Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrolysate Characteristics
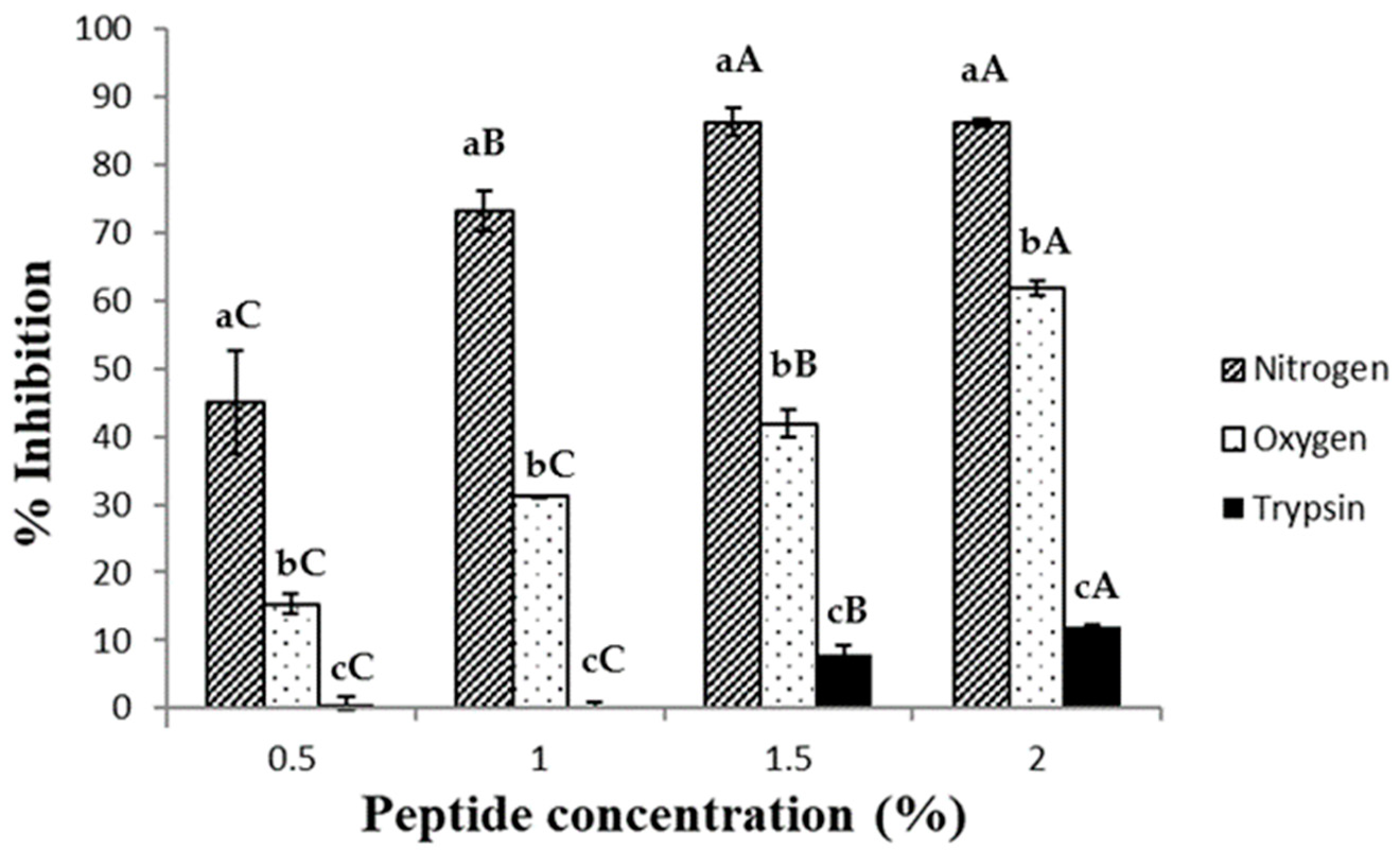
2.2. ABTS•+ Scavenging Assay
2.3. DPPH Radical Scavenging Activity Assay
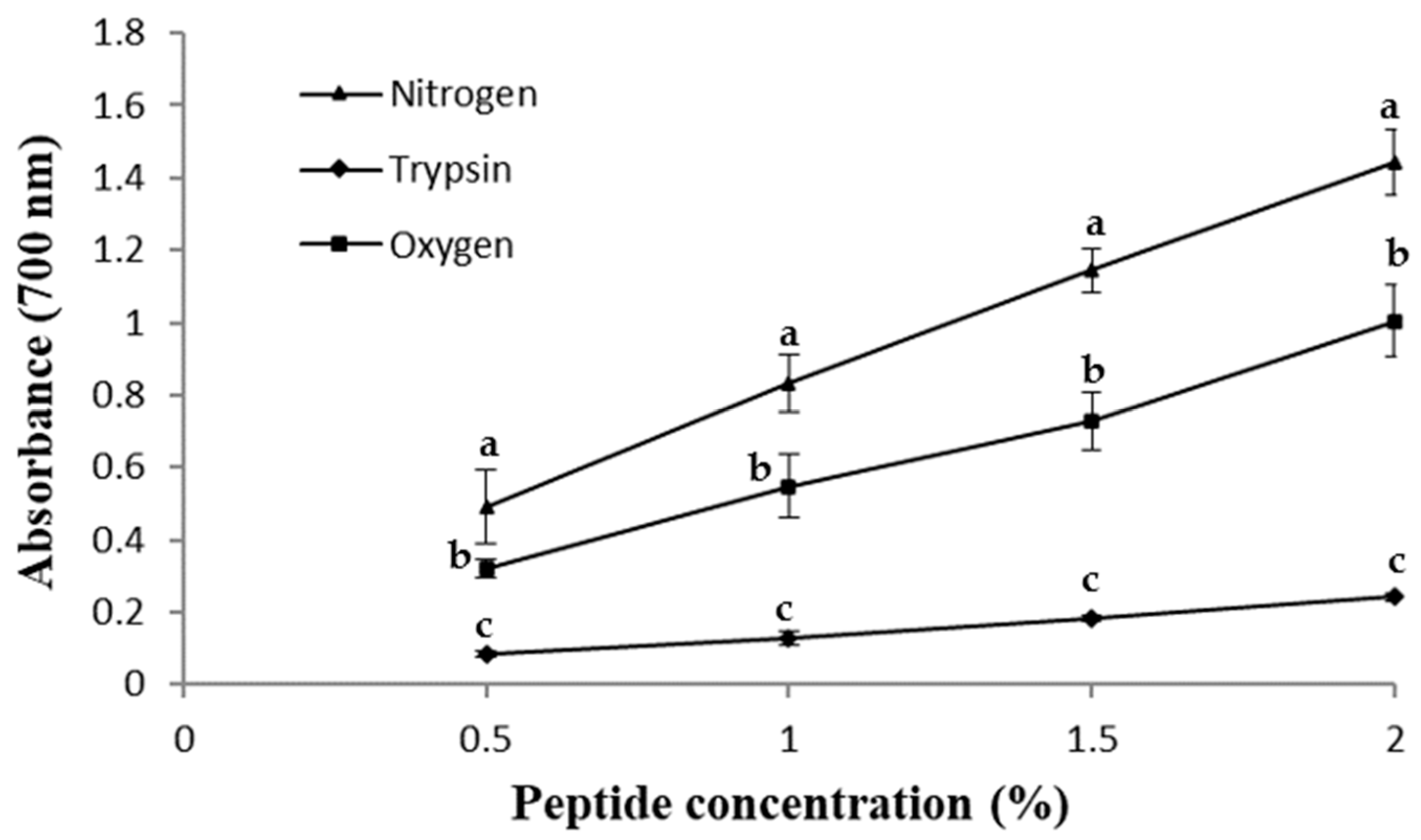
2.4. Peptide Reducing Power (RP)
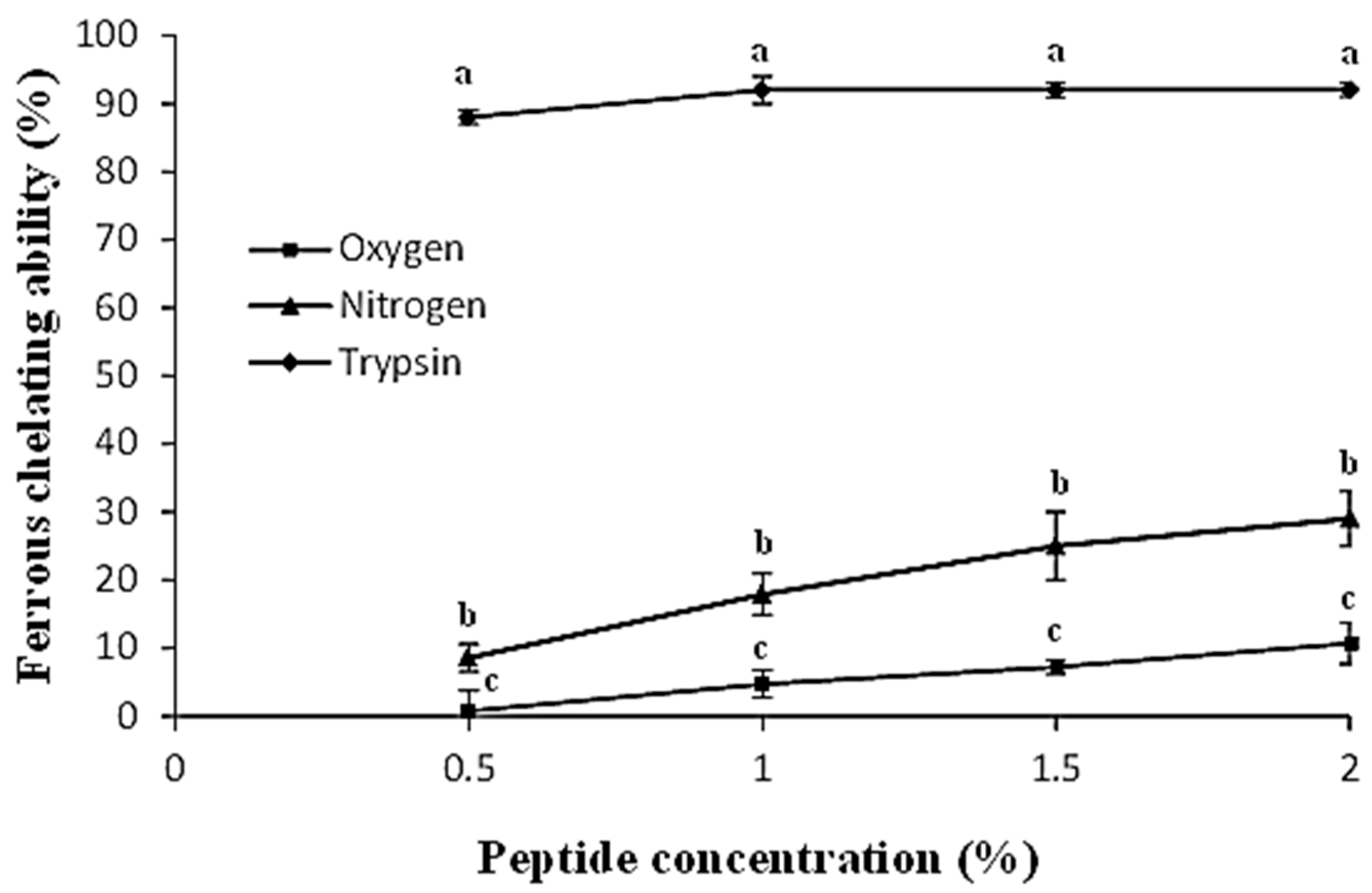
2.5. Ferrous Ion Chelating Assay
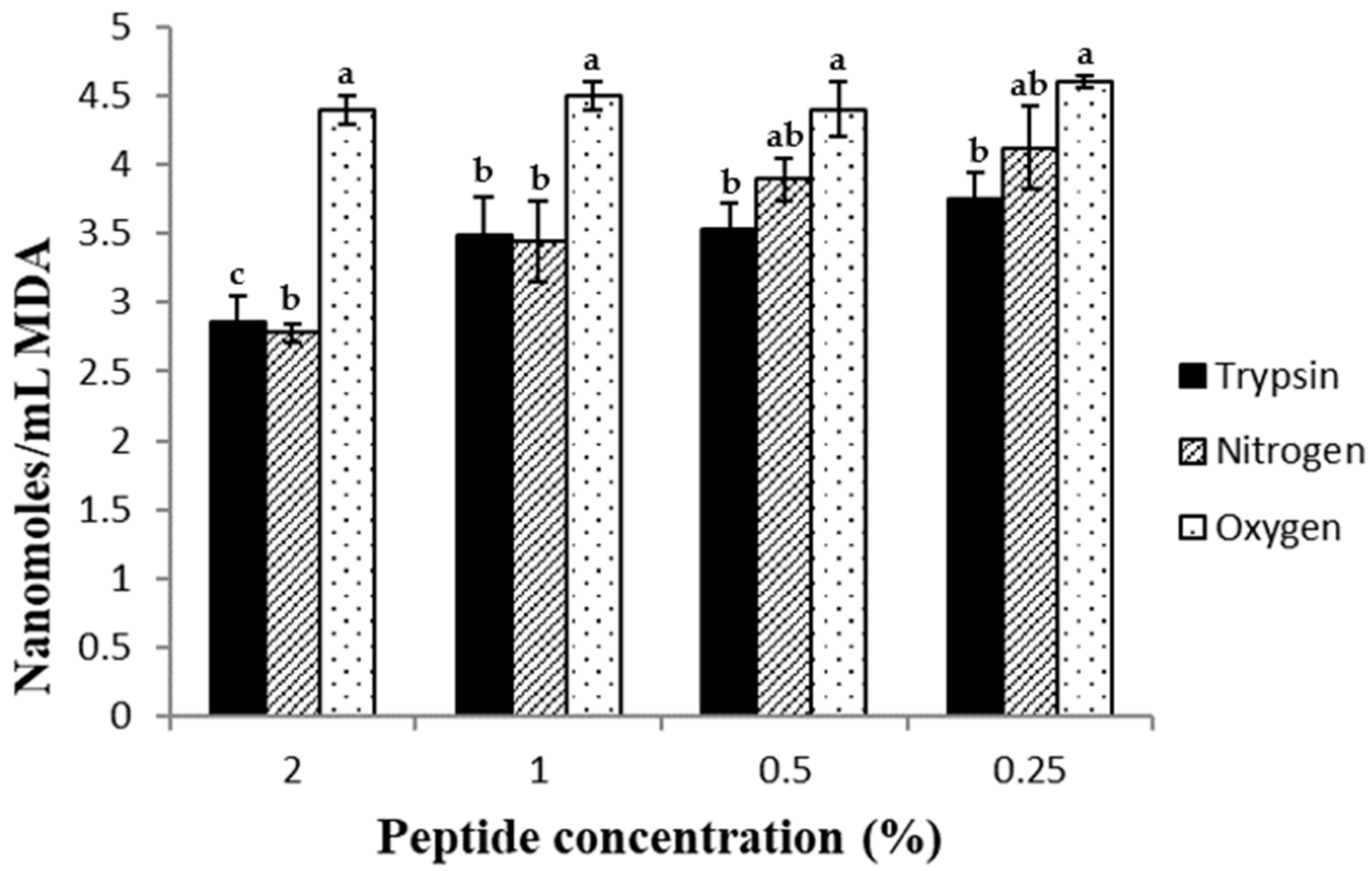
2.6. Antioxidant Effect of the Peptides in Beef Homogenates
2.7. Antimicrobial Test
3. Materials and Methods
3.1. Delipidated Granules Obtention
3.2. Hydrolysates Production
3.3. Amino Acid Analyses
3.4. ABTS•+ Scavenging Assay
3.5. DPPH Radical Scavenging Activity Assay
3.6. Peptide Reducing Power (RP)
3.7. Ferrous Ion Chelating Assay
3.8. Antioxidant Effect of the Peptides on Beef Homogenates
3.9. Antimicrobial Test
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, B.R.; Pearson, A.M.; Shorland, F.B. Effect of Total Lipids and Phospholipids on Warmed-over Flavor in Red and White Muscle from Several Species as Measured by Thiobarbituric Acid Analysis. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf60203a040 (accessed on 7 September 2023).
- Abeyrathne, E.D.N.S.; Nam, K.; Huang, X.; Ahn, D.U. Plant- and Animal-Based Antioxidants’ Structure, Efficacy, Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Marcet, I.; Delgado, J.; Díaz, N.; Rendueles, M.; Díaz, M. Peptides Recovery from Egg Yolk Lipovitellins by Ultrafiltration and Their in Silico Bioactivity Analysis. Food Chem. 2022, 379, 132145. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Luo, Z.; Ban, Z.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.; Li, X.; Li, L. Bioactive Peptides of Plant Origin: Distribution, Functionality, and Evidence of Benefits in Food and Health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Toldrá, F. Advanced Enzymatic Hydrolysis of Food Proteins for the Production of Bioactive Peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-Converting Enzyme Inhibitory Activity and Antioxidant Capacity of Bioactive Peptides Derived from Enzymatic Hydrolysis of Buffalo Milk Proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Production of Bioactive Peptides Using Enzymatic Hydrolysis and Identification Antioxidative Peptides from Patin (Pangasius Sutchi) Sarcoplasmic Protein Hydolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Durak, A.; Baraniak, B.; Jakubczyk, A.; Świeca, M. Biologically Active Peptides Obtained by Enzymatic Hydrolysis of Adzuki Bean Seeds. Food Chem. 2013, 141, 2177–2183. [Google Scholar] [CrossRef]
- Marcet, I.; Álvarez, C.; Paredes, B.; Díaz, M. The Use of Sub-Critical Water Hydrolysis for the Recovery of Peptides and Free Amino Acids from Food Processing Wastes. Review of Sources and Main Parameters. Waste Manag. 2016, 49, 364–371. [Google Scholar] [CrossRef]
- Miller, D.J.; Hawthorne, S.B. Solubility of Liquid Organic Flavor and Fragrance Compounds in Subcritical (Hot/Liquid) Water from 298 K to 473 K. J. Chem. Eng. Data 2000, 45, 315–318. [Google Scholar] [CrossRef]
- Marques, B.; Nunes, R.; Araújo-Rodrigues, H.; Pintado, M.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Solubilization and Hydrolysis of Porcine Coagulated Blood Protein Using Sub-Critical Solvent Extraction. Food Bioprocess Technol. 2023, 1–15. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Sanz, M.T.; Benito-Román, O.; Beltrán, S.; Trigueros, E. Subcritical Water as Hydrolytic Medium to Recover and Fractionate the Protein Fraction and Phenolic Compounds from Craft Brewer’s Spent Grain. Food Chem. 2021, 351, 129264. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, A.; Goosen, N.J. A Greener Alternative Using Subcritical Water Extraction to Valorize the Brown Macroalgae Ecklonia Maxima for Bioactive Compounds. J. Appl. Phycol. 2020, 32, 2307–2319. [Google Scholar] [CrossRef]
- Le Denmat, M.; Anton, M.; Beaumal, V. Characterisation of Emulsion Properties and of Interface Composition in O/W Emulsions Prepared with Hen Egg Yolk, Plasma and Granules. Food Hydrocoll. 2000, 14, 539–549. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Boskou, D.; Kiosseoglou, V. Stabilization of Olive Oil–Lemon Juice Emulsion with Polysaccharides. Food Chem. 2005, 90, 627–634. [Google Scholar] [CrossRef]
- Causeret, D.; Matringe, E.; Lorient, D. Ionic Strength and PH Effects on Composition and Microstructure of Yolk Granules. J. Food Sci. 1991, 56, 1532–1536. [Google Scholar] [CrossRef]
- Marcet, I.; Álvarez, C.; Paredes, B.; Díaz, M. Inert and Oxidative Subcritical Water Hydrolysis of Insoluble Egg Yolk Granular Protein, Functional Properties, and Comparison to Enzymatic Hydrolysis. J. Agric. Food Chem. 2014, 62, 8179–8186. [Google Scholar] [CrossRef]
- Pińkowska, H.; Oliveros, E. Application of the Doehlert Matrix for the Determination of the Optimal Conditions of Hydrolysis of Soybean Protein in Subcritical Water. Ind. Eng. Chem. Res. 2014, 53, 1320–1326. [Google Scholar] [CrossRef]
- Esteban, M.B.; García, A.J.; Ramos, P.; Márquez, M.C. Sub-Critical Water Hydrolysis of Hog Hair for Amino Acid Production. Bioresour. Technol. 2010, 101, 2472–2476. [Google Scholar] [CrossRef]
- Di Domenico Ziero, H.; Ampese, L.C.; Sganzerla, W.G.; Torres-Mayanga, P.C.; Timko, M.T.; Mussatto, S.I.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Poultry Feathers for Amino Acids Production. J. Supercrit. Fluids 2022, 181, 105492. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free Radical-Mediated Oxidation of Free Amino Acids and Amino Acid Residues in Proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Lissi, E.A. Reactions of the Radical Cation Derived from 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS·+) with Amino Acids. Kinetics and Mechanism. Can. J. Chem. 2000, 78, 1052–1059. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical Problems When Using ABTS Assay to Assess the Radical-Scavenging Activity of Peptides: Importance of Controlling Reaction PH and Time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Tang, X.; He, Z.; Dai, Y.; Xiong, Y.L.; Xie, M.; Chen, J. Peptide Fractionation and Free Radical Scavenging Activity of Zein Hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y. Active Oxygen Scavenging Activity of Egg-Yolk Protein Hydrolysates and Their Effects on Lipid Oxidation in Beef and Tuna Homogenates. Food Chem. 2006, 95, 243–249. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wu, K.-C.; Chiang, S.-H. Antioxidant Properties and Protein Compositions of Porcine Haemoglobin Hydrolysates. Food Chem. 2007, 100, 1537–1543. [Google Scholar] [CrossRef]
- He, X.; Cao, W.; Zhao, Z.; Zhang, C. Analysis of Protein Composition and Antioxidant Activity of Hydrolysates from Paphia Undulate. J. Food Nutr. Res. 2013, 1, 30–36. [Google Scholar]
- Zambrowicz, A.; Pokora, M.; Eckert, E.; Szołtysik, M.; Anna, D.Ä.; Chrzanowska, J.; Trziszka, T. Antioxidant and Antimicrobial Activity of Lecithin Free Egg Yolk Protein Preparation Hydrolysates Obtained with Digestive Enzymes. Funct. Foods Health Dis. 2012, 2, 487. [Google Scholar] [CrossRef]
- Abe, Y.; Itoh, T.; Adachi, S. Fractionation and Characterization of Hen’s Egg Yolk Phosvitin. J. Food Sci. 1982, 47, 1903–1907. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ağagündüz, D. Bioactivities of Hen’s Egg Yolk Phosvitin and Its Functional Phosphopeptides in Food Industry and Health. J. Food Sci. 2020, 85, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Ben Sassi, C.; Marcet, I.; Rendueles, M.; Díaz, M.; Fattouch, S. Egg Yolk Protein as a Novel Wall Material Used Together with Gum Arabic to Encapsulate Polyphenols Extracted from Phoenix Dactylifera L Pits. LWT 2020, 131, 109778. [Google Scholar] [CrossRef]
- Xu, X.; Katayama, S.; Mine, Y. Antioxidant Activity of Tryptic Digests of Hen Egg Yolk Phosvitin. J. Sci. Food Agric. 2007, 87, 2604–2608. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; García, V.; Rendueles, M.; Díaz, M. Functional Properties of Isolated Porcine Blood Proteins Modified by Maillard’s Reaction. Food Hydrocoll. 2012, 28, 267–274. [Google Scholar] [CrossRef]
- Lee, B.J.; Hendricks, D.G. Antioxidant Effects of L-Carnosine on Liposomes and Beef Homogenates. J. Food Sci. 1997, 62, 931–1000. [Google Scholar] [CrossRef]
- Chaisuwan, B.; Supawong, S. Physicochemical and Antioxidative Characteristics of Rice Bran Protein Extracted Using Subcritical Water as a Pretreatment and Stability in a Functional Drink Model during Storage. Biocatal. Agric. Biotechnol. 2022, 44, 102466. [Google Scholar] [CrossRef]
- Mine, Y.; Ma, F.; Lauriau, S. Antimicrobial Peptides Released by Enzymatic Hydrolysis of Hen Egg White Lysozyme. J. Agric. Food Chem. 2004, 52, 1088–1094. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, S.; Xia, J.; Shao, P.; Sun, P.; Xiang, N. Enhanced Antibacterial Activity of Hen Egg-White Lysozyme against Staphylococcus Aureus and Escherichia Coli Due to Protein Fibrillation. Biomacromolecules 2021, 22, 890–897. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Méndez, P.; Reyes, W.; Romero, H.; Pinto, A.; Carrillo, W. Antibacterial Activity of Hen Egg White Lysozyme Denatured by Thermal and Chemical Treatments. Sci. Pharm. 2018, 86, 48. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A Novel Antioxidant and Antimicrobial Peptide from Hen Egg White Lysozyme Hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Marcet, I.; Sáez, S.; Rendueles, M.; Díaz, M. Edible Films from Residual Delipidated Egg Yolk Proteins. J. Food Sci. Technol. 2017, 54, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Watchararuji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Value-Added Subcritical Water Hydrolysate from Rice Bran and Soybean Meal. Bioresour. Technol. 2008, 99, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf00093a019 (accessed on 11 September 2023).

| Amino Acid | Granules | Enzyme Hydrolysis | Sub-Critical Water Hydrolysis | |
|---|---|---|---|---|
| Trypsin | Nitrogen | Oxygen | ||
| Gly | 5.4 | 5.3 | 9 | 13 |
| Thre | 4.5 | 4.5 | 2.6 | 4 |
| Ser | 12 | 11.8 | 4.4 | 6.3 |
| Cys | 1.6 | 1.6 | 0.5 | 2 |
| Tyr | 2.8 | 2.7 | 2.9 | 0.6 |
| Asp | 9.1 | 9.2 | 3.7 | 6 |
| Glu | 11.3 | 12 | 14.5 | 21 |
| Ala | 7.2 | 7 | 12.3 | 12 |
| Met | 2 | 2 | 2.8 | 1.2 |
| Val | 6.1 | 5.9 | 8 | 9.6 |
| Ile | 5 | 4.9 | 5.2 | 4.7 |
| Leu | 7.9 | 8 | 10 | 7.5 |
| Pro | 5 | 4.9 | 6 | 1.2 |
| Phe | 2.8 | 2.7 | 3.4 | 1.3 |
| His | 2.6 | 2.5 | 2.8 | 1.1 |
| Lys | 7.4 | 7.2 | 5.8 | 3.5 |
| Arg | 7 | 7 | 6.2 | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcet, I.; Carpintero, M.; Rendueles, M.; Díaz, M. Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis. Molecules 2023, 28, 7836. https://doi.org/10.3390/molecules28237836
Marcet I, Carpintero M, Rendueles M, Díaz M. Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis. Molecules. 2023; 28(23):7836. https://doi.org/10.3390/molecules28237836
Chicago/Turabian StyleMarcet, Ismael, María Carpintero, Manuel Rendueles, and Mario Díaz. 2023. "Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis" Molecules 28, no. 23: 7836. https://doi.org/10.3390/molecules28237836
APA StyleMarcet, I., Carpintero, M., Rendueles, M., & Díaz, M. (2023). Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis. Molecules, 28(23), 7836. https://doi.org/10.3390/molecules28237836








