Analysis of Volatile Components in Rosa roxburghii Tratt. and Rosa sterilis Using Headspace–Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. VOCs in RR and RS
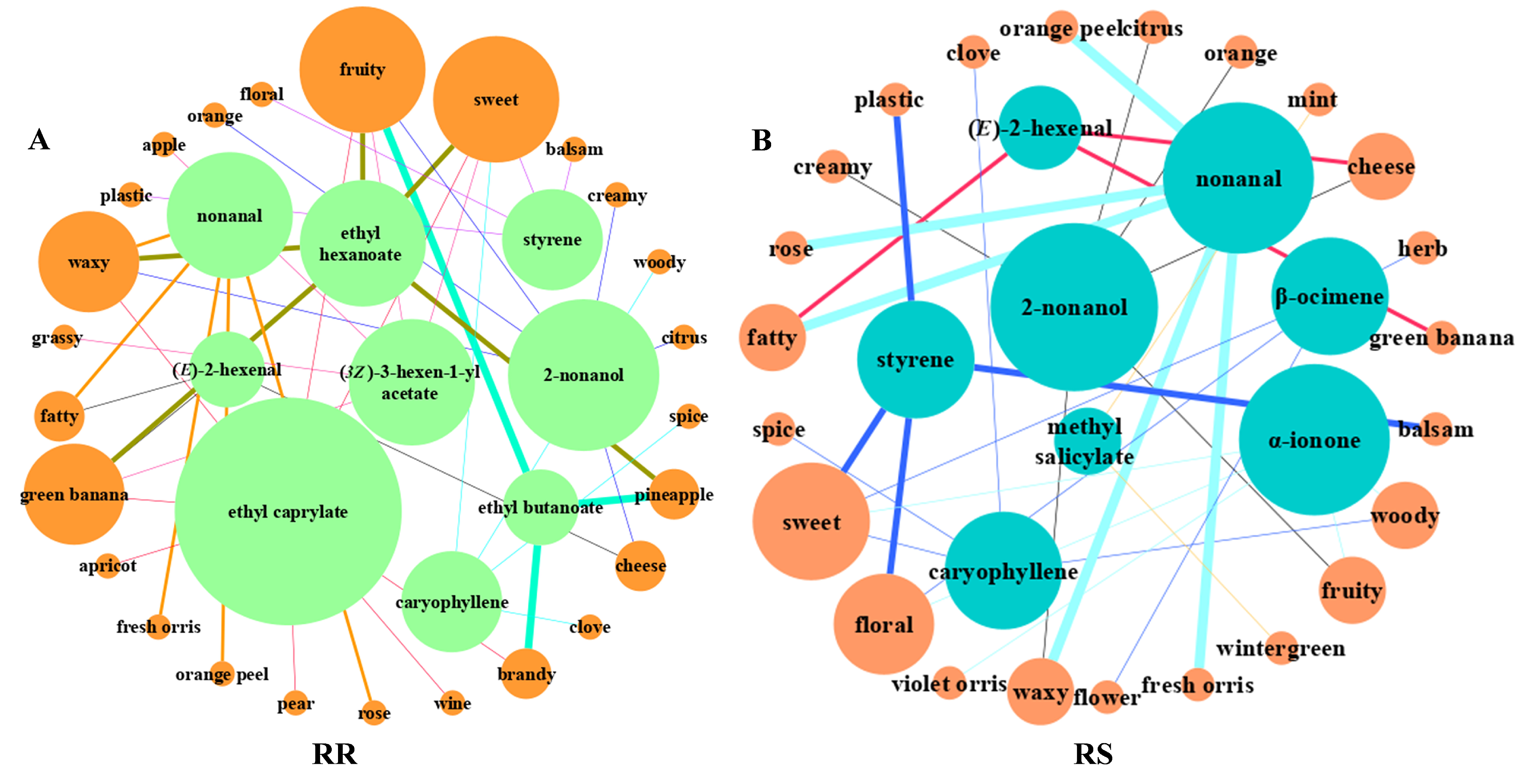
2.2. ROAVs Analyses in RR and RS
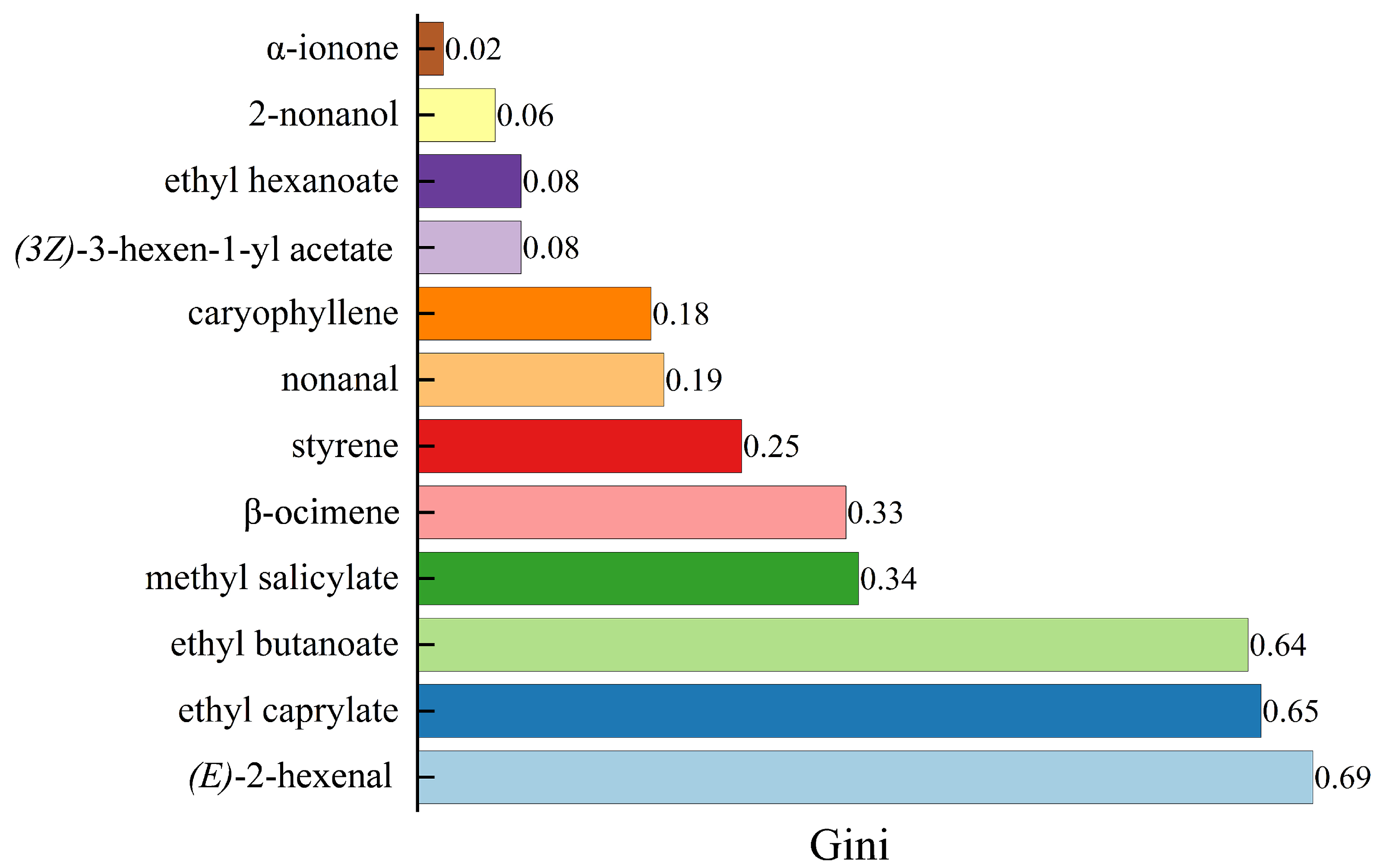
2.3. Screening of Signature Difference Flavor Components
3. Discussion
4. Materials and Methods
4.1. RR and RS Samples
4.2. HS-SPME Conditions
4.3. GC-MS Conditions
4.4. Qualitative Analyses of GC-MS
4.5. Calculation of Relative Odor Activity Value
4.6. Calculation of OPLS-DA and RF
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.Z.; Li, Y.F.; Yu, Z.H.; Liu, X.H.; Hardie, W.J.; Huang, M.Z. Screening and characterisation of β-glucosidase production strains from Rosa roxburghii Tratt. Int. J. Food Eng. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Xu, P.; Liu, X.X.; Xiong, X.W.; Zhang, W.B.; Cai, X.H.; Qiu, P.Y.; Hao, M.H.; Wang, L.J.; Lu, D.D.; Zhang, X.H.; et al. Flavonoids of Rosa roxburghii Tratt. exhibit anti-apoptosis properties by regulating PARP-1/AIF. J. Cell. Biochem. 2017, 118, 3943–3952. [Google Scholar] [CrossRef]
- Xu, J.W.; Vidyarthi, S.K.; Bai, W.B.; Pan, Z.L. Nutritional constituents, health benefits and processing of Rosa Roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Ao, H.P.; Liu, W.H.; Zheng, J.X.; Li, X.J.; Ren, D.F. Physicochemical and structural properties of dietary fiber from Rosa roxburghii pomace by steam explosion. J. Food Sci. Technol. 2022, 59, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, T.T.; Zheng, L.; Jiang, F.F.; Ma, W.T.; Lu, M.; Wu, X.M.; An, H. Recent advances on main active ingredients, pharmacological activities of Rosa roxbughii and its development and utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Li, L.Q.; Peng, M.; Li, Y.M.; Niu, Z.P.; Li, Q.J.; Wang, T.; Yang, X.S. Immunocompetence of total triterpenoids from Rosa roxburghii Tratt fruit. J. Northwest A F Univ. 2022, 50, 11–19. [Google Scholar] [CrossRef]
- Wang, L.Q.; Li, Y.L.; Xia, R.; Zheng, X.Y.; Li, X.J.; Wu, S.X.; Zhang, Q.Y.; Li, S.; Deng, Y.L.; Yao, Y.Q.; et al. Component analysis and anti-pulmonary fibrosis effects of Rosa sterilis juice. Food Funct. 2022, 13, 12915–12924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Zhang, D.; Farha, A.K.; Yang, X.; Li, H.B.; Kong, K.W.; Zhang, J.R.; Chan, C.L.; Lu, W.Y.; Corke, H.; et al. Phytochemicals, essential oils, and bioactivities of an underutilized wild fruit Cili (Rosa roxburghii). Ind. Crops Prod. 2020, 143, 111928. [Google Scholar] [CrossRef]
- Wang, L.T.; Lv, M.J.; An, J.Y.; Fan, X.H.; Dong, M.Z.; Zhang, S.D.; Wang, J.D.; Wang, Y.Q.; Cai, Z.H.; Fu, Y.J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, L. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Han, S.C.H.; Wu, M.H. Beneficial effects of hydroalcoholic extract from Rosa roxburghii Tratt. fruit on hyperlipidemia in high-fat-fed rats. Acta Cardiol. Sin. 2020, 36, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.W.; Wang, R.L.; Xiao, Z.B.; Sun, X.X.; Wang, P.P.; Zhu, J.C.; Cao, X.Y. Characterization of volatile compounds of Rosa roxburghii Tratt by gas chromatography-olfactometry, quantitative measurements, odor activity value, and aroma intensity. Molecules 2021, 26, 6202. [Google Scholar] [CrossRef] [PubMed]
- Li, F.H.; Yang, S.H.; Liu, L.H.; Fu, H.Z.; Ming, J. Variations of bioactive compounds, physicochemical and sensory properties of Rosa roxburghii Tratt juice after high pressure processing. LWT-Food Sci. Technol. 2023, 184, 114932. [Google Scholar] [CrossRef]
- Wang, J.Q.; Du, Q.; You, X.R.; Lv, Y.Q.; Bi, W.T.; Li, H.L.; Chen, D.D.Y. Solvent-free high-throughput analysis of herbicides in environmental water. Anal. Chim. Acta 2019, 1071, 8–16. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.H.; Liu, Y.; Wang, D.W. Analyzing volatile compounds of young and mature Docynia delavayi fruit by HS-SPME-GC-MS and rOAV. Foods 2023, 12, 59. [Google Scholar] [CrossRef]
- Devi, M.L.; Singh, N.B.; Sharma, K.C.; Rajashekar, Y.; Mishra, A.; Das, S. Volatile compound profile analysis of seasonal flower, fruit, leaf, and stem of Zanthoxylum armatum DC. from Manipur using HS-SPME-GC-MS. Chemosensors 2023, 11, 273. [Google Scholar] [CrossRef]
- Li, C.X.; Li, X.L.; Liang, G.L.; Xiang, S.Q.; Han, G.H. Volatile composition changes in lemon during fruit maturation by HS-SPME-GC-MS. J. Sci. Food Agric. 2022, 102, 3599–3606. [Google Scholar] [CrossRef]
- Azam, M.; Song, M.; Fan, F.J.; Zhang, B.; Xu, Y.Y.; Xu, C.J.; Chen, K.S. Comparative analysis of flower volatiles from nine citrus at three blooming stages. Int. J. Mol. Sci. 2013, 14, 22346–22367. [Google Scholar] [CrossRef]
- Azam, M.; Jiang, Q.; Zhang, B.; Xu, C.J.; Chen, K.S. Citrus leaf volatiles as affected by developmental stage and genetic type. Int. J. Mol. Sci. 2013, 14, 17744–17766. [Google Scholar] [CrossRef]
- Hu, L.L.; Liu, R.; Wang, X.H.; Zhang, X.Y. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wan, Y.M.; Sun, Z.H.; Li, T.Q.; Liu, X.F.; Ma, H.; Liu, X.X.; He, R.; Ma, Y.; Li, Z.H. Floral scent chemistry of Luculia yunnanensis (Rubiaceae), a species endemic to China with sweetly fragrant flowers. Molecules 2017, 22, 879. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.F.; Gao, P.; Gao, X.F. Deeper insight into the volatile profile of Rosa willmottiae with headspace solid-phase microextraction and GC–MS analysis. Molecules 2022, 27, 1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Li, Y.; Ren, Z.H.; Cong, Z.C.; Chen, M.J.; Shi, L.; Han, X.; Pei, J. Optimization of the microwave-assisted enzymatic extraction of Rosa roxburghii Tratt. polysaccharides using response surface methodology and its antioxidant and α-d-glucosidase inhibitory activity. Int. J. Biol. Macromol. 2018, 112, 473–482. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Zhang, Y.H.; Ma, N.; Zhang, X.L.; Liu, M.H.; Fu, W.M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Jiang, L.L.; Lu, M.; Rao, T.Z.; Liu, Z.Y.; Wu, X.M.; An, H.M. Comparative analysis of fruit metabolome using widely targeted metabolomics reveals nutritional characteristics of different Rosa roxburghii genotypes. Foods 2022, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Li, N.Y.; Jiang, L.L.; Liu, Y.Y.; Zou, S.M.; Lu, M.; An, H.M. Metabolomics combined with transcriptomics analysis revealed the amino acids, phenolic acids, and flavonol derivatives biosynthesis network in developing Rosa roxburghii fruit. Foods 2022, 11, 1639. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.F.; Huang, M.Z.; Li, T.T.; Li, X.; Cen, S.Y.; Li, Q.Y.; Huang, Q.; Tang, W.Y. Characterization of aroma compounds in Rosa roxburghii Tratt using solvent-assisted flavor evaporation headspace-solid phase microextraction coupled with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. X 2023, 18, 100632. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Chen, J.; Chen, X.J.; Chen, D.Z.; Deng, S.G. Use of relative odor activity value (ROAV) to link aroma profiles to volatile compounds: Application to fresh and dried eel (Muraenesox cinereus). Int. J. Food Prop. 2020, 23, 2257–2270. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans, Punter & Partners BV: Utrecht, The Netherlands, 2011; Chapter 2; pp. 207–359. [Google Scholar]
- Strozier, E.D.; Mooney, D.D.; Friedenberg, D.A.; Klupinski, T.P.; Triplett, C.A. Use of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection and random forest pattern recognition techniques for classifying chemical threat agents and detecting chemical attribution signatures. Anal. Chem. 2016, 88, 7068–7075. [Google Scholar] [CrossRef]
- Kang, C.D.; Zhang, Y.Y.; Zhang, M.Y.; Qi, J.; Zhao, W.T.; Gu, J.; Guo, W.P.; Li, Y.Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Meng, L.J.; Lu, Z.M.; Chai, L.J.; Wang, S.T.; Shi, J.S.; Shen, C.H.; Xu, Z.H. Identification of age-markers based on profiling of baijiu volatiles over a two-year maturation period: Case study of Lu-flavor baijiu. LWT-Food Sci. Technol. 2021, 141, 110913. [Google Scholar] [CrossRef]
- Chen, X.; Quek, S.Y. Free and glycosidically bound aroma compounds in fruit: Biosynthesis, transformation, and practical control. Crit. Rev. Food. Sci. 2022, 63, 9052–9073. [Google Scholar] [CrossRef] [PubMed]
- Zellner, B.A.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Zhao, R.F.; Zhang, H.X.; Jin, J.X.; Cheng, J.S. Comparison of volatile components of Rosa roxburghii Tratt. and Rosa sterilis based on gas chromatography-ion mobility spectrometry. J. Food Saf. Food Qual. 2022, 13, 6198–6204. [Google Scholar] [CrossRef]
- Rajendran, S.; Silcock, P.; Bremer, P. Flavour volatiles of fermented vegetable and fruit substrates: A review. Molecules 2023, 28, 3236. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, F.; Wen, X.L.; Chen, S.N.; Wang, K.X.; Wang, F.Q.; Zhang, J.M.; Wu, Y.Y.; He, P.M.; Tu, Y.Y.; et al. Characterization of a new (Z)-3:(E)-2-hexenal isomerase from tea (Camellia sinensis) involved in the conversion of (Z)-3-hexenal to (E)-2-hexenal. Food Chem. 2022, 383, 132463. [Google Scholar] [CrossRef]
- Ma, W.B.; Zhao, L.L.; Xie, Y.L. Inhibitory effect of (E)-2-hexenal as a potential natural fumigant on Aspergillus flavus in stored peanut seeds. Ind. Crops Prod. 2017, 107, 206–210. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, J.G.; Yang, K.Y.; Lim, S.; Lee, E.J. Postharvest fumigation of (E)-2-hexenal on kiwifruit (Actinidia chinensis cv. ‘Haegeum’) enhances resistance to Botrytis cinerea. Postharvest Biol. Tec. 2022, 187, 111854. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhao, J.R.; Liu, X.; Zhang, C.S.; Zhao, Z.G.; Li, X.T.; Sun, B.G. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef]
- Ma, Y.R.; Deng, Q.B.; Du, Y.J.; Ren, J.Y.; Chen, Y.F.; Liu, X.H.; Guo, X.W.; Xiao, D.G. Biosynthetic pathway for ethyl butyrate production in Saccharomyces cerevisiae. J. Agric. Food Chem. 2020, 68, 4252–4260. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, Q.W.; Guo, W.; Ai, L.Z.; Chen, C.; Tian, H.X. Unraveling the difference in flavor characteristics of Huangjiu fermented with different rice varieties using dynamic sensory evaluation and comprehensive two-dimensional gas chromatography-quadrupole mass spectrometry. Front. Nutr. 2023, 10, 1160954. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Yang, S.P.; Zhang, R.; Liu, S.Y.; Zhang, C.Y.; Li, Y.; Li, J.X. Characterization of honey peach (Prunus persica (L.) Batsch) aroma variation and unraveling the potential aroma metabolism mechanism through proteomics analysis under abiotic stress. Food Chem. 2022, 386, 132720. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.H.; Xiao, H.B.; Chen, J.N.; Zhu, J.M.; Fu, Y.J.; Ouyang, S.Y.; Chen, Y.Q.; Chen, D.; Su, J.Q.; Xue, T. Metabolome and whole-transcriptome analyses reveal the molecular mechanisms underlying hypoglycemic nutrient metabolites biosynthesis in Cyclocarya paliurus Leaves during Different Harvest Stages. Front. Nutr. 2022, 9, 851569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, J.; Duan, X.Y.; Wang, Y.; Wen, J.; Li, W.; Li, Z.G.; Wang, G.D.; Xu, H.Y. Plastidial engineering with coupled farnesyl diphosphate pool reconstitution and enhancement for sesquiterpene biosynthesis in tomato fruit. Metab. Eng. 2023, 77, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shang, X.F.; Li, B.; Zhou, X.Z.; Wen, H.; Zhang, J.Y. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compund, δ-cadinene against Psoroptes cuniculi. Vet. Parasitol. 2017, 236, 51–54. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Li, B.B.; Newman, J.; Liu, T.; Ren, Y.L. Evaluation of d-limonene and β-ocimene as attractants of Aphytis melinus (hymenoptera: Aphelinidae), a parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef]
- Sousa, J.M.S.D.; Nunes, T.A.D.L.; Rodrigues, R.R.L.; Sousa, J.P.A.D.; Val, M.D.C.A.; Coelho, F.A.D.R.; Santos, A.L.S.D.; Maciel, N.B.; Souza, V.M.R.D.; Machado, Y.A.A.; et al. Cytotoxic and antileishmanial effects of the monoterpene β-Ocimene. Pharmaceuticals 2023, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, R.; Tao, N.P. Identification of volatile compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) using SDE and HS-SPME combined with GC-MS. Adv. Mater. Res. 2013, 706–708, 399–402. [Google Scholar] [CrossRef]
- How, M.S.; Hamid, N.; Liu, Y.; Kantono, K.; Oey, I.; Wang, M.F. Using OPLS-DA to fingerprint key free amino and fatty acids in understanding the influence of high pressure processing in New Zealand clams. Foods 2023, 12, 1162. [Google Scholar] [CrossRef]
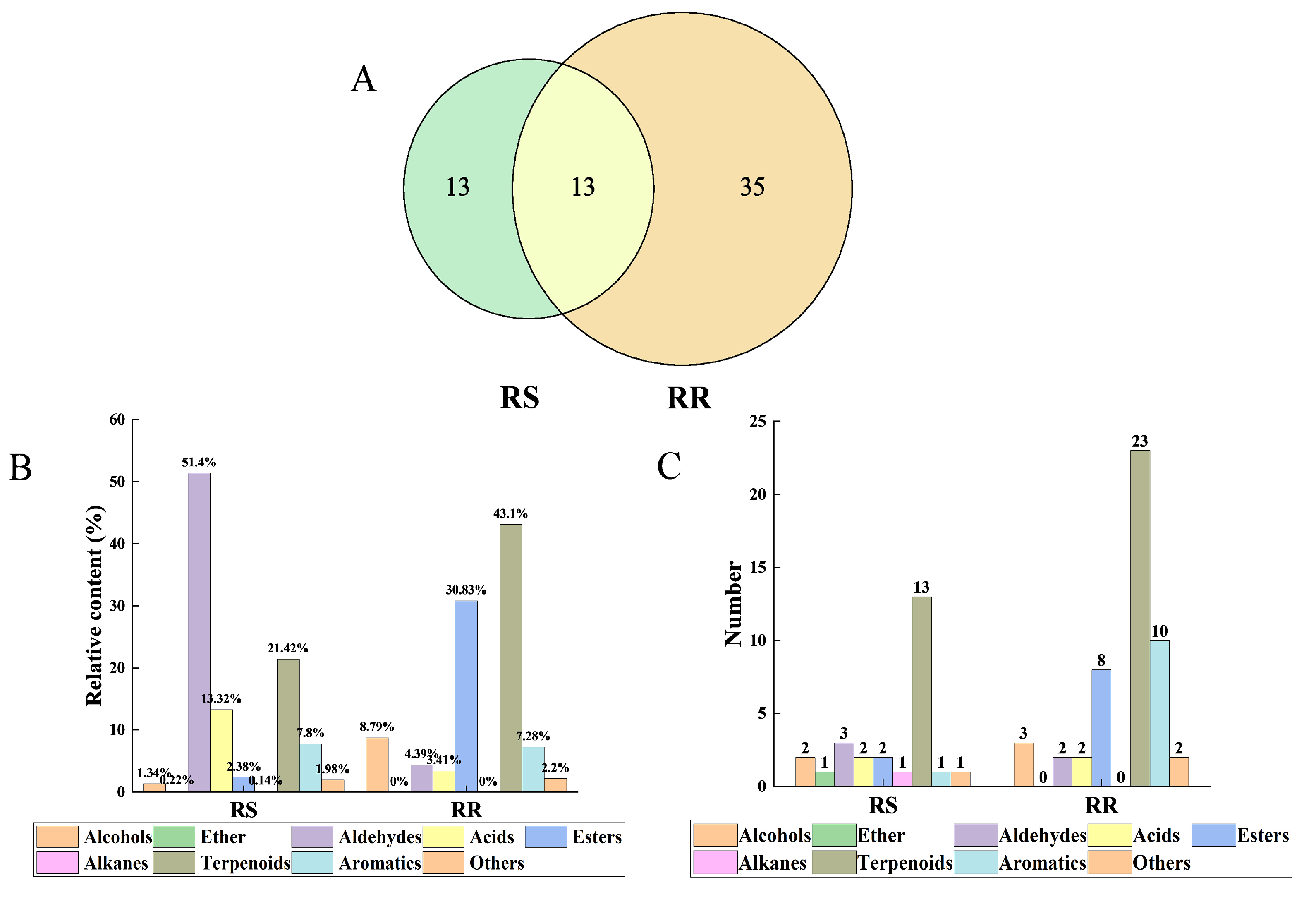


| No. | Compound | Formula | Retention Time (min) | CAS | Relative Content (%) A | |
|---|---|---|---|---|---|---|
| RS | RR | |||||
| Alcohols (4 kinds) | ||||||
| C1 | ethanol | C2H6O | 0.444 | 64-17-5 | - | 3.74 ± 1.42 |
| C2 | 2-nonanol | C9H20O | 9.817 | 628-99-9 | 0.91 ± 0.74 | 1.10 ± 0.74 |
| C3 | α-copaene | C9H11ClO | 19.948 | 1000360-33-0 | - | 4.18 ± 3.81 |
| C4 | dihydro-β-ionol | C13H24O | 22.150 | 3293-47-8 | 0.43 ± 0.51 | - |
| Ether (1 kind) | ||||||
| C5 | (–)-dihydroedulan ii | C13H22O | 17.029 | 41678-32-4 | 0.22 ± 0.18 | - |
| Aldehydes (3 kinds) | ||||||
| C6 | (E)-2-hexenal | C6H10O | 1.150 | 6728-26-3 | 47.88 ± 13.87 | 3.51 ± 0.58 |
| C7 | benzaldehyde | C7H6O | 5.610 | 100-52-7 | 2.71 ± 1.58 | - |
| C8 | nonanal | C9H18O | 10.611 | 124-19-6 | 0.81 ± 0.87 | 1.00 ± 0.63 |
| Acids (3 kinds) | ||||||
| C9 | hexanoic acid | C6H12O2 | 6.591 | 142-62-1 | 11.71 ± 9.80 | - |
| C10 | butane-2,3-diyl diacetate | C8H14O4 | 8.14 | 1114-92-7 | - | 2.03 ± 1.09 |
| C11 | octanoic acid | C8H16O2 | 13.184 | 124-07-2 | 1.61 ± 1.34 | 1.47 ± 0.99 |
| Esters (10 kinds) | ||||||
| C12 | ethyl butanoate | C6H12O2 | 0.297 | 105-54-4 | - | 3.97 ± 8.06 |
| C13 | ethyl acetate | C4H8O2 | 0.751 | 141-78-6 | - | 14.46 ± 5.7 |
| C14 | ethyl tiglate | C7H12O2 | 4.911 | 5837-78-5 | - | 0.84 ± 1.34 |
| C15 | ethyl hexanoate | C8H16O2 | 6.774 | 123-66-0 | - | 5.46 ± 2.89 |
| C16 | (3Z)-3-hexen-1-yl acetate | C8H14O2 | 7.425 | 3681-71-8 | - | 2.85 ± 1.38 |
| C17 | hex-2-enoic acid ethyl ester | C8H14O2 | 8.352 | 1552-67-6 | - | 0.18 ± 0.17 |
| C18 | sec-heptyl acetate | C9H18O2 | 8.547 | 5921-82-4 | 1.92 ± 0.96 | - |
| C19 | ethyl benzoate | C9H10O2 | 13.07 | 93-89-0 | - | 0.47 ± 0.15 |
| C20 | ethyl caprylate | C10H20O2 | 13.557 | 106-32-1 | - | 3.44 ± 2.21 |
| C21 | methyl salicylate | C8H8O3 | 13.855 | 119-36-8 | 0.46 ± 0.98 | - |
| Alkanes (1 kind) | ||||||
| C22 | tetradecane | C14H30 | 20.732 | 629-59-4 | 0.14 ± 0.08 | - |
| Terpenoids (28 kinds) | ||||||
| C23 | β-ocimene | C10H16 | 8.569 | 3338-55-4 | 0.94 ± 0.48 | - |
| C24 | theaspirane | C13H22O | 17.306 | 36431-72-8 | 0.46 ± 1.33 | - |
| C25 | α-cubebene | C15H24 | 18.948 | 17699-14-8 | 0.09 ± 0.21 | 1.47 ± 2.69 |
| C26 | ylangene | C15H24 | 19.805 | 14912-44-8 | - | 0.11 ± 0.07 |
| C27 | α-ionol | C13H22O | 20.019 | 25312-34-9 | 0.77 ± 0.69 | - |
| C28 | (–)-β-bourbonene | C15H24 | 20.131 | 5208-59-3 | - | 0.30 ± 0.91 |
| C29 | germacrene d | C15H24 | 20.430 | 23986-74-5 | - | 1.06 ± 1.25 |
| C30 | β-copaene | C15H24 | 20.445 | 18252-44-3 | - | 1.17 ± 0.33 |
| C31 | β-maaliene | C15H24 | 21.039 | 489-29-2 | - | 0.18 ± 0.12 |
| C32 | (–)-α-gurjunene | C15H24 | 21.051 | 489-40-7 | - | 0.56 ± 0.31 |
| C33 | caryophyllene | C15H24 | 21.234 | 87-44-5 | 3.56 ± 2.28 | 0.61 ± 1.62 |
| C34 | α-ionone | C13H20O | 21.593 | 127-41-3 | 0.33 ± 0.26 | - |
| C35 | (+)-epi-bicyclosesquiphellandrene | C15H24 | 21.64 | 54274-73-6 | - | 0.42 ± 0.44 |
| C36 | valencene | C15H24 | 22.111 | 4630-07-3 | 1.67 ± 1.60 | 0.46 ± 2.55 |
| C37 | cubenene | C15H24 | 22.188 | 16728-99-7 | - | 0.30 ± 0.20 |
| C38 | cis-muurola-4(15),5-diene | C15H24 | 22.308 | 157477-72-0 | - | 0.38 ± 0.33 |
| C39 | γ-muurolene | C15H24 | 22.658 | 30021-74-0 | - | 0.38 ± 0.41 |
| C40 | epizonarene | C15H24 | 23.038 | 41702-63-0 | - | 1.45 ± 0.44 |
| C41 | δ-cadinene | C15H24 | 23.085 | 483-76-1 | - | 16.16 ± 8.15 |
| C42 | selina-4,11-dien | C15H24 | 23.328 | 103827-22-1 | 7.83 ± 6.99 | 7.21 ± 3.15 |
| C43 | β-selinene | C15H24 | 23.414 | 17066-67-0 | 1.78 ± 1.38 | 1.84 ± 0.50 |
| C44 | α-vetivenen | C15H22 | 23.529 | 28908-26-1 | 1.02 ± 0.83 | - |
| C45 | 3,5,11-eudesmatriene | C15H22 | 23.543 | 193615-07-5 | 1.47 ± 0.70 | 0.85 ± 0.24 |
| C46 | α-muurolene | C15H24 | 23.843 | 31983-22-9 | - | 2.53 ± 1.84 |
| C47 | (r)-γ-cadinene | C15H24 | 24.262 | 39029-41-9 | - | 1.11 ± 0.74 |
| C48 | (–)-α-panasinsen | C15H24 | 24.364 | 56633-28-4 | 1.37 ± 1.85 | 2.01 ± 1.35 |
| C49 | cadinadiene | C8H4 | 24.80 | 29837-12-5 | - | 1.17 ± 0.39 |
| C50 | α-agarofuran | C15H24O | 25.190 | 5956-12-7 | 0.14 ± 0.11 | - |
| Aromatics (9 species) | ||||||
| C51 | styrene | C8H8 | 3.411 | 100-42-5 | 7.80 ± 1.91 | 3.75 ± 3.22 |
| C52 | 4-methoxystyrene | C9H10O | 12.353 | 637-69-4 | - | 0.31 ± 0.28 |
| C53 | estragole | C10H12O | 14.198 | 140-67-0 | - | 1.77 ± 0.89 |
| C54 | anethole | C10H12O | 17.112 | 104-46-1 | - | 0.30 ± 0.20 |
| C55 | α-calacorene | C15H20 | 25.118 | 21391-99-1 | - | 0.81 ± 0.71 |
| C56 | elemicin | C12H16O3 | 25.495 | 487-11-6 | - | 0.06 ± 0.04 |
| C57 | β-calacorene | C15H20 | 25.712 | 50277-34-4 | - | 0.09 ± 0.05 |
| C58 | α-corocalene | C15H20 | 27.422 | 20129-39-9 | - | 0.09 ± 0.06 |
| C59 | cadalin | C15H18 | 28.887 | 483-78-3 | - | 0.15 ± 0.15 |
| Others (2 kinds) | ||||||
| C60 | cis-muurola-3,5-diene | C17H22N4O | 22.185 | 1000365-95-4 | - | 0.26 ± 0.19 |
| C61 | Z,Z,Z-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene | C13H10O | 22.402 | 1000062-61-9 | 1.98 ± 2.31 | 2.01 ± 1.86 |
| No. | Compound | T (mg/kg) | Odor Description | ROAVs | |
|---|---|---|---|---|---|
| RS | RR | ||||
| 1 | 2-nonanol | 0.07 | Waxy, creamy, citrus, orange, cheese, fruity | 1.76 | 0.36 |
| 2 | (E)-2-hexenal | 0.4286 | green banana, fatty, cheesy | 15.15 | 0.19 |
| 3 | nonanal | 0.0011 | Waxy, rose, fresh orris, orange peel, fatty | 100.00 | 20.64 |
| 4 | ethyl butanoate | 0.0009 | fruity, pineapple, brandy | <0.1 | 100.00 |
| 5 | ethyl hexanoate | 0.005 | sweet, fruity, pineapple, waxy, green banana | <0.1 | 24.76 |
| 6 | (3Z)-3-hexen-1-yl acetate | 0.031 | sweet, fruity, green banana, apple, grassy | <0.1 | 2.08 |
| 7 | ethyl caprylate | 0.0193 | Fruity, wine, waxy, sweet, apricot, green banana, brandy, pear | <0.1 | 4.04 |
| 8 | methyl salicylate | 0.04 | Wintergreen, mint | 1.57 | <0.1 |
| 9 | β-ocimene | 0.034 | Floral, herb, flower, sweet | 3.75 | <0.1 |
| 10 | caryophyllene | 0.064 | sweet, woody, spice, clove | 7.54 | 0.22 |
| 11 | α-ionone | 0.0106 | Sweet, woody, floral, violet orris, fruity | 4.19 | <0.1 |
| 12 | styrene | 0.065 | sweet, balsam, floral, plastic | 16.26 | 1.31 |
| No. | Compound | OPLS-DA | RF | XA |
|---|---|---|---|---|
| VIP | Gini | |||
| C1 | (E)-2-hexenal | 1.52633 | 0.69 | 1.00 |
| C2 | ethyl caprylate | 1.50715 | 0.65 | 0.96 |
| C3 | β-ocimene | 1.22437 | 0.33 | 0.62 |
| C4 | ethyl butanoate | 1.12642 | 0.64 | 0.82 |
| C5 | styrene | 0.945676 | 0.25 | 0.46 |
| C6 | (3Z)-3-hexen-1-yl acetate | 0.92031 | 0.08 | 0.33 |
| C7 | methyl salicylate | 0.908079 | 0.34 | 0.52 |
| C8 | ethyl hexanoate | 0.905574 | 0.08 | 0.32 |
| C9 | nonanal | 0.760134 | 0.19 | 0.35 |
| C10 | α-ionone | 0.628868 | 0.02 | 0.18 |
| C11 | caryophyllene | 0.502894 | 0.18 | 0.25 |
| C12 | 2-nonanol | 0.137078 | 0.06 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Kan, H.; Li, Y.; Liu, Y.; Qiu, X. Analysis of Volatile Components in Rosa roxburghii Tratt. and Rosa sterilis Using Headspace–Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry. Molecules 2023, 28, 7879. https://doi.org/10.3390/molecules28237879
Deng Y, Kan H, Li Y, Liu Y, Qiu X. Analysis of Volatile Components in Rosa roxburghii Tratt. and Rosa sterilis Using Headspace–Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry. Molecules. 2023; 28(23):7879. https://doi.org/10.3390/molecules28237879
Chicago/Turabian StyleDeng, Yuhang, Huan Kan, Yonghe Li, Yun Liu, and Xu Qiu. 2023. "Analysis of Volatile Components in Rosa roxburghii Tratt. and Rosa sterilis Using Headspace–Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry" Molecules 28, no. 23: 7879. https://doi.org/10.3390/molecules28237879
APA StyleDeng, Y., Kan, H., Li, Y., Liu, Y., & Qiu, X. (2023). Analysis of Volatile Components in Rosa roxburghii Tratt. and Rosa sterilis Using Headspace–Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry. Molecules, 28(23), 7879. https://doi.org/10.3390/molecules28237879







