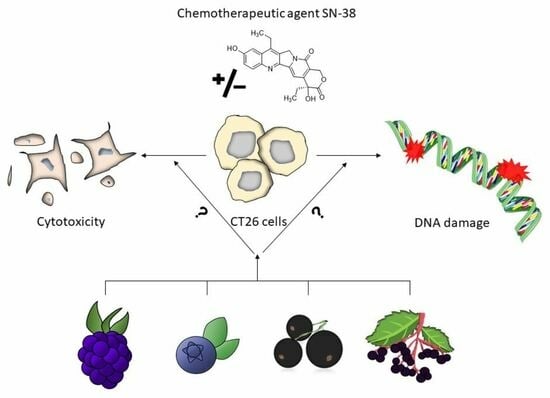
In Vitro Inhibitory Potential of Different Anthocyanin-Rich Berry Extracts in Murine CT26 Colon Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Anthocyanin Content of Berry Extracts
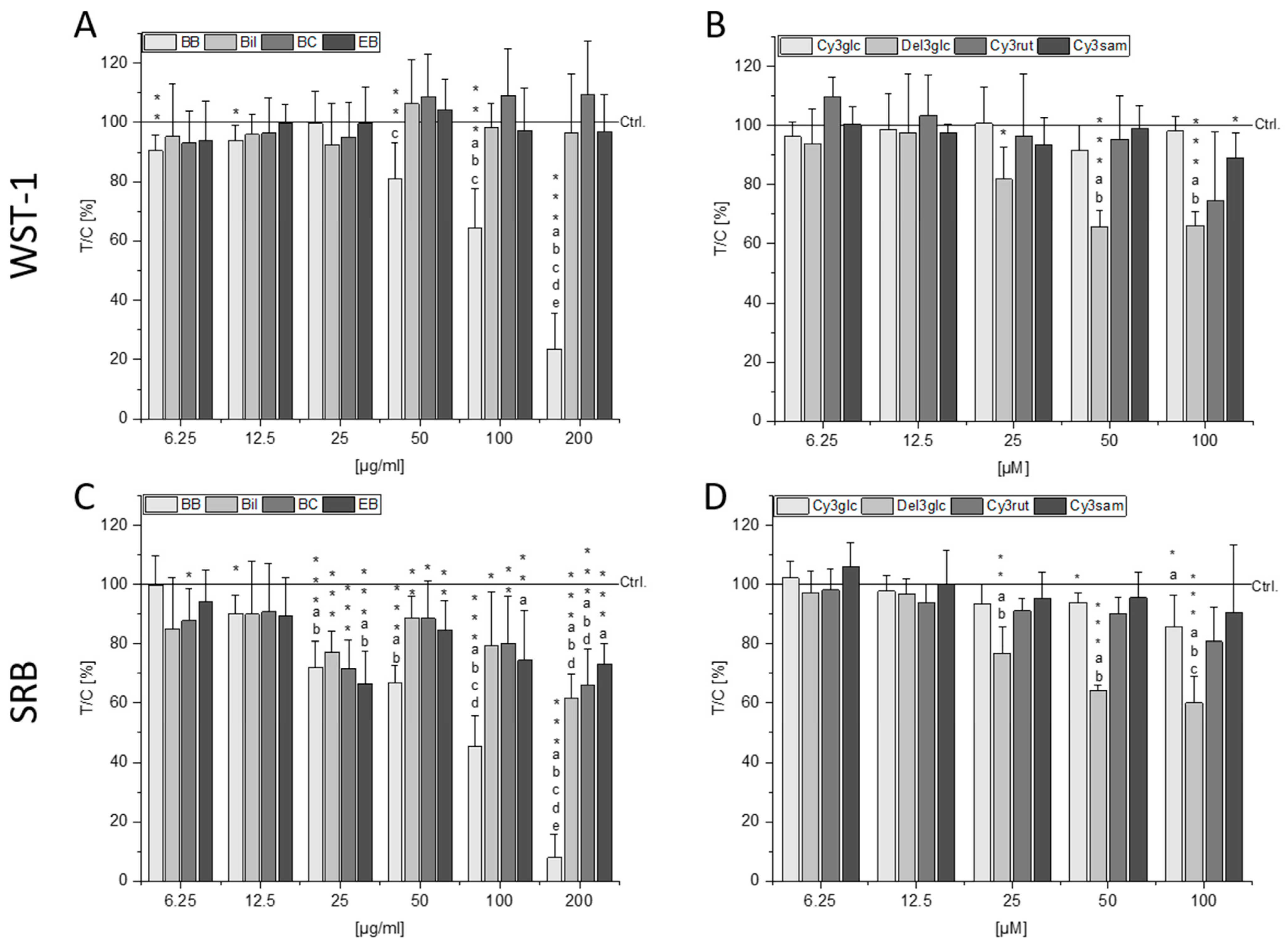
2.2. Cytotoxic Effects of Berry Extracts and Single Anthocyanins
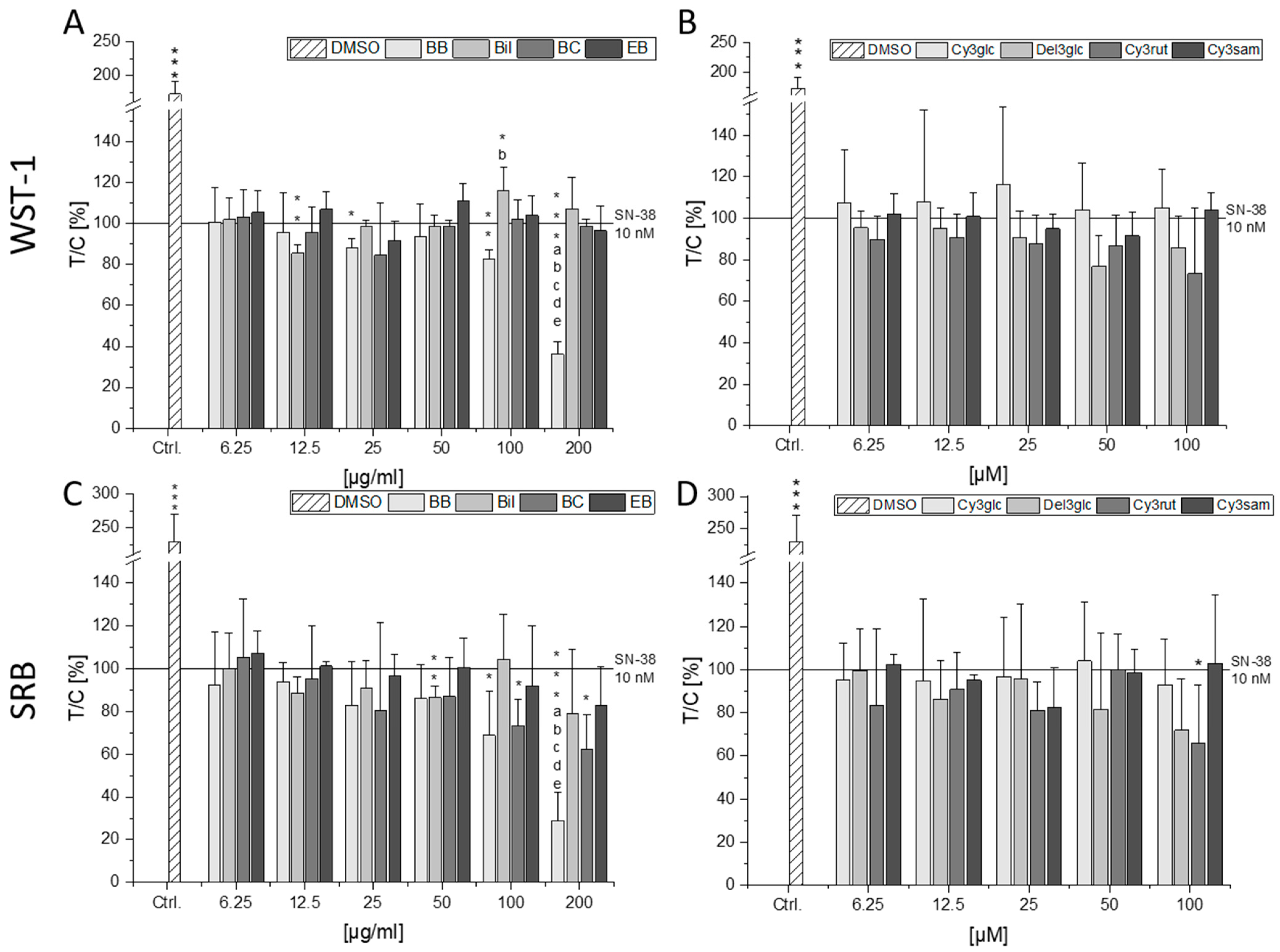
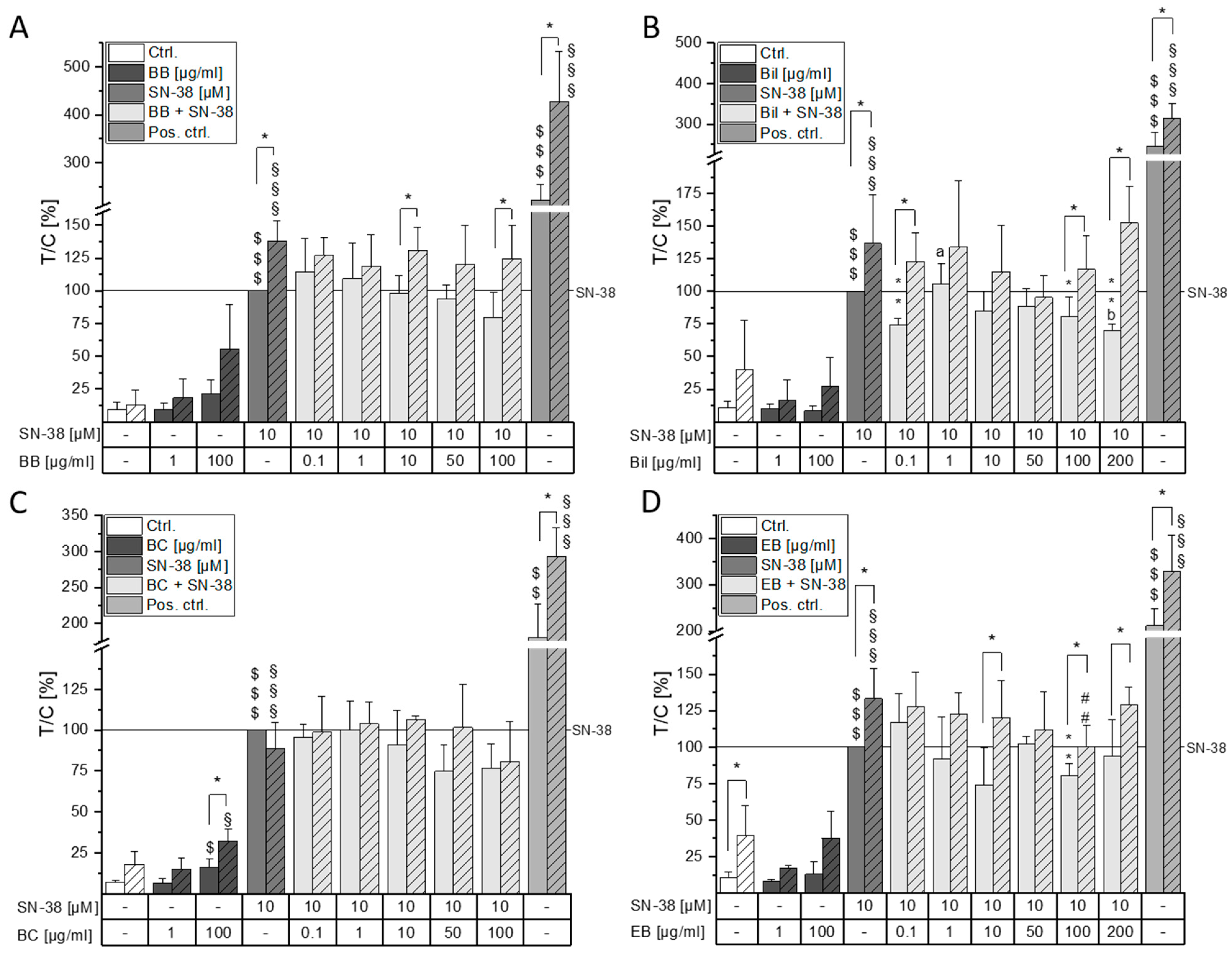
2.3. Combinatory Cytotoxic Effects
2.4. Impact on DNA Damaging Properties of SN-38
2.5. Impact on Cell Adhesion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Juice Manufacturing
4.3. Extract Manufacturing
4.4. HPLC Analysis of Anthocyanins
4.5. Electrospray Ionization (ESI)-MS of Anthocyanins
4.6. Cell Culture
4.7. Treatment and Dosage Information
4.8. Coupled WST-1 and SRB Assay
4.9. Interaction Analysis
4.10. Comet Assay
4.11. Cell Adhesion Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables and Grains; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A.; et al. Pilot Study of Oral Anthocyanins for Colorectal Cancer Chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry Anthocyanins as Novel Antioxidants in Human Health and Disease Prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of Anthocyanins on the Prevention and Treatment of Cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Inhibitory Potential of Anthocyanin-Rich Purple and Red Corn Extracts on Human Colorectal Cancer Cell Proliferation in Vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Comparison of the Effect of Chemical Composition of Anthocyanin-Rich Plant Extracts on Colon Cancer Cell Proliferation and Their Potential Mechanism of Action Using in Vitro, in Silico, and Biochemical Assays. Food Chem. 2018, 242, 378–388. [Google Scholar] [CrossRef]
- Caponio, G.R.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-Proliferative and Pro-Apoptotic Effects of Digested Aglianico Grape Pomace Extract in Human Colorectal Cancer Cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Caliskan, Z.; Yucel, M.F.; Celikok, Y.; Guler, V.; Duranay, S. A Preliminary Study of the Anti-Proliferative Effect of Aronia Melanocarpa Extract on Human Colon Cancer Cells and Its Relation with Human TERT Protein. Exp. Biomed. Res. 2023, 6, 88–98. [Google Scholar] [CrossRef]
- Kahle, K.; Kraus, M.; Scheppach, W.; Ackermann, M.; Ridder, F.; Richling, E. Studies on Apple and Blueberry Fruit Constituents: Do the Polyphenols Reach the Colon after Ingestion? Mol. Nutr. Food Res. 2006, 50, 418–423. [Google Scholar] [CrossRef]
- Stoner, G.D.; Wang, L.S.; Zikri, N.; Chen, T.; Hecht, S.S.; Huang, C.; Sardo, C.; Lechner, J.F. Cancer Prevention with Freeze-Dried Berries and Berry Components. Semin. Cancer Biol. 2007, 17, 403–410. [Google Scholar] [CrossRef]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef]
- Paillas, S.; Boissière, F.; Bibeau, F.; Denouel, A.; Mollevi, C.; Causse, A.; Denis, V.; Vezzio-Vié, N.; Marzi, L.; Cortijo, C.; et al. Targeting the P38 MAPK Pathway Inhibits Irinotecan Resistance in Colon Adenocarcinoma. Cancer Res. 2011, 71, 1041–1049. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Saltz, L.B.; Douillard, J.-Y.; Pirotta, N.; Alakl, M.; Gruia, G.; Awad, L.; Elfring, G.L.; Locker, P.K.; Miller, L.L. Irinotecan Plus Fluorouracil/Leucovorin for Metastatic Colorectal Cancer: A New Survival Standard. Oncologist 2001, 6, 81–91. [Google Scholar] [CrossRef]
- Habermeyer, M.; Fritz, J.; Barthelmes, H.U.; Christensen, M.O.; Larsen, M.K.; Boege, F.; Marko, D. Anthocyanidins Modulate the Activity of Human DNA Topoisomerases I and II and Affect Cellular DNA Integrity. Chem. Res. Toxicol. 2005, 18, 1395–1404. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Jung, H.; Lee, H.J.; Cho, H.; Hwang, K.T. Anti-Inflammatory Activities of Rubus Fruit Anthocyanins in Inflamed Human Intestinal Epithelial Cells. J. Food Biochem. 2015, 39, 300–309. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from Black Soybean Seed Coats Preferentially Inhibit TNF-α-Mediated Induction of VCAM-1 over ICAM-1 through the Regulation of GATAs and IRF-1. J. Agric. Food Chem. 2009, 57, 7324–7330. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I Inhibitors: Camptothecins and Beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Nakajima, J.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. LC/PDA/ESI-MS Profiling and Radical Scavenging Activity of Anthocyanins in Various Berries. J. Biomed. Biotechnol. 2004, 5, 241–247. [Google Scholar] [CrossRef]
- Ştefǎnuţ, M.N.; Cǎta, A.; Pop, R.; Moşoarcǎ, C.; Zamfir, A.D. Anthocyanins HPLC-DAD and MS Characterization, Total Phenolics, and Antioxidant Activity of Some Berries Extracts. Anal. Lett. 2011, 44, 2843–2855. [Google Scholar] [CrossRef]
- Müller, D.; Schantz, M.; Richling, E. High Performance Liquid Chromatography Analysis of Anthocyanins in Bilberries (Vaccinium myrtillus L.), Blueberries (Vaccinium corymbosum L.), and Corresponding Juices. J. Food Sci. 2012, 77, C340–C345. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human Intervention Study to Investigate the Intestinal Accessibility and Bioavailability of Anthocyanins from Bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry Anthocyanins: Isolation, Identification and Antioxidant Activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Cenk, E.; Schmutz, C.; Pahlke, G.; Oertel, A.; Kollarova, J.; Mock, H.P.; Matros, A.; Marko, D. Immunomodulatory Properties of Blackberry Anthocyanins in THP-1 Derived Macrophages. Int. J. Mol. Sci. 2021, 22, 10483. [Google Scholar] [CrossRef]
- Évora, A.; De Freitas, V.; Mateus, N.; Fernandes, I. The Effect of Anthocyanins from Red Wine and Blackberry on the Integrity of a Keratinocyte Model Using ECIS. Food Funct. 2017, 8, 3989–3998. [Google Scholar] [CrossRef]
- Fan-Chiang, H.-J.; Wrolstad, R.E. Anthocyanin Pigment Composition of Blackberries. J. Food Sci. 2005, 70, 198–202. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Medivodovic-Kosanovic, M.; Novak, I. Anthocyanin Content and Antioxidant Activity of Various Red Fruit Juices. Dtsch. Lebensm.-Rundsch. 2007, 2, 58–64. [Google Scholar]
- Iversen, C.K. Black Currant Nectar: Effect of Processing and Storage on Anthocyanin and Ascorbic Acid Content. J. Food Sci. 1999, 64, 37–41. [Google Scholar] [CrossRef]
- Pahlke, G.; Ahlberg, K.; Oertel, A.; Janson-Schaffer, T.; Grabher, S.; Mock, H.P.; Matros, A.; Marko, D. Antioxidant Effects of Elderberry Anthocyanins in Human Colon Carcinoma Cells: A Study on Structure–Activity Relationships. Mol. Nutr. Food Res. 2021, 65, 2100229. [Google Scholar] [CrossRef]
- Da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin Profile of Elderberry Juice: A Natural-Based Bioactive Colouring Ingredient with Potential Food Application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Bridle, P.; Garcia-Viguerab, C. Analysis of Anthocyanins in Strawberries and Elderberries. A Comparison of Capillary Zone Electrophoresis and HPLC. Anal. Nutr. Clin. Methods Sect. 1997, 59, 299–304. [Google Scholar] [CrossRef]
- Aichinger, G.; Pahlke, G.; Nagel, L.J.; Berger, W.; Marko, D. Bilberry Extract, Its Major Polyphenolic Compounds, and the Soy Isoflavone Genistein Antagonize the Cytostatic Drug Erlotinib in Human Epithelial Cells. Food Funct. 2016, 7, 3628–3636. [Google Scholar] [CrossRef]
- Esselen, M.; Fritz, J.; Hutter, M.; Marko, D. Delphinidin Modulates the DNA-Damaging Properties of Topoisomerase II Poisons. Chem. Res. Toxicol. 2009, 22, 554–564. [Google Scholar] [CrossRef]
- Higgins, J.A.; Zainol, M.; Brown, K.; Jones, G.D.D. Anthocyans as Tertiary Chemopreventive Agents in Bladder Cancer: Anti-Oxidant Mechanisms and Interaction with Mitomycin C. Mutagenesis 2014, 29, 227–235. [Google Scholar] [CrossRef]
- Okegawa, T.; Pong, R.-C.; Li, Y.; Hsieh, J.-T. The Role of Cell Adhesion Molecule in Cancer Progression and Its Application in Cancer Therapy. Acta Biochim. Pol. 2004, 51, 445–457. [Google Scholar] [CrossRef]
- Rudolf, E.; John, S.; Cervinka, M. Irinotecan Induces Senescence and Apoptosis in Colonic Cells in Vitro. Toxicol. Lett. 2012, 214, 1–8. [Google Scholar] [CrossRef]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Kuo, W.H.; Chiang, C.L.; Hsieh, Y.S. Mulberry Anthocyanins, Cyanidin 3-Rutinoside and Cyanidin 3-Glucoside, Exhibited an Inhibitory Effect on the Migration and Invasion of a Human Lung Cancer Cell Line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef]
- Xia, M.; Ling, W.; Zhu, H.; Ma, J.; Wang, Q.; Hou, M.; Tang, Z.; Guo, H.; Liu, C.; Ye, Q. Anthocyanin Attenuates CD40-Mediated Endothelial Cell Activation and Apoptosis by Inhibiting CD40-Induced MAPK Activation. Atherosclerosis 2009, 202, 41–47. [Google Scholar] [CrossRef]
- Lamdan, H.; Garcia-Lazaro, R.S.; Lorenzo, N.; Caligiuri, L.G.; Alonso, D.F.; Farina, H.G. Anti-Proliferative Effects of a Blueberry Extract on a Panel of Tumor Cell Lines of Different Origin. Exp. Oncol. 2020, 42, 101–108. [Google Scholar] [CrossRef]
- Antolak, H.; Czyzowska, A.; Sakač, M.; Mišan, A.; Đuragić, O.; Kregiel, D. Phenolic Compounds Contained in Little-Known Wild Fruits as Antiadhesive Agents against the Beverage-Spoiling Bacteria Asaia spp. Molecules 2017, 22, 1256. [Google Scholar] [CrossRef]
- Aichinger, G.; Beisl, J.; Marko, D. Genistein and Delphinidin Antagonize the Genotoxic Effects of the Mycotoxin Alternariol in Human Colon Carcinoma Cells. Mol. Nutr. Food Res. 2017, 61, 1600462. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Webb, J.L. Effect of More than One Inhibitor. Enzym. Metab. Inhib. 1963, 1, 487–512. [Google Scholar]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]


| Anthocyanins | Bilberry | Black Currant | Blackberry | Elderberry | ||||
|---|---|---|---|---|---|---|---|---|
| (g/kg) | % | (g/kg) | % | (g/kg) | % | (g/kg) | % | |
| cyanidin-3-O-glucoside | 13.8 | 13.5 | 2.4 | 5.3 | 67.3 | 88.5 | - | - |
| cyanidin-3-O-arabinoside | 7.5 | 7.3 | - | - | - | - | - | - |
| cyanidin-3-O-galactoside | 9.1 | 8.9 | - | - | - | - | - | - |
| cyanidin-3-O-rutinoside | - | - | 18.2 | 39.4 | 2.3 | 3.0 | - | - |
| cyanidin-3-O-sambubioside | - | - | - | - | - | - | 56.6 | 72.9 |
| cyanidin-3-O-sambubioside-5-O-glucoside | - | - | - | - | - | - | 21.0 | 27.1 |
| cyanidin-3-O-xyloside | - | - | - | - | 1.5 | 1.9 | - | - |
| cyanidin-3-O-(6″-O-malonyl)-glucoside | - | - | - | - | 1.5 | 2.0 | - | - |
| cyanidin-3-O-(6″-O-dioxalyl)-glucoside | - | - | - | - | 3.5 | 4.6 | - | - |
| delphinidin-3-O-glucoside | 17.1 | 16.7 | 5.1 | 11.0 | - | - | - | - |
| delphinidin-3-O-arabinoside | 10.5 | 10.2 | - | - | - | - | - | - |
| delphinidin-3-O-galactoside | 12.6 | 12.3 | - | - | - | - | - | - |
| delphinidin-3-O-rutinoside | - | - | 19.1 | 41.3 | - | - | - | - |
| malvidin-3-O-glucoside | 7.8 | 7.6 | - | - | - | - | - | - |
| malvidin-3-O-arabinoside | 1.2 | 1.1 | - | - | - | - | - | - |
| malvidin-3-O-galactoside | 2.6 | 2.5 | - | - | - | - | - | - |
| peonidin-3-O-glucoside | 4.2 | 4.1 | - | - | - | - | - | - |
| peonidin-3-O-galactoside | 0.6 | 0.6 | - | - | - | - | - | - |
| peonidin-3-O-rutinoside | - | - | 0.5 | 1.1 | - | - | - | - |
| petunidin-3-O-glucoside | 9.5 | 9.3 | - | - | - | - | - | - |
| petunidin-3-O-arabinoside | 2.0 | 2.0 | - | - | - | - | - | - |
| petunidin-3-O-galactoside | 3.8 | 3.7 | - | - | - | - | - | - |
| petunidin-3-O-rutinoside | - | - | 0.9 | 1.9 | - | - | - | - |
| Total | 102.5 | 100 | 46.3 | 100 | 76.0 | 100 | 77.6 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmutz, C.; Will, F.; Varga, E.; Jaunecker, C.; Pahlke, G.; Berger, W.; Marko, D. In Vitro Inhibitory Potential of Different Anthocyanin-Rich Berry Extracts in Murine CT26 Colon Cancer Cells. Molecules 2023, 28, 7684. https://doi.org/10.3390/molecules28237684
Schmutz C, Will F, Varga E, Jaunecker C, Pahlke G, Berger W, Marko D. In Vitro Inhibitory Potential of Different Anthocyanin-Rich Berry Extracts in Murine CT26 Colon Cancer Cells. Molecules. 2023; 28(23):7684. https://doi.org/10.3390/molecules28237684
Chicago/Turabian StyleSchmutz, Cornelia, Frank Will, Elisabeth Varga, Carola Jaunecker, Gudrun Pahlke, Walter Berger, and Doris Marko. 2023. "In Vitro Inhibitory Potential of Different Anthocyanin-Rich Berry Extracts in Murine CT26 Colon Cancer Cells" Molecules 28, no. 23: 7684. https://doi.org/10.3390/molecules28237684
APA StyleSchmutz, C., Will, F., Varga, E., Jaunecker, C., Pahlke, G., Berger, W., & Marko, D. (2023). In Vitro Inhibitory Potential of Different Anthocyanin-Rich Berry Extracts in Murine CT26 Colon Cancer Cells. Molecules, 28(23), 7684. https://doi.org/10.3390/molecules28237684