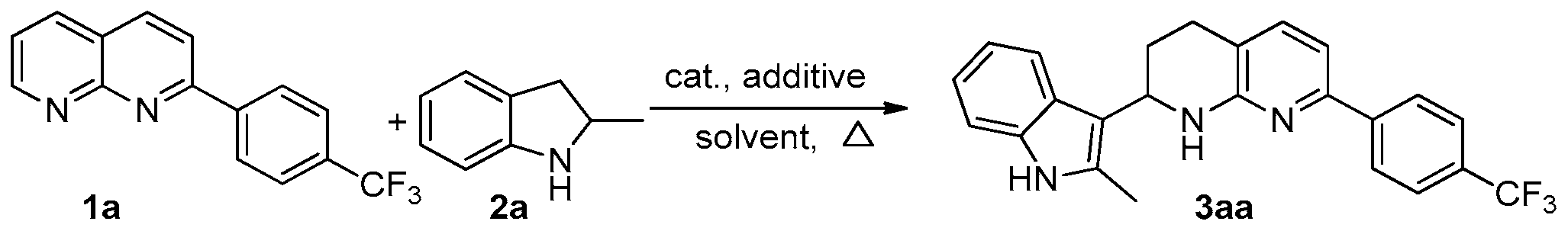
Abstract
An iridium-catalysed hydrogen transfer strategy, enabling straightforward access to tetrahydro pyridine derivatives from aryl-1,8-naphthyridines and indolines, was developed. This method proceeds with unprecedented synthetic effectiveness including high step-economic fashion together with the advantages of having no by-product and no need for external high-pressure H2 gas, offering an important basis for the transformation of 1,8-naphthyridines and indolines into functionalized products.
1. Introduction
Coupling of two components by transfer hydrogenation is an attractive but challenging task in organic chemistry, materials and medicinal science. Its significance lies in the applications in both creation of a wide array of functional products and hydrogen energy storage [1,2,3,4,5,6]. In general, transfer hydrogenation (TH) is a fundamental tool in organic chemistry, to which great efforts have been made because it does not need flammable high-pressure H2 gas, and offers more convenient and safe production processes. Pioneered by Benkeser [7,8] and Birch [9,10] reduction, much attention has been focused on the transfer hydrogenation with specific reducing agents [11,12,13,14] and the hydrogenation with high-pressure H2 gas [7,8]. Moreover, the Krische group has reported distinguished contributions on the coupling between alcohols and C–C double/triple bonds [15,16,17,18,19,20]. Li and co-workers have demonstrated significant achievements converting phenol derivatives into amines in the presence of NaCO2H [21,22] as the hydrogen donor. Despite these valuable contributions, the utilization of such a strategy to construct functionalized N-heterocycles is rarely explored.
1,2,3,4-Tetrahydronaphthyridines (THNADs) constitute the core structure of numerous functionalized molecules, exhibiting diversely interesting biological and therapeutic activities [11,12,13,14,23,24,25]. Traditionally, procedures for these compounds required the use of organometallic hydrides, strong acids or alcohols. However, the preparation of such compounds has to date presented a difficult goal. We have been committed to the ongoing study of N-heterocyclic generation through transfer hydrogenation coupling strategies [26,27,28,29,30,31,32,33,34,35], and we have reported C(sp3)-H bond alkylation using tetrahydro-n-heterarene as a coupling partner and hydrogen donor. Initially, our motivation was to test the transfer hydrogenation coupling of indoline with 2-phenyl-1,8-naphthidine. However, after repeated attempts at this reaction, we did not achieve the expected product reported in this paper, and a dehydrogen-coupled compound was detected as the only product at a yield of 12%. Considering that the preparation of indoline feedstock involves the prefabrication step of catalytic hydrogenation of indole derivatives, we then tested the direct coupling of indole with 2-phenyl-1,8-naphthidine under the same conditions. Interestingly, by releasing hydrogen, it produced an even higher yield of 18%. To our knowledge, although a range of approaches have been well explored for functionalization of different sites of the indole and pyrrole skeletons, direct arylation of indole without directing group assistance, prefunctionalization, or consumption of external oxidants remains an unaddressed goal [36,37,38,39,40,41,42,43,44]. After further investigation of these new findings, we reported a new method for directly obtaining nitrogen-containing biological heterocyclic aromatic hydrocarbons by iridium-catalyzed cross-coupling of the β-site of indole/pyrrole with the α-site of N-heterocyclic aromatic hydrocarbons (Scheme 1(1)) [34], we went back and continued to be motivated to test the transfer hydrogenative by using indoline (b) as both the coupling partner and the hydrogen donor, coupling with 2-phenyl-1,8-naphthyridine (1g) in the presence of iridium NaOTf and tert-amyl alcohol (1.0 mL) at 110 °C for 16 h under N2 protection. To our delight, the reaction produced the expected product 1gb in 5% yield and a tetrahydro-1,8-naphthyridine 1g’ was detected (Scheme 1(2)). Upon a thorough investigation of this observation, we herein report an iridium-catalyzed transfer hydrogenative coupling reaction of indolines and 1,8-naphthyridines, offering a practicable approach for the construction of an α-functionalized tetrahydro-1,8-naphthyridines structurally unique product.
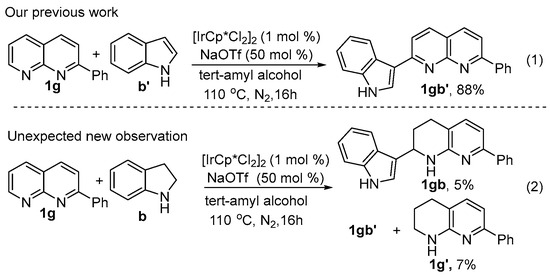
Scheme 1.
Unexpected New Observation.
2. Results and Discussion
Our initial studies focused on developing a more efficient catalytic system for the coupling of 2-(4-(trifluoromethyl)phenyl)-1,8-naphthyridine 1a with 2-methylindoline 2a as a model system. First, the reaction was performed at 130 °C for 16 h by using [Cp*IrCl2]2 (1 mol %) as a catalyst and NaOTf (50 mol %) as an additive, which afforded the product 3aa in 35% yield (entry 1). Then, a series of acids and bases were evaluated (50 mol %, entries 2–7); unfortunately, they were totally ineffective or less effective. Gratifyingly, no use of additive led to an improved yield (entry 9, 68%), and the absence of catalyst failed to yield any product (entry 10), indicating that the iridium catalyst plays a crucial role in affording the product. Further, serval ligands (entries 12–13) did not show any activity under the studied reaction conditions. Moreover, other palladium and iridium catalysts employed for the reaction showed less reactivity for the transformation. Finally, change of reaction temperatures (entry 10) or solvents (entries 14–15) were not fruitful since no increase of product yield was obtained. Thus, the optimal conditions are as indicated in entry 9 of Table 1.

Table 1.
Screening of the Optimal Conditions a.
With the optimal conditions in hand, we next examined the generality and the limitation of the synthetic protocol. First, a series of 1,8-nphthyridines [1a-1l] and N-heteroaromatics (1m-1p) with 2-methylindoline 2a, for their structures (see Supporting Information (SI) Table S1) were tested. As shown in Scheme 2, all the reactions proceeded smoothly and furnished the desired products in moderate to good isolated yields. The results indicated that the substituents on the aryl ring of reactant 1 significantly affected the reactions. Specially, electron-withdrawing substitutents afforded the products (3aa-ca) in much higher yields than those of electro-rich substitutents. This observation might be attributed to the electron-withdrawing groups that could enhance the electrophilicity of the in situ formed imine intermediate, thus favoring the coupling process. Gratifyingly, 2-(1-methyl-1H-pyrrol-2-yl)-1,8-naphthyridine (1m) proved to be an effective coupling partner, yielding the products in reasonable yields (see 3ma). Moreover, Substrate 1o and 2p, nitrogen-modified 1,8-naphthyridines, effectively coupled with 1a to give compound 3oa and 3pa in 56% and 52% yield, respectively; this example demonstrates the potential of the methodology to be applied to other heterocyclic scaffolds. It is worth mentioning that a series of functional groups such as -CF3, OH, -Cl, -Br, -NO2, and -CN are well tolerated in the synthetic protocol which would offer the potential for molecular complexity via further chemical transformation.
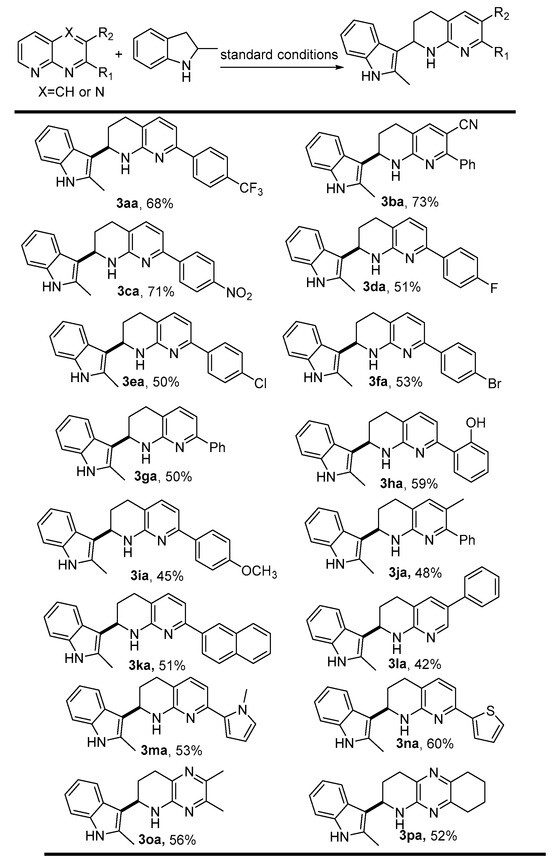
Scheme 2.
Variations of 1,8-nphthyridines. Standard conditions: all the reaction in t-amyl alcohol (1.0 mL) was performed with 1 (0.2 mmol), 2 (0.3 mmol), [Cp*IrCl2]2 (1 mol %), at 110 °C for 16 h under N2 protection.
Subsequently, we focused on the variation of both coupling partners. Thus, various combinations of 1 with indolines 2 were tested. Similar to the results described in Scheme 2, all the reactions afforded the desired products in moderate-to-excellent isolated yields (Scheme 3). Gratifyingly, a series of indolines underwent efficient transfer hydrogen evolution cross-coupling reactions, and the reactions of electron-rich indolines (2b-c) with electron-poor 1,8-nphthyridines (1a,1h) could give satisfactory yields, presumably because the electron-donating group could enhance the nucleophilicity of the indole skeleton, which is also beneficial for the coupling step.
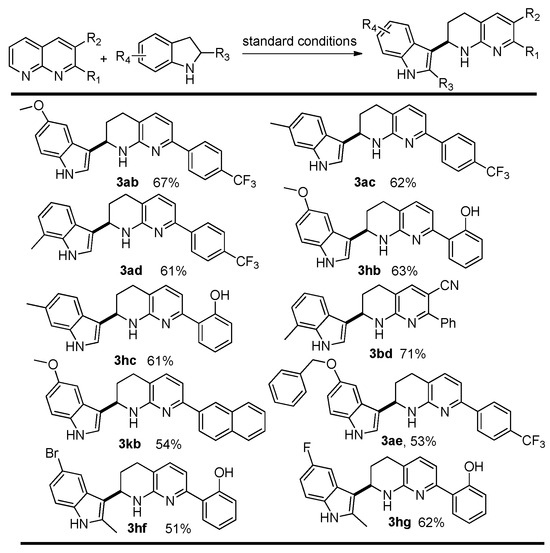
Scheme 3.
Variation of indolines.
To gain insights into the possible mechanism, several verification experiments were performed. The model reaction was subsequently interrupted after 3 h to analyse the intermediates. By means of GC analyses, product 3ga, tetra- and di-hydronaphthyridine were detected in 5%, 5% and 2% yields, respectively (Scheme 4). Then, the reaction 1g’, 2a’ and 2-methyl-1,2,3,4-tetrahydroquinoline as the hydrogen donor under standard conditions produced product 3aa in 21% yield. Further it was found that 2a’ failed to directly couple with the di-hydronaphthyridine 1g’ to give product 3ga, indicating that tetrahydronaphthyridine 1g’ is not the reaction intermediate; finally, treating 3ga’ with equimolar amount of 2a or e was not able to afford 3ga (Scheme 4), showing that c-1g may be the key intermediate and 3ga’ as the intermediate of the reaction is less likely.
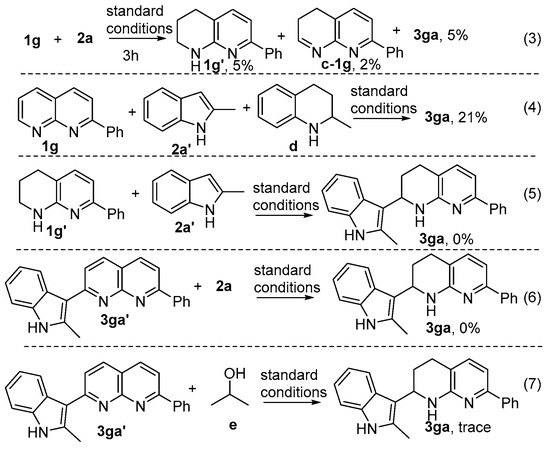
Scheme 4.
Control Experiments.
Although the mechanism of this reaction has not been fully elucidated, on the basis of the above-observed findings, a hydrogen transfer is proposed in Scheme 5. Based on metal-catalyzed transfer hydrogen mechanism reported in the literature [45,46] and the above control experiments, a plausible reaction pathway is proposed in Scheme 5. First, IrCp*Cl2 and NaOTf proceed in a ligand exchange process to generate complex A which thereupon reacts with 2-methylindoline 2a to give B followed by β-H elimination to form 2-methyl-1H-indole 2a’ and metal hydride C. Next, 2-phenyl-1,8-naphthyridine 1g undergoes coordination with C and then experiences hydrometallation to afford intermediate E. Subsequently, with the alcoholysis of E, transfer hydrogenation intermediate c-1g or its tautomerism c-1g’ is produced, and A is regenerated to accomplish the catalytic cycle. Finally, the formed c-1g and 2-methyl-1H-indole 2a’ undergoes the classic nucleophilic addition to provide the desired tetrahydro α-functionalized product 3ga.
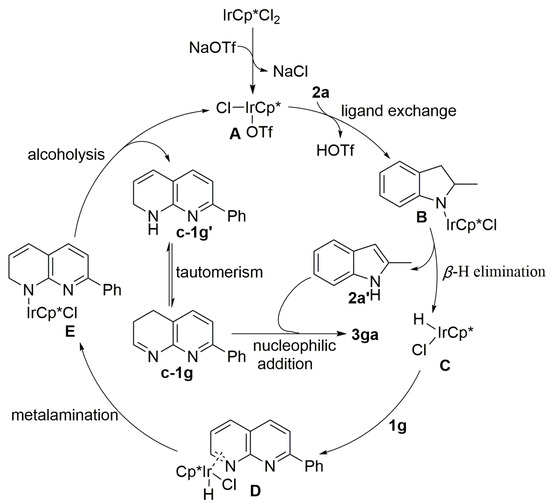
Scheme 5.
Plausible Catalytic Cycle.
3. Experimental
3.1. General Information
All the obtained products were characterized by melting points (m.p.), 1H-NMR, 13C-NMR and infrared spectra (IR). Melting points were measured on an Electrothemal W-X4 microscopy digital melting point apparatus and are uncorrected; IR spectra were recorded on a FTLA2000 spectrometer; 1H-NMR and 13C-NMR spectra were obtained on Bruker-400 and referenced to CHCl3 (7.26 ppm for 1H, and 77.2 ppm for 13C) or DMSO-d6 (2.50 ppm for 1H, and 39.5 ppm for 13C). Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns are described as singlet (s), doublet (d), triplet (t), multiplet (m); TLC was performed using commercially prepared 100–400 mesh silica gel plates (GF254), and visualization was effected at 254 nm; Unless otherwise stated, all the reagents were purchased from commercial sources (J&KChemic, TCI, Fluka, Acros, SCRC, Shanghai, China), used without further purification.
3.2. Substrates Preparation
The preparation of 1,8-naphthyridines 2. 2-aminonicotinaldehyde 4 (5 mmol), ketones 5 (5 mmol), t-BuOK (20 mol %) and ethanol (10 mL) were introduced in a flask (50 mL). Then, it was stirred at 50 °C under atmosphere for 2 h. After cooling down to room temperature, the resulting mixture was filtered and washed with ethyl acetate, and then concentrated by removing the solvent under vacuum. Finally the residue was purified by preparative TLC on silica, eluting with petroleum ether (60–90 °C): ethyl acetate (10:1, v/v) to give 1,8-naphthyridines, all the substrates used in our reaction are listed in Table S1. All the reagents were purchased from Bide Pharmatech Ltd. and Energy Chemical, all the solvents were purchased from Greagent (Shanghai Titansci incorporated company, Shanghai, China) and used without further purification. All the reactions were heated by metal sand bath (WATTCAS, LAB-500, https://www.wattcas.com (accessed on 17 May 2017)).
3.3. Typical Procedure for the Synthesis of Ester 3aa
The mixture of 2-phenyl-1,8-naphthyridine 1a (0.2 mmol), 2-methylindoline 2a (0.3 mmol), [Cp*IrCl2]2 and t-amyl alcohol (1.0 mL) were added to the Schlenk tube (50 mL) successively under nitrogen protection; then, the reaction tube was closed and placed in an oil bath at a temperature of 110 °C, where the reaction took place for 16 h. After that, the Schlenk tube was then removed from the oil bath and placed in the air to cool. After cooling down to room temperature, the reaction mixture was concentrated by removing the solvent under vacuum. Finally, the residue was purified by preparative TLC on silica, eluting with ethyl acetate: petroleum ether (60–90 °C) = 1:5, to give the desired product 3aa.
4. Conclusions
In summary, by employing hydrogen transfer strategy and indoline as both hydrogen donor and the reactant, we have developed a novel straightforward synthesis of functionalized N-heterocycles. This method proceeds with unprecedented synthetic effects including high step-economic fashion together with the advantages of being without any by-product and having no need for external high pressure H2, offering a practicable approach for the construction of an α-functionalized tetrahydro-1,8-naphthyridines structurally unique product. Further investigation applying the hydrogen transfer coupling strategy in other hetero cyclic systems as well as the asymmetric synthesis is ongoing in our laboratory and will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28237886/s1, Table S1: Synthesis of substrates 1,8-naphthyridines; Scheme S1: Substrates employed. Refs. [47,48,49,50,51,52,53] are cited in Supplementary Materials.
Author Contributions
Data curation, J.Z., Y.F. and C.C.; Writing—original draft, C.Z.; Writing—review & editing, C.C. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by support by 2023 Jiangsu Degree and Postgraduate Education Teaching Reform project (JGKT23_C085), 2023 Jiangsu Graduate Research and Practice Innovation plan (KYCX23_3469), the Funding for School-level Research projects of Yancheng Institute of Technology (No. xjr2019007 and No. xjr2023031), 2023 Excellent Graduation Project (Thesis) Cultivation project of Yancheng Institute of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malancona, S.; Donghi, M.; Ferrara, M.; Hernando, M.J.I.; Pompei, M.; Pesci, S.; Ontoria, J.M.; Koch, U.; Rowley, M.; Summa, V. Allosteric inhibitors of hepatitis C virus NS5B polymerase thumb domain site II: Structure-based design and synthesis of new templates. Bioorg. Med. Chem. 2010, 18, 2836. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Ikeda, C.; Takahashi, Y.; Fujita, K.I. Homogeneous Catalytic System for Reversible Dehydrogenation-Hydrogenation Reactions of Nitrogen Heterocycles with Reversible Interconversion of Catalytic Species. J. Am. Chem. Soc. 2009, 131, 8410. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Kim, M.H.; Kim, J. Cu-Catalyzed Aerobic Oxidation of Di-tert-butyl Hydrazodicarboxylate to Di-tert-butyl Azodicarboxylate and Its Application on Dehydrogenation of 1,2,3,4-Tetrahydroquinolines under Mild Conditions. Org. Lett. 2016, 18, 6300. [Google Scholar] [CrossRef] [PubMed]
- Iosub, A.V.; Stahl, V. Catalytic Aerobic Dehydrogenation of Nitrogen Heterocycles Using Heterogeneous Cobalt Oxide Supported on Nitrogen-Doped Carbon. Org. Lett. 2015, 17, 4404. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Hu, L.; Wang, J.; Li, X.; Qi, F.; Lu, J.; Cao, X.; Gu, H. Reversible Hydrogenation-Oxidative Dehydrogenation of Quinolines over a Highly Active Pt Nanowire Catalyst under Mild Conditions. ChemCatChem 2013, 5, 2183. [Google Scholar] [CrossRef]
- Chakraborty, S.; Brennessel, W.W.; Jones, W.D. A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-Heterocycles. J. Am. Chem. Soc. 2014, 136, 8564. [Google Scholar] [CrossRef] [PubMed]
- Benkeser, R.A.; Robinson, R.E.; Landesman, H. The True Identity of the Solvated Free Radical “Triphenylsilicyl Ethylammine.” The Multiple Addition of Lithium to an Aromatic System in Ethylamine. J. Am. Chem. Soc. 1952, 74, 5699. [Google Scholar] [CrossRef]
- Benkeser, R.A.; Robinson, R.E.; Sauve, D.M.; Thomas, O.H. Reduction of Organic Compounds by Lithium in Low Molecular Weight Amines. I. Selective Reduction of Aromatic Hydrocarbons to Monoölefins. J. Am. Chem. Soc. 1955, 77, 3230. [Google Scholar] [CrossRef]
- Birch, A.J. Reduction by dissolving metals. Nature 1946, 158, 585. [Google Scholar] [CrossRef]
- Zimmerman, H.E. A Mechanistic Analysis of the Birch Reduction. Acc. Chem. Res. 2012, 45, 164. [Google Scholar] [CrossRef]
- Li, H.X.; Yin, H.; Zhang, F.; Li, H.; Huo, Y.N.; Lu, Y.F. Water-Medium Clean Organic Reactions over an Active Mesoporous Ru(II) Organometallic Catalyst. Environ. Sci. Technol. 2009, 43, 188. [Google Scholar] [CrossRef]
- He, W.; Ge, Y.C.; Tan, C.H. Halogen-Bonding-Induced Hydrogen Transfer to C=N Bond with Hantzsch Ester. Org. Lett. 2014, 16, 3244. [Google Scholar] [CrossRef] [PubMed]
- Grobas, J.; Bolivar, C.; Scott, C.E. Hydrodesulfurization of Benzothiophene and Hydrogenation of Cyclohexene, Biphenyl, and Quinoline, Assisted by Ultrasound, Using Formic Acid as Hydrogen Precursor. Energy Fuels 2007, 21, 19. [Google Scholar] [CrossRef]
- Chatterjee, I.; Oestreich, M. B(C6F5)3-Catalyzed Transfer Hydrogenation of Imines and Related Heteroarenes Using Cyclohexa-1,4-dienes as a Dihydrogen Source. Angew. Chem. Int. Ed. 2015, 54, 1965. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Perez, F.; Krische, M.J. Ruthenium(0)-Catalyzed [4+2] Cycloaddition of Acetylenic Aldehydes with α-Ketols: Convergent Construction of Angucycline Ring Systems. Angew. Chem. Int. Ed. 2016, 55, 1493. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Kasun, Z.A.; Krische, M.J. Enantioselective Alcohol C-H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis. J. Am. Chem. Soc. 2016, 138, 5467. [Google Scholar] [CrossRef] [PubMed]
- Garza, V.J.; Krische, M.J. Hydroxymethylation beyond Carbonylation: Enantioselective Iridium-Catalyzed Reductive Coupling of Formaldehyde with Allylic Acetates via Enantiotopic π-Facial Discrimination. J. Am. Chem. Soc. 2016, 138, 3655. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Krische, M.J. Iridium-Catalyzed C-C Coupling of a Simple Propargyl Ether with Primary Alcohols: Enantioselective Homoaldol Addition via Redox-Triggered (Z)-Siloxyallylation. J. Am. Chem. Soc. 2015, 137, 16024. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Herkommerand, D.; Krische, M.J. Ruthenium-BINAP Catalyzed Alcohol C-H tert-Prenylation via 1,3-Enyne Transfer Hydrogenation: Beyond Stoichiometric Carbanions in Enantioselective Carbonyl Propargylation. J. Am. Chem. Soc. 2016, 138, 5238. [Google Scholar] [CrossRef]
- Perez, F.; Waldeck, A.R.; Krische, M.J. Total Synthesis of Cryptocaryol A by Enantioselective Iridium-Catalyzed Alcohol C-H Allylation. Angew. Chem. Int. Ed. 2016, 55, 5049. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, H.; Girard, S.A.; Wang, F.; Chen, N.; Li, C.J. Formal Direct Cross-Coupling of Phenols with Amines. Angew. Chem. Int. Ed. 2015, 54, 14487. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, H.; Gong, H.; Wang, H.; Li, C.J. Palladium-catalyzed reductive coupling of phenols with anilines and amines: Efficient conversion of phenolic lignin model monomers and analogues to cyclohexylamines. Chem. Sci. 2015, 6, 4174. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Breslin, M.J.; Coleman, P.J.; Duggan, M.E.; Hunt, C.A.; Hutchinson, J.H.; Leu, C.T.; Rodan, S.B.; Rodan, G.A.; Duong, L.T.; et al. Non-peptide αvβ3 antagonists. Part 7: 3-Substituted tetrahydro-1, 8naphthyridine derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Seefeld, M.A.; Miller, W.H.; Newlander, K.A.; Burgess, W.J.; DeWolf, W.E.; Elkins, P.A.; Head, M.S.; Jakas, D.R.; Janson, C.A.; Keller, P.M.; et al. Indole Naphthyridinones as Inhibitors of Bacterial Enoyl-ACP Reductases FabI and FabK. J. Med. Chem. 2003, 46, 1627. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Rector, C.L.; Kim, H.Y.; Sonnen, A.F.P.; Meyer, R.; Nau, W.M.; Atkinson, J.; Rintoul, J.; Pratt, D.A.; Porter, N.A. Tetrahydro-1,8-naphthyridinol Analogues of α-Tocopherol as Antioxidants in Lipid Membranes and Low-Density Lipoproteins. J. Am. Chem. Soc. 2007, 129, 10211. [Google Scholar] [CrossRef]
- Lv, W.; Xiong, B.; Jiang, H.F.; Zhang, M. Synthesis of 2-Alkylaminoquinolines and 1,8-Naphthyridines by Successive Ruthenium-Catalyzed Dehydrogenative Annulation and N-Alkylation Processes. Adv. Synth. Catal. 2017, 359, 1202. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, S.D.; Jiang, H.F.; Zhang, M. Hydrogen-Transfer-Mediated Direct β-Alkylation of Aryl-1,8-naphthyridines with Alcohols under Transition Metal Catalyst Free Conditions. Org. Lett. 2016, 18, 724. [Google Scholar] [CrossRef]
- Tan, Z.D.; Jiang, H.F.; Zhang, M. Ruthenium-Catalyzed Dehydrogenative β-Benzylation of 1,2,3,4-Tetrahydroquinolines with Aryl Aldehydes: Access to Functionalized Quinolines. Org. Lett. 2016, 18, 3174. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, S.D.; Chen, L.; Li, B.; Jiang, H.F.; Zhang, M. An annulative transfer hydrogenation strategy enables straightforward access to tetrahydro fused-pyrazine derivatives. Chem. Commun. 2016, 52, 10636. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, M.; Jiang, H.F.; Chen, M.M.; Lv, W.; Zheng, A.B.; Jian, X.J. Efficient synthesis of quinoxalines from 2-nitroanilines and vicinal diols via a ruthenium-catalyzed hydrogen transfer strategy. Green Chem. 2015, 17, 279. [Google Scholar] [CrossRef]
- Xiong, B.; Li, Y.; Lv, W.; Tan, Z.D.; Jiang, H.F.; Zhang, M. Ruthenium-Catalyzed Straightforward Synthesis of 1,2,3,4-Tetrahydronaphthyridines via Selective Transfer Hydrogenation of Pyridyl Ring with Alcohols. Org. Lett. 2015, 17, 4054. [Google Scholar] [CrossRef]
- Tan, Z.D.; Jiang, H.F.; Zhang, M. A novel iridium/acid co-catalyzed transfer hydrogenative C(sp3)–H bond alkylation to access functionalized N-heteroaromatics. Chem. Commun. 2016, 52, 9359. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, M.; Chen, M.M.; Lv, W.; Jiang, H.F. Convenient Synthesis of Quinolines from α-2-Nitroaryl Alcohols and Alcohols via a Ruthenium-catalyzed Hydrogen Transfer Strategy. ChemCatChem 2015, 7, 349. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Zhao, H.; Jiang, H.; Zhang, M. Direct Access to Nitrogen Bi-heteroarenes via Iridium-Catalyzed Hydrogen-Evolution Cross-Coupling Reaction. Org. Lett. 2017, 19, 3390. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Xiong, B.; Yang, J.; Ci, C.; Jiang, H.; Zhang, M. Selective reductive cross-coupling of N-heteroarenes by an unsymmetrical PNP-ligated manganese catalyst. J. Catal. 2020, 392, 135. [Google Scholar] [CrossRef]
- Modha, S.G.; Greaney, M.F. Atom-Economical Transformation of Diaryliodonium Salts: Tandem C-H and N-H Arylation of Indoles. J. Am. Chem. Soc. 2015, 137, 1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.H.; Zhang, B.; Sun, Y.; Wang, L.; Chen, J.B.; Cheng, J. Copper-mediated C3-cyanation of indoles by the combination of amine and ammonium. Chem. Commun. 2014, 50, 2315. [Google Scholar] [CrossRef]
- Chen, S.P.; Liao, Y.F.; Zhao, F.; Qi, H.R.; Liu, S.W.; Deng, G.J. Palladium-Catalyzed Direct Arylation of Indoles with Cyclohexanones. Org. Lett. 2014, 16, 1618. [Google Scholar] [CrossRef]
- Li, Y.; Yan, T.; Junge, K.; Beller, M. Catalytic Methylation of C–H Bonds Using CO2 and H2. Angew. Chem. Int. Ed. 2014, 126, 10644. [Google Scholar] [CrossRef]
- Wu, J.C.; Song, R.J.; Wang, Z.Q.; Huang, X.C.; Xie, Y.X.; Li, J.H. Copper-Catalyzed C-H Oxidation/Cross-Coupling of α-Amino Carbonyl Compounds. Angew. Chem. Int. Ed. 2012, 51, 3453. [Google Scholar] [CrossRef]
- Leskinen, M.V.; Yip, K.T.; Valkonen, A.; Pihko, P.M. Palladium-Catalyzed Dehydrogenative β′-Functionalization of β-Keto Esters with Indoles at Room Temperature. J. Am. Chem Soc. 2012, 134, 5750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Rawal, V.H. Palladium-Catalyzed C3-Benzylation of Indoles. J. Am. Chem. Soc. 2012, 134, 111. [Google Scholar] [CrossRef]
- Wu, W.; Su, W. Mild and Selective Ru-Catalyzed Formylation and Fe-Catalyzed Acylation of Free (N-H) Indoles Using Anilines as the Carbonyl Source. J. Am. Chem. Soc. 2011, 133, 11924. [Google Scholar] [CrossRef]
- Zeng, Z.; Deng, Y.; Li, L.; Li, C.; Zhong, M. Hydrogen Transfer Coupling with 100% Atom Economy: Synthesis of 2-Indolyltetrahydronaphthyridine Derivatives. J. Org. Chem. 2022, 87, 12257. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wang, X.; Tong, Y.; Qiu, X.; Zeng, X.; Xiong, B. Ruthenium-catalyzed acceptorless dehydrogenative coupling of amino alcohols and ynones to access 3-acylpyrroles. Chem. Commun. 2022, 58, 2379. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Wang, Y.; Liu, Y.; Bao, Y.; Liu, Z.; Zhang, Y.; Ling, Y. Straightforward synthesis of quinolines from enones and 2-aminobenzyl alcohols using an iridium-catalyzed transfer hydrogenative strategy. Org. Biomol. Chem. 2018, 16, 5707. [Google Scholar] [CrossRef] [PubMed]
- Moya, S.A.; Gajardo, J.; Araya, J.C.; Cornejo, J.J.; Guerchais, V.; Le Bozec, H.; Bayón, J.C.; Pardey, A.J.; Aguirre, P. Synthesis and characterization of new complexes of the type [Ru(CO)2Cl2(2-phenyl-1,8-naphthyridine-kN) (2-phenyl-1,8-naphthyridine-kN′)]. Preliminary applications in homogeneous catalysis. Appl. Organomet. Chem. 2008, 22, 471–478. [Google Scholar] [CrossRef]
- Hawes, E.M.; Gorecki, D.K.J.; Gedir, R.G. 2,3-Disubstituted 1,8-naphthyridines as potential diuretic agents. 2. 5,7-Dimethyl derivatives. J. Med. Chem. 1977, 20, 838–841. [Google Scholar] [CrossRef]
- Reddy, K.V.; Sreenivasulu, B. A Facile One-Step Synthesis of 2-Aryl and Heteryl-1,8-Naphthyridines. Curr. Sci. 1977, 46, 597–598. [Google Scholar]
- Mogilaiah, K.; Kumar, K.S.; Reddy, N.V. Potassium triiodide catalyzed Friedlander synthesis of 1,8-naphthyridines in aqueous media. Indian J. Chem. 2010, 49, 253–255. [Google Scholar]
- Galatsis, P.; Yamagata, K.; Wendt, J.A.; Connolly, C.J.; Mickelson, J.W.; Milbank, J.B.J.; Bove, S.E.; Knauer, C.S.; Brooker, R.M.; Augelli-Szafran, C.E.; et al. Synthesis and SAR comparison of regioisomeric aryl naphthyridines as potent mGlu5 receptor antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 6525–6528. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhao, H.; Xiong, B.; Jiang, H.F.; Dixneuf, P.H.; Zhang, M. Selective synthesis of nitrogen bi-heteroarenes by a hydrogen transfer-mediated direct α,β-coupling reaction. Org. Biomol. Chem. 2017, 15, 6093–6097. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Zhao, H.; Chen, C.L.; Jiang, H.F.; Zhang, M. Hydrogen-Transfer-Mediated α-Functionalization of 1,8-Naphthyridines by a Strategy Overcoming the Over-Hydrogenation Barrier. Angew. Chem. Int. Ed. 2017, 56, 14232–14236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).