Seyed Mohammad Davachi, Kayli Dimas, Yulianna Laurencin, Lesly Carmona, Pablo Zarate Avalos, and Mahmood Karimi Abdolmaleki. 2023. "Modified Diatomaceous Earth in Heparin Recovery from Porcine Intestinal Mucosa" Molecules 28, no. 24: 7982. https://doi.org/10.3390/molecules28247982
APA StyleDas, A., Khambhati, D. P., Longoria, N. D., Tabibi, A., Davachi, S. M., Dimas, K., Laurencin, Y., Carmona, L., Avalos, P. Z., & Karimi Abdolmaleki, M. (2023). Modified Diatomaceous Earth in Heparin Recovery from Porcine Intestinal Mucosa. Molecules, 28(24), 7982. https://doi.org/10.3390/molecules28247982






