Characteristics and Absorption Rate of Whey Protein Hydrolysates Prepared Using Flavourzyme after Treatment with Alcalase and Protamex
Abstract
:1. Introduction
2. Results
2.1. Molecular Weight Distribution of WPC and LMWPH
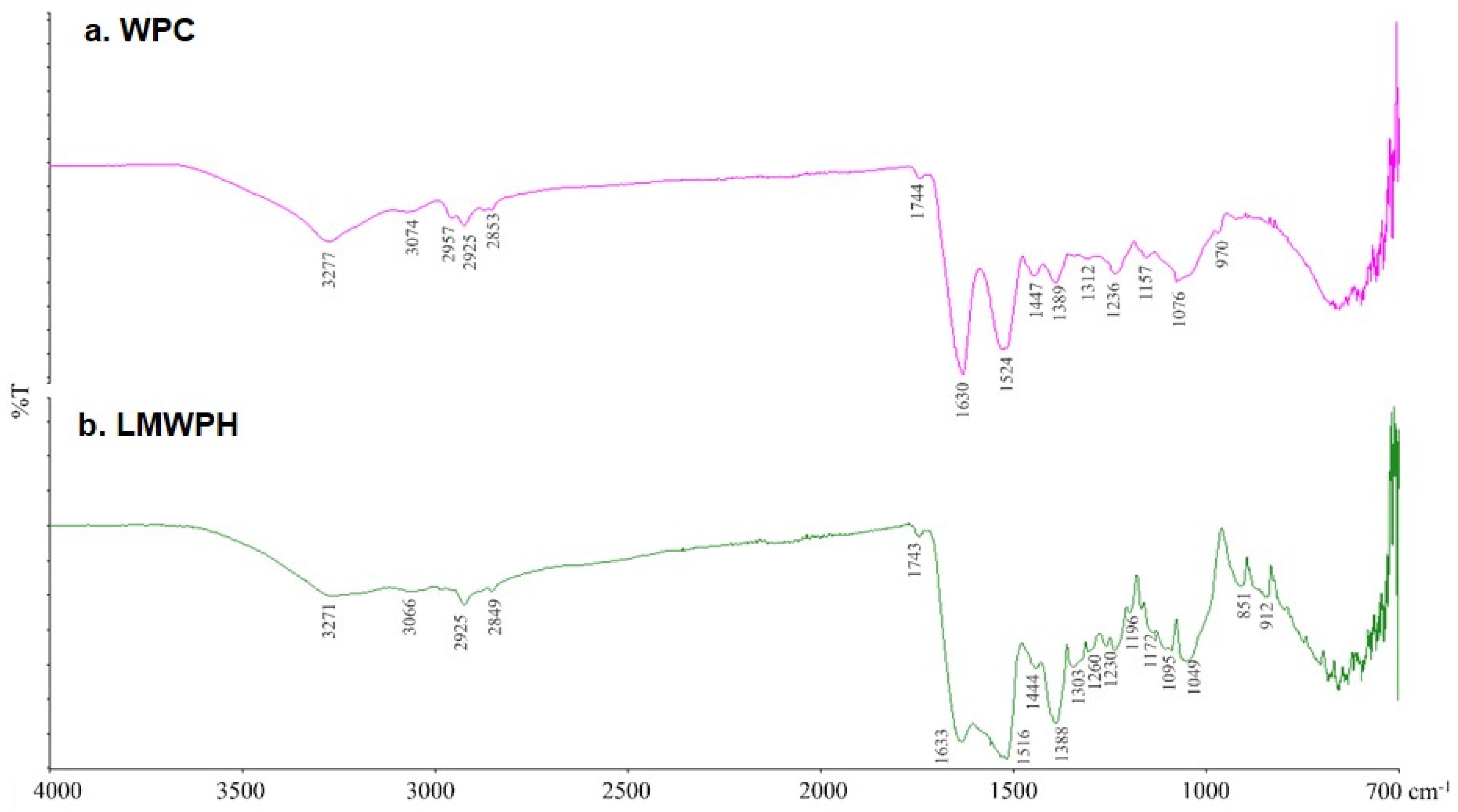
2.2. FT-IR Spectroscopy Analysis of WPC and LMWPH
2.3. Thermal Properties of WPC and LMWPH
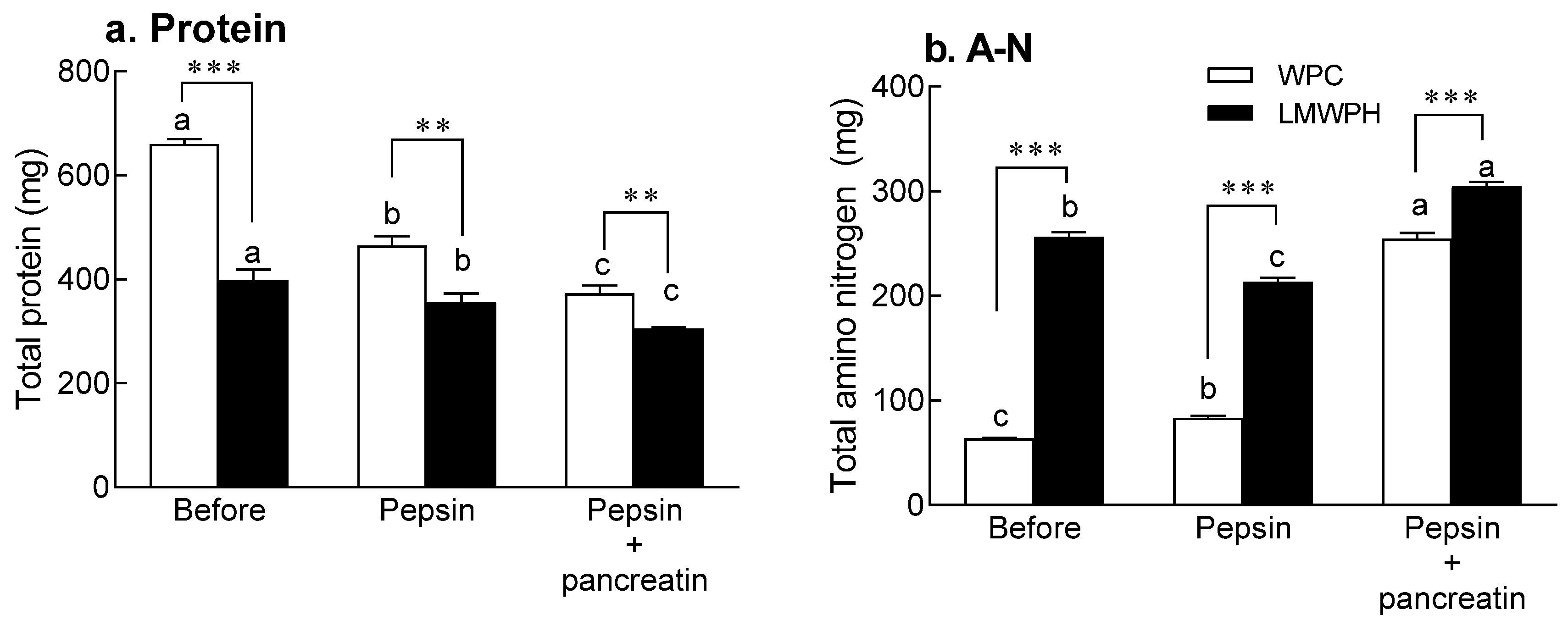
2.4. In Vitro Digestion
2.5. Analysis of Intestinal Permeability of WPC and LMWPH before and after Digestive Enzyme Treatment Using Caco-2 Cells
2.6. Intestinal Permeability of WPC and LMWPH after Digestive Enzyme Treatment Using the Intestinal Sac
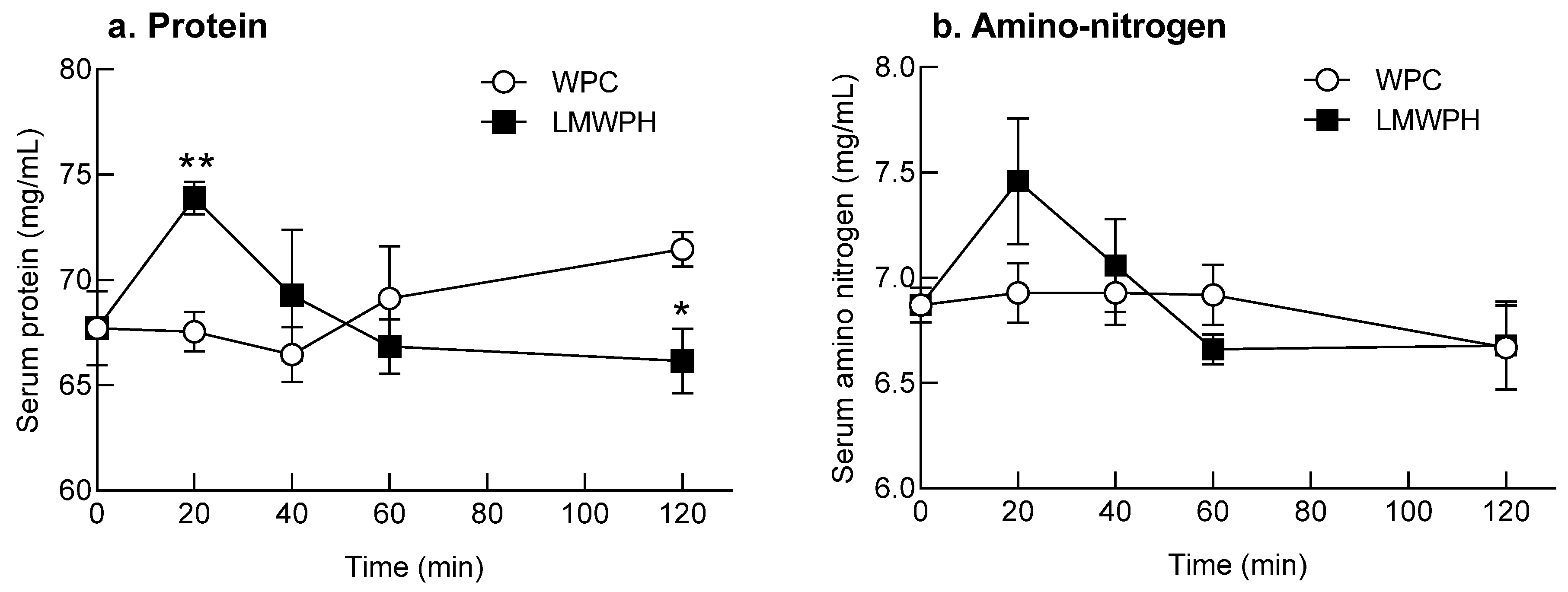
2.7. Evaluation of the Absorption Rate of the Samples after a Single High-Dose Administration to SD Rats
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Molecular Weight Distribution Analysis of WPC and LMWPH Using High-Performance Liquid Chromatography (HPLC)
4.3. DSC Analysis of WPC and LMWPH
4.4. FT-IR Spectroscopy Analysis of WPC and LMWPH
4.5. In Vitro Digestion
4.6. Intestinal Permeability Assay Using Caco-2 Cell Line
4.7. Analysis of Protein and Amino Nitrogen Content
4.8. Intestinal Permeability Analysis Using the Intestinal Sac
4.9. Evaluation of the Absorption Rate after a Single High-Dose Administration of Samples to SD Rats
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Boscaini, S.; Skuse, P.; Nilaweera, K.N.; Cryan, J.F.; Cotter, P.D. The ‘Whey’to good health: Whey protein and its beneficial effect on metabolism, gut microbiota and mental health. Trends Food Sci. Technol. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Kadian, D.; Dularia, C.; Chander, M. Potential Aspects of Whey Proteins in Dairy Products: Chemistry, Bio-Functional Characteristics, and Their Applications. In The Chemistry of Milk and Milk Products; Academic Press: Cambridge, MA, USA, 2023; pp. 251–274. [Google Scholar]
- Izquierdo, F.J.; Peñas, E.; Baeza, M.L.; Gomez, R. Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int. Dairy J. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Yigit, A.; Bielska, P.; Cais-Sokolinska, D.; Samur, G. Whey proteins as a functional food: Health effects, functional properties, and applications in food. J. Am. Nutr. Assoc. 2023, 42, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Minj, S.; Anand, S. Whey proteins and its derivatives: Bioactivity, functionality, and current applications. Dairy 2020, 1, 233–258. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Whey proteins and peptides in health-promoting functions–A review. Int. Dairy J. 2022, 126, 105269. [Google Scholar] [CrossRef]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef]
- Broer, S. Intestinal amino acid transport and metabolic health. Annu. Rev. Nutr. 2023, 43, 73–99. [Google Scholar] [CrossRef]
- Shin, J.E.; Park, S.J.; Ahn, S.I.; Choung, S.Y. Soluble Whey Protein Hydrolysate Ameliorates Muscle Atrophy Induced by Immobilization via Regulating the PI3K/Akt Pathway in C57BL/6 Mice. Nutrients 2020, 12, 3362. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: A systematic review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Fernandez-Musoles, R.; Salom, J.B.; Castelló-Ruiz, M.; del Mar Contreras, M.; Recio, I.; Manzanares, P. Bioavailability of antihypertensive lactoferricin B-derived peptides: Transepithelial transport and resistance to intestinal and plasma peptidases. Int. Dairy J. 2013, 32, 169–174. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Zhao, C.; Ashaolu, T.J. Bioactivity and safety of whey peptides. LWT 2020, 134, 109935. [Google Scholar] [CrossRef]
- Knipping, K.; Simons, P.J.; Buelens-Sleumer, L.S.; Cox, L.; den Hartog, M.; de Jong, N.; Teshima, R.; Garssen, J.; Boon, L.; Knippels, L.M. Development of β-lactoglobulin-specific chimeric human IgEκ monoclonal antibodies for in vitro safety assessment of whey hydrolysates. PLoS ONE 2014, 9, e106025. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Berliner, L.J. Alpha-lactalbumine: Structure and function. FEBS Lett. 2000, 473, 269–274. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Streicher, C.; Anema, S.G. The effect of thiol reagents on the denaturation of the whey protein in milk and whey protein concentrate solutions. Int. Dairy J. 2018, 85, 285–293. [Google Scholar] [CrossRef]
- Ha, W.-K.; Lee, J.; Kim, K.-E. Development and properties of hypoallergenic infant formula. Allergy Asthma Respir. Dis. 2017, 5, 63–72. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta BBA-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef]
- Fan, H.; Jiang, X.; Zhang, T.; Jin, Q. Peptide-induced fluorescence quenching of conjugated polyelectrolyte for label-free, ultrasensitive and selective assay of protease activity. Biosens. Bioelectron. 2012, 34, 221–226. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Creamer, L.K.; Parry, D.A.; Malcolm, G.N. Secondary structure of bovine beta-lactoglobulin B. Arch. Biochem. Biophys. 1983, 227, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Sun, Y.; Zhou, Z.; Cheng, J.; Guo, M. Effects of enzymatic hydrolysis on physicochemical properties and solubility and bitterness of milk protein hydrolysates. Foods 2021, 10, 2462. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.A.; Cornec, M.; Narsimhan, G. Effect of thermal treatment on interfacial properties of beta-lactoglobulin. J. Colloid. Interface Sci. 2005, 285, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Gruppi, A.; Dermiki, M.; Spigno, G.; FitzGerald, R.J. Impact of Enzymatic Hydrolysis and Heat Inactivation on the Physicochemical Properties of Milk Protein Hydrolysates. Foods 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, D.; Taylor, M.; Singh, H. Effect of preheating and other process parameters on whey protein reactions during skim milk powder manufacture. Int. Dairy J. 2005, 15, 501–511. [Google Scholar] [CrossRef]
- Ludescher, R.D. Physical and chemical properties of amino acids and proteins. In Food Proteins: Properties and Characterization; VCH Publishers: Hoboken, NJ, USA, 1996; pp. 23–70. [Google Scholar]
- Sandberg, A.S. Methods and options in vitro dialyzability; benefits and limitations. Int. J. Vitam. Nutr. Res. 2005, 75, 395–404. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, Y.S.; Shadchehr, A.; Garrido, A.; Macgregor, I.L.; Sleisenger, M.H. Protein digestion and absorption in human small intestine. Gastroenterology 1979, 76, 1415–1421. [Google Scholar] [CrossRef]
- Dalgalarrondo, M.; Dufour, E.; Chobert, J.-M.; Bertrand-Harb, C.; Haertlé, T. Proteolysis of β-lactoglobulin and β-casein by pepsin in ethanolic media. Int. Dairy J. 1995, 5, 1–14. [Google Scholar] [CrossRef]
- Stapelfeldt, H.; Petersen, P.H.; Kristiansen, K.R.; Qvist, K.B.; Skibsted, L.H. Effect of high hydrostatic pressure on the enzymic hydrolysis of beta-lactoglobulin B by trypsin, thermolysin and pepsin. J. Dairy Res. 1996, 63, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine alpha-lactalbumin molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kontopidis, G.; Holt, C.; Sawyer, L. Invited review: Beta-lactoglobulin: Binding properties, structure, and function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, T.; Liang, X.; Yuan, Q.; Zeng, X.; Wu, Z.; Pan, D.; Tao, M.; Guo, Y. Production and transepithelial transportation of casein-derived peptides and identification a novel antioxidant peptide LHSMK. LWT 2021, 151, 112194. [Google Scholar] [CrossRef]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Rahbek, S.K.; Storm, A.C.; Klitgaard, S.; Jorgensen, H.; Bibby, B.M.; Serena, A.; Vissing, K. Effect of degree of hydrolysis of whey protein on in vivo plasma amino acid appearance in humans. Springerplus 2016, 5, 382. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-A.; Kim, I.-Y. Review on Exercise Training and Protein Intake in Skeletal Muscle Protein Metabolism. Exerc. Sci. 2017, 26, 103–114. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Børsheim, E.; Wolfe, R.R. Potential ergogenic effects of arginine and creatine supplementation. J. Nutr. 2004, 134, 2888S–2894S. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, S.; Lee, H.J.; Suh, H.J.; Jo, K. Stimulating effect of whey protein hydrolysate on bone growth in MC3T3-E1 cells and a rat model. Food Funct. 2021, 12, 5109–5117. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physico-chemical and functional properties of flours from Indian kidney bean (Phaseolus vulgaris L.) cultivars. LWT 2013, 53, 278–284. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Dore, P.; Nucara, A. Application of Fourier transform infrared spectroscopy to legume seed flour analysis. Food Chem. 2008, 108, 361–368. [Google Scholar] [CrossRef]
- Zaguri, M.; Kandel, S.; Rinehart, S.A.; Torsekar, V.R.; Hawlena, D. Protein quantification in ecological studies: A literature review and empirical comparisons of standard methodologies. Methods Ecol. Evol. 2021, 12, 1240–1251. [Google Scholar] [CrossRef]
- Singh, T.P.; Siddiqi, R.A.; Sogi, D.S. Statistical optimization of enzymatic hydrolysis of rice bran protein concentrate for enhanced hydrolysate production by papain. LWT 2019, 99, 77–83. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Experimental evidence for in vitro fluid transport in the presence of a traditional medicinal fruit extract across rat everted intestinal sacs. Fundam. Clin. Pharmacol. 2005, 19, 87–92. [Google Scholar] [CrossRef]



| Sample | α-Helix | β-Sheet | β-Turn | Random Coil |
|---|---|---|---|---|
| WPC | 23.83 ± 7.60 | 36.41 ± 7.86 | 26.58 ± 8.08 | 13.18 ± 7.98 |
| LMWPH | 35.61 ± 10.74 | 28.20 ± 9.79 | 24.90 ± 10.37 | 11.29 ± 10.88 |
| Sample | Peak 1 | Peak 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ts1 (°C) | Tmax1 (°C) | Te1 (°C) | ∆H1 (J/g) | Ts2 (°C) | Tmax2 (°C) | Te2 (°C) | ∆H2 (J/g) | |
| WPC | 41.73 ± 1.24 | 53.23 ± 1.32 | 65.49 ± 2.04 | 1.555 ± 0.07 | 76.18 ± 2.04 | 120.81 ± 3.07 | 164.07 ± 2.74 | 129.3 ± 5.64 |
| LMWPH | 56.96 ± 0.66 | 94.99 ± 2.48 | 127.8 ± 2.17 | 114.1 ± 5.91 | 143.49 ± 3.97 | 164.14 ± 4.11 | 178.85 ± 3.21 | 7.375 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.B.; Kim, H.; Lee, S.K.; Kim, H.-J.; Jeong, A.-H.; Suh, H.J.; Ahn, Y. Characteristics and Absorption Rate of Whey Protein Hydrolysates Prepared Using Flavourzyme after Treatment with Alcalase and Protamex. Molecules 2023, 28, 7969. https://doi.org/10.3390/molecules28247969
Chang YB, Kim H, Lee SK, Kim H-J, Jeong A-H, Suh HJ, Ahn Y. Characteristics and Absorption Rate of Whey Protein Hydrolysates Prepared Using Flavourzyme after Treatment with Alcalase and Protamex. Molecules. 2023; 28(24):7969. https://doi.org/10.3390/molecules28247969
Chicago/Turabian StyleChang, Yeok Boo, Hyeongyeong Kim, Se Kyung Lee, Hye-Jin Kim, A-Hyun Jeong, Hyung Joo Suh, and Yejin Ahn. 2023. "Characteristics and Absorption Rate of Whey Protein Hydrolysates Prepared Using Flavourzyme after Treatment with Alcalase and Protamex" Molecules 28, no. 24: 7969. https://doi.org/10.3390/molecules28247969
APA StyleChang, Y. B., Kim, H., Lee, S. K., Kim, H.-J., Jeong, A.-H., Suh, H. J., & Ahn, Y. (2023). Characteristics and Absorption Rate of Whey Protein Hydrolysates Prepared Using Flavourzyme after Treatment with Alcalase and Protamex. Molecules, 28(24), 7969. https://doi.org/10.3390/molecules28247969







