Cationic Surfactant-Modified Tetraselmis sp. for the Removal of Organic Dyes from Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
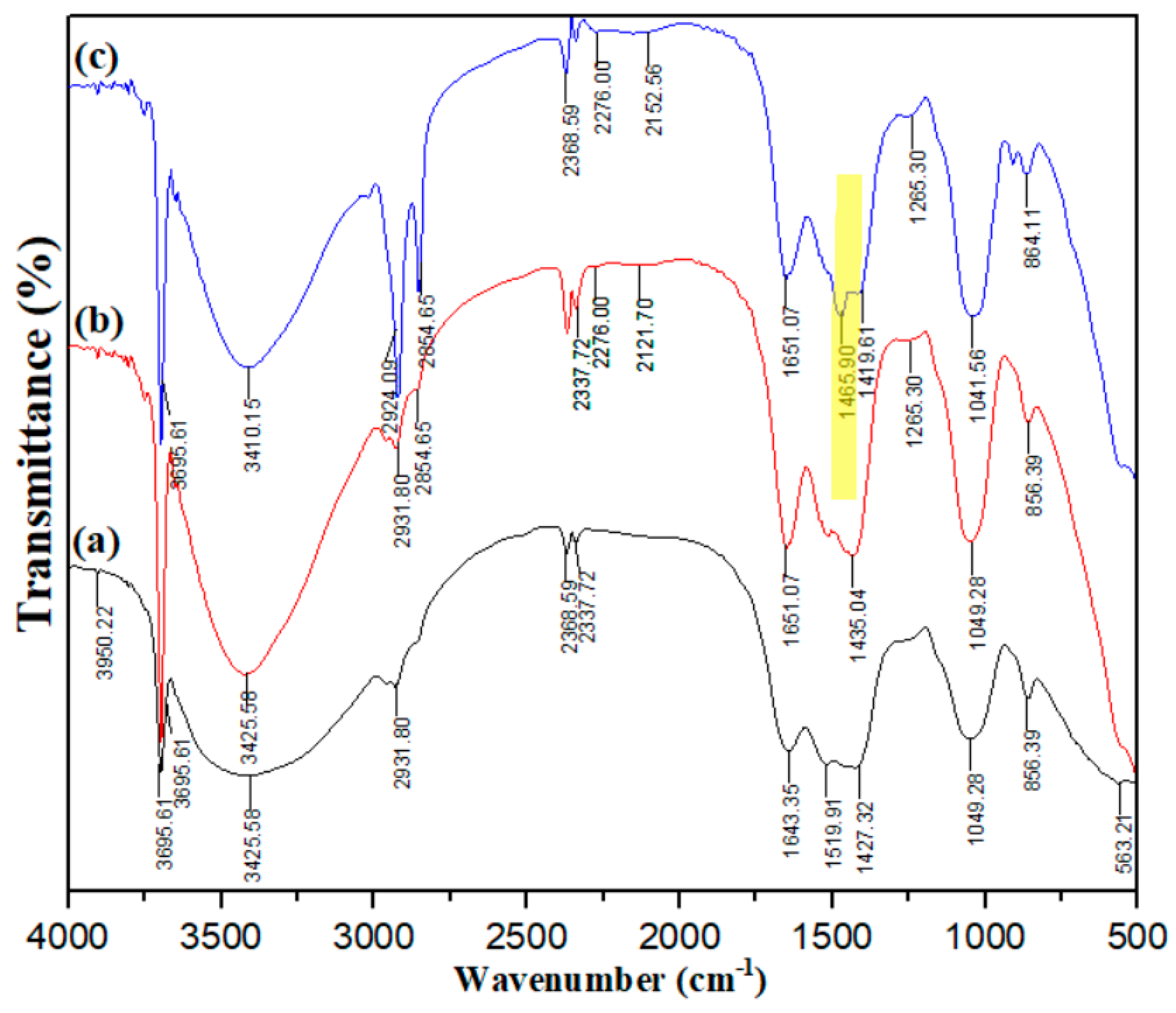
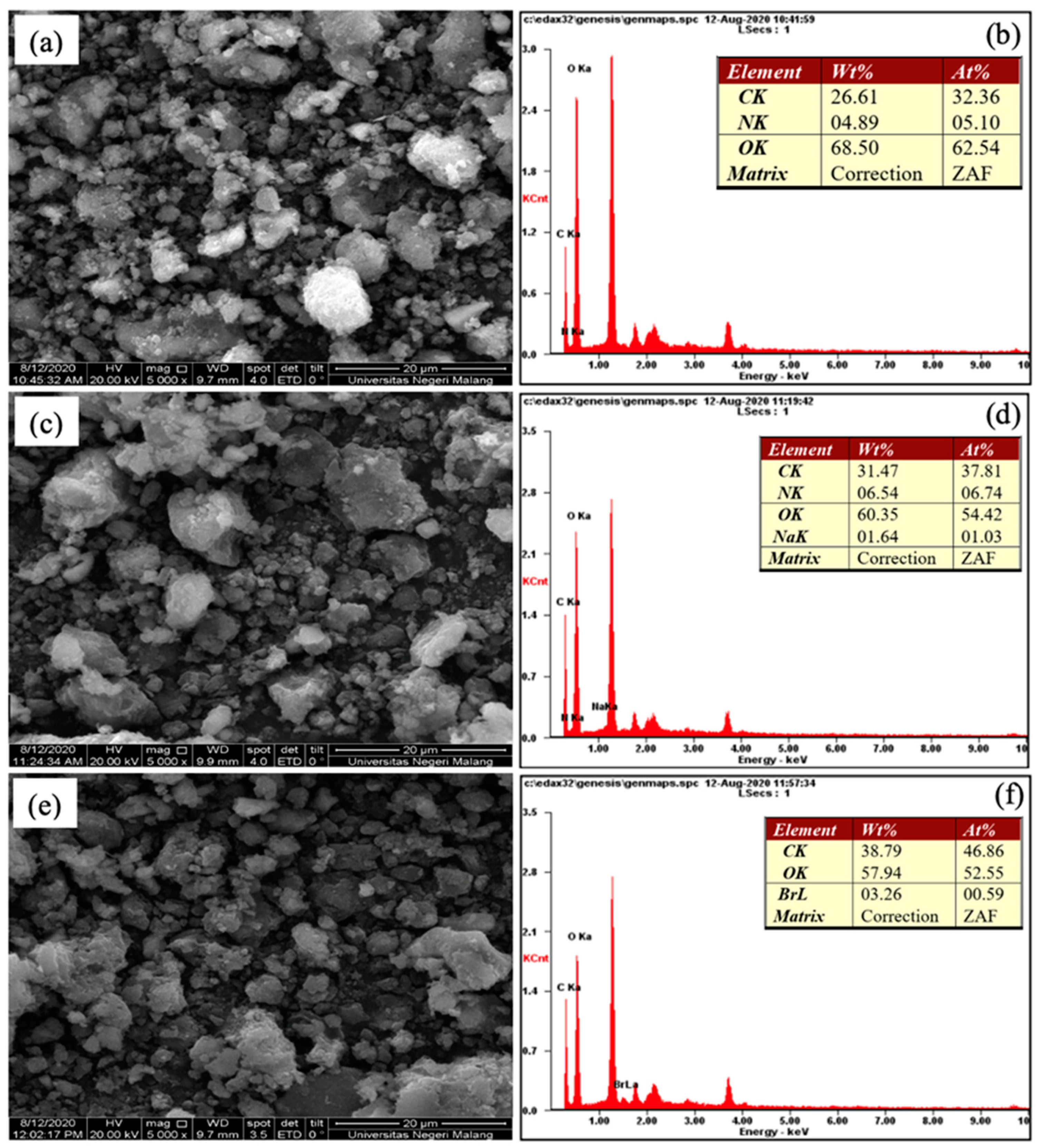
2.1. Adsorbent Characterization
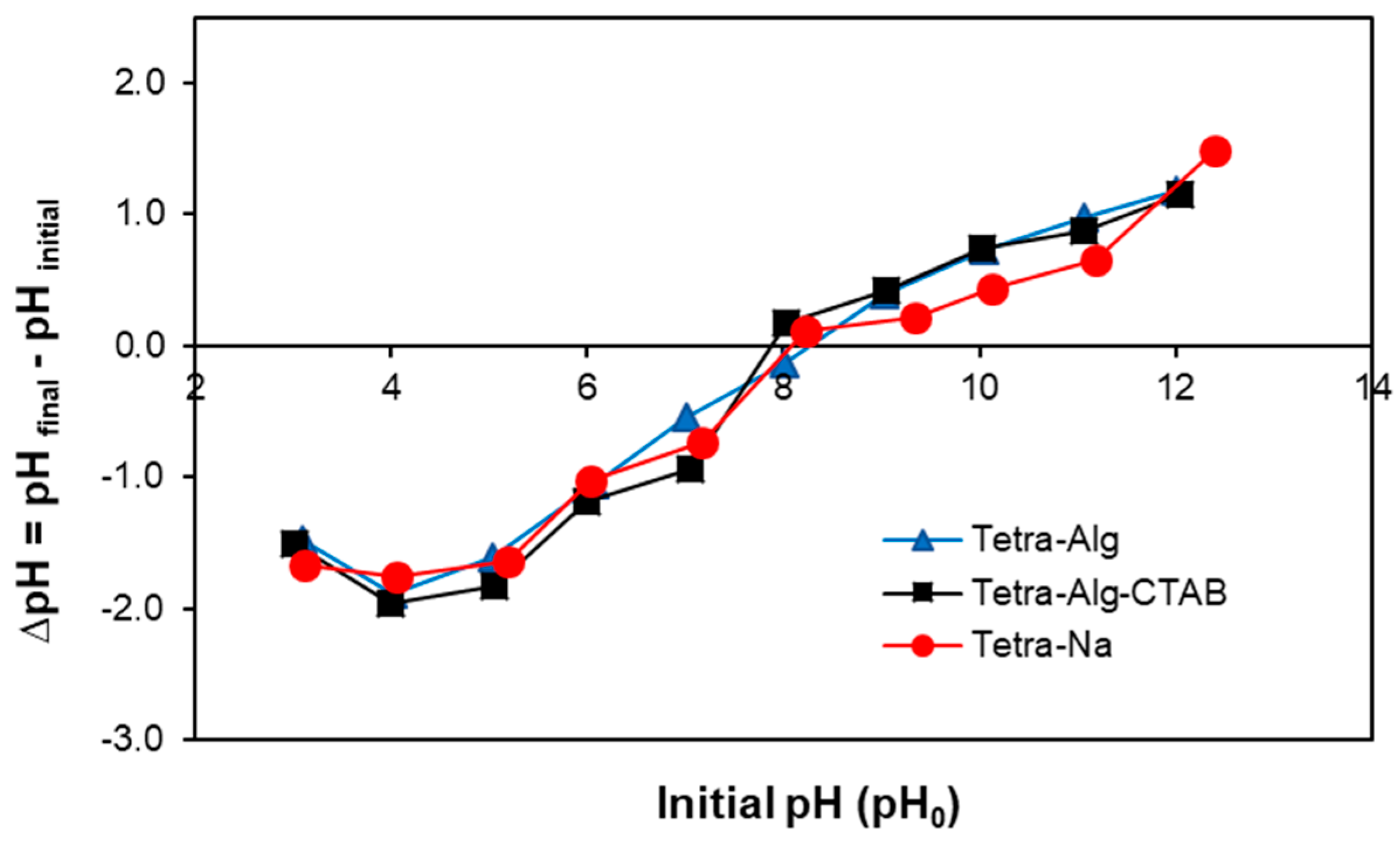
2.2. pHpzc of Adsorbent
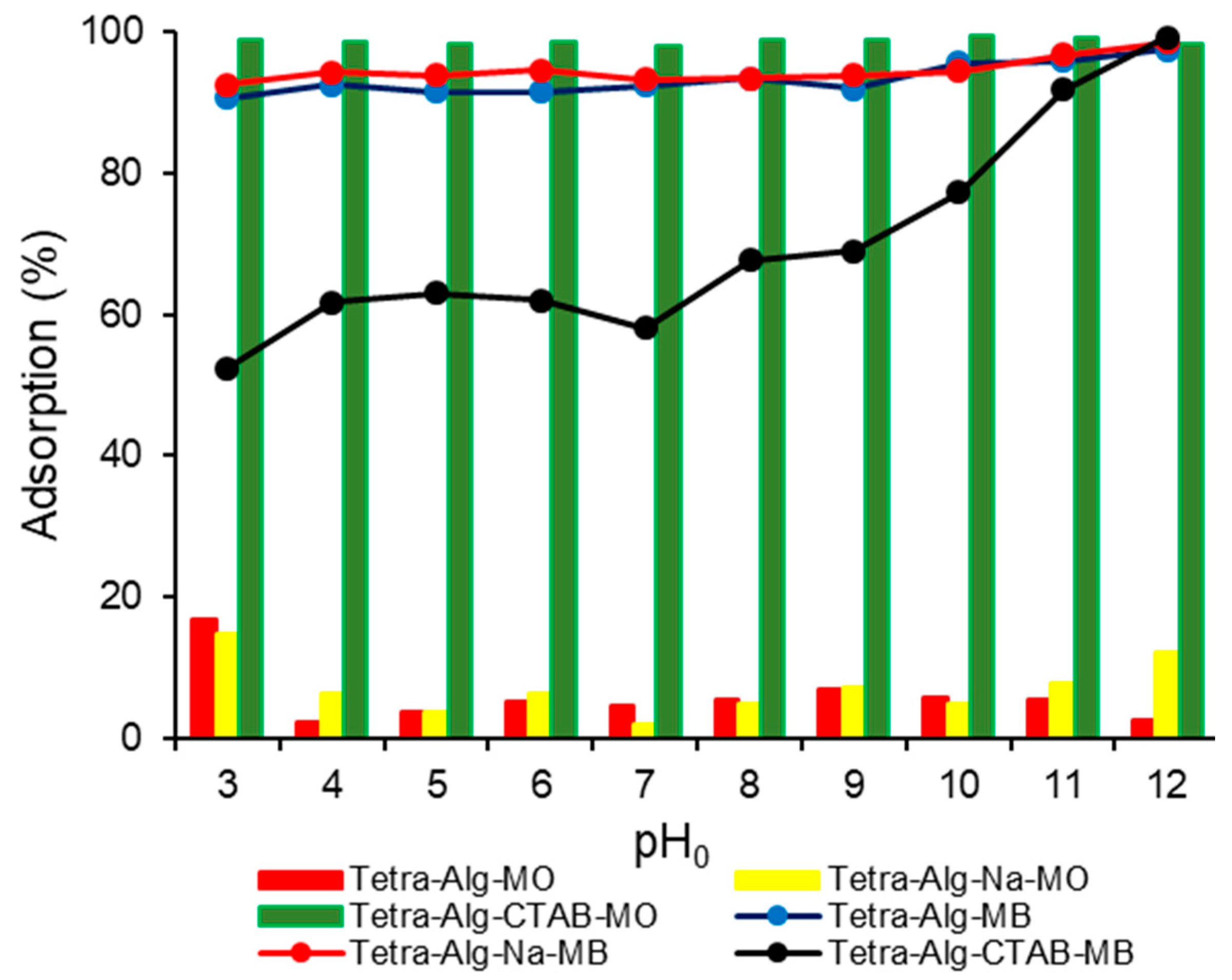
2.3. Effect of Adsorption Initial pH
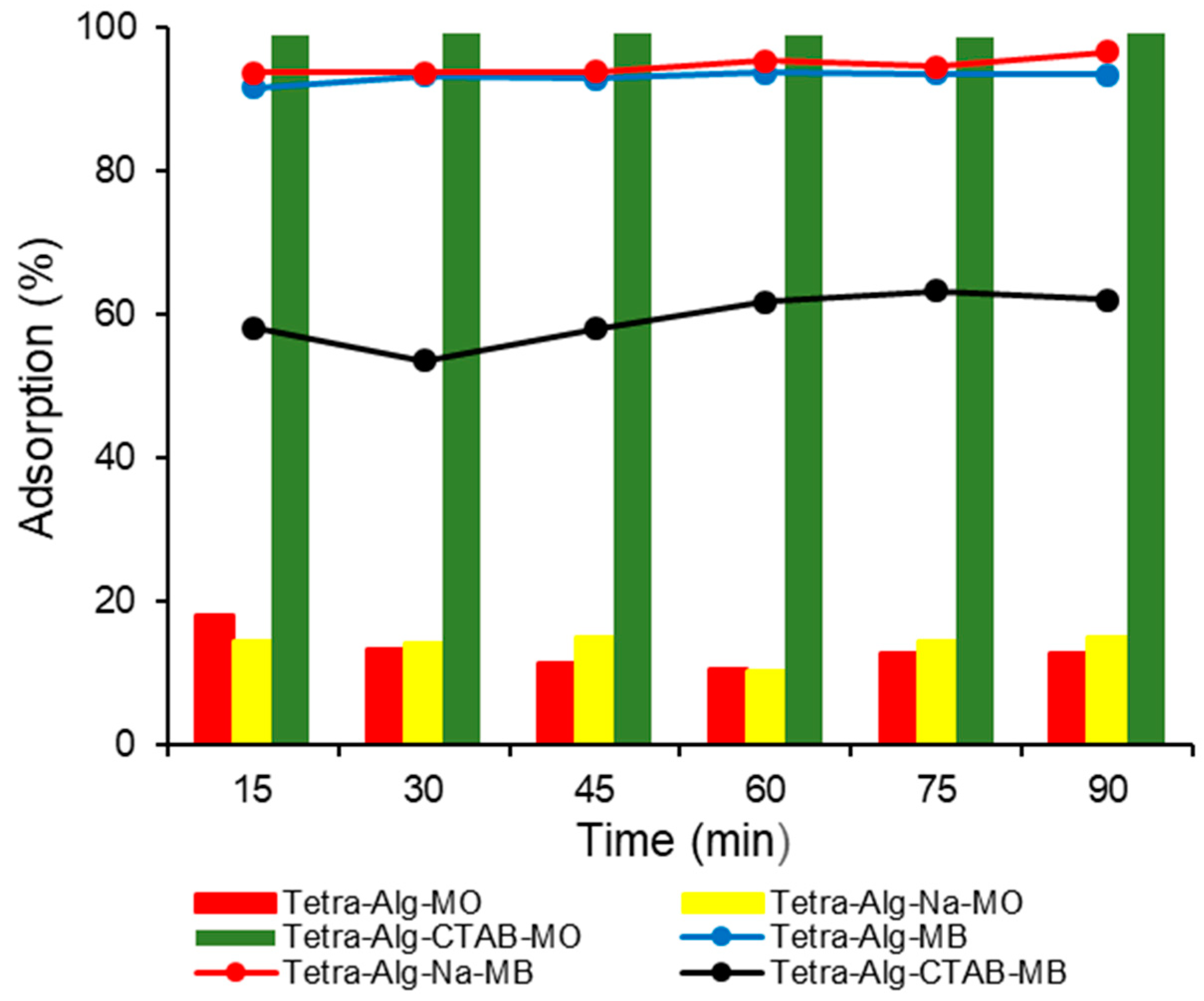
2.4. Effect of Contact Time
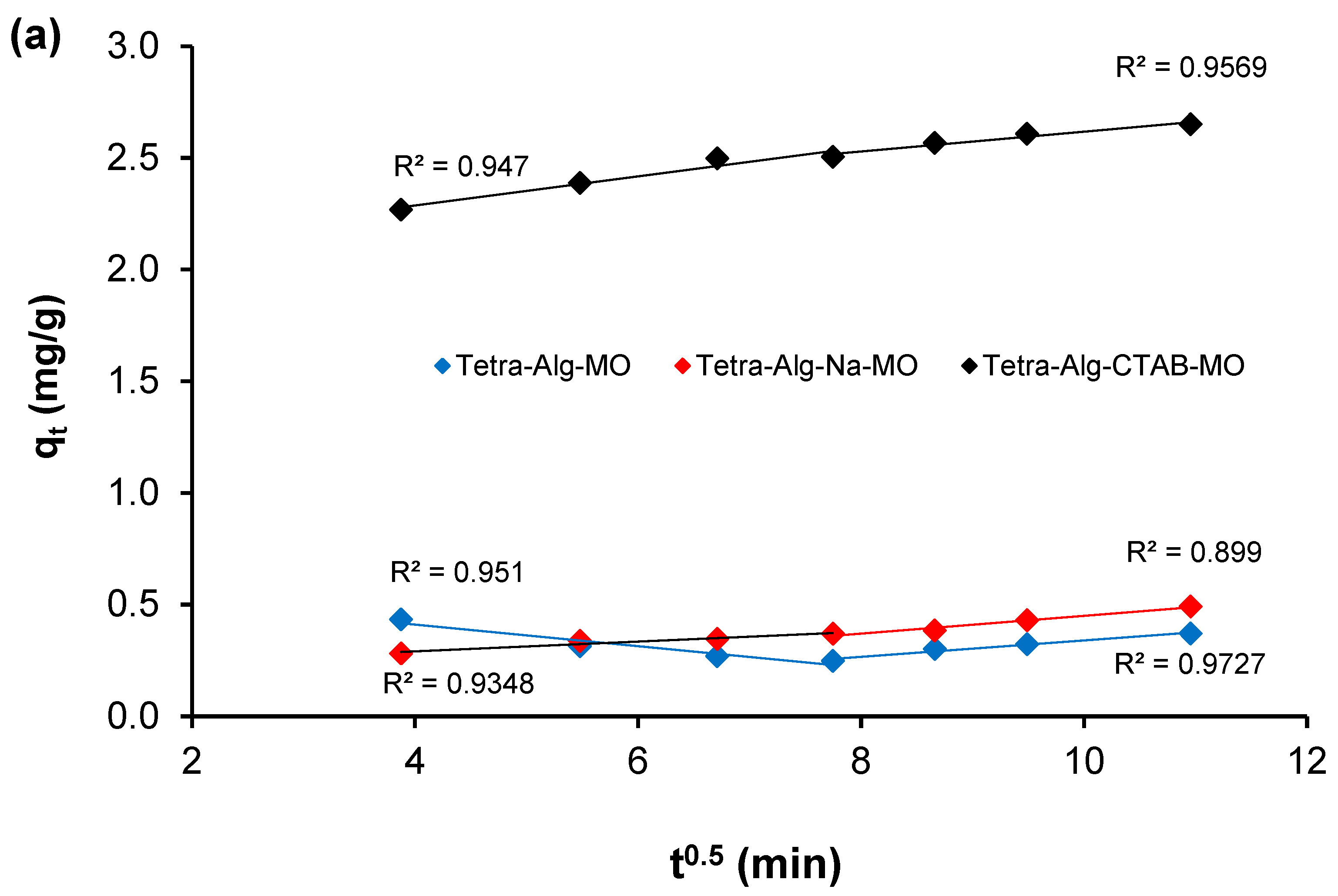
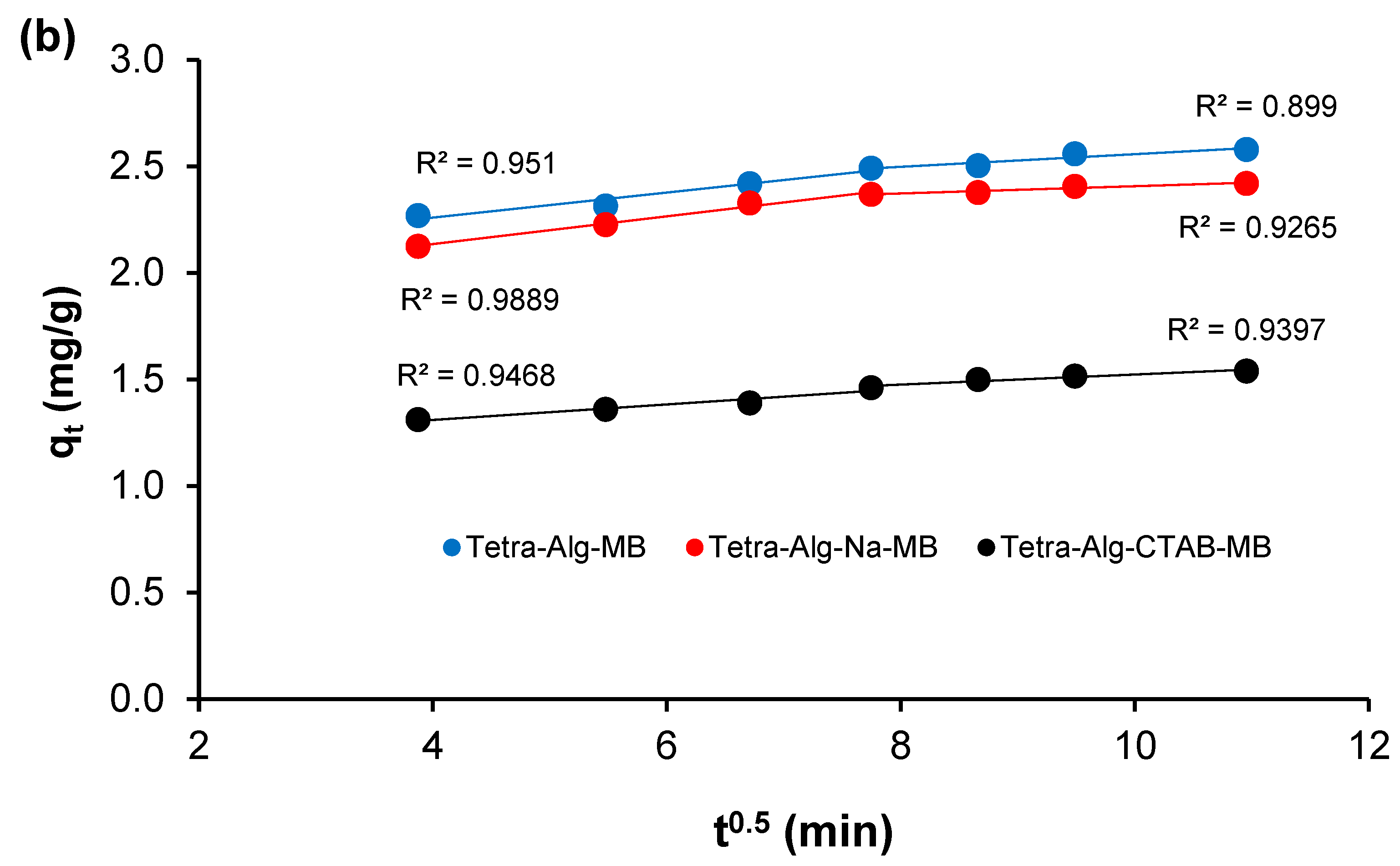
2.5. Adsorption Kinetics
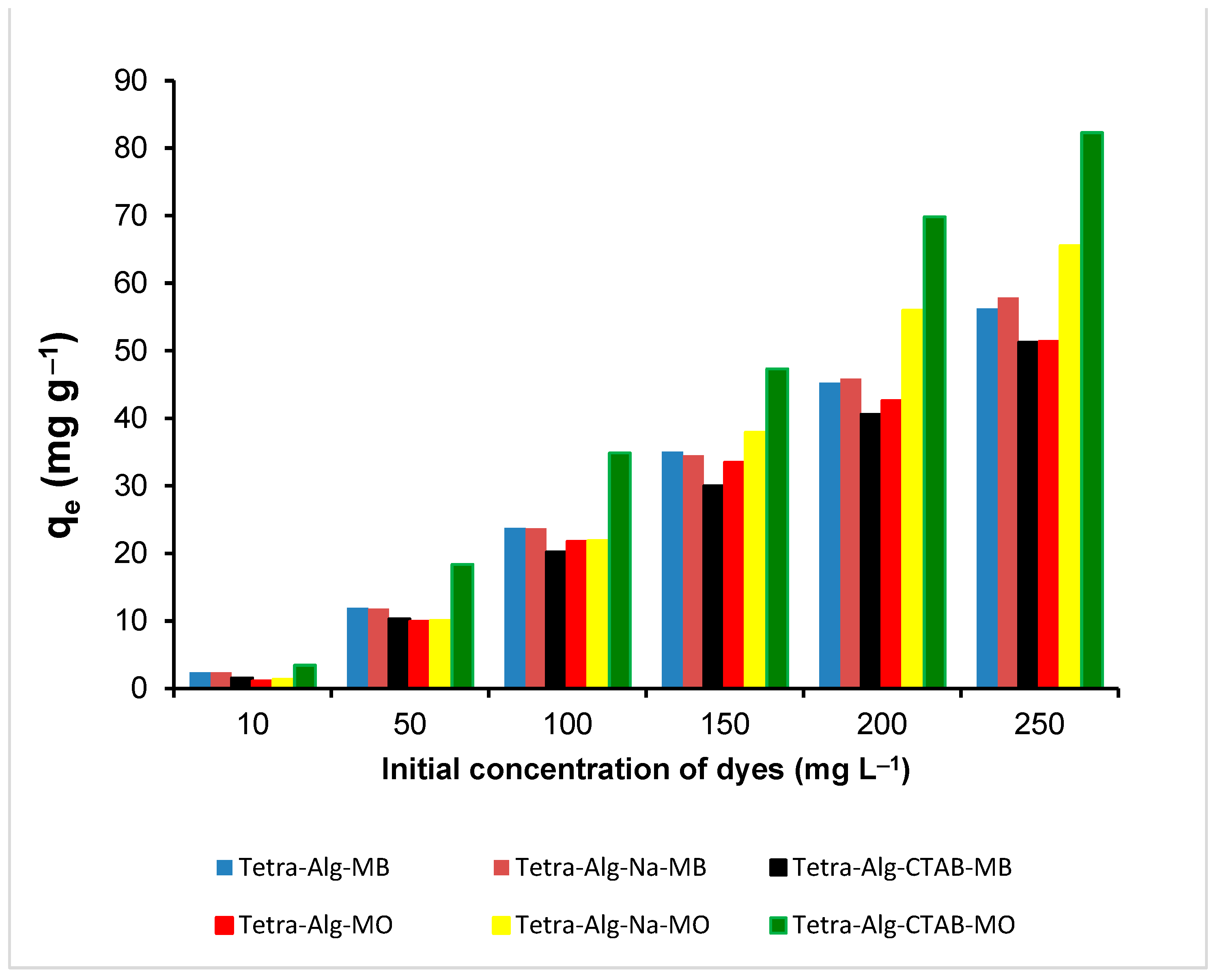
2.6. Adsorption Isotherm
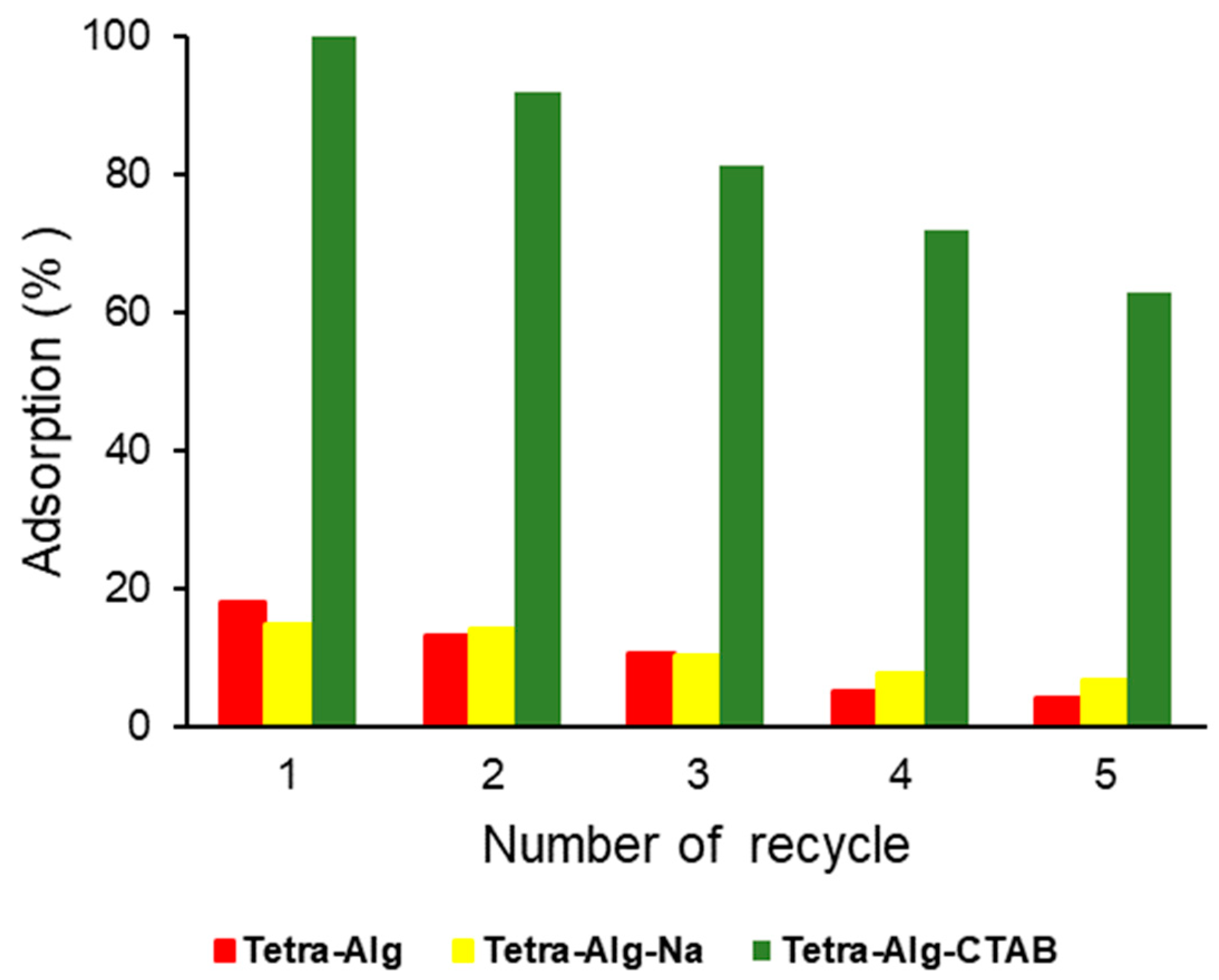
2.7. Adsorbent Reuse
3. Materials and Methods
3.1. Materials and Instruments
3.2. Adsorbent Preparation
3.3. Determination of pHpzc of Adsorbent
3.4. Dye Adsorption Batch Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çelekli, A.; Bozkurt, H. Predictive modeling of an azo metal complex dye sorption by pumpkin husk. Environ. Sci. Pollut. Res. 2013, 20, 7355–7366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Haque, F.; Rahman, M.A.; Parvez, M.A.K.; Mou, T.J. Screening of Methyl Red Degrading Bacteria Isolated from Textile Effluents of Savar Area, Dhaka, Bangladesh. Adv. Biosci. Biotechnol. 2020, 11, 301–318. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Buhani; Halimah, S.N.; Suharso; Sumadi. Utilization of activated carbon from candlenut shells (Aleurites moluccana) as methylene blue adsorbent. Rasayan J. Chem. 2022, 15, 124–131. [Google Scholar] [CrossRef]
- Ma, S.; Lee, S.; Kim, K.; Im, J.; Jeon, H. Purification of organic pollutants in cationic thiazine and azo dye solutions using plasma-based advanced oxidation process via submerged multi-hole dielectric barrier discharge. Sep. Purif. Technol. 2021, 255, 117715. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Heynderickx, P.M.; Verpoort, F. Engineered synthesis of hierarchical porous organic polymers for visible light and natural sunlight induced rapid degradation of azo, thiazine and fluorescein based dyes in a unique mechanistic pathway. Appl. Catal. B 2018, 227, 102–113. [Google Scholar] [CrossRef]
- Teng, M.; Qiao, J.; Li, F.; Bera, P.K. Electrospun mesoporous carbon nanofibers produced from phenolic resin and their use in the adsorption of large dye molecules. Carbon 2012, 50, 2877–2886. [Google Scholar] [CrossRef]
- Khan, T.A.; Khan, E.A. Removal of basic dyes from aqueous solution by adsorption onto binary iron-manganese oxide coated kaolinite: Non-linear isotherm and kinetics modeling. Appl. Clay Sci. 2015, 107, 70–77. [Google Scholar] [CrossRef]
- Gupta, V.; Khamparia, S.; Tyagi, I.; Jaspal, D.; Malviya, A. Decolorization of mixture of dyes: A critical review. Glob. J. Environ. Sci. Manag. 2015, 1, 71–94. [Google Scholar]
- Sana, D.; Jalila, S. A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chin. J. Chem. Eng. 2017, 25, 1282–1287. [Google Scholar] [CrossRef]
- Umpierres, C.S.; Prola, L.D.; Adebayo, M.A.; Lima, E.C.; Dos Reis, G.S.; Kunzler, D.D.; Dotto, G.L.; Arenas, L.T.; Benvenutti, E.V. Mesoporous Nb2O5/SiO2 material obtained by sol-gel method and applied as adsorbent of crystal violet dye. Environ. Technol. 2017, 38, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Kanmani, M.; Debnath, A.; Bhowmik, K.L.; Saha, B. Ultrasonic assisted enhanced adsorption of methyl orange dye onto polyaniline impregnated zinc oxide nanoparticles: Kinetic, isotherm and optimization of process parameters. Ultrason. Sonochem. 2019, 54, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Alventosa-deLara, E.; Barredo-Damas, S.; Alcaina-Miranda, M.; Iborra-Clar, M. Ultrafiltration technology with a ceramic membrane for reactive dye removal: Optimization of membrane performance. J. Hazard. Mater. 2012, 209, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Paul, J.; Rawat, K.; Sarma, K.; Sabharwal, S. Decoloration and degradation of Reactive Red-120 dye by electron beam irradiation in aqueous solution. Appl. Radiat. Isot. 2011, 69, 982–987. [Google Scholar] [CrossRef]
- Ashokan, P.; Asaithambi, M.; Sivakumar, V.; Sivakumar, P. Adsorptive removal of basic orange 21 dye by an adsorbent prepared from Adenanthera paronina L seeds. Rasayan J. Chem. 2022, 15, 1596–1607. [Google Scholar] [CrossRef]
- Vatandoostarani, S.; Lotfabad, T.B.; Heidarinasab, A.; Yaghmaei, S. Degradation of azo dye methyl red by Saccharomyces cerevisiae ATCC 9763. Int. Biodeterior. Biodegrad. 2017, 125, 62–72. [Google Scholar] [CrossRef]
- Buhani; Wijayanti, T.A.; Suharso; Sumadi; Ansori, M. Application of modified green algae Nannochloropsis sp. as adsorbent in the simultaneous adsorption of Methylene Blue and Cu(II) cations in solution. Sustain. Environ. Res. 2021, 31, 17. [Google Scholar] [CrossRef]
- Buhani; Suharso; Aditiya, I.; Kausar, R.A.; Sumadi; Rinawati. Production of a Spirulina sp. algae hybrid with a silica matrix as an effective adsorbent to absorb crystal violet and methylene blue in a solution. Sustain. Environ. Res. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Guler, U.A.; Ersan, M.; Tuncel, E.; Dügenci, F. Mono and simultaneous removal of crystal violet and safranin dyes from aqueous solutions by HDTMA-modified Spirulina sp. Process Saf. Environ. Prot. 2016, 99, 194–206. [Google Scholar] [CrossRef]
- Buhani; Suharso; Miftahza, N.; Permatasari, D.; Sumadi. Improved adsorption capacity of Nannochloropsis sp. through modification with cetyltrimethylammonium bromide on the removal of methyl orange in solution. Adsorpt. Sci. Technol. 2021, 2021, 1641074. [Google Scholar] [CrossRef]
- Angelova, R.; Baldikova, E.; Pospiskova, K.; Maderova, Z.; Safarikova, M.; Safarik, I. Magnetically modified Sargassum horneri biomass as an adsorbent for organic dye removal. J. Clean. Prod. 2016, 137, 189–194. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Yadav, S.; Sharma, A.K.; Kumar, S. Adsorptive studies on the removal of dyes from single and binary systems using Saccharum munja plant-based novel functionalized CNT composites. Environ. Technol. Innov. 2021, 24, 102015. [Google Scholar] [CrossRef]
- Buhani; Rinawati; Suharso; Yuliasari, D.P.; Yuwono, S.D. Removal of Ni (II), Cu (II), and Zn (II) ions from aqueous solution using Tetraselmis sp. biomass modified with silica-coated magnetite nanoparticles. Desalin. Water Treat. 2017, 80, 203–213. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Harris, P.O.; Ramelow, G.J. Binding of metal ions by particulate biomass derived from Chlorella vulgaris and Scenedesmus quadricauda. Environ. Sci. Technol. 1990, 24, 220–228. [Google Scholar] [CrossRef]
- Veglio’, F.; Beolchini, F.; Toro, L. Kinetic modeling of copper biosorption by immobilized biomass. Ind. Eng. Chem. Res. 1998, 37, 1107–1111. [Google Scholar] [CrossRef]
- Chandra, T.S.; Mudliar, S.; Vidyashankar, S.; Mukherji, S.; Sarada, R.; Krishnamurthi, K.; Chauhan, V. Defatted algal biomass as a non-conventional low-cost adsorbent: Surface characterization and methylene blue adsorption characteristics. Bioresour. Technol. 2015, 184, 395–404. [Google Scholar] [CrossRef]
- Jing, X.; Cao, Y.; Zhang, X.; Wang, D.; Wu, X.; Xu, H. Biosorption of Cr (VI) from simulated wastewater using a cationic surfactant modified spent mushroom. Desalination 2011, 269, 120–127. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr (VI) and As (V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Bingol, A.; Aslan, A.; Cakici, A. Biosorption of chromate anions from aqueous solution by a cationic surfactant-modified lichen (Cladonia rangiformis (L.)). J. Hazard. Mater. 2009, 161, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dong, J.; Li, B.; Xue, C.; Tetteh, P.A.; Li, D.; Gao, K.; Deng, X. Using a freshwater green alga Chlorella pyrenoidosa to evaluate the biotoxicity of ionic liquids with different cations and anions. Ecotoxicol. Environ. Saf. 2020, 198, 110604. [Google Scholar] [CrossRef] [PubMed]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kuang, Y.; Liu, N.; Ge, F. Extracellular polymeric substrates of Chlorella vulgaris F1068 weaken stress of cetyltrimethyl ammonium chloride on ammonium uptake. Sci. Total Environ. 2019, 661, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Maged, A.; Elwakeel, K.Z.; El-Gohary, F.; El-Qelish, M. Tuning cationic/anionic dyes sorption from aqueous solution onto green algal biomass for biohydrogen production. Environ. Res. 2023, 216, 114522. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ. Sci. Pollut. Res. 2019, 26, 22704–22722. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Abdel-Wahab, Z.H.; Elwakeel, K.Z. Extraction and separation studies of silver(I) and copper(II) from their aqueous solution using chemically modified melamine resins. Hydrometallurgy 2009, 96, 27–34. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Mostafa, H.Y.; Zaki, E.G.; ElSaeed, S.M.; Elwakeel, K.Z.; Akhdhar, A.; Guibal, E. Methylene blue removal from aqueous solutions using a biochar/gellan gum hydrogel composite: Effect of agitation mode on sorption kinetics. Int. J. Biol. Macromol. 2023, 232, 123355. [Google Scholar] [CrossRef]
- Liang, Y.-D.; He, Y.-J.; Wang, T.-T.; Lei, L.-H. Adsorptive removal of gentian violet from aqueous solution using CoFe2O4/activated carbon magnetic composite. J. Water Process. Eng. 2019, 27, 77–88. [Google Scholar] [CrossRef]
- AbdEl-Salam, A.; Ewais, H.; Basaleh, A. Silver nanoparticles immobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions. A kinetic study. J. Mol. Liq. 2017, 248, 833–841. [Google Scholar] [CrossRef]
- Ebadollahzadeh, H.; Zabihi, M. Competitive adsorption of methylene blue and Pb (II) ions on the nano-magnetic activated carbon and alumina. Mater. Chem. Phys. 2020, 248, 122893. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Bao, C.; Ma, J.; Liu, M.; Wang, F. Magnetic responsive metal–organic frameworks nanosphere with core–shell structure for highly efficient removal of methylene blue. Chem. Eng. J. 2016, 283, 1127–1136. [Google Scholar] [CrossRef]
- Xin, X.; Wei, Q.; Yang, J.; Yan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Larraza, I.; López-Gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid materials: Magnetite–polyethylenimine–montmorillonite, as magnetic adsorbents for Cr (VI) water treatment. J. Colloid Interface Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef]
- Buhani, B.; Suharso, S.; Fitriyani, A.Y. Comparative Study of Adsorption Ability of Ni(II) and Zn(II) Ionic Imprinted Amino-Silica Hybrid Toward Target Metal in Solution. Asian J. Chem. 2013, 25, 2875–2880. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Q.; Zhang, L.; Yang, B. Utilization of cross-linked chitosan/bentonite composite in the removal of methyl orange from aqueous solution. Water Sci. Technol. 2015, 71, 174–182. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Ou, L. Kinetic, isotherm, and thermodynamic studies of the adsorption of methyl orange from aqueous solution by chitosan/alumina composite. J. Chem. Eng. Data 2012, 57, 412–419. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Y.; Zheng, X. Comparison of adsorption behaviors of Fe-La oxides co-loaded MgO nanosheets for the removal of methyl orange and phosphate in single and binary systems. J. Environ. Chem. Eng. 2020, 8, 104252. [Google Scholar] [CrossRef]
- Su, Y.; Jiao, Y.; Dou, C.; Han, R. Biosorption of methyl orange from aqueous solutions using cationic surfactant-modified wheat straw in batch mode. Desalin. Water Treat. 2014, 52, 6145–6155. [Google Scholar] [CrossRef]
- Hambisa, A.A.; Regasa, M.B.; Ejigu, H.G.; Senbeto, C.B. Adsorption studies of methyl orange dye removal from aqueous solution using Anchote peel-based agricultural waste adsorbent. Appl. Water Sci. 2023, 13, 24. [Google Scholar] [CrossRef]
- Jamwal, H.S.; Kumari, S.; Chauhan, G.S.; Reddy, N.; Ahn, J.-H. Silica-polymer hybrid materials as methylene blue adsorbents. J. Environ. Chem. Eng. 2017, 5, 103–113. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Uddin, M.K.; Isaac, R.; Aldosari, O.F. An Effective Biomass for the Adsorption of Methylene Blue Dye and Treatment of River Water. Adsorpt. Sci. Technol. 2022, 2022, 4143138. [Google Scholar] [CrossRef]
- Varghese, S.P.; Babu, A.T.; Babu, B.; Antony, R. γ-MnOOH nanorods: Efficient adsorbent for removal of methylene blue from aqueous solutions. J. Water Process. Eng. 2017, 19, 1–7. [Google Scholar] [CrossRef]
| Adsorbent | Pseudo-Second-Order (PSO) | |||||||
|---|---|---|---|---|---|---|---|---|
| MO | MB | |||||||
| qe-exp (mg g−1) | qe-PSO (mg g−1) | k2 × 10−3 (g mg−1 min−1) | R2 | qe-exp (mg g−1) | qe-PSO (mg g−1) | k2 × 10−3 (g mg−1 min−1) | R2 | |
| Tetra-Alg | 0.301 | 0.290 | 6.032 | 0.999 | 2.307 | 2.340 | 0.758 | 0.999 |
| Tetra-Alg-Na | 0.357 | 0.357 | 9.551 | 0.994 | 2.407 | 2.363 | 0.749 | 0.999 |
| Tetra-Alg-CTAB | 2.479 | 2.475 | 12.640 | 0.999 | 1.467 | 1.445 | 0.473 | 0.997 |
| Adsorbent–Adsorbate | Initial Linear Portion | Second Linear Portion | ||||
|---|---|---|---|---|---|---|
| ki1 | C1 | R12 | ki2 | C2 | R22 | |
| (mg g−1 min0.5) | (mg g−1) | (mg g−1 min0.5) | (mg g−1) | |||
| Tetra-Alg-MO | 0.005 | 0.603 | 0.951 | 0.037 | 0.029 | 0.899 |
| Tetra-Alg-Na-MO | 0.022 | 0.203 | 0.935 | 0.04 | 0.053 | 0.973 |
| Tetra-Alg-CTAB-MO | 0.065 | 2.028 | 0.947 | 0.044 | 2.172 | 0.957 |
| Tetra-Alg-MB | 0.059 | 2.024 | 0.951 | 0.030 | 2.261 | 0.900 |
| Tetra-Alg-Na-MB | 0.065 | 1.874 | 0.989 | 0.017 | 2.238 | 0.926 |
| Tetra-Alg-CTAB-MB | 0.037 | 1.164 | 0.947 | 0.024 | 1.287 | 0.940 |
| Adsorbent–Adsorbate | Adsorption Isotherm Parameters | |||||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| qm (mg g−1) | KL × 10−2 (L mg−1) | R2 | KF × 10−2 ((mg g−1) (L mg−1)1/n) | n | R2 | |
| Tetra-Alg-MO | 51.631 | 0.361 | 0.946 | 14.894 | 0.310 | 0.985 |
| Tetra-Alg-Na-MO | 94.389 | 0.209 | 0.914 | 13.364 | 0.362 | 0.990 |
| Tetra-Alg-CTAB-MO | 128.369 | 3.520 | 0.933 | 18.059 | 0.235 | 0.994 |
| Tetra-Alg-MB | 50.055 | 3.395 | 0.915 | 0.407 | 1.187 | 0.995 |
| Tetra-Alg-Na-MB | 58.155 | 2.423 | 0.919 | 0.6.68 | 1.124 | 0.990 |
| Tetra-Alg-CTAB-MB | 51.013 | 0.239 | 0.933 | 0.224 | 0.707 | 0.991 |
| Adsorbent | Dyes | qm (mg g−1) | References |
|---|---|---|---|
| Cross-linked chitosan | MO | 89.29 | [48] |
| Chitosan/alumina composite | MO | 33.00 | [49] |
| Fe-La oxides co-loaded MgO (Fe-La/MgO) nanosheets | MO | 30.38 | [50] |
| Modified wheat straw 3.0 | MO | 50.40 | [51] |
| Anchote peel | MO | 103.03 | [52] |
| Tetra-Alg-CTAB | MO | 128.37 | This research |
| Magnetite-loaded multi-wall carbon nanotubes | MB | 48.00 | [39] |
| Fe3O4@MIL-100(Fe) magnetite composite | MB | 74.00 | [44] |
| Silica-Polymer hybrid | MB | 87.00 | [53] |
| Spirulina sp. algae hybrid with a silica matrix | MB | 74.00 | [19] |
| Neolamarckia cadamba leaves | MB | 101.40 | [54] |
| Tetra-Alg-CTAB | MB | 58.15 | This research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buhani; Istikomah; Suharso; Sumadi; Sutarto; Alghamdi, H.M.; Elwakeel, K.Z. Cationic Surfactant-Modified Tetraselmis sp. for the Removal of Organic Dyes from Aqueous Solution. Molecules 2023, 28, 7839. https://doi.org/10.3390/molecules28237839
Buhani, Istikomah, Suharso, Sumadi, Sutarto, Alghamdi HM, Elwakeel KZ. Cationic Surfactant-Modified Tetraselmis sp. for the Removal of Organic Dyes from Aqueous Solution. Molecules. 2023; 28(23):7839. https://doi.org/10.3390/molecules28237839
Chicago/Turabian StyleBuhani, Istikomah, Suharso, Sumadi, Sutarto, Huda M. Alghamdi, and Khalid Z. Elwakeel. 2023. "Cationic Surfactant-Modified Tetraselmis sp. for the Removal of Organic Dyes from Aqueous Solution" Molecules 28, no. 23: 7839. https://doi.org/10.3390/molecules28237839
APA StyleBuhani, Istikomah, Suharso, Sumadi, Sutarto, Alghamdi, H. M., & Elwakeel, K. Z. (2023). Cationic Surfactant-Modified Tetraselmis sp. for the Removal of Organic Dyes from Aqueous Solution. Molecules, 28(23), 7839. https://doi.org/10.3390/molecules28237839






