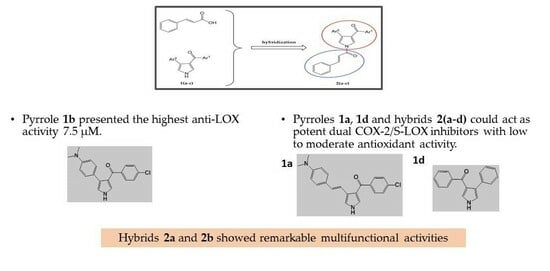
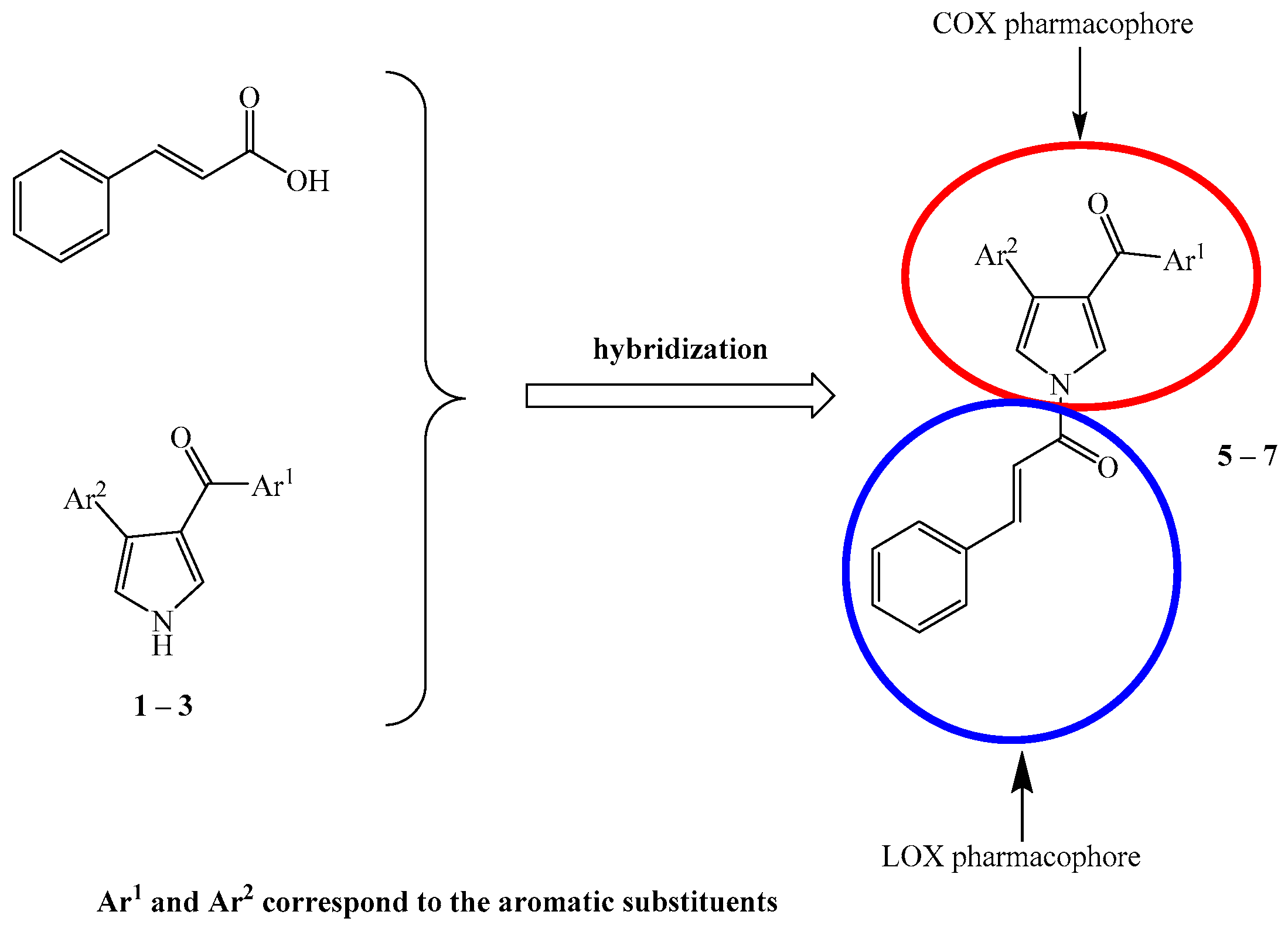
Development of Novel Pyrrole Derivatives and Their Cinnamic Hybrids as Dual COX-2/LOX Inhibitors
Abstract
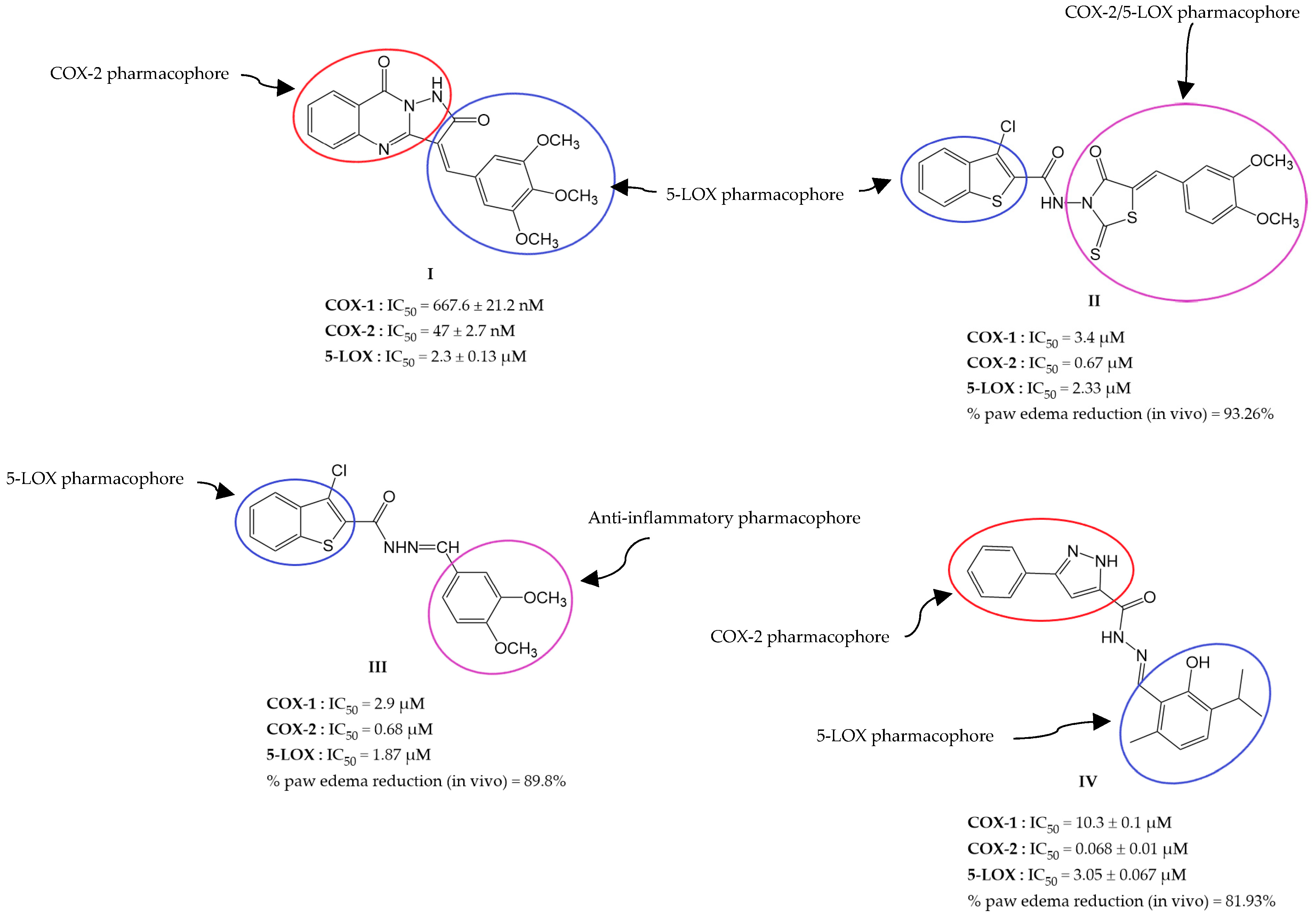
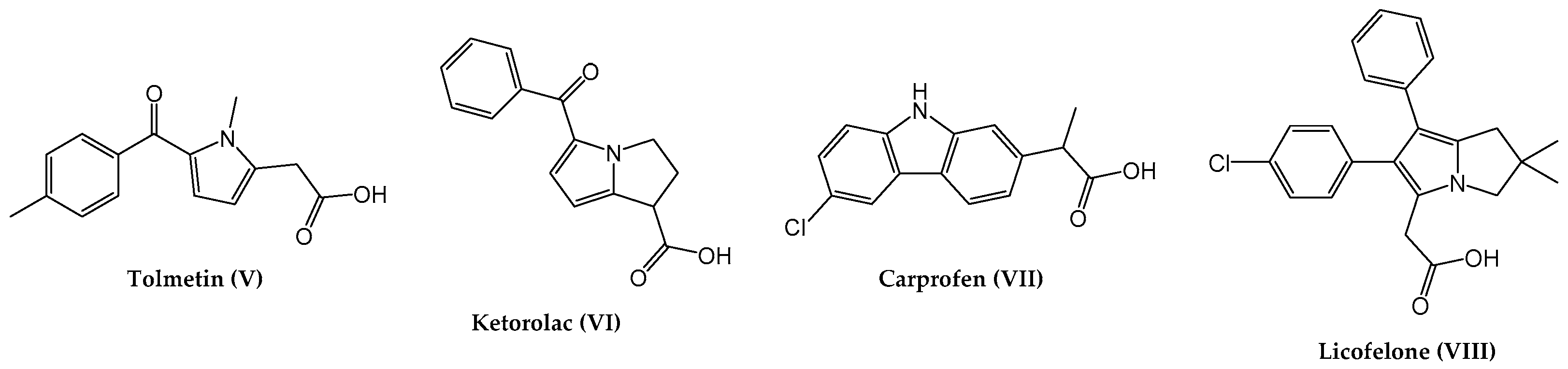
:1. Introduction
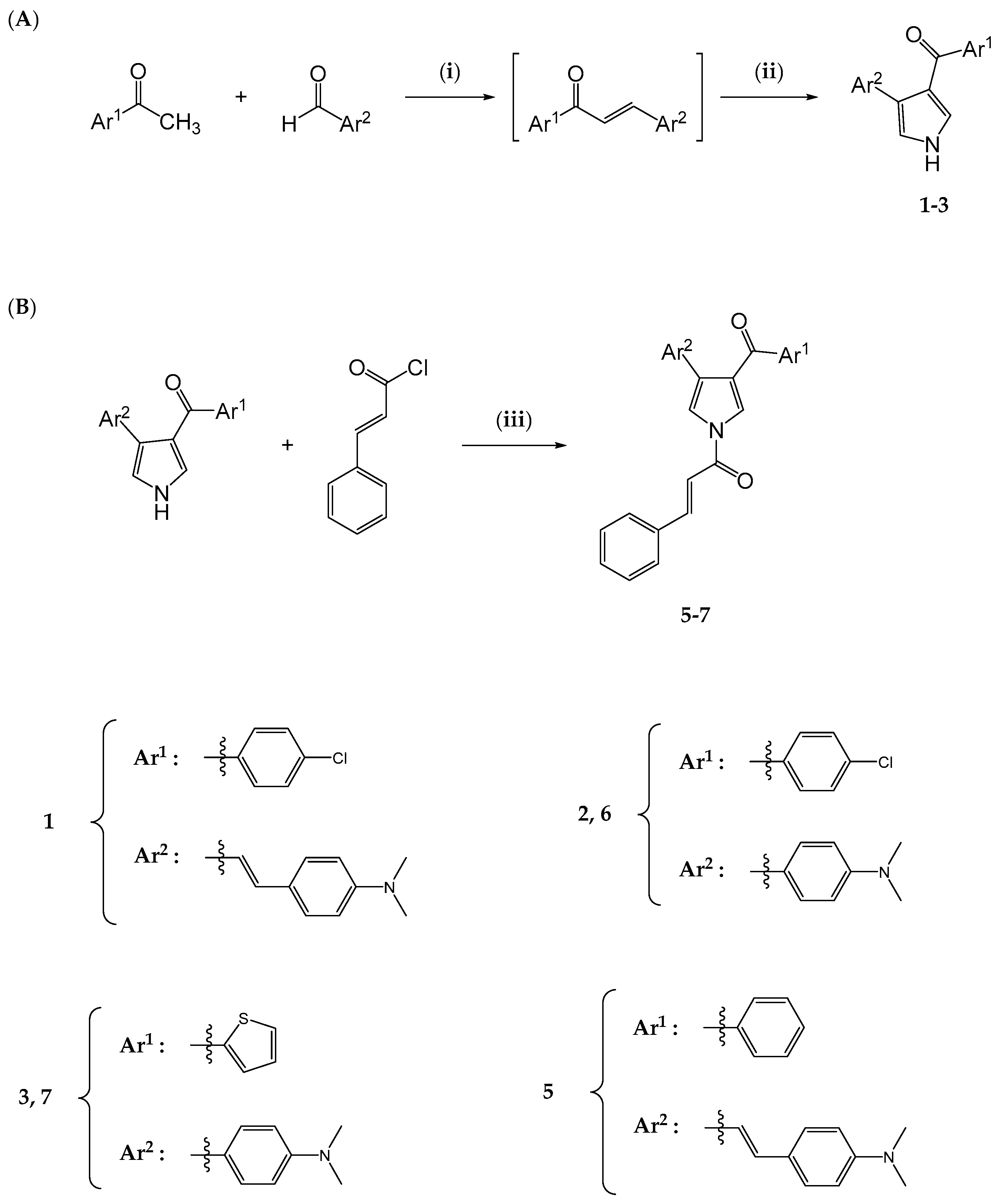
2. Results and Discussion
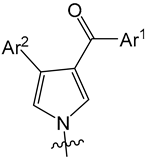
2.1. Chemistry
2.2. Physicochemical Studies
2.2.1. Experimental Determination of Lipophilicity as RM Values
2.2.2. In Silico Determination of ADMET Properties and Drug-Likeness
2.3. Biological Evaluation
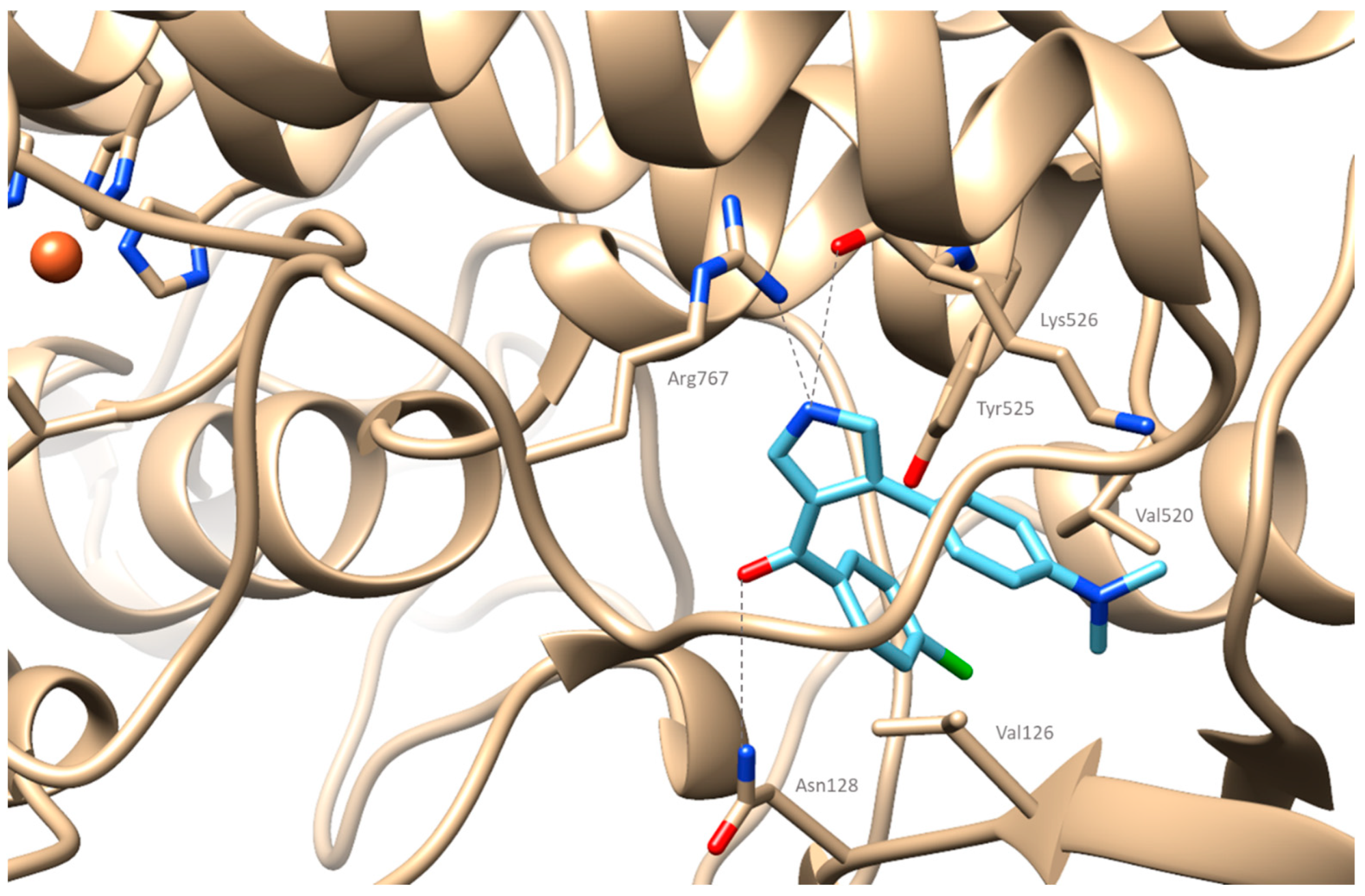
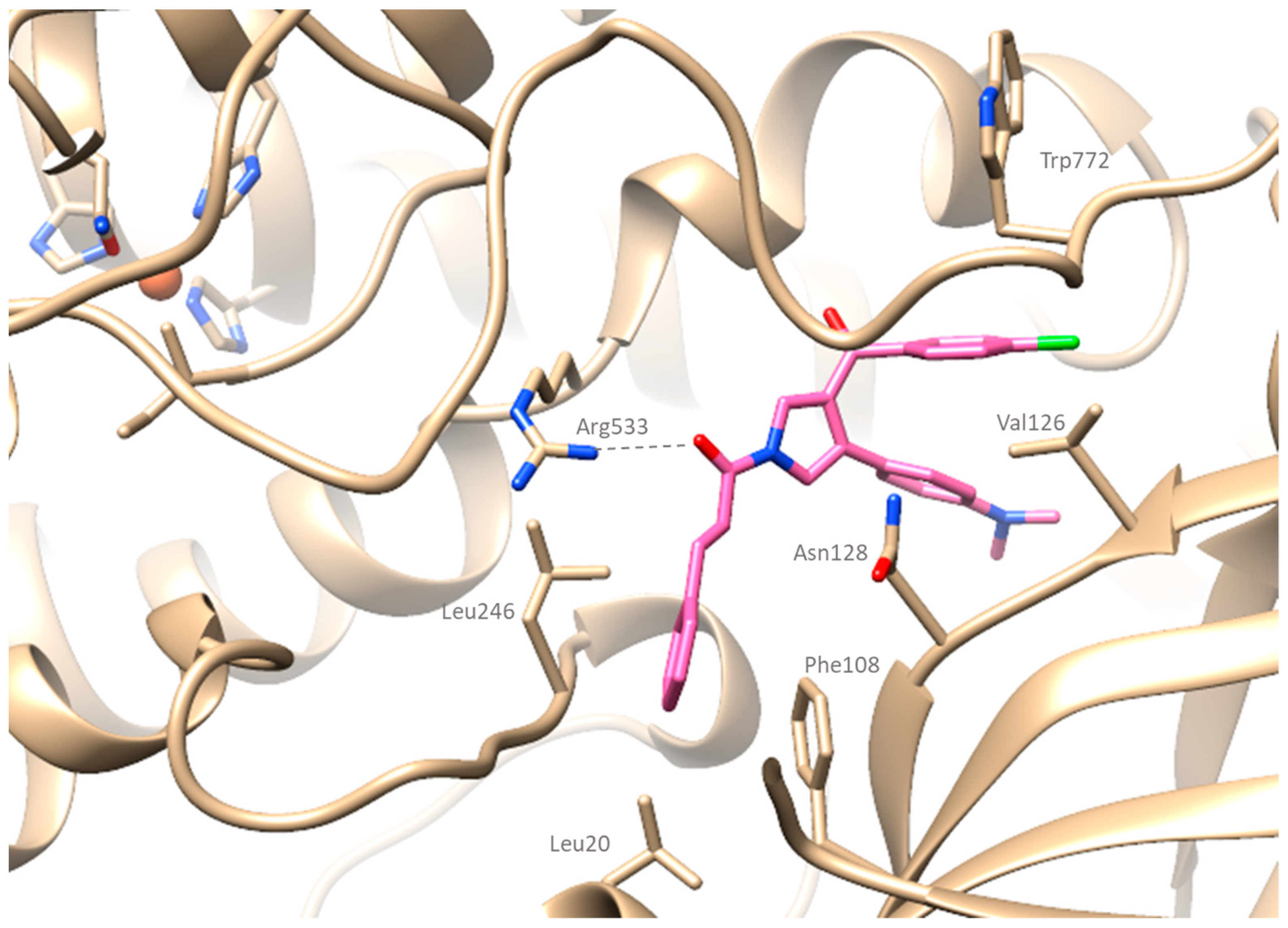
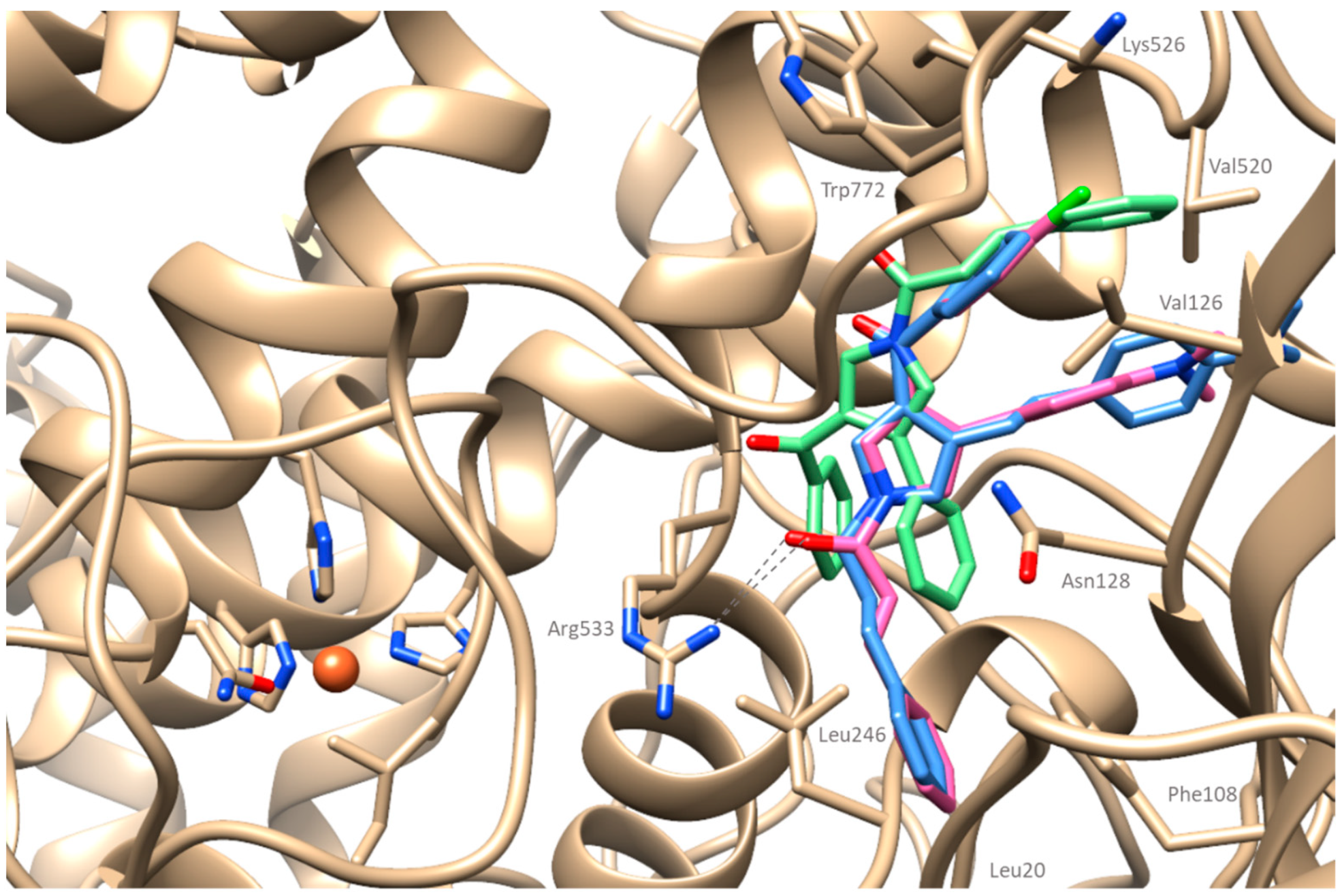
2.4. Computational Studies
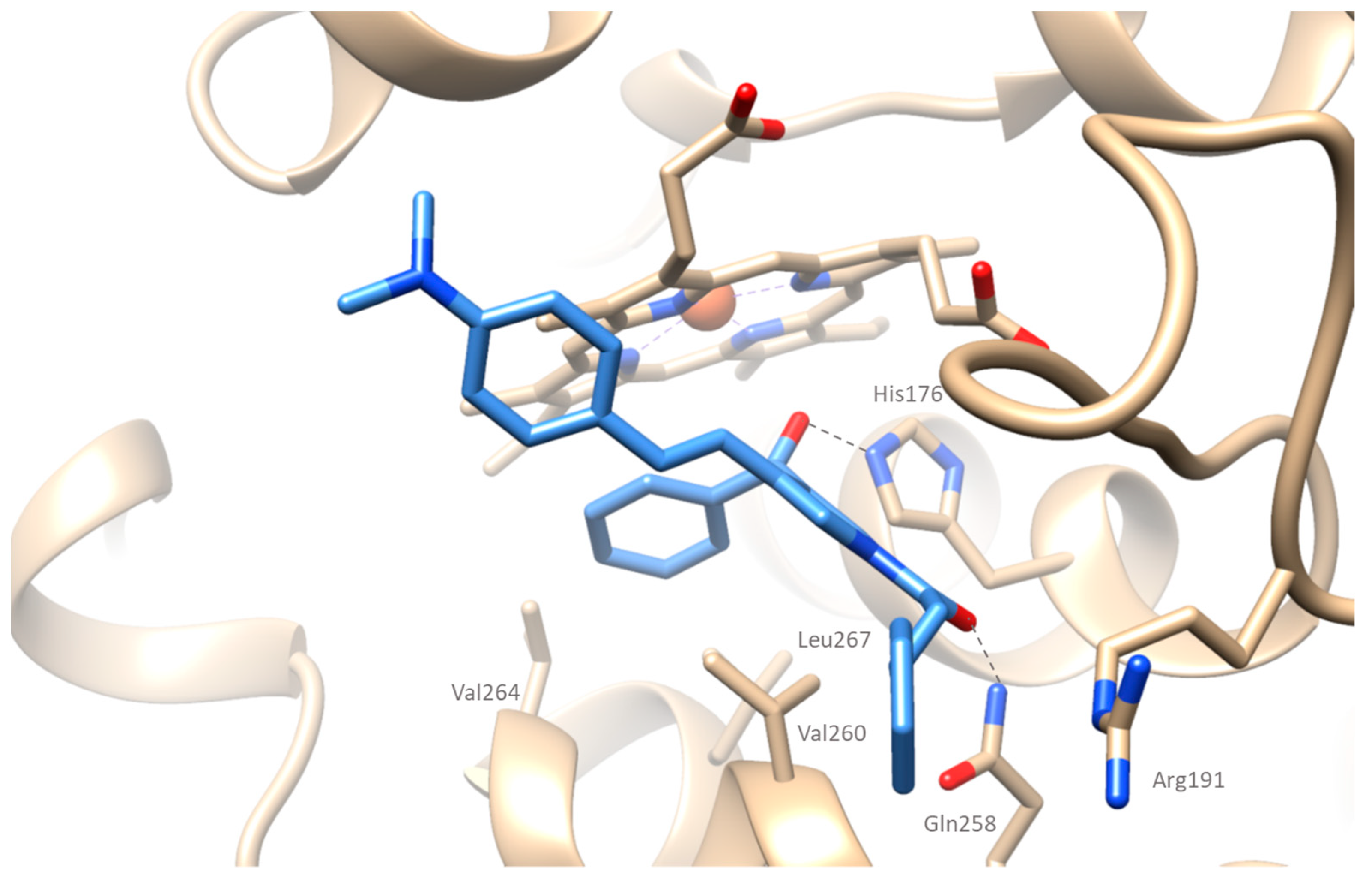
2.4.1. Docking Simulations on Soybean Lipoxygenase
2.4.2. Docking Simulations on Cyclooxygenase-2
3. Materials and Methods
3.1. General Information
3.2. Chemistry General Procedures
3.2.1. General Procedure for the Synthesis of Pyrroles
3.2.2. General Procedure for the Synthesis of Pyrrole–Cinnamate Hybrids
3.3. Physicochemical Studies
3.3.1. Experimental Determination of Lipophilicity as RM Values
3.3.2. In Silico Determination of Lipophilicity as ClogP and miLogP Values
3.3.3. In Silico Determination of ADMET Properties and Drug-Likeness
3.4. Biological In Vitro Assays
3.4.1. Inhibition of Linoleic Acid Lipid Peroxidation
3.4.2. Inhibition of Soybean Lipoxygenase In Vitro
3.4.3. Inhibition of Ovine Cyclooxygenase-2 (COX-2)
3.5. Computational Studies
3.5.1. Molecular Docking Studies on Soybean Lipoxygenase
3.5.2. Molecular Docking Studies on Cyclooxygenase-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Hsieh, L.-F. 31-Rheumatologic Rehabilitation. In Braddom’s Rehabilitation Care: A Clinical Handbook; Cifu, D.X., Lew, H.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 208–213.e3. ISBN 978-0-323-47904-2. [Google Scholar]
- Han, X.; Krempski, J.W.; Nadeau, K. Advances and Novel Developments in Mechanisms of Allergic Inflammation. Allergy 2020, 75, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, K.; Dong, F.; Qumu, S.; Zhao, Q.; Niu, H.; Ren, X.; Gu, X.; Yu, T.; Pan, L.; et al. The Heterogeneity of Inflammatory Response and Emphysema in Chronic Obstructive Pulmonary Disease. Front. Physiol. 2021, 12, 783396. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Wolfe, M.M.; Lichtenstein, D.R.; Singh, G. Gastrointestinal Toxicity of Nonsteroidal Antiinflammatory Drugs. N. Engl. J. Med. 1999, 340, 1888–1899. [Google Scholar] [CrossRef]
- Varga, Z.; rafay ali Sabzwari, S.; Vargova, V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus 2017, 9, e1144. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Cairns, J.A. The Coxibs and Traditional Nonsteroidal Anti-Inflammatory Drugs: A Current Perspective on Cardiovascular Risks. Can. J. Cardiol. 2007, 23, 125–131. [Google Scholar] [CrossRef]
- Qandeel, N.A.; El-Damasy, A.K.; Sharawy, M.H.; Bayomi, S.M.; El-Gohary, N.S. Synthesis, in Vivo Anti-Inflammatory, COX-1/COX-2 and 5-LOX Inhibitory Activities of New 2,3,4-Trisubstituted Thiophene Derivatives. Bioorg. Chem. 2020, 102, 103890. [Google Scholar] [CrossRef]
- Jaismy, J.P.; Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer Anti-Inflammatory Therapy through Dual COX-2/5-LOX Inhibitors: A Structure-Based Approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Shaaban, M.A.; Kamal, A.M.; Faggal, S.I.; Farag, N.A.; Aborehab, N.M.; Elsahar, A.E.; Mohamed, K.O. Design, Synthesis, and Biological Evaluation of New Pyrazoloquinazoline Derivatives as Dual COX-2/5-LOX Inhibitors. Arch. Pharm. (Weinheim) 2020, 353, 2000027. [Google Scholar] [CrossRef]
- El-Miligy, M.M.M.; Hazzaa, A.A.; El-Messmary, H.; Nassra, R.A.; El-Hawash, S.A.M. New Hybrid Molecules Combining Benzothiophene or Benzofuran with Rhodanine as Dual COX-1/2 and 5-LOX Inhibitors: Synthesis, Biological Evaluation and Docking Study. Bioorg. Chem. 2017, 72, 102–115. [Google Scholar] [CrossRef] [PubMed]
- El-Miligy, M.M.; Hazzaa, A.A.; El-Messmary, H.; Nassra, R.A.; El-Hawash, S.A. New Benzothiophene Derivatives as Dual COX-1/2 and 5-LOX Inhibitors: Synthesis, Biological Evaluation and Docking Study. Future Med. Chem. 2017, 9, 443–468. [Google Scholar] [CrossRef] [PubMed]
- El-Miligy, M.M.M.; Al-Kubeisi, A.K.; Bekhit, M.G.; El-Zemity, S.R.; Nassra, R.A.; Hazzaa, A.A. Towards Safer Anti-Inflammatory Therapy: Synthesis of New Thymol–Pyrazole Hybrids as Dual COX-2/5-LOX Inhibitors. J. Enzyme Inhib. Med. Chem. 2023, 38, 294–308. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter Two-Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H. Tolmetin. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Mahmoodi, A.N.; Kim, P.Y. Ketorolac. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fox, S.M.; Johnston, S.A. Use of Carprofen for the Treatment of Pain and Inflammation in Dogs. J. Am. Vet. Med. Assoc. 1997, 210, 1493–1498. [Google Scholar]
- Kumar, V. Licofelone in Osteoarthritis: Is This the Awaited Drug? A Systematic Review. Int. J. Basic Clin. Pharmacol. 2021, 10, 564–571. [Google Scholar] [CrossRef]
- Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. The Pyrrole Moiety as a Template for COX-1/COX-2 Inhibitors. Eur. J. Med. Chem. 2000, 35, 499–510. [Google Scholar] [CrossRef]
- Konstantinidou, M.; Gkermani, A.; Hadjipavlou-Litina, D. Synthesis and Pharmacochemistry of New Pleiotropic Pyrrolyl Derivatives. Molecules 2015, 20, 16354–16374. [Google Scholar] [CrossRef]
- Gislason, N.E.; Currie, B.L.; Waterhouse, A.L. Novel Antioxidant Reactions of Cinnamates in Wine. J. Agric. Food Chem. 2011, 59, 6221–6226. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, O.H.; Owere, P.O.; Anthony, P.C. Acetylation of Cinnamic Acid and Evaluation of Antioxidant Activity of the Resultant Derivative. Biomed. J. Sci. Tech. Res. 2021, 39, 31085–31088. [Google Scholar] [CrossRef]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef] [PubMed]
- Noti, V.; Hadjipavlou-Litina, D. (E)-1-(3-Benzoyl-4-Phenyl-1H-Pyrrol-1-Yl)-3-Phenylprop-2-En-1-One. Molbank 2022, 2022, M1314. [Google Scholar] [CrossRef]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef]
- Martins, T.; Fonseca, B.M.; Rebelo, I. Antioxidant Effects of Chalcones during the Inflammatory Response: An Overall Review. Curr. Med. Chem. 2021, 28, 7658–7713. [Google Scholar] [CrossRef]
- ur Rashid, H.; Xu, Y.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising Anti-Inflammatory Effects of Chalcones via Inhibition of Cyclooxygenase, Prostaglandin E2, Inducible NO Synthase and Nuclear Factor Κb Activities. Bioorg. Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef]
- Tekale, S.; Mashele, S.; Pooe, O.; Thore, S.; Kendrekar, P.; Pawar, R.; Tekale, S.; Mashele, S.; Pooe, O.; Thore, S.; et al. Biological Role of Chalcones in Medicinal Chemistry; IntechOpen: London, UK, 2020; ISBN 978-1-83880-022-2. [Google Scholar]
- Arnott, J.A.; Planey, S.L. The Influence of Lipophilicity in Drug Discovery and Design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the Links between in Vitro Potency, ADMET and Physicochemical Parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- C-QSAR and Clog P. Biobyte Corp. 201 W. Fourth Str. Suite 204 Claremont, CA 91711, USA. Available online: https://www.biobyte.com (accessed on 7 September 2023).
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com/ (accessed on 2 May 2023).
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Maunz, A.; Gütlein, M.; Rautenberg, M.; Vorgrimmler, D.; Gebele, D.; Helma, C. Lazar: A Modular Predictive Toxicology Framework. Front. Pharmacol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- PreADMET|Prediction of ADME/Tox. Available online: https://preadmet.webservice.bmdrc.org/ (accessed on 2 May 2023).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Teague, S.J.; Davis, A.M.; Leeson, P.D.; Oprea, T. The Design of Leadlike Combinatorial Libraries. Angew. Chem. Int. Ed. 1999, 38, 3743–3748. [Google Scholar] [CrossRef]
- Oprea, T.I. Property Distribution of Drug-Related Chemical Databases*. J. Comput. Aided Mol. Des. 2000, 14, 251–264. [Google Scholar] [CrossRef]
- Brown, R.D.; Hassan, M.; Waldman, M. Tools for Designing Diverse, Druglike, Cost-Effective Combinatorial Libraries. In Combinatorial Library Design and Evaluation; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-0-429-18097-2. [Google Scholar]
- Clark, D.E. Rapid Calculation of Polar Molecular Surface Area and Its Application to the Prediction of Transport Phenomena. 2. Prediction of Blood–Brain Barrier Penetration. J. Pharm. Sci. 1999, 88, 815–821. [Google Scholar] [CrossRef]
- Ahmed Juvale, I.I.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-Glycoprotein: New Insights into Structure, Physiological Function, Regulation and Alterations in Disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Review in Quantitative Structure Activity Relationships on Lipoxygenase Inhibitors. Mini Rev. Med. Chem. 2003, 3, 487–499. [Google Scholar] [CrossRef]
- Fotopoulos, I.; Pontiki, E.; Litina, D.H. Targeting Inflammation with Conjugated Cinnamic Amides, Ethers and Esters. Lett. Drug Des. Discov. 2020, 17, 3–11. [Google Scholar] [CrossRef]
- Yadav, U.C.S. Oxidative Stress-Induced Lipid Peroxidation: Role in Inflammation. In Free Radicals in Human Health and Disease; Rani, V., Yadav, U.C.S., Eds.; Springer India: New Delhi, India, 2015; pp. 119–129. ISBN 978-81-322-2035-0. [Google Scholar]
- Niki, E. Free Radical Initiators as Source of Water- or Lipid-Soluble Peroxyl Radicals. In Methods in Enzymology; Oxygen Radicals in Biological Systems Part B: Oxygen Radicals and Antioxidants; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 100–108. [Google Scholar]
- Petrovic, N.; Murray, M. Using N,N,N′,N′-Tetramethyl-p-Phenylenediamine (TMPD) to Assay Cyclooxygenase Activity In Vitro. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 129–140. ISBN 978-1-60761-411-1. [Google Scholar]
- Kasthuri, J.K.; Singh Jadav, S.; Thripuram, V.D.; Gundabolu, U.R.; Ala, V.b.; Kolla, J.N.; Jayaprakash, V.; Ahsan, M.J.; Bollikolla, H.B. Synthesis, Characterization, Docking and Study of Inhibitory Action of Some Novel C-Alkylated Chalcones on 5-LOX Enzyme. Chem. Select 2017, 2, 8771–8778. [Google Scholar] [CrossRef]
- Mavridis, E.; Bermperoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D. 5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules. Molecules 2020, 25, 3173. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Polyzos, N.-I.; Karadendrou, M.-A.; Kritsi, E.; Pontiki, E.; Liargkova, T.; Hadjipavlou-Litina, D.; Zoumpoulakis, P.; Detsi, A. Exploring the 2′-Hydroxy-Chalcone Framework for the Development of Dual Antioxidant and Soybean Lipoxygenase Inhibitory Agents. Molecules 2021, 26, 2777. [Google Scholar] [CrossRef] [PubMed]
- Rekker, R.F. The Hydrophobic Fragmental Constant, Its Derivation and Application: A Means of Characterizing Membrane Systems; Pharmacochemistry Library; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands; New York, NY, USA, 1977; ISBN 978-0-444-41548-6. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. In Methods in Enzymology; Macromolecular Crystallography, Part D; Academic Press: Cambridge, MA, USA, 2003; Volume 374, pp. 461–491. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck Molecular Force Field. I. Basis, Form, Scope, Parameterization, and Performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE-AnteChamber PYthon Parser InterfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber Ff99SB Protein Force Field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [CrossRef] [PubMed]
| No. | Compounds | RM | ClogP | miLogP |
|---|---|---|---|---|
| 1 | 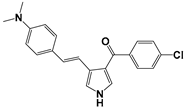 | 0.3076 | 5.7 | 5.32 |
| 2 | 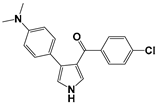 | 0.0116 | 5.05 | 4.74 |
| 3 | 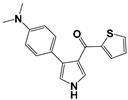 | −0.3340 | 3.98 | 3.96 |
| 4 [31] | 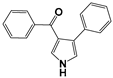 | −0.4102 | 4.08 | 3.96 |
| 5 | 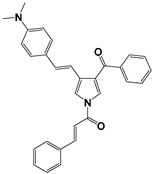 | −0.4174 | 6.68 | 6.94 |
| 6 | 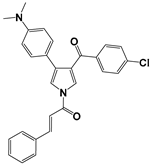 | −0.4771 | 6.87 | 7.04 |
| 7 | 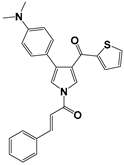 | 0.6488 | 5.81 | 6.26 |
| 8 [31] | 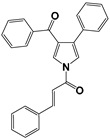 | −0.4771 | 5.85 | 6.26 |
 | ||||||
|---|---|---|---|---|---|---|
| No. | ILP % at 100 μM | LOX Inh. % at 100 μM or IC50 (μM) | COX-2 Inh. % at 100 μM or IC50 (μM) | MV | MR (Ar1) | MR (Ar2) |
| 1 | 33.8 ± 1.3 | 51.5 ± 1.7 μM | 10 ± 0.8 μM | 317.69 | 3.077 | 4.860 |
| 2 | 32.8 ± 0.8 | 7.5 ± 0.5 μM | 17.5% ± 1.1 | 290.27 | 3.077 | N/A |
| 3 | no | 43.5% ± 1.3 | 12.5% ± 0.9 | 267.45 | 2.395 | Ν/A |
| 4 [31] | 44.3 ± 1.1 | 100 ± 3.3 μM | 0.65 ± 0.1 μM | 230.83 | 2.586 | 2.586 |
| 5 | 36.4 ± 0.4 | 30 ± 1.6 μM | 0.55 ± 0.3 μM | 422.34 | 2.586 | 4.860 |
| 6 | 61.6 ± 2.3 | 27.5 ± 1.3 μM | 7.0 ± 0.6 μM | 408.46 | 3.077 | N/A |
| 7 | 31.1 ± 1.1 | 35 ± 1.6 μM | 7.2 ± 0.3 μM | 385.64 | 2.395 | N/A |
| 8 [31] | 58.1 ± 1.8 | 39 ± 2.3 μM | 6 ± 0.1 μM | 349.02 | 2.586 | 2.586 |
| 9 [27] | no | no | 47% ± 0.7 | 304.15 | 2.586 | 4.860 |
| Cinnamic acid [30] | 78 ± 3.2 | 56 ± 1.8 μM | - | - | - | - |
| Trolox | 93 ± 2.5 | - | - | - | - | - |
| NDGA | - | 0.45 ± 0.2μM | - | - | - | - |
| Indomethacin | - | - | 2.12 ± 0.6 μM | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noti, V.; Pontiki, E.; Hadjipavlou-Litina, D. Development of Novel Pyrrole Derivatives and Their Cinnamic Hybrids as Dual COX-2/LOX Inhibitors. Molecules 2023, 28, 7958. https://doi.org/10.3390/molecules28247958
Noti V, Pontiki E, Hadjipavlou-Litina D. Development of Novel Pyrrole Derivatives and Their Cinnamic Hybrids as Dual COX-2/LOX Inhibitors. Molecules. 2023; 28(24):7958. https://doi.org/10.3390/molecules28247958
Chicago/Turabian StyleNoti, Viola, Eleni Pontiki, and Dimitra Hadjipavlou-Litina. 2023. "Development of Novel Pyrrole Derivatives and Their Cinnamic Hybrids as Dual COX-2/LOX Inhibitors" Molecules 28, no. 24: 7958. https://doi.org/10.3390/molecules28247958
APA StyleNoti, V., Pontiki, E., & Hadjipavlou-Litina, D. (2023). Development of Novel Pyrrole Derivatives and Their Cinnamic Hybrids as Dual COX-2/LOX Inhibitors. Molecules, 28(24), 7958. https://doi.org/10.3390/molecules28247958